Abstract
Objectives
STRIDE assessed whether a lifestyle intervention, tailored for individuals with serious mental illnesses, reduced weight and diabetes risk.
Methods
A multi-site, parallel, two-arm randomized controlled trial in community settings and an integrated health plan. Inclusion criteria: Age ≥18; taking antipsychotic medication for ≥30 days; BMI ≥27. Exclusions: significant cognitive impairment; pregnancy/breastfeeding; recent psychiatric hospitalization, bariatric surgery, cancer, heart attack or stroke. The intervention emphasized moderate caloric reduction, DASH diet, and physical activity. Blinded staff collected data at baseline, 6, and 12 months.
Results
Participants (56 men, 144 women), mean age = 47.2(SD =10.6), were randomized to usual care (n =96) or a 6-month weekly group intervention plus 6 monthly maintenance sessions (n =104). 181 participants (90.5%) completed 6-month, and 170 (85%) completed 12-month assessments, without differential attrition. Participants attended 14.5 of 24 sessions over 6 months. Intent-to-treat analyses found intervention participants lost 4.4 kg more than control participants from baseline to 6 months (95% CI [−6.96 kg, −1.78 kg]), and 2.6 kg more than controls (95% CI −5.14 kg, −0.07 kg] from baseline to 12 months. At 12 months, fasting glucose levels in controls had increased from 106.0 mg/dL to 109.5 mg/dL and decreased in intervention participants, from 106.3 mg/dL to 100.4 mg/dL. No serious adverse events were study-related; medical hospitalizations were reduced in the intervention group (6.7%) compared to controls (18.8%)(χ2= 6.66, p = 0.01).
Conclusions
Individuals taking antipsychotic medications can lose weight and improve fasting glucose levels. Increasing reach of the intervention is an important future step.
Funding Source
National Institute of Diabetes and Digestive and Kidney Diseases, Grant R18DK076775
Trial Registration
Clinical Trials.gov, NCT00790517; http://clinicaltrials.gov/ct2/show/NCT00790517?term=STRIDE&rank=1
Individuals with serious mental illnesses (SMI) are at high risk of common medical comorbidities and metabolic disturbances that lead to early morbidity and mortality (1–3) and are attributable to obesity-related conditions and risk factors (1, 4, 5). Additional contributing factors include metabolic consequences of antipsychotic medications (6, 7), limited access to medical care (8), poor nutrition (9), hyperlipidemia (10), sedentary lifestyles (5), smoking (11), and substance abuse (12).
Lifestyle-modification programs (13, 14) are the basis for recent efforts to assist individuals with SMI in improving health and reducing cardiometabolic risks (15, 16). These programs apply behavioral approaches to weight loss and management, including education and behavioral self-management skills (17). While most focus on weight loss, reductions have typically been modest (16). We are unaware of programs that have effectively reduced diabetes risk among people with SMI. Given the burden of medical morbidity and premature mortality in this group, methods for reducing obesity and cardiometabolic risk factors are urgently needed.
We evaluated a comprehensive lifestyle intervention (STRIDE) for individuals taking antipsychotic medications. STRIDE was based on the PREMIER lifestyle intervention with DASH dietary pattern (18–24), both developed for people without mental illnesses. PREMIER successfully reduces weight and blood pressure (25); DASH diet can increase HDL cholesterol, lower triglycerides, reduce fasting blood glucose levels, and improve insulin resistance (23, 26). We hypothesized the STRIDE intervention would be more effective than usual care in reducing weight and improving glucose metabolism.
Method
Study Design
STRIDE was a multi-site, parallel, two-arm (balanced 1:1), randomized controlled trial implemented in community mental health centers (CMHCs) and a not-for-profit integrated health plan. A description of the protocol is available elsewhere (27).
We included adults (age ≥18) taking antipsychotic agents for ≥30 days prior to enrollment and BMIs ≥27. Planned BMI inclusion criteria (>25 to <45) were adjusted after pilot results (24) and following safety consultations with clinicians after individuals with BMIs over 44.9 asked to participate. Study exclusion criteria included: current or planning pregnancy/breastfeeding; inpatient psychiatric hospitalization within ≤30 days (deferred participation allowed); history or planning bariatric surgery; history of cancer (prior two years); heart attack or stroke within 6 months; and cognitive impairment that might interfere with consenting/participation.
All sites and procedures were reviewed, approved, and monitored by the Kaiser Permanente Northwest (KPNW) Institutional Review Board (IRB).
Settings
The study took place in Pacific Northwest CMHCs (Cascadia Behavioral Healthcare [Cascadia] and LifeWorks Northwest [LifeWorks]) and a not-for-profit integrated health system (KPNW). All settings provide comprehensive mental health and addiction treatment; KPNW also provides medical care. Most individuals served by Cascadia and LifeWorks are low-income. KPNW’s membership is insured and demographically representative of the surrounding metropolitan area.
Recruitment, Screening, and Randomization
Recruitment began in July 2009 and ended in August 2011; the trial ended when follow-up visits were completed in October 2013. Using electronic medical records at two sites (KPNW, Cascadia) and clinician referral at LifeWorks, we identified potential participants based on medication use, diagnoses, and BMI (when available). We sent letters for each potential participant to primary care or psychiatry providers to review for suitability/safety and to co-sign if participation was deemed appropriate. Staff mailed recruitment letters and telephoned to answer questions and conduct a brief screening. Eligible participants were scheduled for full screening and orientation.
Potential participants attended a group orientation session and consented to height and weight measurements. Staff reviewed inclusion criteria to ensure eligibility before requesting full written consent. The second visit included a fasting blood sample, blood pressure and waist circumference measurement, and randomization.
Participants were assigned to intervention or usual-care using a stratified blocked (on gender and BMI [27–34.9 and ≥35]) randomization procedure, within sites. We used computer and paper-based randomization systems; sequence generated by author NAP. Staff not involved in data collection informed participants about randomization. Others were blinded to assignment and participants were routinely reminded not to discuss assignment during assessments. Usual-care participants were free to pursue alternative weight-loss efforts.
Staff informed participants of blood pressure and laboratory results and referred them to primary care if outside normal ranges. If results indicated immediate danger, participants were instructed to go to urgent care or visit clinicians immediately. At baseline, 31 participants (15.5%) were referred for blood pressure above 120 mmHg systolic or 80 mmHg diastolic (urgent = systolic ≥ 220 mmHg or diastolic ≥ 120 mmHg); 89 (44.5%) were referred for triglyceride levels >150 (urgent if >400); 44 (22%) were referred for fasting glucose levels ≥100 (urgent if >125 without diabetes mellitus diagnosis or >300 with diagnosis); and 35 (17.5%) for cholesterol levels >200. No cholesterol levels were considered urgent.
Intervention
We based STRIDE on the PREMIER lifestyle intervention with DASH diet (19, 21), and guidelines for obesity treatment for individuals at risk for cardiovascular disease (13). STRIDE was designed to reduce weight and obesity-related risks through dietary changes, moderate calorie restriction, and increased energy expenditure via moderate physical activity. The goal was weight loss of 4.5–6.8 kg (10–15 pounds) over 6 months. Adaptations made to tailor intervention content and implementation approaches for people with SMI included using two facilitators (mental health counselor, nutritional interventionist) and managing cognitive barriers by using repetition, multiple teaching modalities (e.g., verbal, visual), skill building exercises, practice assignments, and tying materials to mental health. Added sessions addressed effects of psychiatric medications on weight, planning for psychiatric symptom exacerbation, improving sleep, eating healthfully on a budget, and stress management. We also increased tailoring for individual and group needs and case management (28). Intervention materials are available for download: http://www.kpchr.org/stridepublic/.
Initial Intervention
STRIDE’s core was a series of weekly 2-hour group meetings with 20 minutes of physical activity, delivered over 6 months. Participants were taught to keep records of 1) food, beverages, and calories consumed; 2) servings of fruits, vegetables, and low-fat dairy products; 3) fiber and fat intake; 4) daily minutes exercised; and 5) nightly hours slept. Goals included: ≥25 minutes of moderate physical activity per day, primarily through walking, increased fruit, vegetable and low-fat dairy consumption, and improved sleep quality. Food and other monitoring records were used to assess progress and identify barriers to lifestyle change. Interventionists reviewed records to help participants evaluate and modify goals and plans. Participants received a workbook, the Calorie King book (29), and a resistance band for strength training. The intervention relied on engaging sessions and small-group activities to facilitate acquisition and practice of behavioral self-management, problem-solving skills, and to foster social support and program ownership. Core components included: increasing awareness of health-related practices through self-monitoring; creating personalized plans; reducing energy intake by reducing portions; increasing consumption of low-calorie density foods; increasing physical activity; managing high-risk eating situations; graphing progress; and addressing effects of mental health on change efforts.
Maintenance intervention
The maintenance phase included 6 months of group sessions focused on maintaining weight loss through problem-solving and motivational enhancement. Sessions were supplemented with monthly individual telephone sessions with group leaders. Contacts were collaborative, discussed lifestyle change efforts, and included guided problem-solving.
Assessment, Data Collection, and Measurement
Blinded staff collected assessment data at baseline, 6, and 12 months. Height was measured to the nearest .1 cm (baseline only) and weight to the nearest .1 kg; BMI was calculated using the Quetelet index (kg/m2). Blood samples were collected after a minimum 8-hour fast (we used reminder post-cards and telephone calls, then questioned participants about consumption prior to obtaining samples). Those not fasting were rescheduled. Fasting tests included: insulin, plasma glucose, triglycerides, and cholesterol. Blood pressure was measured after a 5-minute rest period and again after an additional 30-second rest. Measurement protocols and questionnaires are described elsewhere (27).
Statistical Analyses
We examined distributions for outcomes to determine whether transformations or different models were needed. We used generalized estimating equations (GEE) (30) for primary analyses because they allow estimation of population-averaged models in repeated-measures data. Time was dummy coded and models run switching the reference category from baseline (first model) to 6 months (second model) to obtain effect estimates for each period. Control vs. intervention interaction terms assessed changes between groups over time; Wald tests determined whether joint effects of time-by-group equaled zero (omnibus tests for interactions). Age and study site were included as time-invariant covariates; time-varying covariates included whether or not outcome-related medications were being taken at a given time (see supplementary tables of medications included in analyses). We used GEE models with a normal distribution and identity link; the working covariance matrix was specified as exchangeable. We report covariate-adjusted results using robust estimates of standard errors (unadjusted results available as supplementary material).
We conducted sensitivity analyses for each outcome using transformations that improved the normality of the outcome, a different family and link (e.g., negative binomial with log link) where appropriate, and unstructured working covariance matrices. For time-varying covariates, we also ran models that specified them as time-invariant (i.e., baseline only). In most cases, there were no substantive differences among models; we report differences below. Covariates in all analyses included age, site, medications known to affect outcomes, and treatment referral.
We examined between-group differences for percentage weight change, proportion of participants at or below baseline weight, proportion of participants who lost at least 5% or 10% of baseline weight and proportion of participants who had fasting glucose values <100. In contrast to other analyses, these were not intent-to-treat results because computations require complete data to compute change scores. We then tested differences in percentage weight change between intervention and control groups using one-way ANCOVAs, and used multiple logistic regression to test whether the proportion of participants at or below baseline weight at follow-up differed between intervention and control groups. We also examined Pearson correlations between attendance, food and sleep logs kept, and weight and glucose change at 6 months, for participants with complete data.
Sample Size
Using effect size estimates based on PREMIER (25), a two-tailed alpha level of .05, and a target sample size of 252 participants, we estimated 96% power to detect a time-by-group effect on weight at 6 months; 87% power at 12 months (27). We experienced recruitment difficulties, including one CMHC that significantly downsized, and lack of interest in physical health among some patients and providers (28), resulting in 200 enrolled participants. Using the same a priori effect-size estimates, 200 participants provided 91% power to detect a weight change at 6 months and 77% power to detect change at 12 months.
Results
Participants
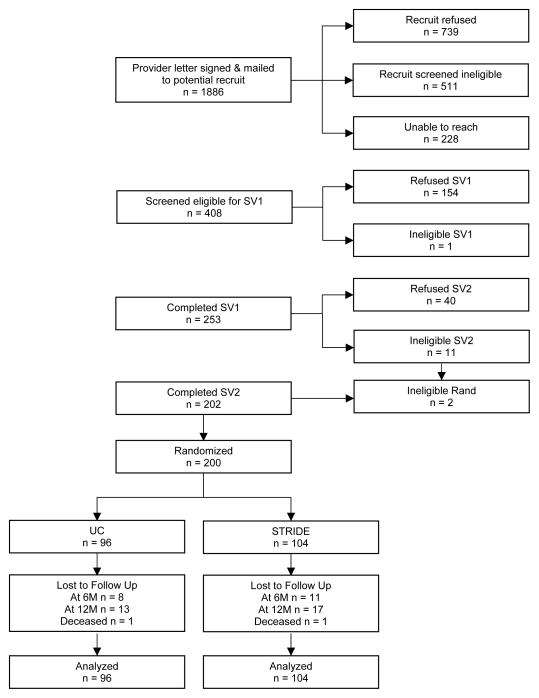
Research project staff sent 1,886 letters to potential participants, followed with telephone calls. The most common reasons for refusal were lack of interest in weight loss, scheduling conflicts, or perceptions that the intervention required too much time. The most common reasons for ineligibility were BMI below threshold or not taking antipsychotic medications. Four-hundred-and-eight (21.6%) passed a preliminary eligibility screen and scheduled an orientation/screening visit— 253 (62%) attended. Of these, 202 completed both screening visits and 200 individuals aged ≥18 (mean age = 47.2, SD = 10.6) were enrolled and randomized: 96 to usual care and 104 to intervention (56 men and 144 women). Figure 1 shows participant flow; Table 1 shows demographic and descriptive information.
Figure 1.
Study flow and full disposition of potential and randomized participants.
Table 1.
Baseline Characteristics.
| Characteristic | Total (N = 200) | Intervention Group (n = 104) | Control Group (n = 96) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Mean | SD | Mean | SD | Mean | SD | |
|
| ||||||
| Age, years | 47.2 | 10.6 | 46.2 | 11.4 | 48.3 | 9.7 |
|
| ||||||
| Weight, kg | 107.7 | 25.1 | 108.6 | 27.2 | 106.6 | 22.7 |
|
| ||||||
| Body-mass index | 38.3 | 8.3 | 38.3 | 9.1 | 38.2 | 7.3 |
|
| ||||||
| Female waist circumference, cm | 114.5 | 19.2 | 114.6 | 20.5 | 114.4 | 17.7 |
|
| ||||||
| Male waist circumference, cm | 112.4 | 17.5 | 113.8 | 19.6 | 110.9 | 15.1 |
|
| ||||||
| Systolic blood pressure, mmHg | 119.2 | 14.7 | 117.5 | 14.2 | 121.0 | 15.2 |
|
| ||||||
| Diastolic blood pressure, mmHg | 79.4 | 10.1 | 78.5 | 9.7 | 80.4 | 10.5 |
|
| ||||||
| Fasting triglycerides, mg/dL | 188.0 | 138.6 | 188.0 | 130.3 | 188.0 | 147.8 |
|
| ||||||
| Fasting LDL, mg/dL | 101.4 | 32.9 | 101.4 | 31.3 | 101.4 | 34.7 |
|
| ||||||
| Fasting HDL, mg/dL | 45.8 | 12.7 | 46.6 | 14.0 | 45.0 | 11.0 |
|
| ||||||
| Fasting total cholesterol, mg/dL | 181.6 | 39.7 | 183.2 | 38.7 | 179.9 | 40.9 |
|
| ||||||
| Fasting plasma glucose, mg/dL | 108.9 | 32.5 | 107.6 | 31.2 | 110.3 | 34.1 |
|
| ||||||
| Fasting insulin, μU/mL | 13.0 | 11.9 | 11.2 | 7.8 | 15.1 | 15.0 |
|
| ||||||
| No. of psychiatric medications | 3.2 | 1.5 | 3.1 | 1.4 | 3.3 | 1.5 |
|
| ||||||
| Modified Colorado Symptom Index score | 19.3 | 11.4 | 18.3 | 11.2 | 20.4 | 11.6 |
|
| ||||||
| BASIS-24 score | 1.37 | 0.68 | 1.29 | 0.70 | 1.47 | 0.64 |
|
| ||||||
| SF-36v2 General Health | 42.08 | 9.99 | 42.79 | 10.94 | 41.33 | 8.84 |
|
| ||||||
| N | % | n | % | n | % | |
|
| ||||||
| Female sex | 144 | 72.0 | 75 | 72.1 | 69 | 71.9 |
|
| ||||||
| Race | ||||||
|
| ||||||
| White | 174 | 87.7 | 90 | 88.2 | 81 | 87.1 |
|
| ||||||
| Non-white | 26 | 12.3 | 12 | 11.8 | 12 | 12.9 |
|
| ||||||
| Hispanic ethnicity | 4 | 2.0 | 3 | 2.9 | 1 | 1.1 |
|
| ||||||
| Education level | ||||||
| <high school graduate/GED | 15 | 7.5 | 6 | 5.8 | 9 | 9.4 |
| High school graduate/GED | 46 | 23.0 | 26 | 25.0 | 20 | 20.8 |
| Some college | 87 | 43.5 | 44 | 42.3 | 43 | 44.8 |
| College graduate | 52 | 26.0 | 28 | 26.9 | 24 | 25.0 |
|
| ||||||
| Never married | 57 | 28.5 | 37 | 35.6 | 20 | 20.8 |
|
| ||||||
| Currently working | 59 | 29.5 | 27 | 26.0 | 32 | 33.3 |
|
| ||||||
| Receiving disability income | 90 | 45 | 46 | 44.2 | 44 | 45.8 |
|
| ||||||
| Individual Monthly Income | 33 | |||||
| <$500 | 66 | 16.5 | 20 | 20.0 | 13 | 13.7 |
| $500–1000 | 36 | 33.8 | 35 | 35.0 | 31 | 32.6 |
| $1000–1499 | 17 | 18.5 | 20 | 20.0 | 16 | 16.8 |
| $1500–1999 | 18 | 8.7 | 6 | 6.0 | 11 | 11.6 |
| $2000–2499 | 25 | 9.2 | 8 | 8.0 | 10 | 10.5 |
| ≥$2500 | 12.9 | 11 | 11.0 | 14 | 14.8 | |
|
| ||||||
| Mental health diagnoses (from medical records) | ||||||
|
| ||||||
| Schizophrenia spectrum disorder | 58 | 29.0 | 31 | 29.8 | 27 | 28.1 |
|
| ||||||
| Bipolar disorder or affective psychosis | 138 | 69.0 | 71 | 68.2 | 67 | 69.8 |
|
| ||||||
| Post-traumatic stress disorder | 4 | 2.0 | 2 | 1.9 | 2 | 2.1 |
|
| ||||||
| Current Medications | ||||||
|
| ||||||
| Blood pressure medications | 59 | 29.5 | 30 | 28.8 | 29 | 30.2 |
|
| ||||||
| Diabetes medications | 30 | 15.0 | 14 | 13.5 | 16 | 16.7 |
|
| ||||||
| Cholesterol medications | 49 | 24.5 | 27 | 26.0 | 22 | 22.9 |
|
| ||||||
| Atypical antipsychotic medications | 182 | 91.0 | 95 | 91.3 | 87 | 90.6 |
|
| ||||||
| Lithium or anticonvulsant medications | 97 | 48.5 | 51 | 49.0 | 46 | 47.9 |
|
| ||||||
| Antidepressant medications | 33 | 16.5 | 15 | 14.4 | 18 | 18.8 |
|
| ||||||
| Current psychiatric medications classified according to weight loss/gain profile (see supplementary materials for details of medications included in each category) | ||||||
|
| ||||||
| 1+ slight/moderate weight loss | 77 | 38.5 | 39 | 37.5 | 38 | 39.6 |
|
| ||||||
| 1+ weight neutral | 168 | 84.0 | 87 | 83.7 | 81 | 84.4 |
|
| ||||||
| 1+ slight/moderate weight gain | 21 | 10.5 | 10 | 9.6 | 11 | 11.5 |
|
| ||||||
| 1+ severe weight gain | 128 | 64.0 | 68 | 65.4 | 60 | 62.5 |
Study Retention and Intervention Attendance
Follow-up data collection was completed for 91% of participants (n = 181) at 6 months, and 85% (n = 170) at 12-months, with no differential attrition (χ2 = 0.01, p=.995). Missing data were thus unlikely to be conditional on group assignment. Average sessions attended during the initial intervention was 14.5 (SD=7.2) of 24 (60.2%) among intervention participants; average maintenance sessions attended was 2.7 (SD= 2.17) of 6 (44.5%).
Analyses
Table 2 presents adjusted time-by-group coefficients and confidence intervals for intent-to-treat analyses.
Table 2.
Adjusted results of intervention on primary study outcomes
| Δ Baseline-6 mo. | Δ 6 mo.-12 mo. | Δ Baseline-12 mo. | |||||
|---|---|---|---|---|---|---|---|
| Coef. a | 95% CI (lower, upper) | Coef. | 95% CI (lower, upper) | Coef. | 95% CI (lower, upper) | p valueb | |
| Weight, kg | −4.37 | −6.96, −1.78 | 1.77 | −0.87, 4.40 | −2.60 | −5.14, −0.07 | 0.004 |
| BMI, kg/m2 | −1.55 | −2.47, −0.63 | 0.58 | −0.35, 1.50 | −0.97 | −1.88, −0.06 | 0.004 |
| Systolic blood pressure, mmHg | −1.60 | −5.21, 2.02 | 1.68 | −1.91, 5.27 | 0.09 | −3.36, 3.53 | 0.5960 |
| Diastolic blood pressure, mmHg | −1.21 | −3.58, 1.17 | 0.82 | −1.59, 3.23 | −0.38 | −2.89, 2.12 | 0.590 |
| Fasting Glucose, mg/dL | −0.02 | −0.08, 0.05 | −0.08 | −0.14, −0.01 | −0.09 | −0.16, −0.02 | 0.020 |
| Fasting insulin, μU/mL | 0.11 | −0.09, 0.31 | −0.12 | −0.58, 0.34 | −0.01 | −0.46, 0.43 | 0.560 |
| HOMA-IRc | 0.09 | −0.12,0.30 | −0.15 | −0.37,0.07 | −0.24 | −0.48, 0.01 | 0.163 |
| Diabetes riskd | 0.17 | −1.55, 1.89 | −1.56 | −3.39, 0.26 | −1.39 | −3.09, 0.31 | 0.171 |
| Fasting Triglycerides, mg/dL | 3.72 | −21.57, 29.01 | 0.67 | −21.52, 22.86 | 4.39 | −24.18, 32.96 | 0.949 |
| Fasting LDL, mg/dL | −1.65 | −8.52, 5.22 | 1.68 | −5.25, 8.61 | 0.03 | −7.58, 7.64 | 0.852 |
| Fasting HDL, mg/dL | 1.23 | −0.70, 3.16 | 1.05 | −1.14, 3.24 | 2.28 | −0.14, 1.05 | 0.172 |
Coef=Coefficient for the time-by-group indicators estimated from the GEE models.
Omnibus Wald test assessing whether the joint effect of the time-by-group indicators = 0.
The Homeostasis Model Assessment Index for Insulin Resistance (HOMA-IR) is calculated as follows: fasting glucose [mmol/L] x fasting insulin [μU/mL]/22.5. Lower scores indicate lower risk for developing insulin resistance. Coefficients represent the change in the natural log of the HOMA-IR index.
Based on the Framingham Diabetes Risk Scale. Lower scores represent decreased risk of developing diabetes.
Primary Outcomes
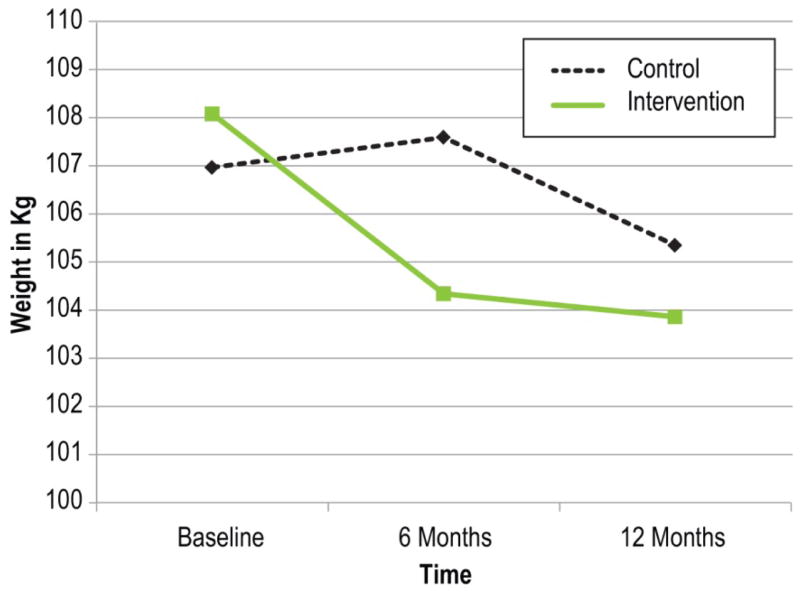
There was a significant time-by-group effect for weight and BMI. The intervention group lost 4.4 kg more than the control group (95% CI [−6.96 kg, −1.78 kg] at 6 months, and 2.6 kg more than the control group 95% CI [−5.14 kg, −0.07 kg] at 12 months. As expected, there was no significant difference in weight change between the groups (1.77 kg, 95% CI [−0.87 kg, 4.40 kg]) during maintenance (6–12 months). Figure 2 and Figure 3 show estimated marginal means; results for BMI parallel weight results.
Figure 2.
Adjusted mean weights of intervention and control groups from baseline to 12 months
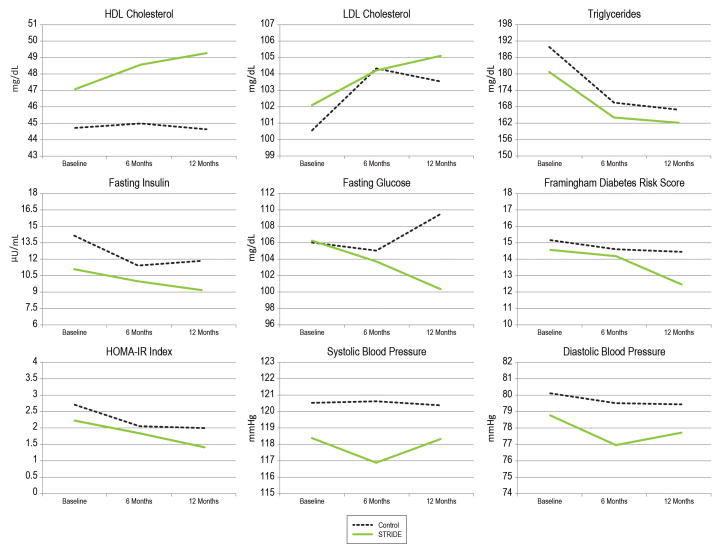
Figure 3.
Adjusted mean outcomes of intervention and control groups from baseline to 12 months.
Among participants with complete data at baseline and 6-month follow-up, the intervention group (n=93) lost an average of 3.9%, and the control group (n=85) gained 0.9%, of their baseline weight F(1,171)=11.9, p=.001. From baseline to 12 months, the intervention group (n=87) lost a greater percentage of their baseline weight (4.5%) than the control group (n=81; 1.7%), F(1,161)=4.9, p=.029. There was marginal evidence that the intervention group was more likely to be at or below their baseline weight at 6 months (odds ratio = 1.69; 95% CI [0.91, 3.14], p=.096) and 12 months (odds ratio = 1.88; 95% CI [0.98, 3.64], p=.059), compared to the control group. The intervention group had 3.78 times greater odds (95% CI [1.82, 7.84], p<.001) of more than 5% loss of baseline weight by 6 months compared to controls—40% of intervention participants achieved at least 5% of baseline weight loss compared with 17% of controls. This effect was not significant at 12 months (odds ratio = 1.64; 95% CI [0.87, 3.08], p=.124), although nearly half (47%) of intervention participants with complete data lost at least 5% of baseline body weight, compared with 36% of controls. At 6 months, the intervention group had 5.14 times greater odds of achieving ≥10% loss of baseline weight than controls (95% CI [1.62, 16.30], p=.005). At 12 months, the intervention group had 3.08 times greater odds of a ≥10% weight loss (95% CI [1.20, 7.91], p=.019). At 6 months, 18% of intervention participants and 5% of control participants had lost at least 10% of baseline body weight, while 22% of intervention participants and 9% of controls met this threshold at 12 months. Consistent with findings of weight reduction in both groups, 21.5% of intervention participants reported additional weight loss activities, compared to weight loss efforts among 41.7% of controls (χ2=8.38, p=0.004).
Distributions for fasting glucose, insulin, and HOMA-IR index were positively skewed, thus we fitted log transformations for these outcomes using a Gaussian-based GEE model, and a GEE model using the negative binomial distribution and log link (except for HOMA). For fasting glucose and insulin, we report results of the negative binomial GEE. There were no significant time-by-group interactions for fasting insulin, Framingham Diabetes Risk Score, or HOMA-IR. There was, however, a significant time-by-group interaction for fasting glucose (p=.020). From baseline to 12 months, the intervention group showed a greater decline (log of the incidence rate ratio = −.089, p=.012) compared to controls. Fasting glucose among controls increased from 106.0 mg/dL at baseline to 109.5 mg/dL at 12 months, whereas the intervention group declined from 106.3 mg/dL at baseline to 100.4 mg/dL at 12 months. The difference in change from 6 months to 12 months was also significant (log of incidence rate ratio=−.075, p=.016). Controls increased from 105.1 mg/dL at 6 months to 109.5 mg/dL at 12 months, whereas the intervention group declined from 103.7 mg/dL at 6 months to 100.4 mg/dL at 12 months. Difference in change from baseline to 6 months was not significant (p=.64). The proportion of control arm participants who had fasting glucose values <100 at baseline, 6 months, and 12 months were .45, .46, and .42, respectively, and in the intervention arm .59, .60, and .68, respectively. While there was no difference in the proportion of participants with glucose <100 at 6 months (p=.59), participants in the intervention had 2.39 (95% CI [1.12, 5.12]) times greater odds of glucose<100 at 12 months compared to controls (p=.025).
Secondary Outcomes
Changes in systolic and diastolic blood pressure from pre- to post-intervention were not significant, likely because average values were within normal ranges at baseline. Time-by-group interactions were not significantly different for triglycerides, LDL, or HDL cholesterol, although average LDL cholesterol was also within normal range at baseline. Correlations between changes in weight at 6 months and food logs kept (r = −.45, p < .001), sleep logs kept (r = −.39, p < .001) and number of sessions attended (r = −.43, p < .001) were significant. The greater the number of food and sleep logs kept and the higher the attendance, the greater the weight loss. No significant correlations were found between logs or attendance and glucose levels.
Acute Service Use and Adverse Events
There were significantly fewer medical hospitalizations in intervention than control arms over the 12-month period: 6.7% of intervention participants reported medical hospitalizations compared to 18.8% of controls (χ2= 6.66, p = 0.01). There were no differences in psychiatric hospitalizations: 15.6% of control participants vs. 15.4% of intervention participants had hospitalizations (χ2= 0.97, p = 0.32). There were no differences in emergency department visits that did not result in hospitalizations for either medical or psychiatric problems. There was one death in each arm, neither related to study participation.
Discussion
Our results support recent findings (14) suggesting that behavioral lifestyle-change programs can help individuals with SMI to lose weight, and extend these findings by showing that lifestyle interventions can produce changes in fasting glucose levels among individuals taking antipsychotic medications—drugs known to disrupt glucose metabolism (7, 31). Consistent with other interventions (16), STRIDE spurred clinically significant weight loss of ≥5% of initial body weight among 40% of participants. Weight loss of ≥10% was achieved by 18% and intervention participants were 2.39 times as likely as controls to have normal fasting glucose levels at 12 months. In addition, we observed substantially fewer medical hospitalizations in the intervention group than the control group. If these results are replicated, reduced hospital costs could be an added benefit of offering these interventions.
Our goal was to produce an average weight loss of 4.5–6.8kg (10–15 pounds), consistent with the original PREMIER intervention goal for people without SMI. STRIDE participants lost an average of 4.2 kg (9.3 pounds, adjusted means). Unadjusted means (for those with full data only) showed losses of 5.8 kg (12.8 pounds) in the intervention group. Participants in the PREMIER intervention, DASH diet arm, lost an average of 4.7 kg more than the control group, while STRIDE intervention participants lost 4.4 kg more than the control group. The similarity of the STRIDE and PREMIER outcomes is remarkable given known barriers to weight loss among individuals with SMI. Moreover, STRIDE participants were heavier than PREMIER participants at baseline, with BMIs of 38.3 and 33.6, respectively. Thus, individuals in STRIDE needed to lose many more pounds to achieve a “clinically significant” 5% loss compared to PREMIER participants. Control-group participants also lost weight, although much less than intervention participants. This likely stems from referrals to primary care following study assessments for at-risk values, and because individuals who joined the trial were motivated to lose weight and attempted to do so after assignment to the control group, including using other formal weight-loss methods and programs.
Our results parallel ACHIEVE study results (32) and are consistent with those of other randomized controlled trials in showing positive results of weight-loss interventions in this population (16). Other than ACHIEVE, however, RCTs assessing similar lifestyle interventions in similar populations have been of short duration (e.g., 12–16 weeks), so are not directly comparable. The In SHAPE program (33) was similar in target population, length and intensity, but the intervention was focused on exercise (12 months of weekly meetings with a fitness trainer and fitness club membership). In SHAPE was associated with a clinically significant reduction in cardiovascular risk in 49% of participants and produced improvements in fitness and diet, but not in weight when compared to an active control consisting of a health club membership and fitness education. In another study of similar length and intensity, Wu and colleagues (34) implemented a 6-month diet and exercise program for obese adults with schizophrenia taking clozapine, reporting 6-month weight loss quite similar (−4.2 kg) to what we found in STRIDE, but under highly controlled inpatient circumstances.
Thus, the most useful comparisons for STRIDE results are with ACHIEVE, which implemented a similar lifestyle intervention in an outpatient population. A notable difference, however, was that the setting for ACHIEVE was within psychiatric rehabilitation programs that participants attended for several hours daily. The intervention capitalized on the setting by including group weight-management and exercise sessions, and individual sessions as part of daily programming. Additionally, programs routinely provided two meals/day for participants, and researchers worked with staff to include more healthy offerings. ACHIEVE participants lost weight steadily over 18 months, with an average loss of 3.4 kg. In contrast, STRIDE participants traveled to stand-alone groups weekly, achieved a 4.4 kg loss over six months, but gained some of this weight back during the maintenance phase. Process evaluation data suggested that STRIDE participants who were engaged in the intervention wanted the weekly contact to continue, and the relationship between keeping food and sleep logs and greater weight loss indicate that increasing the length of the more intensive intervention could be beneficial. ACHIEVE results support this contention, showing that sustained support can result in continued lifestyle improvements. This may indicate that providing access to STRIDE for longer periods could result in substantial additional improvements in weight and cardiometabolic outcomes. Observed reductions in fasting glucose, and trends toward improvements in several other outcomes (fasting insulin, HOMA-IR, Framingham Diabetes Risk, HDL cholesterol), are consistent with this conclusion. Moreover, STRIDE study subjects had average baseline fasting glucose levels of 109mg/dl (35), and were therefore similar to subjects enrolled in diabetes prevention trials with multi-year lifestyle interventions (36, 37). Long-term follow up in these trials has shown sustained diabetes risk-reduction (35, 38, 39).
Limitations and Opportunities
Our results and the study’s limitations suggest opportunities for improving intervention and outcomes. First, attendance during the initial intervention was lower than desired (about 60%). Although this is similar to ACHIEVE attendance (32), and not unexpected given instability in the lives of people with SMI, it represents a study limitation, an implementation challenge, and an opportunity to improve outcomes (17), particularly given the relationship we found between greater attendance and weight loss. Reach of the intervention was also a limitation: Average age of participants was ~47 years, thus health-related risks were well-established; only 28% of participants were men; and only 14% were members of racial or ethnic minority groups despite efforts to recruit equal numbers of men and women and to oversample minority group members. Although this pattern is typical of lifestyle change programs (25, 33, 40, 40, 41), it nevertheless suggests that special efforts are needed to make interventions more appealing to these groups. In terms of design limitations, although this was an RCT (with the advantage of achieving balance on unmeasured covariates compared to other research designs), we neither measured nor controlled for medical severity or comorbidity, as these data were not available from participating CMHCs and beyond our capacity to measure at study assessments.
Conclusions
Individuals taking antipsychotic medications can lose significant amounts of weight and improve fasting glucose levels in a tailored comprehensive weight-loss and lifestyle-change program. Increasing the length of the intervention and the number of sessions attended holds potential to support additional weight loss and glucose control and address other cardiometabolic risk factors. Increasing the reach of the intervention is an important step in advancing research on health interventions for people with serious mental illnesses.
Supplementary Material
Patient Perspectives.
Interviewed participants reported that helpful features included camaraderie and support resulting from shared mental health experiences and health-related goals:
I really enjoyed the group setting. And I could sit next to anybody in the class and be perfectly comfortable. Because we all shared this kind of common mental health issue… I really liked the support… You know, how did you do this week? What were your successes? What were your failures? The part I didn’t like was when the group setting ended. That was hard for me. I tried to go out and find another group…like Weight Watchers, and I couldn’t find a group that I clicked with. So it was really frustrating to have that camaraderie and then lose it.
Also appreciated was support of self-determination to make broad lifestyle changes:
I thought it was wonderful, because it didn’t box you in that you had to do anything rigid. It stressed lifestyle changes… I felt no immediate pressure that I had to lose forty pounds or fifty pounds in a year… The offering was there: We’re here to help you. And so what I decided to do made the difference, so I got committed because I decided to do it.
Some aspects of participation were easier than others:
…one thing that was also hard…being weighed weekly and being reminded weekly that… you go two, three, four weeks where there’s no change, or you might have went up …That’s really hard.
_______
…the thing that just bothers me right now is…the daily journal. It’s gotten to be a bit of a grind after awhile…but I can see where I’m going with what I’ve been eating, counting up the calories. [Is that helpful to you?] Yeah. Real helpful.
Acknowledgments
Disclosures and Acknowledgements
Dr. Green has received grant support from NIH, NIDA, NIMH, NIDDK, AHRQ, Purdue Pharma, the Kaiser Permanente Center for Safety and Effectiveness Research, and the Kaiser Permanente Community Benefit Initiative. Dr. Green has also provided research consultation for the Industry PMR, a consortium of nine companies who are working together to conduct FDA-required post-marketing studies that assess known risks related to extended-release, long-acting opioid analgesics. The Industry PMR consortium is comprised of Pfizer, Purdue Pharma, Roxane Laboratories, Janssen Pharmaceuticals, Mallinckrodt, Actavis, Endo Pharmaceuticals, Rhodes Pharmaceuticals, and Zogenix. Dr. Yarborough has received grant support from NIDA, NIMH, NIDDK, NCCAM, AHRQ, the Kaiser Permanente Center for Safety and Effectiveness Research, Purdue Pharma, and the Kaiser Permanente Community Benefit Initiative. Dr. Leo has received grant support from the NIH, NIMH, the American Cancer Society, National Cancer Institute, Robert Wood Johnson Foundation, National Institute of Nursing Research, American Heart Association, NHLBI, NIDDK, the Veterans Administration, NIDCR, NICHD, Amgen, HRSA, NCCAM, Pfizer, and GenomeDx. Mr. Yarborough has received grant support from NIMH, NIDDK, Purdue Pharma, and the Kaiser Permanente Community Benefit Initiative. Mr. Stumbo has received grant support from the Health Resources & Services Administration, Maternal and Child Health Bureau, the Lucile Packard Foundation for Children’s Health, NCCAM, AHRQ, Purdue Pharma, the Kaiser Permanente Community Benefit Initiative, NIDDK, and NIMH. Ms. Janoff has received grant support from NIMH, NIDDK, NIDA, AHRQ, the Kaiser Permanente Center for Safety and Effectiveness Research, and Purdue Pharma. Dr. Perrin has received grant support from NIMH, NIDDK, NIDA, NCCAM, AHRQ, NICHD, NHLBI, NCMHD, the Veterans Administration, NCI, CDC, NINR, NIA, Kaiser Permanente Northwest, NIOSH, Merck & Co., the Kaiser Permanente Community Benefit Initiative, and Purdue Pharma. Dr. Nichols has received grant support from AHRQ, NIH, NIDDK, NHLBI, AstraZeneca, Bristol-Myers Squibb, Novartis Pharmaceuticals, GlaxoSmithKline, and Merck & Co. Dr. Stevens has received grant support from NHLBI, NIDDK, NICHD, AHRQ, NCI, NCRR, NIH, NCCAM, and Kaiser Permanente Northwest.
Funding for this study was provided by the National Institute of Diabetes and Digestive and Kidney Diseases, Grant R18DK076775, entitled “Reducing Weight and Diabetes Risk in an Underserved Population.”
The authors would like to thank our scientific collaborators and the clinical and project management staff that supported this trial. Without their efforts, this project would not have been possible. We would also like to thank STRIDE participants, who gave their precious time and effort.
Footnotes
Previous Presentations or Publications: None
Contributor Information
Carla A. Green, Email: carla.a.green@kpchr.org.
Bobbi Jo H. Yarborough, Email: bobbijo.h.yarborough@kpchr.org.
Michael C. Leo, Email: michael.c.leo@kpchr.org.
Micah T. Yarborough, Email: micah.yarborough@kpchr.org.
Scott P. Stumbo, Email: scott.p.stumbo@kpchr.org.
Shannon L. Janoff, Email: shannon.l.janoff@kpchr.org.
Nancy A. Perrin, Email: nancy.perrin@kpchr.org.
Greg A. Nichols, Email: gregory.nichols@kpchr.org.
Victor J. Stevens, Email: victor.j.stevens@kpchr.org.
Reference List
- 1.Parks J, Svendsen D, Singer P, Foti ME, editors. National Association of State Mental Health Program Directors (NASMHPD) Medical Directors Council. Morbidity and mortality in people with serious mental illness. Alexandria, VA: 2007. [Google Scholar]
- 2.Kilbourne AM, Cornelius JR, Han X, Pincus HA, Shad M, Salloum I, Conigliaro J, Haas GL. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 2004;6:368–373. doi: 10.1111/j.1399-5618.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 3.Druss BG, Zhao L, Von ES, Morrato EH, Marcus SC. Understanding excess mortality in persons with mental illness: 17-year follow up of a nationally representative US survey. Med Care. 2011;49:599–604. doi: 10.1097/MLR.0b013e31820bf86e. [DOI] [PubMed] [Google Scholar]
- 4.Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, Cope MB, Riley WT, Vreeland B, Hibbeln JR, Alpert JE. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36:341–350. doi: 10.1016/j.amepre.2008.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Kilbourne AM, Morden NE, Austin K, Ilgen M, McCarthy JF, Dalack G, Blow FC. Excess heart-disease-related mortality in a national study of patients with mental disorders: Identifying modifiable risk factors. Gen Hosp Psychiatry. 2009;31:555–563. doi: 10.1016/j.genhosppsych.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaggar PS, Shaw SM, Williams SG. Effect of antipsychotic medications on glucose and lipid levels. J Clin Pharmacol. 2011;51:631–638. doi: 10.1177/0091270010368678. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes: Consensus statement. Diabetes Care. 2004;27:596–601. doi: 10.2337/diacare.27.2.596. [DOI] [PubMed] [Google Scholar]
- 8.Kilbourne AM, McCarthy JF, Post EP, Welsh D, Pincus HA, Bauer MS, Blow FC. Access to and satisfaction with care comparing patients with and without serious mental illness. Int J Psychiatry Med. 2006;36:383–399. doi: 10.2190/04XR-3107-4004-4670. [DOI] [PubMed] [Google Scholar]
- 9.Casagrande SS, Anderson CA, Dalcin A, Appel LJ, Jerome GJ, Dickerson FB, Gennusa JV, Daumit GL. Dietary intake of adults with serious mental illness. Psychiatr Rehabil J. 2011;35:137–140. doi: 10.2975/35.2.2011.137.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D’Agostino RB, Stroup TS, Davis S, Lieberman JA. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80:45–53. doi: 10.1016/j.schres.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 11.LeCook B, Wayne G, Kafali E, Liu Z, Shu C, Flores M. Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA. 2014;311:172–182. doi: 10.1001/jama.2013.284985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickey B, Normand SL, Weiss RD, Drake RE, Azeni H. Medical morbidity, mental illness, and substance use disorders. Psychiatr Serv. 2002;53:861–867. doi: 10.1176/appi.ps.53.7.861. [DOI] [PubMed] [Google Scholar]
- 13.Jensen MD, Ryan DH, Apovian CM, Loria CM, Ard JD, Millen BE, Comuzzie AG, Nonas CA, Donato KA, Pi-Sunyer FX, Hu FB, Stevens J, Hubbard VS, Stevens VJ, Jakicic JM, Wadden TA, Kushner RF, Wolfe BM, Yanovski SZ. AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. J Am Coll Cardiol. 2013;2013 doi: 10.1016/j.jacc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Gierisch JM, Nieuwsma JA, Bradford DW, Wilder CM, Mann-Wrobel MC, McBroom AJ, Wing L, Musty MD, Chobot MM, Hasselblad V, Williams JW. Comparative Effectiveness Review No. 105. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-2007-10066-I.) AHRQ Publication No. 13-EHC063-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Interventions To Improve Cardiovascular Risk Factors in People With Serious Mental Illness. [PubMed] [Google Scholar]
- 15.Alvarez-Jimenez M, Hetrick SE, Gonzalez-Blanch C, Gleeson JF, McGorry PD. Non-pharmacological management of antipsychotic-induced weight gain: Systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry. 2008;193:101–107. doi: 10.1192/bjp.bp.107.042853. [DOI] [PubMed] [Google Scholar]
- 16.Bartels S, Desilets R. Health Promotion Programs for People with Serious Mental Illness (Prepared by the Dartmouth Health Promotion Research Team) Washington, D.C: SAMHSA-HRSA Center for Integrated Health Solutions; 2012. [Google Scholar]
- 17.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012;125:1157–1170. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elmer PJ, Obarzanek E, Vollmer WM, Simons-Morton D, Stevens VJ, Young DR, Lin PH, Champagne C, Harsha DW, Svetkey LP, Ard J, Brantley PJ, Proschan MA, Erlinger TP, Appel LJ. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 19.Funk KL, Elmer PJ, Stevens VJ, Harsha DW, Craddick SR, Lin PH, Young DR, Champagne CM, Brantley PJ, McCarron PB, Simons-Morton DG, Appel LJ. PREMIER--A trial of lifestyle interventions for blood pressure control: Intervention design and rationale. Health Promot Pract. 2006;9:271–280. doi: 10.1177/1524839906289035. [DOI] [PubMed] [Google Scholar]
- 20.McGuire HL, Svetkey LP, Harsha DW, Elmer PJ, Appel LJ, Ard JD. Comprehensive lifestyle modification and blood pressure control: A review of the PREMIER trial. J Clin Hypertens (Greenwich) 2004;6:383–390. doi: 10.1111/j.1524-6175.2004.03147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svetkey LP, Harsha DW, Vollmer WM, Stevens VJ, Obarzanek E, Elmer PJ, Lin PH, Champagne C, Simons-Morton DG, Aickin M, Proschan MA, Appel LJ. Premier: A clinical trial of comprehensive lifestyle modification for blood pressure control: Rationale, design and baseline characteristics. Ann Epidemiol. 2003;13:462–471. doi: 10.1016/s1047-2797(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 22.Obarzanek E, Sacks FM, Vollmer WM, Bray GA, Miller ER, III, Lin PH, Karanja NM, Most-Windhauser MM, Moore TJ, Swain JF, Bales CW, Proschan MA. Effects on blood lipids of a blood pressure-lowering diet: the Dietary Approaches to Stop Hypertension (DASH) Trial. Am J Clin Nurt. 2001;74:80–89. doi: 10.1093/ajcn/74.1.80. [DOI] [PubMed] [Google Scholar]
- 23.Ard JD, Grambow SC, Liu D, Slentz CA, Kraus WE, Svetkey LP. The effect of the PREMIER interventions on insulin sensitivity. Diabetes Care. 2004;27:340–347. doi: 10.2337/diacare.27.2.340. [DOI] [PubMed] [Google Scholar]
- 24.Green CA, Janoff SL, Yarborough BJ, Yarborough MT. A 12-Week weight reduction intervention for overweight individuals taking antipsychotic medications. Community Ment Health J. 2014 doi: 10.1007/s10597-014-9716-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Appel LJ, Champagne CM, Harsha DW, Cooper LS, Obarzanek E, Elmer PJ, Stevens VJ, Vollmer WM, Lin PH, Svetkey LP, Stedman SW, Young DR PREMIER Collaborative Research Group. Effects of comprehensive lifestyle modification on blood pressure control: Main results of the PREMIER clinical trial. JAMA. 2003;289:2083–2093. doi: 10.1001/jama.289.16.2083. [DOI] [PubMed] [Google Scholar]
- 26.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a dietary approaches to stop hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28:2823–2831. doi: 10.2337/diacare.28.12.2823. [DOI] [PubMed] [Google Scholar]
- 27.Yarborough BJ, Leo MC, Stumbo S, Perrin NA, Green CA. STRIDE: a randomized trial of a lifestyle intervention to promote weight loss among individuals taking antipsychotic medications. BMC Psychiatry. 2013;13:238. doi: 10.1186/1471-244X-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yarborough BJ, Janoff SL, Stevens VJ, Kohler D, Green CA. Delivering a lifestyle and weight loss intervention to individuals in real-world mental health settings: Lessons and opportunities. Transl Behav Med. 2011;1:406–415. doi: 10.1007/s13142-011-0056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borushek A. The Calorie King, Calorie, Fat, & Carbohydrate Counter 2013. Costa Mesa, Family Health Publications; 2012. [Google Scholar]
- 30.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 31.De Hert M, Detraux J, van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8:114–126. doi: 10.1038/nrendo.2011.156. [DOI] [PubMed] [Google Scholar]
- 32.Daumit GL, Dickerson FB, Wang NY, Dalcin A, Jerome GJ, Anderson CA, Young DR, Frick KD, Yu A, Gennusa JV, III, Oefinger M, Crum RM, Charleston J, Casagrande SS, Guallar E, Goldberg RW, Campbell LM, Appel LJ. A Behavioral Weight-Loss Intervention in Persons with Serious Mental Illness. N Engl J Med. 2013;17:1594–1602. doi: 10.1056/NEJMoa1214530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bartels SJ, Pratt SI, Aschbrenner KA, Barre LK, Jue K, Wolfe RS, Xie H, McHugo G, Santos M, Williams GE, Naslund JA, Mueser KT. Clinically significant improved fitness and weight loss among overweight persons with serious mental illness. Psychiatr Serv. 2013;64:729–736. doi: 10.1176/appi.ps.003622012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu MK, Wang CK, Bai YM, Huang CY, Lee SD. Outcomes of obese, clozapine-treated inpatients with schizophrenia placed on a six-month diet and physical activity program. Psychiatr Serv. 2007;58:544–550. doi: 10.1176/ps.2007.58.4.544. [DOI] [PubMed] [Google Scholar]
- 35.American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2014;37:s81–s90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 36.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM The Diabetes Prevention Program (DPP) Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 38.Lindstrom J, Ilanne-Parikka P, Peltonen M, Aunola S, Eriksson JG, Hemio K, Hamalainen H, Harkonen P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Mannelin M, Paturi M, Sundvall J, Valle TT, Uusitupa M, Tuomilehto J. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368:1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 39.Li G, Zhang P, Wang J, Gregg EW, Yang W, Gong Q, Li H, Li H, Jiang Y, An Y, Shuai Y, Zhang B, Zhang J, Thompson TJ, Gerzoff RB, Roglic G, Hu Y, Bennett PH. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet. 2008;371:1783–1789. doi: 10.1016/S0140-6736(08)60766-7. [DOI] [PubMed] [Google Scholar]
- 40.Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Bowman JD, Pronk NP. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107:1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 41.Happell B, Davies C, Scott D. Health behaviour interventions to improve physical health in individuals diagnosed with a mental illness: a systematic review. Int J Ment Health Nurs. 2012;21:236–247. doi: 10.1111/j.1447-0349.2012.00816.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.