Abstract
Purpose
High-dose methotrexate (HD-MTX) has been used to treat children with central nervous system tumors. Accumulation of MTX within pleural, peritoneal, or cardiac effusions has led to delayed excretion and increased risk of systemic toxicity. This retrospective study analyzed the association of intracranial post-resection fluid collections with MTX plasma disposition in infants and young children with brain tumors.
Methods
Brain MRI findings were analyzed for postoperative intracranial fluid collections in 75 pediatric patients treated with HD-MTX and for whom serial MTX plasma concentrations ([MTX]) were collected. Delayed plasma excretion was defined as [MTX] ≥1μM at 42 hours (h). Leucovorin was administered at 42 h and then every 6 h until [MTX] <0.1μM. Population and individual MTX pharmacokinetic parameters were estimated by nonlinear mixed-effects modeling.
Results
Fifty-eight patients had intracranial fluid collections present. Population average (inter-individual variation) MTX clearance was 96.0 ml/min/m2 (41.1 CV%) and increased with age. Of the patients with intracranial fluid collections, 24 had delayed excretion; only 2 of the 17 without fluid collections (p<0.04) had delayed excretion. Eleven patients had grade 3 or 4 toxicities attributed to HD-MTX. No significant difference was observed in intracranial fluid collection, total leucovorin dosing, or hydration fluids between those with and without toxicity.
Conclusions
Although an intracranial fluid collection is associated with delayed MTX excretion, HD-MTX can be safely administered with monitoring of infants and young children with intracranial fluid collections. Infants younger than one year may need additional monitoring to avoid toxicity.
Keywords: Methotrexate, Pharmacokinetics, Pseudocyst, Toxicity, Cerebrospinal Fluid
INTRODUCTION
Embryonal brain tumors in infants and young children carry a poor prognosis, in part because craniospinal irradiation (CSI) is avoided as a treatment option due to unacceptable neurotoxicity [1–5]. Consequently, high-dose intravenous methotrexate (HD-MTX)–based chemotherapy regimens have been used as an alternative to CSI [6–8]. Young children with medulloblastoma treated on MTX-based regimens have shown improvement in progression free and overall survival [6–7, 9]. Methotrexate disposition in infants and very young children with brain tumors, however, is unknown despite similar studies in children with acute leukemia [10–16]. Pharmacokinetic evaluation of MTX in infants with acute lymphoblastic leukemia (ALL) enrolled on Pediatric Oncology Group 9407 study showed that older infants (e.g., 7 to 12 months) had a 25% higher average methotrexate clearance than younger infants (e.g., 0 to 6 months); younger infants, correspondingly, had a higher incidence of renal toxicity during induction [10]. In contrast, retrospective pharmacokinetic analyses of MTX serum concentrations after high dose MTX in children with non-B cell ALL showed MTX system clearance decreased as a function of age (range 0.25 to 15 years), suggesting faster elimination in infants [11–12].
The inclusion of MTX in several ongoing and planned clinical trials for young children with brain tumors warrants further study of MTX disposition in these patients, especially due to methotrexate’s tendency to accumulate within extra-axial spaces and tumor-associated fluid collections, thereby leading to delayed systemic MTX excretion and potential toxicity [17–20]. Patients with brain tumors often develop various post resection fluid collections, such as tumor-associated pseudocysts, subdural collections, or pseudomeningocoeles, which may not communicate freely with normal cerebrospinal fluid (CSF) spaces. The association of these abnormal fluid collections with MTX pharmacokinetics has not been documented in infants and young children with brain tumors who have post resection fluid collections. Therefore, we conducted a retrospective study of MTX pharmacokinetics in 75 infants and young children diagnosed with central nervous system (CNS) tumors and treated with intravenous MTX to determine how the presence of possibly sequestered intracranial fluid collections affects MTX disposition.
PATIENTS AND METHODS
Data set and study sample
The study was approved by the St. Jude Institutional Review Board. Records were reviewed for patients enrolled on institutional protocol St. Jude Young Children 2007 (SJYC07; NCT00602667) from December 1, 2007, until July 31, 2010 (n=65), or treated on non-protocol treatment plans as per SJYC07 (January to November 2007; n= 10). Informed consent was obtained for all invasive procedures. Patient demographics, diagnosis, magnetic resonance (MR) imaging findings, MTX dosage, and results of plasma MTX analysis were reviewed.
Methotrexate administration and monitoring
Each HD-MTX dose was administered as a 24-hour (h) infusion. The total MTX dosage (5000 mg/m2 or 2500 mg/m2, the latter an empiric dose reduction for patients less than 31 days old at treatment start due to the lack of available data regarding MTX disposition in children this young) was diluted in 5% dextrose in water with 40 mEq/L sodium bicarbonate to a concentration of 10 mg/mL; 10% of the total dosage (500 or 250 mg/m2, respectively) was administered as a loading dose over 1 h, followed by a 23 h infusion of the remaining 90% (4500 or 2250 mg/m2, respectively). For clinical purposes, delayed plasma excretion was defined as an MTX concentration ([MTX]) greater than 1.0 μM at 42 h. Prehydration with sodium bicarbonate at 200 mL/m2/h was initiated either at least 2 h before starting MTX and decreased to 125 mL/m2/h after prehydration fluids were completed or started at 125 mL/m2/h for at least 6 h until the urine pH was 7–8. Standard leucovorin rescue was administered at 15 mg/m2/dose (intravenous or oral) starting at 42 h and given every 6 h for a total of 5 doses. For plasma [MTX] ≥ 1 μM at 42 h or clinical toxicity, the dosage, frequency, and duration of leucovorin rescue and/or rate of intravenous fluid hydration was increased.
Magnetic resonance (MR) imaging and analysis for patients
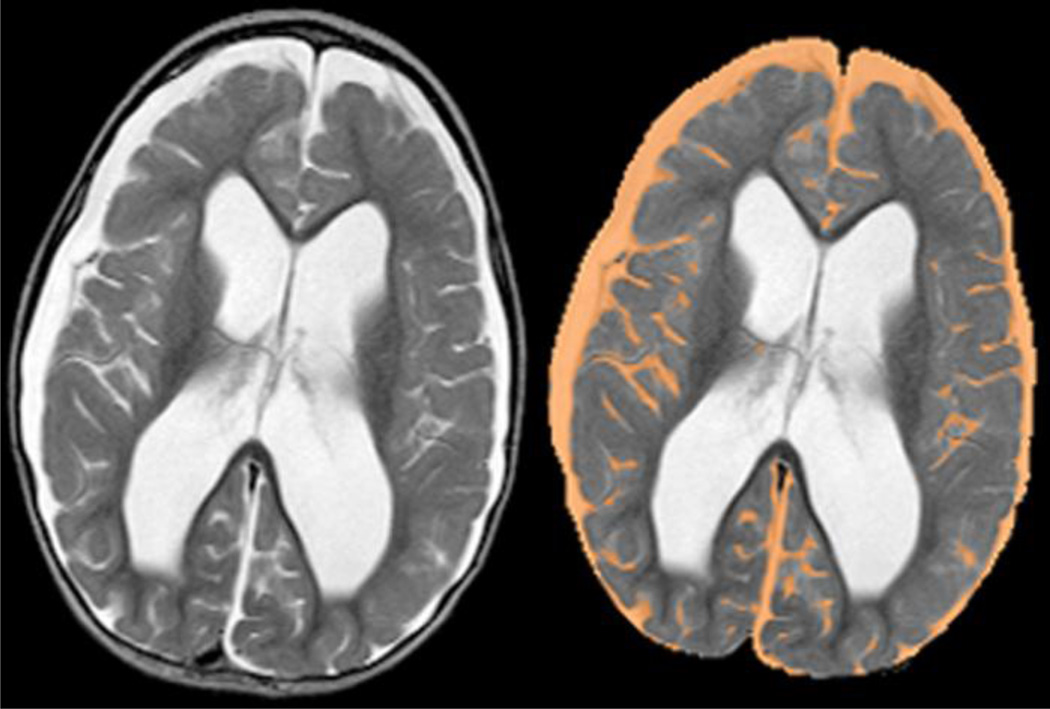
All MR images were acquired on one of two 1.5 T Avanto MR imagers (Siemens Medical Systems, Iselin, NJ) as 4-mm-thick contiguous axial oblique slices with whole-head coverage and a 256 matrix (voxel size 0.82 × 0.82 × 4 mm at 210 mm field-of-view). T2-weighted images were acquired with a spin-echo sequence (TR/TE = 5570/119 ms, 1 acquisition) on an oblique plane defined by the most inferior extent of the genu and splenium of the corpus callosum. T2 images were first processed to extract the brain and surrounding fluid (Fig. 1). An empirically determined threshold was applied to the images to segment the fluid within them. Once a binary image of all T2-hyperintense voxels was created, the ventricles and any residual white matter hyperintensities were manually removed. A histogram analysis across all slices was performed to determine the total number of imaging voxels containing extra-axial fluid within normal subarachnoid space or abnormal collections, such as subdurals, epidurals, pseudomeningocoeles, cysts or other possible extra-axial fluid collections (e.g., arachnoidal cysts). This voxel count was then multiplied by the voxel volume to yield a total fluid volume in milliliters.
Figure 1.
(Left) T2-weighted image as acquired during the MR examination demonstrating bilateral extra-axial fluid collections (subdural hygromas) over cerebral hemispheres and ventriculomegaly. (Right) T2-weighted image after extraction of the brain and surrounding fluid. The segmented fluid volume is shown in orange and demonstrates the exclusion of CSF within the ventricles
Brain MRIs from all patients obtained after tumor resection but before course 1 HD-MTX were reviewed for these abnormal fluid collections. Hydrocephalus was not used as a measurable collection because of its communication with cerebrospinal fluid spaces. In cases in which more than one fluid collection was present, volumes were summed.
Plasma methotrexate bioanalysis
Blood samples (1–2 mL) from patients’ central lines were collected in K2EDTA tubes before MTX infusion and 6, 23, 42, and 66 h from start of the first infusion during course 1. Additional samples were collected if the patient’s plasma [MTX] was not below 0.1 μM at 66 h. Samples were centrifuged at 7000g for 10 min and MTX plasma concentrations determined by fluorescence polarization immunoassay (FPIA, TDx System; Abbott Laboratories, Abbott Park, IL). The lower limit of quantitation for plasma [MTX] was 0.03 μM.
Pharmacokinetic analysis
Nonlinear mixed-effects modeling (Monolix 3.1) was used to determine population and individual post-hoc MTX pharmacokinetic parameters [21]. All data were obtained from course one of treatment. A two-compartment pharmacokinetic model with first-order elimination was fit to the data [22–25]. Parameters estimated included systemic clearance (Cl), volume of distribution for each compartment (V1, V2), and inter-compartmental clearance (Q). The distribution of the parameters was assumed log-normal. Data below the lower limit of quantification were censored and modeled using the likelihood approach described in Monolix. A proportional residual error model was used which assumed normal distribution of the residuals.
The association of both age and post-operative CNS fluid collection volume with the pharmacokinetic parameters was analyzed. These covariates were considered significant in a univariate analysis if their addition to 6 the model reduced the objective function value (OFV) at least 3.84 units (P < 0.05, based on χ2 test for the difference in the −2 log-likelihood between 2 hierarchical models that differ by 1 degree of freedom), and the covariate term was significantly different than zero (t-test, P < 0.05). The percent change in the interindividual variability (IIV) was described by the percent change in the variance estimate (Ω2) between the IIV of the base and covariate model.
Two measures of delayed MTX excretion were considered. We determined whether the individual’s estimated plasma [MTX] was greater than 1.0 μM at 42 h (clinical indicator of whether additional leucovorin or intravenous fluid hydration is given) or greater than 0.1 μM at 66 h. In addition, we estimated the time each individual’s estimated plasma [MTX] remained above 0.1 μM. Analysis of variance was used to determine the effect of post-operative CNS fluid and age on threshold time.
Toxicities
Methotrexate toxicity was monitored and graded according to the Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 3.0. All grade 3, 4 and 5 adverse events were documented. Dose limiting toxicities of MTX are generally bone marrow suppression, ulcerative stomatitis, severe diarrhea and nephrotoxicity. The majority of identified toxicities were considered related to methotrexate if occurring within a week of administration and prior to the start of the next chemotherapy on day 8. The only exception was neurotoxicity, which can be a delayed complication.
Statistical analysis
Kruskal-Wallis (KW) tests were used to compare the differences in various pharmacokinetic and clinical parameters between two groups, e.g., patients with and without toxicities, fluid collections, etc. Associations between categorical variables were explored via Fishers exact test. Correlations between continuous variables were explored via Pearson’s correlation coefficient calculated on the natural log scale (PCC ln) to improve linearity and remove the influence of outliers. P-values presented are not adjusted for multiplicity.
RESULTS
Patient characteristics
Seventy-five infants and young children (45 male, 30 female) were treated with HD–MTX: 65 were enrolled on SJYC07 (for children less than 3 years old at diagnosis) and 10 were treated off protocol. Median age of patients at diagnosis was 1.6 years (range 8 days to 42 months). None of the patients were pretreated; all were newly diagnosed. Table 1 summarizes patient demographics, diagnosis and details of MTX administration.
Table 1.
Patient characteristics, diagnosis, and details of MTX administration
| Parameter | All patients (n=75) |
With intracranial fluid collection (n=58) |
Without intracranial fluid collection (n=17) |
P Value |
|---|---|---|---|---|
| Age (years) [median (range)] | 1.6 (0.022, 3.5) | 1.7 (0.049, 3.5) | 1.0 (0.022, 3.1) | 0.25 |
| BSA (m2) [median (range)] | 0.52 (0.23, 0.73) | 0.53 (0.23, 0.73) | 0.48 (0.26, 0.67) | 0.64 |
| Baseline creatinine [median (range)] | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.4) | |
| Sex | ||||
| Male | 45 | 35 | 10 | |
| Female | 30 | 23 | 7 | |
| Diagnosis | ||||
| ATRTa | 18 | 16 | 2 | |
| MBb | 17 | 10 | 7 | |
| Ependymoma | 10 | 9 | 1 | |
| PNETc | 6 | 6 | 0 | |
| HGGd | 5 | 2 | 3 | |
| AAe | 4 | 2 | 2 | |
| Pineoblastoma | 4 | 3 | 1 | |
| CPCf | 3 | 3 | 0 | |
| GBMg | 2 | 1 | 1 | |
| MGNTh | 2 | 2 | 0 | |
| ETANERi | 2 | 2 | 0 | |
| Medulloepithelioma | 1 | 1 | 0 | |
| MMBj | 1 | 1 | 0 | |
| MTXk dosage (mg/m2) | ||||
| 5000 | 73 | 57 | 16 | |
| 2500 | 2 | 1 | 1 | |
ATRT, atypical teratoid rhabdoid tumor
MB, medulloblastoma
PNET, primitive neuroectodermal tumor
HGG, high grade glioma
AA, anaplastic astrocytoma
CPC, choroid plexus carcinoma
GBM, glioblastoma multiforme
MGNT, malignant glioneuronal tumor
ETANER, embryonal tumor with abundant neuropil and ependymoblastic rosettes
MMB, medullomyoblastoma
MTX, methotrexate
MR Imaging of CNS fluid collections
Abnormal intracranial fluid collections were identified in 58 patients following review of MRIs performed after surgery and prior to the first HD-MTX course. Specifically, there were 29 subdural, 1 epidural, and 13 other extra-axial fluid collections. In addition, four patients’ MRIs demonstrated tumor cysts. Twenty-three patients had pseudomeningoceles. Twelve of the 58 patients had more than one type of fluid collection. The median (range) observed abnormal fluid collection volume was 375.8 mL/m2 (102.8 to 1510.8 mL/m2).
Pharmacokinetic analysis
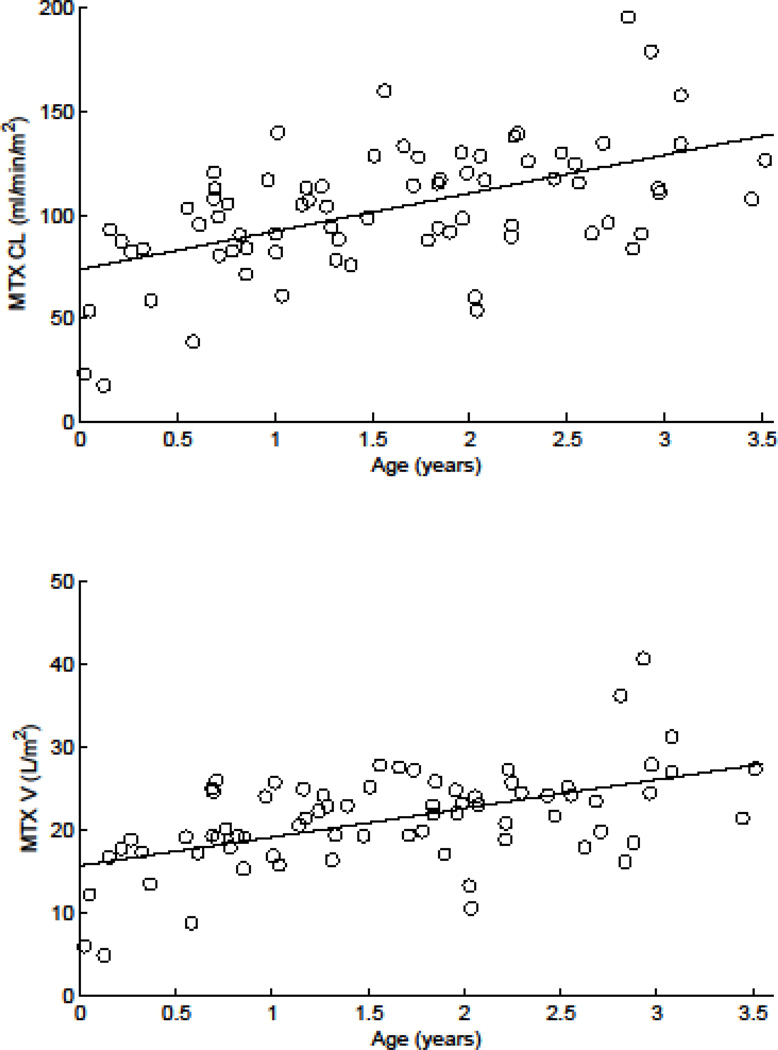
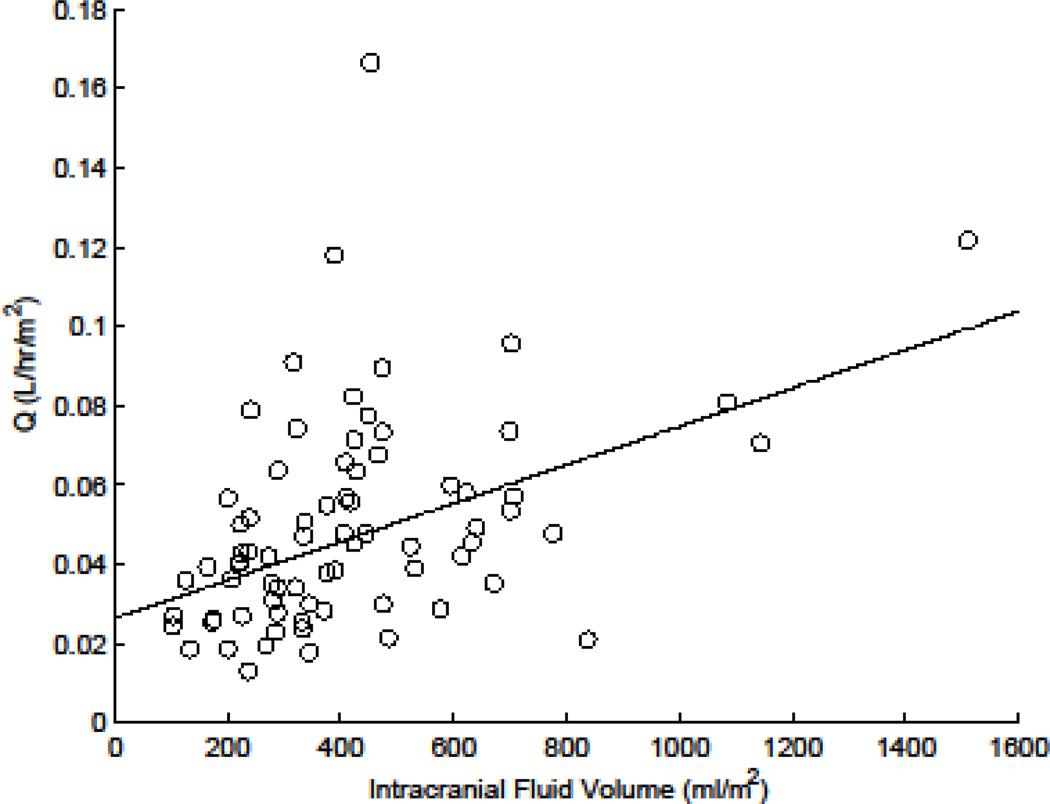
Table 2 summarizes the population MTX pharmacokinetic parameters for these infants and young children. Estimated population average (inter-individual variability) MTX clearance in our patients was 96.0 ml/min/m2 (41.1 CV%). The results of the nonlinear mixed-effects analyses showed age and intracranial fluid volumes were significantly related to MTX pharmacokinetic parameters. MTX clearance and volume of distribution increased with age (P<10−4 and P<0.002, respectively, Fig. 2a and b). As a result, the −2 log likelihood decreased 11.1 units (P<10−3). The addition of age to the model explained 31% and 30% of the interindividual variability (IIV) in clearance and volume of distribution, respectively. In addition, higher intracranial fluid volume correlated with higher inter–compartmental clearance (Q; P<10−3, Fig. 3), thereby decreasing the −2 log likelihood an additional 4.8 units (P<0.03) relative to the model with age as a covariate. Adding intracranial fluid volume as a covariate to the model explained 22% of IIV in inter-compartmental clearance.
Table 2.
Summary of population pharmacokinetic parameters for methotrexate
| Parameter | Base Model | Age Covariate Model | Full Covariate Model | |||
|---|---|---|---|---|---|---|
| Population Estimate [RSEa (%)] |
IIVb Ω2 (CVc%) |
Population Estimate [RSEa (%)] |
IIVb Ω2 (CVc %) |
Population Estimate [RSEa (%)] | IIV Ω2 (CVc%) |
|
| CL (mL/min/m2)d | 96.0 (5) | 0.169 (41.1) | 72.5 e(0.174 age); (8, 24) | 0.116 (34.1) | 74.5 e(0.154 age); (8, 27) | 0.126 (35.5) |
| V1 (L/m2)e | 19.7 (5) | 0.142 (37.7) | 16.4 e(0.124 age); (8, 32) | 0.100 (31.6) | 16.6 e(0.113 age); (8, 35) | 0.106 (32.5) |
| Q (L/hr/m2)f | 0.05 (11) | 0.405 (63.6) | 0.0468 (10) | 0.347 (58.9) | 0.0431 e0.887 (IFC-median IFC) h; (9, 26) | 0.315 (56.1) |
| V2 (L/m2)g | 1.42 (10) | 0.563 (75.0) | 1.34 (10) | 0.540 (73.5) | 1.42 (10) | 0.498 (70.6) |
| Residual | 0.202 (6) | 0.204 (6) | 0.212 (6) | |||
| −2 Log likelihood | 1342.3 | 1331.2 | 1326.4 | |||
RSE, relative standard error
IIV, inter individual variability
CV, coefficient of variation
Cl, systemic clearance
V1, volume of distribution for compartment 1
Q, inter-compartmental clearance
V2, volume of distribution for compartment 2
IFC, intracranial fluid collection
Figure 2.
Variation in methotrexate (MTX) clearance (a) and volume of distribution with age (b)
Figure 3.
Relation between MTX intercompartmental clearance term (Q) and intracranial fluid volume
Delayed Excretion
When considering the 42 h MTX concentration, no significant difference was observed between patients with and without intracranial fluid collections (KW, p=0.09). Patients with intracranial fluid collections, however, had plasma MTX concentrations above a threshold of 0.1 μM longer than those that did not (63.6 vs. 54.6 h; median increase 17%; KW, p=0.01). Of the 58 patients with intracranial fluid collections, 24 (41%) had 66 h MTX concentrations greater than 0.1 μM compared to only 2 of 17 remaining patients (12%; Fisher’s exact test, p<0.04). Our data also suggested that patients with delayed excretion at 66 h had larger median intracranial fluid collections compared to those without abnormal fluid collections (423.1 vs 333.7 ml/m2; KW, p=0.07).
Toxicity
Of the 75 patients studied during course 1, only 11 MTX-related grade 3 or 4 toxicities were observed. These included gastrointestinal toxicities (n=7), dermatologic toxicities (n=2), and metabolic/laboratory toxicities (n=2). No grade 5 toxicities occurred. We investigated whether intracranial fluid collections, delayed MTX excretion, pre or post MTX hydration, or leucovorin rescue was related to the occurrence of these toxicities. In all cases no significant relationships were observed, however, the power to detect such relationships was limited due to only 11/75 patients experiencing grade 3 or 4 MTX related toxicities. At 5% significance level based on Kruskal Wallis test, this sample size has only 30% power to detect an effect size of 0.5 standard deviation and 80% power for an effect size of 1 standard deviation. Interestingly, our data suggested that patients who developed grade 3 or 4 toxicities tended to be younger compared to those who did not though the difference did not reach statistical significance (median 1.1 vs. 1.7 yr; KW, p=0.096).
Supportive Care
We also studied how delayed excretion was affected by supportive care. We observed a significant increase in total post MTX hydration in children with delayed excretion versus normal MTX excretion either defined as [MTX] > 1.0 μM at 42 h (7.0 vs 7.7 L/m2, KW, p=0.055) or as [MTX] > 0.1 μM at 66 h (6.8 vs 8.1 L/m2; KW, p=0.005). With respect to intracranial fluid collections, we did not see a change in total pre or post MTX hydration, but we did observe a positive association between total leucovorin dose and intracranial fluid collection (PCC ln, r=0.25, p=0.03).
DISCUSSION
Intravenous HD-MTX has been recognized as an active agent in treating pediatric brain tumors [6–7], thus its incorporation into treatment regimens for such tumors has led to concern that MTX may accumulate within sizeable, abnormal, sequestered intracranial fluid collections created by neurosurgical procedures. Sequestration of MTX in other third spaces such as pleural fluid and ascites has been documented to impact MTX clearance in lymphoid malignancies and solid tumors [17, 19, 26–27]. Our study investigates the role of post-resection intracranial fluid collections on MTX disposition in infants and young children with brain tumors. The 66 h MTX concentrations were greater than 0.1 μM more than three times as often in patients with intracranial fluid collections compared to those without fluid collections. Our data also suggest that patients with MTX concentrations greater than 0.1 μM at 66 h had larger median intracranial fluid collections compared to those without an abnormal fluid collection. Neither presence of abnormal fluid collection nor delayed MTX excretion was associated with the occurrence of grade 3 or 4 toxicity presumably due to close MTX monitoring that resulted in aggressive fluid management and protocol-guided leucovorin rescue for patients with delayed MTX excretion. Our ability to detect these associations, however, is limited by the small number of patients who experienced grade 3 or 4 toxicity in our cohort.
Clinically relevant CNS fluid collections are relatively rare in other patient populations, but common in patients with primary CNS malignancies, especially in the immediate postoperative setting [27–31]. These collections include postoperative subdural fluid collections (typically hygromas), pseudomeningoceles, or intracranial pseudocysts. Therefore, this population is ideal to examine the potential impact of intracranial fluid collections on MTX pharmacokinetics and toxicity, and to study whether potential modifications to MTX administration (e.g., dosage) or monitoring (e.g., MTX concentrations, increase in fluid hydration, changing leucovorin rescue) are required for these patients.
Several investigators have reported alterations in MTX pharmacokinetics due to presence of fluid collections or third spaces [17–18, 32–34]. Using a physiologically-based pharmacokinetic model, Li and Gwilt assessed factors related to the extent to which methotrexate is sequestered into physiologic third spaces [19]. Results of their simulations showed that the volume of the third space, MTX protein binding in that space, and rate of transport into and out of the space were the key covariates related to methotrexate pharmacokinetic parameters (e.g., volume of distribution at steady-state and half-life). Thus, third spaces with more protein, higher methotrexate protein binding, or larger volumes will have an apparent increased volume of distribution at steady-state and longer terminal plasma half-lives. Protein concentration in the fluid collections observed in our patients was similar to that of CSF (e.g., 15–60 mg/dl), which is very low and should not alter MTX disposition [35]. As the fluid collection volume increased, we observed an increase in the inter-compartment clearance rate (Q), a term which is similar to permeability clearance (PA) used by Li and Gwilt to describe the transport rate into and out of third spaces [19]. The net effect of a higher Q on plasma MTX pharmacokinetics in our patients was to shift the start of the slower terminal elimination phase to a higher MTX concentration. This translated to a terminal phase that remained above 0.1 μM longer, resulting in delayed MTX excretion at 66 h.
At the outset of this clinical trial, little data were available upon which to base the selection of MTX monitoring guidelines for infants with brain tumors receiving HD-MTX. On the basis of unpublished guidelines from previous pediatric trials at our institution incorporating HD-MTX, we included an additional time point for methotrexate monitoring during the infusion at six hours to provide an early indicator of whether a patient might have a low clearance and possibly high MTX systemic exposure, necessitating closer clinical monitoring. The remainder of the methotrexate monitoring was per our institutional guidelines with leucovorin rescue beginning at 42 h, and delayed excretion based upon the methotrexate concentrations at 42 and 66 h. The definition of delayed excretion used in this study differs slightly from other current guidelines summarized in Table 3 [6–7, 36–41].
Table 3.
Summary of definition of delayed methotrexate excretion among various clinical trials
| Clinical Protocol |
MTX Dosage (as available) and Duration of Infusion |
Time Point from Beginning of Infusion (h) |
Time Point from End of Infusion (h) |
MTX Concentration Cutoff (µM) |
References |
|---|---|---|---|---|---|
| ACNS0333 | HD-MTX IV over 4 h day 1 (dose N/A) | 72 | 68 | 0.1 | 32 |
| ACNS0334 | HD-MTX IV over 4 h day 1 (dose N/A) | 72 | 68 | 0.1 | 33 |
| SIOPII | 2 gm/m2 IV over 6 h days 15, 22, 29 | N/Aa | N/Aa | 0.1 | 34 |
| HIT-SSK’92 | 5 gm/m2 IV over 24 h days 15, 22 | N/Aa | N/Aa | 0.25 | 6 |
| HIT – SKK’2000BIS4 | 5 gm/m2 IV over 24 h days 15, 22 | N/Aa | N/Aa | 0.25 | 35 |
| Head Start II | 400 mg/kg IV day 4 × 2 days per cycle | 72 | 68 | 0.1 | 7, 36–37 |
| SJYC07 | 5 gm/m2 IV over 24 h day 1 | 42 | 18 | 1.0 |
N/A, not available
In the Children’s Oncology Group (COG) studies ACNS0333 and ACNS0334, suggested administration guidelines for HD-MTX with leucovorin rescue are provided based upon 24-, 48-, and 72-hour MTX concentrations. Similar to the Head Start II (HS II) trial, these studies define delayed excretion as [MTX] ≥ 0.1 μM at 72 h [7, 36–37, 40–41]. Our patients with intracranial fluid collections had plasma MTX concentrations above the COG and HSII threshold of 0.1 μM at 72 hours longer than those that did not (data not shown). In those patients with MTX plasma concentrations above the threshold of 0.1 μM, the median intracranial fluid volume was greater than those patients with MTX concentrations below the threshold (423 vs. 336 mL/m2; p=0.07).
HD-MTX was well tolerated after the first course of therapy in our patients with grade 3 and 4 toxicities. We did observe an interesting trend for greater toxicities in younger patients similar to that observed by Thompson and colleagues in infants with acute lymphoblastic leukemia [10], but more patients must be studied to fully understand this relationship. Younger children had a longer time above the threshold of 0.1 μM when controlling for the presence of an intracranial fluid collection. Thus, it is possible that age may affect the time for MTX serum concentrations to reach safe concentrations, indirectly reflecting the ontogeny of renal function [42–43]. The tolerability of HD-MTX after one course among the majority of patients, however, should not obviate the need for careful monitoring and further study, especially as renal function may be further compromised by additional courses of therapy. The lack of significant clinical toxicities may be due to adjustments made to the post hydration fluid and leucovorin rescue per supportive care guidelines while being mindful of the presence of intracranial fluid collections.
Our study suggests that HD-MTX can be administered safely to children with transient or permanent postsurgical intracranial fluid collections, using intensive methotrexate monitoring, hydration, and leucovorin rescue. In patients younger than one year who have an intracranial fluid collection or those with compromised renal function, we recommend that plasma MTX concentrations be monitored until they are undetectable.
Acknowledgments
Acknowledgments/Funding: This work was supported in part by the National Cancer Institute grants CA21765, CA098543, CA096832, CA154619, and CA081457; Musicians Against Childhood Cancer; the Noyes Brain Tumor Foundation; and the American Lebanese Syrian Associated Charities.
Footnotes
Ethical Standards: The study was approved by the St. Jude Institutional Review Board and therefore performed with the ethical standards laid down in the 1964 Declaration of Helsinki and it subsequent amendments. All involved patients’ parents gave their informed consents prior to the child’s inclusion in the study.
Conflicts: The authors have no conflicts of interest to disclose and maintain control of all primary data. To that end, the journal may review data as requested.
References
- 1.Duffner PK, Horowitz ME, Krischer JP, Friedman HS, Burger PC, Cohen ME, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 2.Geyer JR, Zeltzer PM, Boyett JM, Rorke LB, Stanley P, Albright AL, et al. Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: a report from the Childrens Cancer Group. J Clin Oncol. 1994;12:1607–1615. doi: 10.1200/JCO.1994.12.8.1607. [DOI] [PubMed] [Google Scholar]
- 3.Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 4.Gajjar A, Mulhern RK, Heideman RL, Sanford RA, Douglass EC, Kovnar EH, et al. Medulloblastoma in very young children: outcome of definitive craniospinal irradiation following incomplete response to chemotherapy. J Clin Oncol. 1994;12:1212–1216. doi: 10.1200/JCO.1994.12.6.1212. [DOI] [PubMed] [Google Scholar]
- 5.Walter AW, Mulhern RK, Gajjar A, Heideman RL, Reardon D, Sanford RA, et al. Survival and neurodevelopmental outcome of young children with medulloblastoma at St Jude Children's Research Hospital. J Clin Oncol. 1999;17:3720–3728. doi: 10.1200/JCO.1999.17.12.3720. [DOI] [PubMed] [Google Scholar]
- 6.Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Sorensen N, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 7.Chi SN, Gardner SL, Levy AS, Knopp EA, Miller DC, Wisoff JH, et al. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22:4881–4887. doi: 10.1200/JCO.2004.12.126. [DOI] [PubMed] [Google Scholar]
- 8.Sands SA, Oberg JA, Gardner SL, Whiteley JA, Glade-Bender JL, Finlay JL. Neuropsychological functioning of children treated with intensive chemotherapy followed by myelopablative consolidation chemotherapy and autologous hematopoietic cell rescue for newly diagnosed CNS tumors: an analysis of the Head Start II survivors. Pediatr Blood Cancer. 2010;54:429–436. doi: 10.1002/pbc.22318. [DOI] [PubMed] [Google Scholar]
- 9.Allen JC, Walker R, Rosen G. Preradiation high-dose intravenous methotrexate with leucovorin rescue for untreated primary childhood brain tumors. J Clin Oncol. 1988;6:649–653. doi: 10.1200/JCO.1988.6.4.649. [DOI] [PubMed] [Google Scholar]
- 10.Thompson PA, Murry DJ, Rosner GL, Lunagomez S, Blaney SM, Berg SL, et al. Methotrexate pharmacokinetics in infants with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 2007;59:847–853. doi: 10.1007/s00280-006-0388-1. [DOI] [PubMed] [Google Scholar]
- 11.Donelli MG, Zucchetti M, Robatto A, Perlangeli V, D'Incalci M, Masera G, et al. Pharmacokinetics of HD-MTX in infants, children, and adolescents with non-B acute lymphoblastic leukemia. Med Pediatr Oncol. 1995;24:154–159. doi: 10.1002/mpo.2950240303. [DOI] [PubMed] [Google Scholar]
- 12.Borsi JD, Moe PJ. A comparative study of pharmacokinetics of methotrexate in a dose range of 0.5 g to 33.6 g/m2 in children with acute lymphoblastic leukemia. Cancer. 1987;60:5–13. doi: 10.1002/1097-0142(19870701)60:1<5::aid-cncr2820600103>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 13.Evans WE, Abromowitch M, Crom WR, Relling MV, Bowman WP, Pui CH, et al. Clinical pharmacodynamic studies of high-dose methotrexate in acute lymphocytic leukemia. NCI Monogr. 1987;5:81–85. [PubMed] [Google Scholar]
- 14.Garre ML, Relling MV, Kalwinsky D, Dodge R, Crom WR, Abromowitch CH, et al. Pharmacokinetics and toxicity of methotrexate in children with Down syndrome and acute lymphocytic leukemia. J Pediatr. 1987;111:606–614. doi: 10.1016/s0022-3476(87)80131-2. [DOI] [PubMed] [Google Scholar]
- 15.Wall Am, Gajjar A, Link A, Mahmoud H, Pui CH, Relling MV. Individualized dosing in children with relapsed acute lymphoblastic leukemia. Leukemia. 2000;14:221–225. doi: 10.1038/sj.leu.2401673. [DOI] [PubMed] [Google Scholar]
- 16.Trevino LR, Shimasaki N, Yang W, Panetta JC, Cheng C, Pei D, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetic and clinical effects. J Clin Oncol. 2009;10:4972–4978. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans WE, Pratt CB. Effect of pleural effusion on high-dose methotrexate kinetics. Clin Pharmacol Ther. 1978;23:68–72. doi: 10.1002/cpt197823168. [DOI] [PubMed] [Google Scholar]
- 18.Pauley JL, Panetta JC, Schmidt J, Kornegay N, Relling MV, Pui CH. Late-onset delayed excretion of methotrexate. Cancer Chemother Pharmacol. 2004;54:146–152. doi: 10.1007/s00280-004-0797-y. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Gwilt P. The effect of malignant effusions on methotrexate disposition. Cancer Chemother Pharmacol. 2002;50:373–382. doi: 10.1007/s00280-002-0512-9. [DOI] [PubMed] [Google Scholar]
- 20.Wan SH, Huffman DH, Azarnoff DL, Stephens R, Hoogstraten B. Effect of route of administration and effusions on methotrexate pharmacokinetics. Cancer Res. 1974;34:3487–3491. [PubMed] [Google Scholar]
- 21.Monolix 3.1: User Guide. 2010 http://software.monolix.org.
- 22.Evans WE, Relling MV, Rodman JH, Crom WR, Boyett JM, Pui CH. Conventional compared with individualized chemotherapy for childhood acute lymphoblastic leukemia. N Engl J Med. 1998;338:499–505. doi: 10.1056/NEJM199802193380803. [DOI] [PubMed] [Google Scholar]
- 23.Dombrowsky E, Jayaraman B, Narayan M, Barrett JS. Evaluating performance of a decision support system to improve methotrexate pharmacotherapy in children and young adults with cancer. Ther Drug Monit. 2011;33:99–107. doi: 10.1097/FTD.0b013e318203b41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aumente D, Buelga DS, Lukas JC, Gomez P, Torres A, Garcia MJ. Population pharmacokinetics of high-dose methotrexate in children with acute lymphoblastic leukaemia. Clin Pharmacokinet. 2006;45:1227–1238. doi: 10.2165/00003088-200645120-00007. [DOI] [PubMed] [Google Scholar]
- 25.Faltaos DW, Hulot JS, Urien S, Morel V, Kaloshi G, Fernandez C, Xuan kH, Leblond V, Lechat P. Population pharmacokinetic study of methotrexate in patients with lymphoid malignancy. Cancer Chemother Pharmacol. 2006;58:626–633. doi: 10.1007/s00280-006-0202-0. [DOI] [PubMed] [Google Scholar]
- 26.Chan H, Evans WE, Pratt CB. Recovery from toxicity associated with high-dose methotrexate: prognostic factors. Cancer Treat Rep. 1977;61:797–804. [PubMed] [Google Scholar]
- 27.Koizumi H, Fukamachi A, Nukui H. Postoperative subdural fluid collections in neurosurgery. Surg Neurol. 1987;27:147–153. doi: 10.1016/0090-3019(87)90286-2. [DOI] [PubMed] [Google Scholar]
- 28.Cabanes J, Vazquez R, Rivas A. Hydrocephalus after posterior fossa operations. Surg Neurol. 1978;9:42–46. [PubMed] [Google Scholar]
- 29.Eguchi S, Aihara Y, Hori T, Okada Y. Postoperative extra-axial cerebrospinal fluid collection –its pathophysiology and clinical management. Pediatr Neurosurg. 2011;47:125–132. doi: 10.1159/000330543. [DOI] [PubMed] [Google Scholar]
- 30.Gnanalingham KK, Lafuente J, Thompson D, Harkness W, Hayward R. Surgical procedures for posterior fossa tumors in children: does carniotomy lead to fewer complications than craniectomy? J Neurosurg. 2002;97:821–826. doi: 10.3171/jns.2002.97.4.0821. [DOI] [PubMed] [Google Scholar]
- 31.Ernestus RI, Ketter G, Klug N. Dura-plasty in intracranial operations. Zentralbl Neurochir. 1995;56:106–110. [PubMed] [Google Scholar]
- 32.Frei E, 3rd, Jaffe N, Tattersall MH, Pitman S, Parker L. New approaches to cancer chemotherapy with methotrexate. N Engl J Med. 1975;292:846–851. doi: 10.1056/NEJM197504172921607. [DOI] [PubMed] [Google Scholar]
- 33.Gandara DR, Edelman MJ, Crowley JJ, Lau DH, Livingston RB. Phase II trial of edatrexate plus carboplatin in metastatic non-small-cell lung cancer: a Southwest Oncology Group Study. Cancer Chemother Pharmacol. 1997;41:75–78. doi: 10.1007/s002800050710. [DOI] [PubMed] [Google Scholar]
- 34.Mahadevan A, Kanegaonkar R, Hoskin PJ. Third space sequestration increases toxicity of fludarabine--a case report. Acta Oncol. 1997;36:441. doi: 10.3109/02841869709001295. [DOI] [PubMed] [Google Scholar]
- 35.Medline Plus: A service of the U.S. National Library of Medicine NIH National Institutes of Health: CSF total protein. http://www.nlm.nih.gov/medlineplus/ency/article/003628.htm.
- 36.National Cancer Institute Clinical Trials (9/8/2012 update) Phase III study of induction therapy comprising vincristine, high-dose methotrexate, leucovorin calcium, etoposide, cisplatin, and cyclophosphamide followed by 3-dimensional conformal radiotherapy and high-dose consolidation therapy comprising carboplatin, thiotepa, and autologous peripheral blood stem cell rescue in pediatric patients with atypical teratoid/rhabdoid tumor of the central nervous system (ACNS0333) http://cancer.gov/clinicaltrials/search/view?cdrid=592812&version=healthprofessional#StudyIdInfo_CDR0000592812.
- 37.National Cancer Institute Clinical Trials (9/8/2012 update) Phase III study of induction therapy comprising vincristine, etoposide, cyclophosphamide, and cisplatin with or without high-dose methotrexate and leucovorin calcium followed by consolidation chemotherapy comprising carboplatin and thiotepa and peripheral blood stem cell rescue in pediatric patients with newly diagnosed supratentorial primitive neuroectodermal tumors or high risk medulloblastoma. http://cancer.gov/clinicaltrials/search/view?cdrid=483683&version=HealthProfessional&protocolsearchid=10902453.
- 38.Bailey CC, Gnekow A, Wellek S, Jones M, Round C, Brown J, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GIOP): SIOP II. Medical and Pediatr Oncol. 1991;25:166–178. doi: 10.1002/mpo.2950250303. [DOI] [PubMed] [Google Scholar]
- 39.von Bueren AO, von Hoff K, Pietsch T, Gerber NU, Warmuth-Metz M, Deinlein F, et al. Treatment of young children with localized medulloblastoma by chemotherapy alone: results of the prospective, multicenter trial HIT 2000 confirming the prognostic impact of histology. Neuro-Onc. 2011;13:669–679. doi: 10.1093/neuonc/nor025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fangusaro J, Finlay J, Sposto R, Ji L, Saly M, Zacharoulis S, et al. Intensive chemotherapy followed by consolidative myeloablative chemotherapy with autologous hematopoietic cell rescue (AuHCR) 24 in young children with newly diagnosed supratentorial primitive neuroectodermal tumors (sPNETs): report of the Head Start I and II experience. Pediatr Blood Cancer. 2008;50:312–318. doi: 10.1002/pbc.21307. [DOI] [PubMed] [Google Scholar]
- 41.Gardner SL, Asgharzadeh S, Green A, Horn B, McCowage G, Finlay J. Intensive induction chemotherapy followed by high dose chemotherapy with autologous heamtopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51:235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 42.Arant BS Jr. Developmental patterns of renal function maturation compared in the human neonate. J Pediatr. 1978;92:705–712. doi: 10.1016/s0022-3476(78)80133-4. [DOI] [PubMed] [Google Scholar]
- 43.Alcorn J, McNamara PJ. Ontogeny of hepatic and renal systemic clearance pathways in infants. Clin Pharmacokinet. 2002;41:1077–1094. doi: 10.2165/00003088-200241130-00005. [DOI] [PubMed] [Google Scholar]