Abstract
Objective
To examine the potential associations between biomarkers of metal exposure and serum testosterone in men of reproductive age in the general U.S. population.
Design
Cross-sectional epidemiology study with adjustment for potential confounders.
Setting
Nationwide survey.
Patient(s)
Men recruited in the U.S. National Health and Nutrition Examination Survey (NHANES).
Intervention(s)
Metal concentrations measured in whole blood, urine, and/or serum samples collected from 484 men.
Main Outcome Measure(s)
Serum testosterone concentration.
Result(s)
Concentrations of the metals were detected in 69-100% of the samples. In adjusted analyses where metals were modeled as a continuous variable, we found significant inverse associations between urinary molybdenum and serum copper and serum testosterone, whereas there were significant positive associations between blood lead and cadmium and serum testosterone. When metals were categorized into quartiles, analyses for serum copper and blood lead and cadmium produced significant associations in the same direction as the continuous measures. A suggestive inverse association was observed between quartiles of urinary molybdenum and serum testosterone, but the association was statistically significant when molybdenum was categorized into quintiles. Significant positive associations were also observed for quartiles of blood Se and serum Zn and serum testosterone.
Conclusion(s)
These findings add to the limited human evidence that exposure to molybdenum and other metals is associated with altered testosterone in men, which may have important implications for male health. More research is needed to confirm the findings of our study.
Keywords: Biomarkers, epidemiology, exposure, testosterone, metals
Introduction
Humans are exposed to metals everyday through naturally-fortified foods, supplements, and contaminated food, air, and/or water. Metals, such as copper (Cu), manganese (Mn), molybdenum (Mo), selenium (Se), and zinc (Zn), at low levels are necessary for homeostasis, but at high levels can be harmful to human health (1). Other metals, such as arsenic (As), cadmium (Cd), lead (Pb), and thallium (Tl) are nonessential and play no role in normal physiology (1,2). Mo is one such essential metal that is ubiquitously found in foods and drinking water, present in multivitamin and multimineral supplements, and used in a variety of industrial operations, such as in the manufacture of stainless steels and other steel alloys (1). Recently, Meeker et al. (1) reported a significant inverse association between blood Mo and serum testosterone in men from infertility clinics in Michigan, as well as a significant inverse association between blood Mo and semen quality parameters in the same cohort (3), raising concern that environmental exposures to Mo in the general population may be linked to reductions in testosterone levels, which may have important implications for male reproductive health. However, the potential human health effects resulting from low testosterone levels are far-reaching (4), having also been linked to metabolic syndrome (5), diabetes (6), cardiovascular disease (7), fractures (8,9), neurodegenerative disorder (10,11), and mortality (12,13). On the other hand, other metals, such as Cd and Pb, may increase testosterone (1,14-17), which is also concerning because elevated testosterone has been linked to both increased risk of cancer (18) and aggressive personality disorders (19).
In this study, we extend the limited human studies conducted on this topic to date and examined the potential associations between biomarkers of Mo and eight other metals (As, Cd, Cu, Pb, Mn, Se, Tl, Zn) and serum testosterone in men aged 18-55 years from the U.S. population. This is the first study of Mo and testosterone in men from the U.S. National Health and Nutrition Examination Survey (NHANES).
Materials and Methods
Study Population
This analysis concerned publicly-available data that was derived from NHANES 2011-2012. NHANES is a cross-sectional survey administered by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention (CDC) that collects data on the health and nutritional status of non-institutionalized civilian residents and conducts more detailed laboratory analyses on a subset of the participants (20). In the present analysis, we analyzed data from a subset of men that were 18-55 years old (n=1851). Men in this age range were selected to be consistent with previous research (1,3). Men with missing data on the metals of interest, serum testosterone, body mass index (BMI), poverty income ratio (PIR), race, serum cotinine, or urinary creatinine were excluded from the analysis (n=1367), resulting in a final sample size of data from 484 men. NHANES received approval from the NCHS Ethics Review Board, and informed consent was obtained for all participants.
Demographic Data
Information on family income and race was obtained from the men in the home by a trained interviewer using a Computer-Assisted Personal Interviewing system (21). The interviews were conducted in English or Spanish or with the assistance of an interpreter if necessary. PIR was calculated by CDC as the ratio of family income to poverty guidelines established by the Department of Health and Human Services (21).
Body Measurements
Height and weight were collected in a Mobile Examination Center (MEC) by trained health technicians with the assistance of a recorder during the body measurements (22). BMI was calculated by CDC as weight in kg divided by height in m2 (22).
Metals, Testosterone, and Other Analytes
Whole venous blood and urine were collected from participants at the MEC, which were then processed, stored, and shipped to either laboratories at the CDC (Atlanta, GA, USA) or the University of Minnesota (Minneapolis, MN, USA) (urinary creatinine only) for analysis (23). Samples were analyzed for blood, urine, and/or serum metals, serum testosterone (total), serum cotinine, and urinary creatinine. Metals in all three matrices were measured using inductively coupled-dynamic reaction-plasma mass spectrometry (24-26), whereas serum testosterone/cotinine and urinary creatinine were measured with isotope dilution-high performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) (4,27) and an enzymatic method (23), respectively. While most of these variables were collected in multiple NHANES cycles, data on testosterone measured by HPLC-MS/MS was only available for the NHANES 2011-2012 cycle, which is why we limited our analysis to those years. Concentrations below the limit of detection (LOD) were assigned a value of LOD divided by the square root of 2. LODs are reported in Table 2.
Table 2. Concentrations of analytes in blood, urine, and/or serum in men aged 18-55 years from NHANES 2011-2012 (n=484).
| Percentiles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Analyte | LOD | N (%) ≥LOD | GM | 10th | 25th | 50th | 75th | 90th | 95th | Max |
| Blood | ||||||||||
| Cadmium (μg/L) | 0.16 | 352 (72.7) | 0.28 | <LOD | <LOD | 0.23 | 0.44 | 1.09 | 1.50 | 6.90 |
| Lead (μg/dL) | 0.25 | 482 (99.6) | 1.06 | 0.52 | 0.71 | 1.00 | 1.59 | 2.39 | 2.89 | 33.67 |
| Manganese (μg/L) | 1.06 | 484 (100) | 8.64 | 5.74 | 7.06 | 8.70 | 10.47 | 12.75 | 14.98 | 45.49 |
| Selenium (μg/L) | 30 | 484 (100) | 194 | 167 | 178 | 192 | 210 | 227 | 238 | 312 |
| Urine | ||||||||||
| Arsenic (μg/L) | 1.25 | 471 (97.3) | 8.83 | 2.26 | 3.98 | 7.75 | 16.73 | 43.58 | 75.72 | 1036 |
| Cadmium (μg/L) | 0.056 | 408 (84.3) | 0.162 | <LOD | 0.083 | 0.161 | 0.298 | 0.577 | 0.873 | 4.830 |
| Lead (μg/L) | 0.08 | 465 (96.1) | 0.39 | 0.13 | 0.22 | 0.40 | 0.69 | 1.05 | 1.58 | 35.00 |
| Manganese (μg/L) | 0.08 | 335 (69.2) | 0.12 | <LOD | <LOD | 0.12 | 0.20 | 0.29 | 0.36 | 4.28 |
| Molybdenum (μg/L) | 0.99 | 484 (100) | 41.54 | 11.80 | 23.50 | 46.05 | 76.70 | 108 | 141 | 541 |
| Thallium (μg/L) | 0.020 | 481 (99.4) | 0.173 | 0.071 | 0.113 | 0.181 | 0.281 | 0.384 | 0.468 | 0.914 |
| Creatinine (mg/dL) | 1 | 484 (100) | 120 | 46 | 77 | 135 | 203 | 274 | 313 | 800 |
| Serum | ||||||||||
| Copper (μg/dL) | 2.5 | 484 (100) | 101 | 80.3 | 89.4 | 100 | 114 | 129 | 141 | 203 |
| Selenium (μg/L) | 4.5 | 484 (100) | 129 | 109 | 118 | 128 | 140 | 151 | 162 | 299 |
| Zinc (μg/dL) | 2.9 | 484 (100) | 83.8 | 67.7 | 74.7 | 83.8 | 94.0 | 103 | 109 | 233 |
| Total testosterone (ng/dL) | 0.36 | 484 (100) | 379 | 218 | 294 | 394 | 497 | 643 | 739 | 1245 |
| Cotinine (ng/mL) | 0.015 | 384 (79.3) | 0.490 | <LOD | 0.018 | 0.073 | 31.650 | 287 | 371 | 857 |
Abbreviations: GM, geometric mean; LO D, limit of detection.
Statistical Analysis
Statistical analysis was performed using SAS version 9.3 for Windows (SAS Institute, Cary, NC, USA). Descriptive statistics of participant demographics and concentrations of the analytes were calculated. Spearman's rank correlation coefficients were also calculated for all possible pairs of metal biomarkers. The associations between metal and testosterone concentrations were first assessed using simple linear regression in unadjusted models. These associations were then examined using multiple linear regression in models that were adjusted for the following potential confounding variables: age, BMI, PIR, and serum cotinine. These variables were included in final models because when individually added to unadjusted models, the beta estimate for the metal biomarker changed by >10% for the majority of metal biomarkers. We also considered controlling for serum iron concentration, but we did not see a relationship with select metals (Mn and Mo) to warrant iron as a potential confounder. Urinary creatinine concentration was additionally added to adjusted models for metals in urine to adjust for variability in urinary output (28). In unadjusted and adjusted models, testosterone was log-transformed because it did not follow a normal distribution. Serum cotinine and age were modeled untransformed, whereas all metal biomarkers were log-transformed to improve model fit. BMI was categorized into <18.50 kg/m2 (underweight), 18.50-24.99 kg/m2 (normal weight), and ≥25.00 kg/m2 (overweight) (29); PIR was categorized into ≤0.999 (below poverty level), 1.000-1.850 (low income), 1.851-3.500 (middle income), and ≥3.501 (high income) (30,31); race was categorized into Mexican American, other Hispanic, non-Hispanic Black, non-Hispanic White, and other/multi-racial; and serum cotinine was categorized into <1 ng/ml (no or little secondhand tobacco smoke (STS) exposure), 1-10 ng/ml (high STS exposure), and >10 ng/ml (likely smoker) (31,32). In adjusted models, categories of underweight and normal weight, Mexican American and other Hispanic, and high STS exposure and likely smoker were each collapsed into single categories to increase sample size per category, resulting in the following categories: underweight/normal weight, Mexican American/other Hispanic, and high STS exposure/likely smoker. In addition to performing regression analyses where metal concentration was modeled as a continuous variable, we also evaluated relationships with testosterone where metal concentration was categorized into quartiles to explore potential non-linear relationships. We chose not to use sampling weights in our analysis because when variables employed in the calculation of sampling weights are also included in statistical models, a weighted analysis can lead to decreased precision of effect estimates (31,33). In NHANES 2011-2012, there was oversampling of certain racial, income, and age groups and, as a result, race, income, and age were also used in the calculation of sampling weights (34). Because we adjusted for race, income, and age in our statistical models, an unweighted analysis was more appropriate than a weighted analysis. For each metal biomarker, we performed four separate regression analyses: 1) unadjusted (continuous), 2) adjusted (continuous), 3) unadjusted (quartiles), and 4) adjusted (quartiles). To facilitate interpretation of the beta coefficients, results were expressed as percent change in testosterone concentration associated with a doubling (i.e., 100% increase) in metal concentration (equation: % change = [2̂beta – 1]*100) where metal concentration was modeled as a continuous variable (Table 3), and percent change in testosterone concentration (equation: % change = [êbeta – 1]*100]) where metal concentration was categorized into quartiles (Figure 1).
Table 3. Percent change in serum testosterone concentration associated with a doubling (100% increase) in metal concentration in men aged 18-55 years from NHANES 2011-2012 (n=484).
| Unadjusted models | Adjusted modelsa | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Metal | %Change | 95% CI | p-value | % Change | 95% CI | p-value |
| Blood | ||||||
| Cadmium | 5.50 | 2.36, 8.73 | 0.001* | 4.66 | 0.62, 8.87 | 0.023* |
| Lead | 5.96 | 1.61, 10.50 | 0.007* | 6.65 | 2.09, 11.41 | 0.004* |
| Manganese | -0.49 | -8.21, 7.89 | 0.906 | -0.64 | -8.22, 7.57 | 0.874 |
| Selenium | -4.68 | -22.77, 17.65 | 0.655 | 12.12 | -8.42, 37.26 | 0.267 |
| Urine | ||||||
| Arsenic | -0.29 | -2.53, 2.00 | 0.802 | -1.25 | -3.73, 1.29 | 0.330 |
| Cadmium | -1.56 | -4.26, 1.21 | 0.265 | 0.03 | -3.68, 3.89 | 0.987 |
| Lead | -0.60 | -3.59, 2.49 | 0.701 | -0.80 | -4.29, 2.81 | 0.659 |
| Manganese | -1.61 | -5.49, 2.43 | 0.429 | -1.58 | -5.35, 2.35 | 0.425 |
| Molybdenum | -2.67 | -5.66, 0.41 | 0.089 | -4.26 | -7.70, -0.69 | 0.020* |
| Thallium | -3.32 | -6.98, 0.49 | 0.087 | -3.82 | -8.14, 0.71 | 0.097 |
| Serum | ||||||
| Copper | -18.27 | -28.23, -6.93 | 0.002* | -16.43 | -26.64, -4.79 | 0.007* |
| Selenium | 1.91 | -15.41, 22.78 | 0.842 | 8.46 | -9.09, 29.39 | 0.367 |
| Zinc | 12.80 | -3.08, 31.28 | 0.120 | 13.43 | -1.68, 30.85 | 0.084 |
Abbreviations: CI, confidence interval
Models adjusted for 1) age [continuous: years], 2) BMI [categorical: overweight, referent=normal weight/underweight], 3) PIR [categorical: low, middle, and high income, referent=below poverty level], 4) race [categorical: Mexican American/other Hispanic, non-Hispanic Black, and non-Hispanic White, referent=other/multi-racial], 5) serum cotinine [categorical: high STS exposure/likely smoker, referent=no or little STS exposure], and 6) urinary creatinine [continuous: mg/dL] (for metals in urine only).
Statistically significant association between metal and testosterone.
Figure 1.
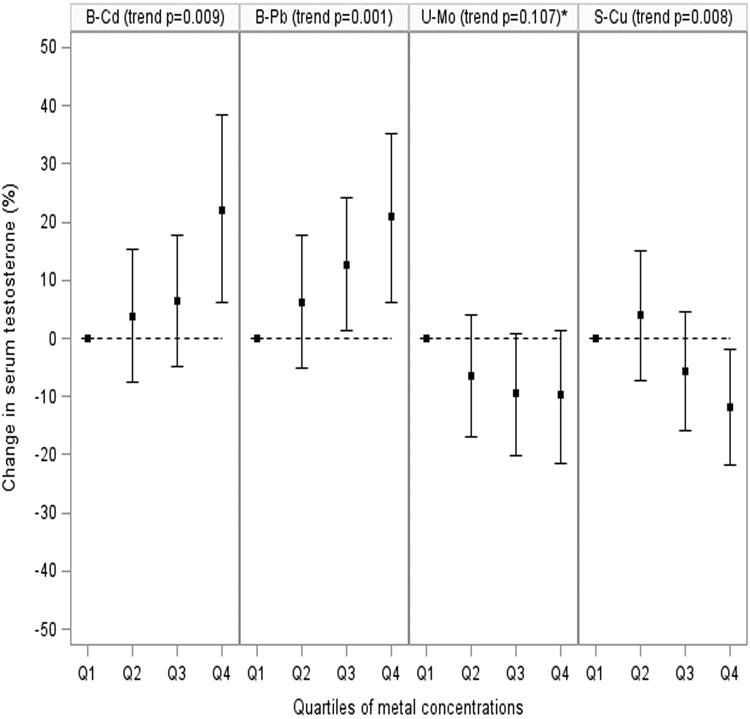
Percent change in serum testosterone concentration associated with metal quartile concentration relative to lowest metal quartile concentration in men aged 18-55 years from NHANES 2011-2012 (n=484). Results adjusted for age, BMI, PIR, race, serum cotinine, and urinary creatinine (for urinary Mo only). B-Cd=blood cadmium; B-Pb=blood lead; U-Mo=urinary molybdenum; and S-Cu=serum copper.
*When categorized into quintiles, the inverse trend was statistically significant (p=0 020).
Results
Table 1 shows the demographic characteristics of the subset of men (n=484) from NHANES 2011-2012 analyzed in our study. Overall, these participants had a median age of 35 years, were predominantly overweight (65.91%), high income (35.91%), and non-Hispanic White (36.16%), and had no to little STS exposure (65.91%). The demographic characteristics of the subset of men were comparable to those of the overall sample of 18-55-year-old men (data not shown).
Table 1. Characteristics of men aged 18-55 years from NHANES 2011-2012 (n=484).
| Variable | Median (IQR)/N (%) |
|---|---|
| Age (years) | 35 (24, 45) |
| BMI (kg/m2) | |
| <18.50 (underweight) | 6 (1.24) |
| 18.50-24.99 (normal weight) | 159 (32.85) |
| ≥25.00 (overweight) | 319 (65.91) |
| PIR | |
| ≤0.999 (below poverty level) | 120 (24.79) |
| 1.000-1.850 (low income) | 109 (22.52) |
| 1.851-3.500 (middle income) | 103 (21.28) |
| ≥3.501 (high income) | 152 (31.40) |
| Race | |
| Mexican American | 56 (11.57) |
| Other Hispanic | 43 (8.88) |
| Non-Hispanic Black | 113 (23.35) |
| Non-Hispanic White | 175 (36.16) |
| Other/multi-racial | 97 (20.04) |
| Serum cotinine (ng/ml) | |
| <1 (no or little STS exposure) | 319 (65.91) |
| 1-10 (high STS exposure) | 30 (6.20) |
| >10 (likely smoker) | 135 (27.89) |
Abbreviations: BMI, body mass index; IQR, interquartile range; PIR, poverty index ratio; STS, secondhand tobacco smoke.
Table 2 shows the distributions of the analyte concentrations in blood, urine, and/or serum. All analytes were detected in greater than 95% of the samples, except for blood Cd (72.7%), urine cadmium (84.3%) and manganese (69.2%), and serum cotinine (79.3%).
Spearman's rank correlation coefficients for all possible pairs of metal biomarkers were very weak to moderate. The strongest correlation coefficient (r=0.54) was observed between urinary Pb and urinary Cd. About 40% of the correlations were statistically significant (data not shown).
Table 3 shows the percent change in testosterone associated with a doubling (100 % increase) in metal concentration where metal was modeled as a continuous variable. In unadjusted models, blood Cd (5.50%; 95% confidence interval (CI): 2.36, 8.73%; p=0.001) and Pb (5.96%; 95% CI: 1.61, 10.50; p=0.007) were positively associated with testosterone, whereas serum Cu (-18.27%; 95% CI: -28.23, -6.93%; p=0.002) was inversely associated with testosterone. When adjusting for potential confounders, urinary Mo (-4.26%; 95% CI: -7.70, -0.69%;p=0.020) was inversely associated with testosterone. Blood Cd and Pb and serum Cu remained statistically significant and in the same directions in adjusted models.
When metal concentrations were modeled as quartiles, blood Cd (trend p=0.0001) and Pb (trend p=0.003) were again positively associated with testosterone, whereas urinary Tl (trend p=0.030) and serum Cu (trend p=0.001) were inversely associated with testosterone in unadjusted models (data not shown). Shown in Figure 1 is the percent change in testosterone associated with quartiles of blood Cd (trend p=0.009) and Pb (trend p=0.001), urinary Mo (trend p=0.107), and serum Cu (trend p=0.008) adjusted for potential confounders. Similar to the continuous measures, blood Cd and Pb were positively associated with testosterone, whereas urinary Mo and serum Cu were inversely associated with testosterone. Although the relationship between urinary Mo and testosterone was only statistically suggestive (trend p=0.107), in a sensitivity analysis the inverse trend was statistically significant (p=0.020) when we categorized urinary Mo into quintiles, which is in line with the results from the continuous measure analysis (data not shown). Positive associations were also observed for quartiles of blood Se (trend p=0.038) and serum Zn (trend p=0.024), but no statistically significant relationship was observed for quartiles of urinary Tl in adjusted models (data not shown).
Discussion
In this study, we evaluated the associations between biomarkers of metals and testosterone in men aged 18-55 years from NHANES 2011-2012. After adjusting for potential confounders, urinary Mo and serum Cu were inversely associated with serum testosterone, whereas blood Cd, Pb, and Se and serum Zn were positively associated with serum testosterone.
To our knowledge, only two previous peer-reviewed epidemiology studies have evaluated the relationship between Mo and testosterone. Similar to our findings, Meeker et al. (1) also reported a significant inverse association between blood Mo and testosterone in 219 men from two infertility clinics in Michigan. A separate analysis of the same cohort found a significant inverse association between blood Mo and semen quality (3), although this finding was independent of testosterone, which is supported by the evidence that testosterone is poorly correlated with semen quality in men from infertility clinics (35). A study of 118 men recruited from an infertility clinic in China also examined the relationship between urinary Mo and testosterone, but reported no significant findings (36). Compared to the men in our study, geometric mean urinary Mo concentrations among the Chinese men were higher (41.54 μg/L vs. 55.39 μg/L), but the most highly exposed participant in the China study had a urinary Mo concentration approximately 75% of that in our study (406 μg/L vs. 541 μg/L). The highest Mo quartile was associated with a decline of 39 ng/dL of testosterone in relation to the lowest Mo quartile, but given the small sample size, confidence intervals were wide and statistical power was weak. Acute human Mo poisoning has also been documented in a male in his late-30s who experienced low testosterone, low libido, and psychosis among other health ailments following excessive consumption of Mo dietary supplements for an 18-day period (cumulative dose=13.5 mg, equivalent to 300-800 μg/day) (37). The evidence in humans is supported by several animal studies describing reduced testosterone, lack of libido, seminiferous tubule degeneration, reduced semen volume and sperm concentration, and poor sperm motility and morphology following the oral administration of Mo (38-43). The biological mechanism explaining the inverse association between Mo and testosterone in humans remains unclear. However, animal studies of Mo treatment have reported the accumulation of Mo in the brain, testes, and adrenal and pituitary glands (42,44,45). This, along with the evidence that Mo interacts with steroid receptors (46), suggests that Mo may alter testosterone levels in humans through one or more pathways or mechanisms, but further research in both humans and animal models is needed. Decreased testosterone levels among men has potentially important public health implications (4), since low testosterone has been linked in humans to metabolic syndrome (5), diabetes (6), cardiovascular disease (7), fractures (8,9), neurodegenerative disorder (10,11), and mortality (12,13).
Pb and Cd are historically some of the most widely studied environmental agents in both occupational and non-occupational contexts (47,48), and specifically the most studied metals in relation to testosterone. Although the results of some human studies have been conflicting with ours (49-54), similar to our study, others have reported significant or suggestive positive associations between biomarkers of Pb and Cd and testosterone in cohorts of men from around the world. Specifically, positive relationships have been noted in U.S. men from infertility clinics (Pb and Cd) (1), Chinese men that worked or lived near a smelter (Cd) (14,15), and Croatian men from an andrology clinic (Pb and Cd) (16,17). Based on animal studies, some have hypothesized that the toxicity of Cd relates to the magnitude and duration of exposure, with acute exposures leading to decreased testosterone from testis necrosis, whereas chronic low exposures resulting in increased testosterone from the induction of protective Cd-binding proteins (15). It is possible that Pb affects testosterone synthesis and secretion much in the same way. Elevated testosterone levels in relation to environmental exposures may also be of concern since high testosterone has been associated with increased risk of cancer (18) and aggressive personality disorders (19).
The evidence for a potential relationship between male testosterone and metals other than Pb and Cd is much more limited. Consistent with our analysis, the smaller Chinese study conducted in an infertility clinic reported a suggestive inverse association between Cu and testosterone, which, as the authors hypothesized, may potentially reflect impaired Leydig cell function (36). However, for several other metals (Pb, Cd, Se, Zn), the results of the Chinese study were inconsistent with those in our study. Meeker et al. (1) also reported a suggestive positive association between blood Zn and testosterone in men from infertility clinics, but no association with blood Se and a significant positive association with blood Cu, which is contrary to the findings of our study.
One interesting finding in the present study was inconsistent results for different biomarkers of the same metal, which may reflect the importance of the selected biomarker and the timing of exposure on altering testosterone levels. For example, blood and urinary Pb reflect short-term exposure to Pb on the order of months (55). Although these biomarkers of Pb are correlated, urinary Pb does not predict blood Pb (55), possibly explaining why we did not find an association between urinary Pb and testosterone. On the other hand, blood Se is believed to reflect long-term exposure better than serum Se (56), possibly explaining why we did not find an association between serum Se and testosterone if long-term exposure is more important in relation to testosterone. The selection of the appropriate exposure biomarker, whether in blood, urine, or other human tissues or fluids, should be carefully considered when conducting future epidemiology studies of these and other metals in relation to testosterone.
Strengths of our study included analysis of serum testosterone by HPLC-MS/MS, which provided greater sensitivity than commonly-used immunoassay-based methods (57), a reasonably large sample size, and a variety of metals analyzed. Some limitations included the cross-sectional study design, which limited conclusions of causality due to temporal ambiguity, and the large number of metal-testosterone comparisons made, which may have led to some significant findings due to chance alone. Because we chose not to use sampling weights in our analysis, the results may not be fully generalizable to men of the same age range from the general U.S. population. While it may be appropriate to adjust statistical models for multiple metals simultaneously (1), in our analysis, metals that were associated with testosterone were weakly correlated with each another, thus the potential for confounding was limited. Metals may also interact with one another to impact testosterone levels in an additive or a multiplicative manner. However, we did not test for effect modification between metals since large sample sizes are needed to detect interactions. Future analyses of NHANES data should explore these interactions if testosterone and metals continue to be measured in future years. Finally, due to homeostatic controls, the concentrations of essential metals that were examined in this study (i.e., Cu, Mn, Mo, Se, and Zn) may not reflect the true exposure. For example, up to 5% of ingested Mn is absorbed through the gastrointestinal tract (58) with the remainder excreted as waste. Thus, the biomarkers of Mn, as well as other essential metals, used in our study may underestimate true exposure.
In conclusion, biomarkers of Mo and several other metals (Cu, Cd, Pb, Se, Zn) were associated with altered testosterone in men of reproductive age from NHANES 2011-2012. Because altered testosterone levels has been linked to a wide variety of adverse health effects, additional epidemiology studies are needed in other populations from the U.S. and other countries to confirm the results of our analysis.
Acknowledgments
R.C.L. is supported by The Rackham Predoctoral Fellowship Award from the University of Michigan.
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meeker JD, Rossano MG, Protas B, Padmanahban V, Diamond MP, Puscheck E, et al. Environmental exposure to metals and male reproductive hormones: circulating testosterone is inversely associated with blood molybdenum. Fertil Steril. 2010;93:130–140. doi: 10.1016/j.fertnstert.2008.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Serafim A, Company R, Lopes B, Rosa J, Cavaco A, Castela G, et al. Assessment of essential and nonessential metals and different metal exposure biomarkers in the human placenta in a population from the south of Portugal. J Toxicol Environ Health A. 2012;75:867–877. doi: 10.1080/15287394.2012.690704. [DOI] [PubMed] [Google Scholar]
- 3.Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Cadmium, lead, and other metals in relation to semen quality: human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect. 2008;116:1473–1479. doi: 10.1289/ehp.11490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. Laboratory procedures manual, total testosterone in serum, NHANES 2011-2012. Atlanta (GA): Centers for Disease Control and Prevention; 2014. Jan, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/TST_G_met.pdf. [Google Scholar]
- 5.Saad F, Gooren L. The role of testosterone in the metabolic syndrome: a review. J Steroid Biochem Mol Biol. 2009;114:40–43. doi: 10.1016/j.jsbmb.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Feinleib M, Zhang L, Rohrmann S, Rifai N, Nelson WG, et al. Androgens and diabetes in men: results from the Third National Health and Nutrition Examination Survey (NHANES III) Diabetes Care. 2007;30:234–238. doi: 10.2337/dc06-1579. [DOI] [PubMed] [Google Scholar]
- 7.Hakimian P, Blute M, Jr, Kashanian J, Chan S, Silver D, Shabsigh R. Metabolic and cardiovascular effects of androgen deprivation therapy. BJU Int. 2008;102:1509–1514. doi: 10.1111/j.1464-410X.2008.07933.x. [DOI] [PubMed] [Google Scholar]
- 8.Tuck SP, Francis RM. Testosterone, bone and osteoporosis. Front Horm Res. 2009;37:123–132. doi: 10.1159/000176049. [DOI] [PubMed] [Google Scholar]
- 9.Hu MI, Gagel RF, Jimenez C. Bone loss in patients with breast or prostate cancer. Curr Osteoporos Rep. 2007;5:170–178. doi: 10.1007/s11914-007-0013-1. [DOI] [PubMed] [Google Scholar]
- 10.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherrier MM. Testosterone effects on cognition in health and disease. Front Horm Res. 2009;37:150–162. doi: 10.1159/000176051. [DOI] [PubMed] [Google Scholar]
- 12.Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93:68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shores MM, Matsumoto AM, Sloan KL, Kivlahan DR. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166:1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 14.Zeng X, Lin T, Zhou Y, Kong Q. Alterations of serum hormone levels in male workers occupationally exposed to cadmium. J Toxicol Environ Health A. 2002;65:513–521. doi: 10.1080/15287390252807975. [DOI] [PubMed] [Google Scholar]
- 15.Zeng X, Jin T, Buchet JP, Jiang X, Kong Q, Ye T, et al. Impact of cadmium exposure on male sex hormones: a population-based study in China. Environ Res. 2004;96:338–344. doi: 10.1016/j.envres.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Jurasovicé J, Cvitkovicé P, Pizent A, Colak B, Telisman S. Semen quality and reproductive endocrine function with regard to blood cadmium in Croatian male subjects. Biometals. 2004;17:735–743. doi: 10.1007/s10534-004-1689-7. [DOI] [PubMed] [Google Scholar]
- 17.Telisman S, Colak B, Pizent A, Jurasovicé J, Cvitkovicé P. Reproductive toxicity of low-level lead exposure in men. Environ Res. 2007;105:256–266. doi: 10.1016/j.envres.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Yu MW, Chen CJ. Elevated serum testosterone levels and risk of hepatocellular carcinoma. Cancer Res. 1993;53:790–794. [PubMed] [Google Scholar]
- 19.von der Pahlen B. The role of alcohol and steroid hormones in human aggression. Vitam Horm. 2005;70:415–437. doi: 10.1016/S0083-6729(05)70014-5. [DOI] [PubMed] [Google Scholar]
- 20.CDC. [Accessed June 9, 2014];NHANES 2011-2012 overview. Available at: http://www.cdc.gov/nchs/nhanes/nhanes2011-2012/overview_g.htm.
- 21.CDC. [Accessed June 9, 2014];National Health and Nutrition Examination Survey, 2011-2012 data documentation, codebook, and frequencies, demographic variables and sample weights. Available at: http://wwwn.cdc.gov/nchs/nhanes/2011-2012/DEMO_G.htm#INDFMPIR.
- 22.CDC. [Accessed June 6, 2014];National Health and Nutrition Examination Survey, 2011-2012 data documentation, codebook, and frequencies, body measures. Available at: http://wwwn.cdc.gov/nchs/nhanes/2011-2012/BMX_G.htm.
- 23.CDC. Laboratory procedures manual, creatinine in urine, NHANES 2011-2012. Atlanta (GA): Centers for Disease Control and Prevention; 2011. Jan, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/ALB_CR_G_met_creatinine.pdf. [Google Scholar]
- 24.CDC. Laboratory procedures manual, antimony, arsenic, barium, beryllium, cadmium, cesium, cobalt, lead, manganese, molybdenum, platinum, strontium, thallium, tin, tungsten, and uranium in urine, NHANES 2011-2012. Atlanta (GA): Centers for Disease Control and Prevention; 2012. Mar, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/UHM_G_met_heavy_metals.pdf. [Google Scholar]
- 25.CDC. Laboratory procedures manual, zinc, copper, and selenium in serum, NHANES 2011-2012. Atlanta (GA): Centers for Disease Control and Prevention; 2013. Oct, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/CUSEZN_G_met_serum_elements.pdf. [Google Scholar]
- 26.CDC. Laboratory procedures manual, cadmium, lead, manganese, mercury, and selenium in whole blood, NHANES 2011-2012. Atlanta (GA): Centers for Disease Control and Prevention; 2012. Mar, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/PbCd_G_met_blood%20metals.pdf. [Google Scholar]
- 27.CDC. Laboratory procedures manual, cotinine in serum, NHANES 2011-2012. Atlanta (GA): Centers for Disease Control and Prevention; 2008. Sep, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/COT_G_met_cotinine.pdf. [Google Scholar]
- 28.Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. [Accessed June 9, 2014];BMI classification. Available at: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 30.NCHS. Analytical reporting guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988-1994) Hyattsville (MD): National Center for Health Statistics Centers for Diseases Control and Prevention; 1996. Oct, Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes3/nh3gui.pdf. [Google Scholar]
- 31.Silver MK, Lozoff B, Meeker JD. Blood cadmium is elevated in iron deficient U.S. Children: a cross-sectional study. Environ Health. 2013;12:117. doi: 10.1186/1476-069X-12-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hukkanen J, Jacob P, 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57:79–115. doi: 10.1124/pr.57.1.3. [DOI] [PubMed] [Google Scholar]
- 33.Korn EL, Graubard BI. Epidemiologic studies utilizing surveys: accounting for the sampling design. Am J Public Health. 1991;81:1166–1173. doi: 10.2105/ajph.81.9.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NCHS. National Health and Nutrition Examination Survey: Analytic Guidelines, 1999-2010. Hyattsville (MD): National Center for Health Statistics, Centers for Disease Control and Prevention; 2013. Sep, Available at: http://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf. [Google Scholar]
- 35.Meeker JD, Godfrey-Bailey L, Hauser R. Relationships between serum hormone levels and semen quality among men from an infertility clinic. J Androl. 2007;28:397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- 36.Zeng Q, Zhou B, Feng W, Wang YX, Liu AL, Yue J, et al. Associations of urinary metal concentrations and circulating testosterone in Chinese men. Reprod Toxicol. 2013;41:109–114. doi: 10.1016/j.reprotox.2013.06.062. [DOI] [PubMed] [Google Scholar]
- 37.Momcilovicé B. A case report of acute human molybdenum toxicity from a dietary molybdenum supplement – a new member of the “Lucor metallicum” family. Arh Hig Rada Toksikol. 1999;50:289–297. [PubMed] [Google Scholar]
- 38.Thomas JW, Moss S. The effect of orally administered molybdenum on growth, spermatogenesis and testes histology of young dairy bulls. J Dairy Sci. 1951;34:929–934. [Google Scholar]
- 39.Jeter MA, Davis GK. The effect of dietary molybdenum upon growth, hemoglobin, reproduction and lactation of rats. J Nutr. 1954;54:215–220. doi: 10.1093/jn/54.2.215. [DOI] [PubMed] [Google Scholar]
- 40.Vyskocil A, Viau C. Assessment of molybdenum toxicity in humans. J Appl Toxicol. 1999;19:185–92. doi: 10.1002/(sici)1099-1263(199905/06)19:3<185::aid-jat555>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Van Niekerk FE, Van Niekerk CH. The influence of experimentally induced copper deficiency on the fertility of rams. I.Semen parameters and peripheral plasma androgen concentration. J S Afr Vet Assoc. 1989;60:28–31. [PubMed] [Google Scholar]
- 42.Lyubimov AV, Smith JA, Rousselle SD, Mercieca MD, Tomaszewski JE, Smith AC, et al. The effects of tetrathiomolybdate (TTM, NSC-714598) and copper supplementation on fertility and early embryonic development in rats. Reprod Toxicol. 2004;19:223–233. doi: 10.1016/j.reprotox.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Pandey R, Singh SP. Effects of molybdenum on fertility of male rats. Biometals. 2002;15:65–72. doi: 10.1023/a:1013193013142. [DOI] [PubMed] [Google Scholar]
- 44.Haywood S, Dincer Z, Jasani B, Loughran MJ. Molybdenum-associated pituitary endocrinopathy in sheep treated with ammonium tetrathiomolybdate. J Comp Pathol. 2004;130:21–31. doi: 10.1016/s0021-9975(03)00065-3. [DOI] [PubMed] [Google Scholar]
- 45.Haywood S, Dincer Z, Holding J, Parry NM. Metal (molybdenum, copper) accumulation and retention in brain, pituitary and other organs of ammonium tetrathiomolybdate-treated sheep. Br J Nutr. 1998;79:329–331. doi: 10.1079/bjn19980056. [DOI] [PubMed] [Google Scholar]
- 46.Dahmer MK, Housley PR, Pratt WB. Effects of molybdate and endogenous inhibitors on steroid-receptor inactivation, transformation, and translocation. Annu Rev Physiol. 1984;46:67–81. doi: 10.1146/annurev.ph.46.030184.000435. [DOI] [PubMed] [Google Scholar]
- 47.ATSDR. Toxicological profile for lead. Atlanta (GA): Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services; 2007. Aug, Available at: http://www.atsdr.cdc.gov/toxprofiles/tp13.pdf. [Google Scholar]
- 48.ATSDR. Toxicological profile for cadmium. Atlanta (GA): Agency for Toxic Substances and Disease Registry, U.S. Department of Health and Human Services; 2012. Sep, Available at: http://www.atsdr.cdc.gov/toxprofiles/tp5.pdf. [PubMed] [Google Scholar]
- 49.Mason HJ. Occupational cadmium exposure and testicular endocrine function. Hum Exp Toxicol. 1990;9:91–94. doi: 10.1177/096032719000900205. [DOI] [PubMed] [Google Scholar]
- 50.Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect. 2000;108:45–53. doi: 10.1289/ehp.0010845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McGregor AJ, Mason HJ. Chronic occupational lead exposure and testicular endocrine function. Hum Exp Toxicol. 1990;9:371–376. doi: 10.1177/096032719000900602. [DOI] [PubMed] [Google Scholar]
- 52.Ng TP, Goh HH, Ng YL, Ong HY, Ong CN, Chia KS, et al. Male endocrine functions in workers with moderate exposure to lead. Br J Ind Med. 1991;48:485–491. doi: 10.1136/oem.48.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alexander BH, Checkoway H, van Netten C, Muller CH, Ewers TG, Kaufman JD, et al. Semen quality of men employed at a lead smelter. Occup Environ Med. 1996;53:411–416. doi: 10.1136/oem.53.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Menke A, Guallar E, Shiels MS, Rohrmann S, Basaria S, Rifai N, et al. The association of urinary cadmium with sex steroid hormone concentrations in a general population sample of US adult men. BMC Public Health. 2008;8:72. doi: 10.1186/1471-2458-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergdahl IA, Skerfving S. Biomonitoring of lead exposure – alternatives to blood. J Toxicol Environ Health A. 2008;71:1235–1243. doi: 10.1080/15287390802209525. [DOI] [PubMed] [Google Scholar]
- 56.Gudmundsdottir EY, Gunnarsdottir I, Thorlacius A, Reykdal O, Gunnlaugsdottir H, Thorsdottir I, et al. Blood selenium levels and contribution of food groups to selenium intake in adolescent girls in Iceland. Food Nutr Res. 2012;56 doi: 10.3402/fnr.v56i0.18476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89:534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 58.Aschner JL, Aschner M. Nutritional aspects of manganese homeostasis. Mol Aspects Med. 2005;26:353–362. doi: 10.1016/j.mam.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


