Abstract
Purpose
Cisplatin induces nausea and emesis, even with antiemetic supportive care. To assess platinum exposure, which could activate nausea and emesis, we quantitated platinum in the brain and various organs, and hindbrain and spinal cord substance P, a key neuropeptide for the neuronal signaling of nausea and emesis.
Methods
Musk shrews, a model species for nausea and emesis research, were dosed intraperitoneally with 20 mg/kg cisplatin and euthanized at up to 72 h after injection. Concentrations of platinum were quantitated in plasma ultrafiltrate, plasma, lung, kidney, combined forebrain and midbrain, hindbrain, and spinal cord by flameless atomic absorption spectrometry. Hindbrains and spinal cords were analyzed for substance P by immunohistochemistry after injection of 20 or 30 mg/kg.
Results
Plasma ultrafilterable platinum concentrations decreased rapidly till 60 min after dosing and then more slowly by 24 h. The concentrations of total platinum in both the fore- and midbrain and the hindbrain were similar at all time points and were at least 20-fold lower than plasma total platinum concentrations. There were no significant changes in substance P immunoreactivity after cisplatin dosing. Histology revealed damage to the renal cortex by 72 h after injection of cisplatin.
Conclusions
This is the first study to examine platinum concentrations in musk shrews after administration of cisplatin, and delineate substance P immunohistochemical staining in the hindbrain and spinal cord of this species. The platinum concentrations detected in the brain could potentially contribute to the neurological side effects of cisplatin, such as nausea and emesis.
Introduction
Cisplatin is considered to be one of the most emetogenic of the therapeutically relevant cancer chemotherapies and as such has been widely used to study the efficacy of antiemetic agents [1,2]. Cisplatin causes emesis in almost all patients if prophylactic therapy is not provided [3]. Cisplatin chemotherapy results in two distinct phases of nausea and vomiting: the acute phase that occurs within the first few hours after cisplatin infusion and the delayed phase that occurs within 48–72 h after the infusion [4]. The two phases are clearly distinct in that currently available antiemetics have efficacy during the acute phase, but much less during the delayed phase [5]. Although acute chemotherapy-induced nausea and emesis are largely controlled by available antiemetics, patients still experience these side effects in the delayed phase, especially nausea [e.g., 6].
The acute phase of chemotherapy-induced nausea and vomiting (CINV) is believed to result from serotonin released from enteroendocrine cells in the gastrointestinal tract which activates local vagal afferent fibers containing 5-HT3 receptors [7,8]. Indeed, 5-HT3 receptor antagonists are effective antiemetics during the acute phase of cisplatin associated CINV, but of limited value during the delayed phase [2]. The biological mechanisms for cisplatin-based CINV remain unclear but studies suggest that NK1 (neurokinin type 1) receptors located in the hindbrain are involved and direct action of cisplatin or a circulating factor on the brain could play a role, because lesions of the area postrema in the hindbrain but not the vagus blocks cisplatin-induced emesis in the ferret [9]. Studies using ferrets [10] and dogs [11] have shown that 5-HT3 antagonists are effective in both the acute and delayed phases of emesis, which is not an accurate reflection of the human response. Studies in musk shrews suggest that 5-HT3 receptor antagonists are effective only in the acute phase and not during the delayed phase of cisplatin-induced emesis or activation of the brain [12,13]. Thus, the musk shrew is potentially a better model of human cisplatin-induced emesis than the ferret or dog.
Musk shrews appear to be a good model to study the biology of cisplatin-induced emesis during acute and delayed phases [12–15]; however, we currently lack critical information on the pharmacokinetics of platinum in musk shrews. Because no studies to date have examined the concentrations of platinum in shrews treated with cisplatin we examined in Study 1 the platinum plasma pharmacokinetics and tissue distribution in lungs, kidneys, fore- and midbrain, hindbrain and spinal cord between 5 min and 72 h after ip administration of cisplatin. In Studies 2 and 3, we assessed the impact of cisplatin on 6 h (acute phase) and 72 h (delayed phase) substance P immunoreactivity in the dorsal vagal complex of the hindbrain and spinal cord. We chose to focus on these areas because they are involved in generating the emetic reflex (hindbrain) and processing of pain signals (spinal cord); cisplatin treatment is known to produce both emesis and pain in patients [e.g., 16]. Substance P is the endogenous ligand for the NK1 receptor [17], and cisplatin injection was reported to increase substance P immunoreactivity in the area postrema in mink [18]. In addition, we performed histological analysis of kidney toxicity at these timepoints, since renal toxicity is a common and clinically relevant side-effect of cisplatin treatment [19].
Materials and Methods
Animals
Experimentally naïve adult female musk shrews were derived from a stock obtained from the Chinese University of Hong Kong; a Taiwanese strain of Suncus murinus [20]. Three studies were performed using a total of 83 female musk shrews (Study 1, n = 45, days of age = 34 to 40, body weight range = 29.3 to 43.9 g and mean ± SD = 37.9 ± 2.9 g; Study 2, n = 16, days of age = 36 to 40, body weight range = 25.8 to 46.2 g and mean ± SD = 36.0 ± 1.9 g; Study 3, n = 18, days of age = 84 to 105, body weight range = 35.8 to 44.2 g and mean ± SD = 30.7 ± 2.4 g). Animals were housed individually in clear plastic cages (28 × 17 × 12 cm; length x width x height), with a filtered air supply, under a 12:12 h light:dark cycle (lights on at 0700 h), in a temperature (~ 23 °C) and humidity (~ 40%) controlled environment. Food consisted of a mixture of 75% Purina Cat Chow Complete Formula and 25% Complete Gro-Fur mink food pellets [21]. Food and drinking water were freely available. All experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Animals were housed in an animal care facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Cisplatin
Cisplatin was obtained from Sigma-Aldrich (cis-Diammineplatinum(II) dichloride; P4394 – crystalline) and was mixed in sterile saline (0.15 M) for intraperitoneal (ip) injection. Intraperitoneal injections were administered as a solution of 20 mg cisplatin per 10 ml saline in Studies 1 and 2, and 30 mg cisplatin per 20 ml saline in Study 3.
Study 1: Pharmacokinetics of cisplatin
On the day prior to the study, 45 animals were stratified into time point groups such that each group contained one small, medium and large-weight shrew. Animals were dosed cisplatin (ip), 20 mg/kg, 0.01 ml/g exact body weight in saline and euthanized at 5, 10, 15, 30, 45 min, or 1, 1.5, 2, 4, 6, 24, 48, and 72 h after administration of cisplatin. Shrews were euthanized using and overdose of CO2 and blood was collected by cardiac puncture using a heparinized 22 gauge needle and 3 ml syringe. The blood was centrifuged at 12,000 x g for 4 min to obtain plasma. Approximately 300 μl of plasma was then immediately transferred to an Amicon Ultra 30k centrifugal filter unit and centrifuged at 14,000 x g for 5 min at 4 °C to obtain ultrafiltrate. The following tissues were quickly removed, placed on ice, weighed and snap frozen in liquid nitrogen: lungs, kidneys, spinal cord, combined forebrain and midbrain, and hindbrain.
To quantitate platinum in the plasma, plasma ultrafiltrate, and tissues, samples were processed as follows. Prior to analysis, organs were homogenized in phosphate buffered saline (PBS), pH 7.4 (tissue + 3 parts PBS, v/g). Brain samples were homogenized using a sonic probe cell disruptor. Platinum quantitation was performed using a Perkin-Elmer model 1100 flameless atomic absorption spectrometer (Perkin-Elmer, Norwalk, CT) as previously described [22]. The platinum quantitation range was 0.0245–0.784 μg/mL, and samples were diluted to within the assay range. Platinum concentrations were calculated from a standard calibration curve prepared fresh daily. Plasma total and ultrafiltrate were run against a standard curve prepared in 0.25% Triton X-100. Lung, kidney, spinal cord, combined midbrain and forebrain, and hindbrain were analyzed against a standard curve prepared in control mouse homogenate prepared in PBS and values adjusted to Pt/g wet tissue weight. Pharmacokinetic parameters for plasma total and ultrafilterable platinum and tissue platinum were extracted from the data by non-compartmental methods with PK Solutions 2.0 (Summit Research Services, Montrose, CO).
Studies 2 and 3: Immunohistochemistry of substance P and kidney toxicity
In Study 2, sixteen experimentally naïve animals were dosed with saline, euthanized at 6 (n = 3) and 72 h (n = 3) later, or 20 mg/kg cisplatin, euthanized at 6 (n = 5) and 72 h (n = 5) later. Study 3 included an additional group of experimentally naïve animals dosed with saline (n = 9) or 30 mg/kg cisplatin (n = 9) and euthanized at 72 h after injection. Body weights were measured daily in Studies 2 and 3. The subjects were euthanized with 0.2 ml of Beuthanasia-D (ip) and transcardially perfused with saline (0.15 M) followed by 4% paraformaldehyde with 1.4% l-lysine and 0.2% sodium metaperiodate in 0.1 ml phosphate buffer. Brains and spinal cords (T8 to T10; the level of gastrointestinal nerves) were post-fixed overnight in 4% paraformaldehyde and cryoprotected in 20% sucrose solution in 0.1 M PBS. After 24 h, the brains and spinal cords were removed from sucrose, frozen on dry ice, and stored at −80 °C. All brains and spinal cords were cut into 35 μm sections using a cryostat (Microm HM 500M) at −21 °C and stored in cryoprotectant [23].
For immunohistochemistry, tissue was rinsed in 0.1 M PBS for 50 min and 0.5% hydrogen peroxide for 15 min. The tissue was transferred to the primary antiserum, 1:5,000 anti-substance P antibody (Millipore AB1566, polyclonal; overnight, room temperature) containing 0.01% normal serum and 0.03% triton X-100. On day two, the tissue was rinsed in 0.1 M PB for 1 h and transferred and incubated for 1 h in the secondary antiserum, 1:500 in PB, 10% triton X-100, and 0.01% normal serum. The tissue was rinsed in 0.1 M PB for 30 min, transferred to an avidin-biotin complex (Elite kit; Vector Laboratories) with 10% triton X-100, and 0.1 M PB for 90 min. The tissue was then rinsed in 0.1 M PB for 30 min, and then 0.1 M sodium acetate buffer for 10 min. The tissue was submerged in diaminobenzidine (DAB) and 2.5 % nickel in sodium acetate buffer with hydrogen peroxide for 5 min. The reaction was stopped with sodium acetate buffer rinse, and then 0.1 M PB rinse for 20 min. The tissue sections were mounted onto Superfrost Plus slides (Fisher Scientific). Microscope slides were allowed to dry overnight and then rinsed in Milli-Q water, 70% ethanol, 95% ethanol, 100% ethanol, and xylenes. Slides were coverslipped with cytoseal mountant and left to harden horizontally overnight.
Histological images for substance P quantification were taken on a Nikon Eclipse E800 microscope with a Nuance FX multispectral imaging system (Nuance 2.10 software; Cambridge Research and Instrumentation Inc.; with filter and setting set to DIA). Two sections of the spinal cord (high, T8, and low, T10) and one section of the middle hindbrain (the region with larger area of the area postrema) were analyzed for the presence of substance P labeling. Regions analyzed included the substantia gelatinosa of the spinal cord, the area postrema (AP), the nucleus of the solitary tract (NTS), and the dorsal motor nucleus (DMN) [24]. For spinal cord sections, a region was drawn that covered approximately 1/3 of the most dorsolateral part of the dorsal horn, a region known to express substance P in other species [25,26]. Values represent the average of staining for the left and right sides of the spinal cord (both sections) and hindbrain. Grayscale values were collected for each region of interest using computer software (ImageJ; http://imagej.nih.gov/ij/). Staining was analyzed by two investigators blinded to the experimental condition and we report the average values of these two assessments. In Study 2, one saline animal did not have an appropriate NTS/DMN/AP section for analysis, and an additional saline animal was missing an AP from the appropriate section; therefore, these values are not included in the analysis.
For renal pathology (Study 2), tissues were collected in 10% formalin at necropsy. Once fixed, tissue sections were processed, embedded in paraffin, sectioned at 4 μm, and stained with hematoxylin and eosin (H&E). Blinded histopathologic examination of the tissue sections was conducted by a veterinary pathologist.
Statistical analysis
Substance P immune-labeling was analyzed using Kruskal-Wallis ANOVA for Study 2 and Mann-Whitney U for Study 3. Study 2 salines (6 and 72 h) were combined into a single group for additional statistical power. Student t-tests and ANOVA were used to compare body and organ weights. Post hoc tests following ANOVA were conducted using the Holm correction for cumulative type I errors. Statistical significance was set at a p-value less than 0.05.
Results
Study 1: Pharmacokinetics of cisplatin
The body weights of the shrews through 72 h post-dosing were not significantly different from pre-dose body weights. There were also no significant differences between groups in the weights of the lungs, kidneys, the hindbrain, forebrain or spinal cord. The kidneys from shrews euthanized 72 h after treatment appeared pale compared to the control shrew kidneys. Similarly, the spleen weights of treated shrews at 72 h were significantly smaller than the spleen weights of controls euthanized at 72 h (t-test, p < 0.05; 0.10 ± 0.02 g vs. 0.18 ± 0.01 g; mean ± SEM).
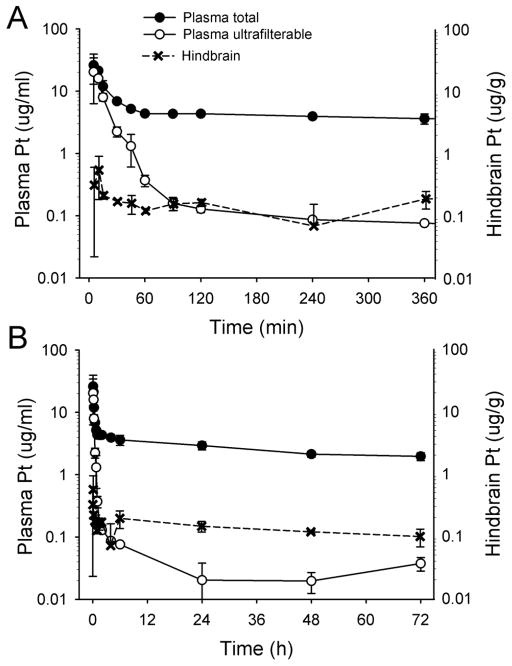
Table 1 shows the concentration of platinum in plasma and organs. The concentration of ultrafilterable platinum and total platinum in shrew plasma compared to hindbrain platinum is presented in Figure 1. The total plasma platinum concentrations dropped rapidly from a mean concentration of 26.1 μg/mL at 5 min to 6.9 μg/ml at 30 min after cisplatin injection, but then decreased more slowly between 1 and 72 h. In contrast, ultrafiltrable platinum decreased much more rapidly from 28.5 μg/ml at 5 min after injection to 0.16 μg/ml at 90 min after injection, indicative of tissue distribution and reaction with macromolecular plasma components. By 24 h the ultrafilterable platinum concentrations dropped below the lower limit of quantitation.
Table 1.
Platinum concentrations in plasma and organs.
| Time | Platinum concentrations (μg/ml (plasma) (μg/g, tissues); mean ± standard deviation) | ||||||
|---|---|---|---|---|---|---|---|
| Plasma Total | Plasma ultrafiltrate | Kidney | Lungs | Forebrain and midbrain | Hindbrain | Spinal Cord | |
| 5 min | 26.1 ± 13.2 | 20.3 ± 14.0 | 26.0 ± 18.2 | 13.3 ± 5.8 | 0.22 ± 0.19 | 0.32 ± 0.30 | 1.36 ± 0.65 |
| 10 min | 21.3 ± 1.2 | 16.0 ± 1.6 | 36.9 ± 5.7 | 23.0 ± 7.9 | 0.29 ± 0.12 | 0.56 ± 0.37 | 0.74 ± 0.36 |
| 15 min | 11.9 ± 2.7 | 7.95 ± 1.0 | 23.1 ± 6.4 | 14.3 ± 7.6 | 0.11 ± 0.03 | 0.22 ± 0.03 | 0.36 ± 0.14 |
| 30 min | 6.86 ± 0.43 | 2.25 ± 0.41 | 24.0 ± 2.9 | 13.2 ± 6.6 | 0.15 ± 0.03 | 0.17 ± 0.01 | 0.51 ± 0.14 |
| 45 min | 5.19 ± 0.53 | 1.31 ± 0.71 | 18.7 ± 4.7 | 6.60 ± 2.65 | 0.19 ± 0.03 | 0.16 ± 0.03 | 0.39 ± 0.16 |
| 1 h | 4.36 ± 0.10 | 0.37 ± 0.08 | 15.1 ± 2.8 | 5.30 ± 1.20 | 0.18 ± 0.06 | 0.12 ± 0.01 | 0.32 ± 0.12 |
| 1.5 h | 4.33 ± 0.05 | 0.16 ± 0.04 | 15.3 ± 2.8 | 3.67 ± 0.65 | 0.21 ± 0.09 | 0.16 ± 0.03 | 0.31 ± 0.21 |
| 2 h | 4.34 ± 0.42 | 0.13 ± 0.02* | 14.7 ± 1.2 | 3.75 ± 1.05 | 0.14 ± 0.03 | 0.17 ± 0.02 | 0.23 ± 0.08 |
| 4 h | 3.93 ± 0.15 | 0.09 ± 0.00* | 14.3 ± 0.3 | 4.44 ± 1.18 | 0.18 ± 0.04 | 0.07 ± 0.09 | 0.42 ± 0.45 |
| 6 h | 3.62 ± 0.67 | 0.08 ± 0.00* | 10.9 ± 1.3 | 4.06 ± 0.81 | 0.14 ± 0.03 | 0.19 ± 0.06 | 0.30 ± 0.12 |
| 24 h | 2.94 ± 0.43 | <LLQ | 9.88 ± 0.23 | 3.07 ± 0.32 | 0.10 ± 0.02 | 0.14 ± 0.03 | 0.18 ± 0.11 |
| 48 h | 2.13 ± 0.24 | <LLQ | 10.5 ± 1.7 | 2.50 ± 0.30 | 0.09 ± 0.01 | 0.12 ± 0.01 | 0.15 ± 0.02 |
| 72 h | 1.97 ± 0.29 | <LLQ | 8.20 ± 1.39 | 2.28 ±0.30 | 0.25 ± 0.06 | 0.10 ± 0.03 | 0.15 ± 0.01 |
| AUC 0-t(mg•h/L) | 200 | 6.28 | 747 | 220 | 9.45 | 9.63 | 14.0 |
| t½ (h) | 83.2 | 0.195 | 178 | 71.6 | 58.7 | 88.0 | 227 |
values were not used for derivation of PK parameters as these concentrations close to the LLQ likely represent small molecule inactive metabolites of carboplatin.
Fig. 1.
Plasma (total and ultrafilterable) and hindbrain platinum (Pt) concentrations (mean ± standard deviation) in musk shrews after injection of cisplatin (20 mg/kg, ip). A, first 360 min, and B, up to 72 h after cisplatin injection.
Distribution of platinum to the lungs and kidneys was rapid and peak concentrations of platinum (23.0 and 37.0 μg/g, respectively) were observed by 10 min after dosing (Table 1). After the initial distribution phase, the time course of lung platinum paralleled the plasma total platinum concentrations, while between 1 and 72 h, kidneys retained approximately 4-fold higher concentrations of total platinum than either lung or plasma. In contrast to the concentrations of total platinum in the rapidly equilibrating lungs and kidneys, peak concentrations of platinum in the hindbrain, forebrain plus midbrain, and spinal cord were much lower. Mean peak concentration in the fore- and midbrain occurred at 10 min and was 0.29 μg/g, slightly lower than the mean peak concentration in the hindbrain, which was 0.56 μg/g (Figure 1). In contrast, the spinal cord mean peak concentration occurred at 5 min and was considerably higher than the brain regions at 1.36 μg/g (Table 1).
The exposures to total platinum reflect these differences. Based on the area under the concentration time curve-values (Table 1), the greatest exposure to platinum in shrews occurs in the kidneys, followed by lung and plasma. Brain exposure was only about 5% of plasma total platinum exposure and spinal cord exposure was only about 7% of the plasma total platinum exposure. Very little platinum appeared to have reached the central nervous system (Table 1).
The non-compartmental analysis of ultrafilterable and total platinum in shrew plasma is summarized in Table 2. The half-life (t½) of ultrafilterable Pt is approximately 12 min while total Pt is retained much longer with a terminal t½ of 83 h. Ultrafilterable Pt is widely distributed to tissues as indicated by the apparent volume of distribution (Vd) of 577 L/kg and the clearance (Cl) of 2.05 L/h/kg.
Table 2.
Non-compartmental analysis of total and ultrafilterable (UF) plasma platinum concentration data and literature values.
| Study | Current | Current | Hennik 1987 [39] | Zamboni 2002 [40] | Kizu 1993[41] | Hardie 1991 [42] | Jacobs 2005 [43] | Urien 2004 [44] |
|---|---|---|---|---|---|---|---|---|
| Species | Shrew | Shrew | Mouse | Mouse | Rabbit | Dog | Rhesus Monkey | Human |
| Dose (mg/kg) | 20 | 20 | 10 | 10 | 3 | 4.5 | 2 | 0.67 |
| Route | IP | IP | IV | IV | IV | IV | 1 h IV | 0.5 h IV |
| Parameter | Total Pt (μg/ml) | UF Pt | UF Pt | UF Pt | UF Pt | UF Pt | UF Pt | UF Pt |
| Cmax (μg/mL) | 26.1 (13.2) | 20.3 (14.0) | 23.4 (2.2) | - | - | - | 2.43 (0.76) | - |
| Tmax (min) | 5 | 5 | - | - | - | - | - | - |
| t1/2 (h) | 83.2 | 0.195 | 0.10 | - | - | - | 0.331 (0.023) | 1.32 |
| AUC0-t (mg•h/L) | 200 | 6.28 | - | - | 4.04 (0.46) | - | 3.58 (1.18) | - |
| AUC0-inf (mg•h/L) | 437 | 6.33 | 4.38 | 3.9 | - | 1.77 | - | 0.737 |
| % AUC Extrapolated | 54 | 0.7 | - | - | - | - | - | - |
| Cl (L/h/kg) | 0.0298 | 2.05 | 2.28 | 1.67 | 0.743 | 1.65 (0.32) | 0.391 (0.130) | 0.588 |
| Vd (L/kg) | 3.57 | 577 | - | - | - | - | - | - |
Assuming typical human body weight of 70 kg. Conversions of parameter normalizations with kg and m2 based on Freireich [45].
Studies 2 and 3: Immunohistochemistry of substance P and kidney toxicity
Body weights
In Study 2, body weights of the 72 h 20 mg/kg cisplatin-injected shrews were significantly lower over the three days after injection [F(3,12) = 4.6, p < 0.03, one-way ANOVA; Pre-injection = 39.6 ± 1.3 g, Day 1 = 39.4 ± 1.4 g, Day 2 = 39. 1 ± 1.3 g, Day 3 = 36.4 g, mean ± SEM; no statistically significant effects with Holm mean comparisons]. Study 3 showed a reduction in body weight after 30 mg/kg cisplatin injection compared to saline control [F(3,48) = 57.7, p < 0.0005, two-way ANOVA, injection condition by day]. This effect was only detected on day 3 (saline = 38.8 ± 0.24 g and cisplatin = 34.5 ± 0.28 g; p < 0.05, Holm test) and not on pre-injection, Day 1, or Day 2 after injection (pre-injection, saline = 39.7 ± 0.25 g and cisplatin = 39.7 ± 0.29 g; Day 1, saline = 39.3 ± 0.29 g and cisplatin = 37.0 ± 0.27 g,; Day 2, saline = 39.3 ± 0.27 g and cisplatin = 36.9 ± 0.28 g; p > 0.05, Holm tests).
Substance P labeling
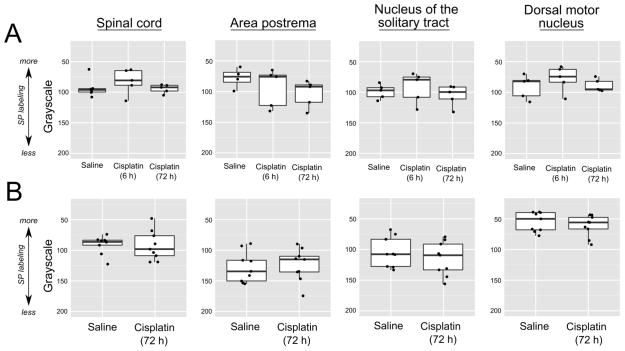
Substance P labeling was clearly evident in the dorsal horn of the spinal cord corresponding to the substantia gelatinosa (Fig. 2), and this was observed bilaterally in the spinal cord and throughout the dorsal vagal complex (AP, NTS, and DMN; Fig. 3). Particularly dense labeling was detected in the DMN (Fig. 3). However, there were no statistically significant differences between the saline conditions and the cisplatin conditions in either Study 2 (20 mg/kg) or Study 3 (30 mg/kg) (p > 0.5, Kruskal-Wallis ANOVA and Mann-Whitney U test; Fig. 4).
Fig. 2.
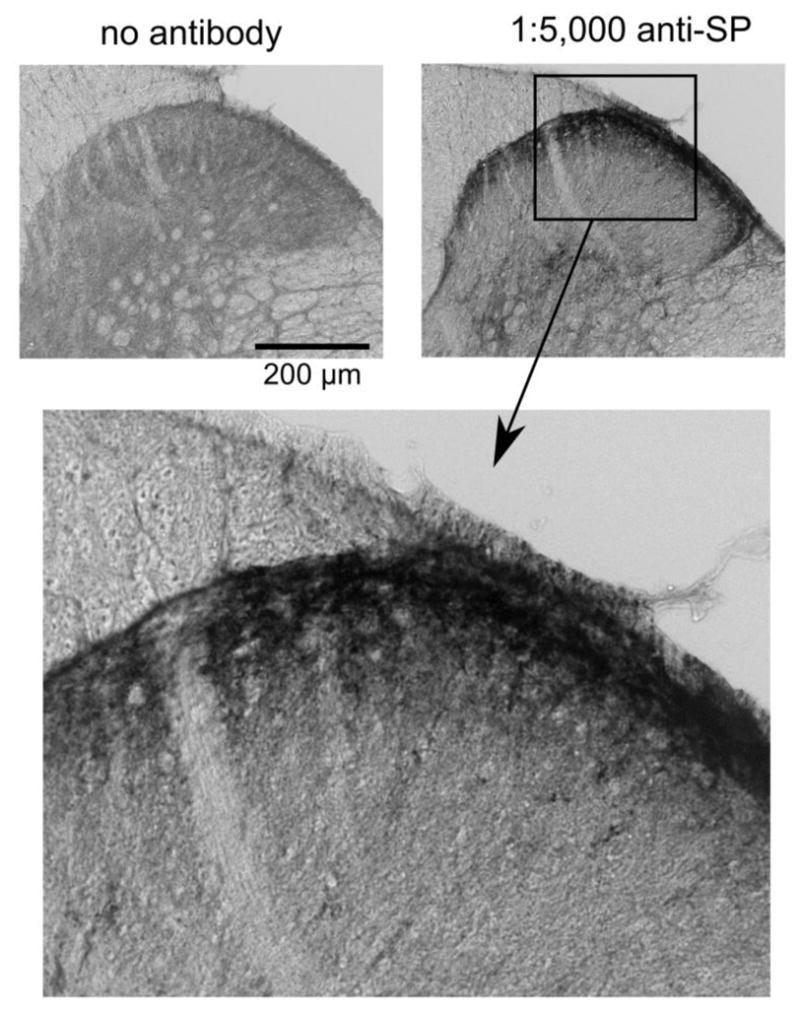
Representative substance P (SP) immunolabeling in the substantia gelatinosa of the musk shrew spinal cord, the terminal location of spinal afferent fibers [46]. The images show the effects of removing the primary antibody from the immunohistochemistry protocol compared to including a concentration of 1:5,000. Lower image is a higher magnification of the SP labeling in the upper right panel.
Fig. 3.

Hindbrain and spinal cord immunohistochemical labeling for substance P. Top panel shows the middle region of the dorsal vagal complex of the hindbrain containing the area postrema (AP), nucleus of the solitary tract (NTS), and dorsal motor nucleus (DMN). Lower panel shows the area of the dorsal horn of the spinal cord. Labeling was quantified on the both sides of the hindbrain and spinal cord but only one half of each transverse section is labeled.
Fig. 4.
Quantification of substance P (SP) labeling after saline and cisplatin (ip) injections in the spinal cord substantia gelatinosa and dorsal vagal complex (area postrema, nucleus of the solitary tract, and dorsal motor nucleus). A) Study 2, effects of saline or 20 mg/kg cisplatin. Tissues were collected at 6 or 72 h after injection. B) Study 3, effects of saline and 30 mg/kg cisplatin. Tissues were collected at 72 h after injection. Data are shown as box and whisker plots with the median, 1st and 3rd quartiles, and whiskers representing 1.5 times the inter-quartile range. A scatterplot of all values is overlayed.
Renal histology
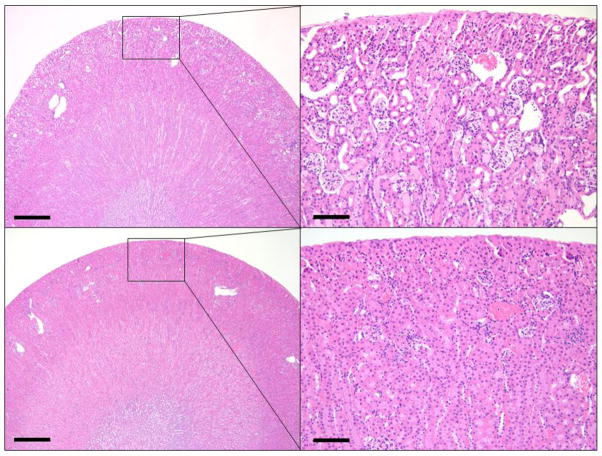
Blind review of the kidney histology from Study 2 (20 mg/kg) indicated that all animals injected with cisplatin and euthanized at 72 h (n = 5) had acute necrosis, degeneration, and attenuation of the renal tubular epithelium throughout the renal cortex which ranged from mild to severe. Prominent loss of the epithelial brush-border was found in affected tubules in addition to multifocal protein casts within the lumen. (Fig. 5). Cisplatin associated lesions were segmental to diffuse and most severe within the outer cortex. In contrast to 72 h, kidneys from saline injected shrews (n = 6) and 20 mg/kg cisplatin treated shrews at 6 h (n = 5) were indistinguishable, with no lesions present on histology.
Fig. 5.
Representative images of the renal cortex from cisplatin (20 mg/kg, ip; Study 2) and saline injected musk shrews. Top row images show acute necrosis, degeneration, and attenuation of the renal tubular epithelium throughout the renal cortex after cisplatin injection compared to saline injection (bottom row). Scale bars: Left images = 500 μm; Right images = 100 μm
Discussion
The musk shrew appears to be an advantageous small animal model to examine the mechanisms involved in cisplatin-induced emesis and exhibits both the acute and delayed emetic phases that are observed in humans [15,14,12]. Musk shrews demonstrate acute and delayed emesis after intraperitoneal injection of 20 to 30 mg/kg cisplatin [14,12]. These doses are approximately equivalent to 80 to 120 mg/m2 [using an estimated body surface area between the body weight of mouse and hamster, 27], which is a dose of cisplatin considered to be highly emetic in humans [2]. 5-HT3 receptor antagonists appear to have similar pharmacodynamic profiles in shrews as in humans, being effective during the acute phase of emesis, but much less effective in the delayed phase [2,12]. Although the shrew appears to be a good model to examine emetic responses to cisplatin, there was no information available on the plasma or tissue platinum pharmacokinetics following doses of cisplatin associated with emesis and no information on the acute toxicity of cisplatin in shrews.
Most of the preclinical studies describing the pharmacokinetics of cisplatin were conducted in rodents, which are not capable of vomiting [28]. Nakashima et al. [29] examined the distribution of cisplatin into intact brain and 9L malignant glioma containing brain after intracarotid infusion of 3.5 mg/kg cisplatin and found that the ratio of CSF platinum tounbound plasma platinum in normal brain (0.04) was much lower than that ratio in brain with glioma (0.69), suggesting that only a small amount of cisplatin derived platinum crosses an intact blood brain barrier, which is in agreement with our observations in the shrews. Moreover, since in our studies whole brain tissues were analyzed, at least part of the observed concentrations may be attributable to platinum residing in the vascular compartment of brain tissues, which makes up between 1–4% of the volume of the brain across species [30]. Similarly, Ramirez-Camacho et al [31] and Fernandez et al [32] examined the concentrations of platinum in Wistar rats following 5 mg/kg cisplatin ip and at 3 days after dosing found dry-weight brain concentrations of platinum of approximately 0.21 μg/g, which is very close to our values of 0.10–0.25 μg/g. When the pharmacokinetics of cisplatin were examined in mice, after ip administration of 3.75, 7.5 and 15 mg/kg cisplatin, Johnsson et al. [33] observed that in plasma and tissues there was an initial elimination phase between 9 and 30 min followed by a slow decline of total platinum concentrations over the next few days, very similar to the results we obtained in the shrews. These investigators [32,33,29,31] all observed that brain concentrations were much lower than the concentrations of total platinum in plasma, suggesting that very little of ultrafilterable platinum is able to penetrate the blood brain barrier. The concentrations of total platinum were highest in the kidneys in both rats and mice in agreement with our observations in the kidneys of shrews. In mice, the terminal t½ for total plasma platinum after administration of cisplatin was 55 h while the terminal t½ in kidneys was 76 h. In shrews, the estimated terminal t½ of total platinum in plasma was 83 h (Table 2) while the terminal half-life in kidneys was 178 h. Thus the shrew pharmacokinetics of platinum after administration of cisplatin appear similar to the results obtained in rodents.
We also evaluated the impact of cisplatin on a critical neurotransmitter for communication to the central nervous system. Substance P serves as a neurotransmitter for vagal and spinal afferent fibers connected to the brain and spinal cord, respectively; and is a signaling factor in vagal and hindbrain circuits for emesis and spinal afferent activation of pain [17]. Notably, another well-known side effect of cisplatin is peripheral neuropathy and pain [34]. Similar to other emesis models, like the ferret [35], we observed substance P immunoreactivity throughout the dorsal vagal complex. In contrast to the reported effect of cisplatin (7.5 mg/kg, ip) to induce an increase in substance P immunoreactivity in the AP of mink by 6 h [18], we did not observe any significant changes in staining in the AP, NTS or DMN of musk shrews, up to 30 mg/kg ip. We also observed no significant difference in substance P immunoreactivity in the spinal cord between saline and cisplatin treated shrews. It is possible that a longer time course is needed to detect changes in the spinal cord (or hindbrain); for example, one report showed increased substance P in the dorsal root ganglia of rats after 4.5 weeks of cisplatin injections (twice a week, 2 mg/kg, ip each injection) [36].
The toxicity of cisplatin in shrews was very similar to that observed in rodents and in humans. Although there were no significant changes in kidney weights in the saline treated control or the cisplatin treated shrews at 72 h, kidneys from the cisplatin treated shrews appeared pale when compared to the saline treated controls and there were gross changes apparent upon longitudinally bisecting of the kidneys. Further, approximately 25% of shrews receiving this dose of cisplatin die between 4 and 7 days after treatment even with administration of saline to increase diuresis (unpublished data). Approximately 20% of patients treated with high dose cisplatin experience dose-limiting renal toxicity [19]. In rats, treatment with a single ip dose of 6 mg/kg cisplatin resulted in marked focal necrosis in the proximal and distal tubules with the greatest lesions in the corticomedullary region [37]. In mice treated with single dose of cisplatin of 40 mg/kg (ip), by 72 h, there was severe tubular injury and only about 25% of the mice survived [38]. The renal pathology observed in shrews in our study appears to be similar to the acute toxicity of cisplatin in rodents, although interestingly, the effects appear to be most severe within the outer cortex and not at the cortico-medulary junction (Fig 5). Further investigation is necessary to determine the cause for this regional difference within the kidney of shrews as compared to rodents.
The spleen weights of the shrews treated with cisplatin and euthanized at 72 h after treatment were also significantly smaller than the spleen weights of shrews treated with saline and killed at the same time point. It is well known that cisplatin treatment is frequently associated with anemia. In rats treated with cisplatin with 6 mg/kg (ip), spleen weights decreased significantly at 72 h after dosing [38].
The results presented support the use of the musk shrew as a small animal model to evaluate the acute nephrotoxicity and the nausea and vomiting associated with a single dose of cisplatin [15,14,12]. Both these toxicities are complex and have many components that are not clearly understood. The shrew may serve as a small animal model to evaluate the mechanisms involved in these complex processes and will also serve as a useful model to evaluate preventive and intervention strategies to decrease the toxicity of the widely used antineoplastic agent cisplatin.
Acknowledgments
This study was supported in part by a pilot grant from the Women’s Cancer Research Center at the University of Pittsburgh Cancer Institute (UPCI) and Magee-Womens Cancer Research and Education Committee. Additional funding was supplied by the UPCI NIH grant P30 CA047904 (Cancer Center Support Grant; CCSG), with core facility support to the Cancer Pharmacokinetic and Pharmacodynamic Facility (CPPF) and the Animal Facility (AF). We wish to thank the University of Pittsburgh, Division of Laboratory Animal Research, especially Dawn Everard, Katie Leschak, Megan Lambert, and Dr. Joseph Newsome for excellent care of the musk shrew colony at the University of Pittsburgh Cancer Institute (UPCI).
Contributor Information
Julie L. Eiseman, Cancer Therapeutics Program, University of Pittsburgh Cancer Institute. Department of Pharmacology and Chemical Biology, School of Medicine, University of Pittsburgh
Jan H. Beumer, Cancer Therapeutics Program, University of Pittsburgh Cancer Institute. Department of Pharmaceutical Sciences, School of Pharmacy, University of Pittsburgh
Lora H. Rigatti, Division of Laboratory Animal Resources, University of Pittsburgh Cancer Institute, University of Pittsburgh
Sandra Strychor, Cancer Therapeutics Program, University of Pittsburgh Cancer Institute.
Kelly Meyers, Biobehavioral Medicine in Oncology Program, University of Pittsburgh Cancer Institute.
Samuel Dienel, Department of Neuroscience, School of Arts and Sciences, University of Pittsburgh. Biobehavioral Medicine in Oncology Program, University of Pittsburgh Cancer Institute.
Charles C. Horn, Biobehavioral Medicine in Oncology Program, University of Pittsburgh Cancer Institute. Department of Medicine, Division of Gastroenterology, Hepatology, and Nutrition, School of Medicine, University of Pittsburgh. Department of Anesthesiology, School of Medicine, University of Pittsburgh, Center for Neuroscience, University of Pittsburgh
References
- 1.Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, Ballatori E, Bria E, Clark-Snow RA, Espersen BT, Feyer P, Grunberg SM, Hesketh PJ, Jordan K, Kris MG, Maranzano E, Molassiotis A, Morrow G, Olver I, Rapoport BL, Rittenberg C, Saito M, Tonato M, Warr D. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 2.Hesketh PJ, Grunberg SM, Herrstedt J, de Wit R, Gralla RJ, Carides AD, Taylor A, Evans JK, Horgan KJ. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: effect of gender on treatment response. Support CarCancer. 2006;14 (4):354–360. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- 3.Kris MG, Cubeddu LX, Gralla RJ, Cupissol D, Tyson LB, Venkatraman E, Homesley HD. Are more antiemetic trials with a placebo necessary? Report of patient data from randomized trials of placebo antiemetics with cisplatin. Cancer. 1996;78 (10):2193–2198. doi: 10.1002/(sici)1097-0142(19961115)78:10<2193::aid-cncr22>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Martin M. The severity and pattern of emesis following different cytotoxic agents. Oncology. 1996;53(Suppl 1):26–31. doi: 10.1159/000227637. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh PJ, Van Belle S, Aapro M, Tattersall FD, Naylor RJ, Hargreaves R, Carides AD, Evans JK, Horgan KJ. Differential involvement of neurotransmitters through the time course of cisplatin-induced emesis as revealed by therapy with specific receptor antagonists. Eur J Cancer. 2003;39 (8):1074–1080. doi: 10.1016/s0959-8049(02)00674-3. [DOI] [PubMed] [Google Scholar]
- 6.Albany C, Brames MJ, Fausel C, Johnson CS, Picus J, Einhorn LH. Randomized, Double-Blind, Placebo-Controlled, Phase III Cross-Over Study Evaluating the Oral Neurokinin-1 Antagonist Aprepitant in Combination With a 5HT3 Receptor Antagonist and Dexamethasone in Patients With Germ Cell Tumors Receiving 5-Day Cisplatin Combination Chemotherapy Regimens: A Hoosier Oncology Group Study. J Clin Oncol. 2012;30(32):3938–4003. doi: 10.1200/JCO.2011.39.5558. [DOI] [PubMed] [Google Scholar]
- 7.Rudd JA, Andrews PLR. Mechanisms of acute, delayed, and anticipatory emesis induced by anticancer therapies. In: Hesketh PJ, editor. Management of nausea and vomiting in cancer and cancer treatment. Jones and Bartlett; Sudbury, MA: 2005. pp. 15–65. [Google Scholar]
- 8.Minami M, Endo T, Hirafuji M, Hamaue N, Liu Y, Hiroshige T, Nemoto M, Saito H, Yoshioka M. Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity. Pharmacol Ther. 2003;99 (2):149–165. doi: 10.1016/s0163-7258(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 9.Percie du Sert N, Rudd JA, Moss R, Andrews PL. The delayed phase of cisplatin-induced emesis is mediated by the area postrema and not the abdominal visceral innervation in the ferret. NeurosciLett. 2009;465 (1):16–20. doi: 10.1016/j.neulet.2009.08.075. [DOI] [PubMed] [Google Scholar]
- 10.Percie du Sert N, Rudd JA, Apfel CC, Andrews PL. Cisplatin-induced emesis: systematic review and meta-analysis of the ferret model and the effects of 5-HT(3) receptor antagonists. Cancer ChemotherPharmacol. 2010;67:667–686. doi: 10.1007/s00280-010-1339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta YK, Sharma SS. Involvement of 5-HT1A and 5-HT2 receptor in cisplatin induced emesis in dogs. Indian J Physiol Pharmacol. 2002;46 (4):463–467. [PubMed] [Google Scholar]
- 12.Sam TS, Cheng JT, Johnston KD, Kan KK, Ngan MP, Rudd JA, Wai MK, Yeung JH. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew) Eur JPharmacol. 2003;472 (1–2):135–145. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- 13.De Jonghe BC, Horn CC. Chemotherapy agent cisplatin induces 48 h Fos expression in the brain of a vomiting species, the house musk shrew (Suncus murinus) Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R902–911. doi: 10.1152/ajpregu.90952.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horn CC, Henry S, Meyers K, Magnusson MS. Behavioral patterns associated with chemotherapy-induced emesis: A potential signature for nausea in musk shrews. Front Neurosci. 2011;5:88. doi: 10.3389/fnins.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang D, Meyers K, Henry S, De la Torre F, Horn CC. Computerized detection and analysis of cancer chemotherapy-induced emesis in a small animal model, musk shrew. J Neurosci Meth. 2011;197 (2):249–258. doi: 10.1016/j.jneumeth.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeimet AG, Reimer D, Radl AC, Reinthaller A, Schauer C, Petru E, Concin N, Braun S, Marth C. Pros and cons of intraperitoneal chemotherapy in the treatment of epithelial ovarian cancer. Anticancer Res. 2009;29(7):2803–2808. [PubMed] [Google Scholar]
- 17.Holzer P. Handbook of experimental pharmacology. Springer; Berlin ; New York: 2004. Tachykinins; p. 164. [Google Scholar]
- 18.Qian QH, Yue W, Wang YX, Yang ZH, Liu ZT, Chen WH. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch Pharm Res. 2009;32 (4):565–573. doi: 10.1007/s12272-009-1413-9. [DOI] [PubMed] [Google Scholar]
- 19.Yao X, Panichpisal K, Kurtzman N, Nugent K. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334 (2):115–124. doi: 10.1097/MAJ.0b013e31812dfe1e. [DOI] [PubMed] [Google Scholar]
- 20.Wang CH. Introduction: A new experimental animal, Suncus murinus. In: Saito H, Wang CH, Chen CY, editors. Proceeding of ROC-Japan Symposium on Suncus murinus: New experimental animal, its speciality and usefulness; Tainan, Taiwan, R.O.C. 1994; Chia Nan, Taiwan ROC: Chia Nan Junior College of Pharmacy Press; [Google Scholar]
- 21.Temple JL. The musk shrew (Suncus murinus): a model species for studies of nutritional regulation of reproduction. ILAR J. 2004;45 (1):25–34. doi: 10.1093/ilar.45.1.25. [DOI] [PubMed] [Google Scholar]
- 22.Colville H, Dzadony R, Kemp R, Stewart S, Zeh HJ, 3rd, Bartlett DL, Holleran J, Schombert K, Kosovec JE, Egorin MJ, Beumer JH. In vitro circuit stability of 5-fluorouracil and oxaliplatin in support of hyperthermic isolated hepatic perfusion. J Extra Corpor Technol. 2010;42 (1):75–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7 (1):155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong DM, Pickel VM, Joh TH, Reis DJ, Miller RJ. Immunocytochemical localization of catecholamine synthesizing enzymes and neuropeptides in area postrema and medial nucleus tractus solitarius of rat brain. JComp Neurol. 1981;196 (3):505–517. doi: 10.1002/cne.901960312. [DOI] [PubMed] [Google Scholar]
- 25.Hokfelt T, Ljungdahl A, Terenius L, Elde R, Nilsson G. Immunohistochemical analysis of peptide pathways possibly related to pain and analgesia: enkephalin and substance P. Proc Natl Acad Sci U S A. 1977;74 (7):3081–3085. doi: 10.1073/pnas.74.7.3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barajon I, Bersani M, Quartu M, Del Fiacco M, Cavaletti G, Holst JJ, Tredici G. Neuropeptides and morphological changes in cisplatin-induced dorsal root ganglion neuronopathy. Exp Neurol. 1996;138 (1):93–104. doi: 10.1006/exnr.1996.0050. [DOI] [PubMed] [Google Scholar]
- 27.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 28.Horn CC, Kimball BA, Wang H, Kaus J, Dienel S, Nagy A, Gathright GR, Yates BJ, Andrews PLR. Why can’t rodents vomit? A comparative behavioral, anatomical, and physiological study. PloS one. 2013;8 (4):e60537. doi: 10.1371/journal.pone.0060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakashima M, Shibata S, Tokunaga Y, Fujita H, Anda T, Arizono K, Tomiyama N, Sasaki H, Ichikawa M. In-vivo microdialysis study of the distribution of cisplatin into brain tumour tissue after intracarotid infusion in rats with 9L malignant glioma. J Pharm Pharmacol. 1997;49 (8):777–780. doi: 10.1111/j.2042-7158.1997.tb06111.x. [DOI] [PubMed] [Google Scholar]
- 30.Brown R, Foran J, Olin S, Robinson D. Physiological Parameter Values for PBPK Models. International Life Sciences Institute: Risk Science Institute; Washington, DC: 1994. [Google Scholar]
- 31.Ramirez-Camacho R, Fernandez DE, Verdaguer JM, Gomez MM, Trinidad A, Garcia-Berrocal JR, Corvillo MA. Cisplatin-induced hearing loss does not correlate with intracellular platinum concentration. Acta Otolaryngol. 2008;128 (5):505–509. doi: 10.1080/00016480701635167. [DOI] [PubMed] [Google Scholar]
- 32.Esteban-Fernandez D, Verdaguer JM, Ramirez-Camacho R, Palacios MA, Gomez-Gomez MM. Accumulation, fractionation, and analysis of platinum in toxicologically affected tissues after cisplatin, oxaliplatin, and carboplatin administration. J Anal Toxicol. 2008;32 (2):140–146. doi: 10.1093/jat/32.2.140. [DOI] [PubMed] [Google Scholar]
- 33.Johnsson A, Olsson C, Nygren O, Nilsson M, Seiving B, Cavallin-Stahl E. Pharmacokinetics and tissue distribution of cisplatin in nude mice: platinum levels and cisplatin-DNA adducts. Cancer Chemother Pharmacol. 1995;37 (1–2):23–31. doi: 10.1007/BF00685625. [DOI] [PubMed] [Google Scholar]
- 34.Sakaeda T, Kadoyama K, Okuno Y. Adverse event profiles of platinum agents: data mining of the public version of the FDA adverse event reporting system, AERS, and reproducibility of clinical observations. Int J Med Sci. 2011;8 (6):487–491. doi: 10.7150/ijms.8.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boissonade FM, Davison JS, Egizii R, Lucier GE, Sharkey KA. The dorsal vagal complex of the ferret: anatomical and immunohistochemical studies. Neurogastroenterol Motil. 1996;8 (3):255–272. doi: 10.1111/j.1365-2982.1996.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 36.Barajon I, Bersani M, Quartu M, Del Fiacco M, Cavaletti G, Holst JJ, Tredici G. Neuropeptides and morphological changes in cisplatin-induced dorsal root ganglion neuronopathy. Exp Neurol. 1996;138 (1):93–104. doi: 10.1006/exnr.1996.0050. [DOI] [PubMed] [Google Scholar]
- 37.Choie DD, Longnecker DS, del Campo AA. Acute and chronic cisplatin nephropathy in rats. Lab Invest. 1981;44 (5):397–402. [PubMed] [Google Scholar]
- 38.Liu M, Chien CC, Burne-Taney M, Molls RR, Racusen LC, Colvin RB, Rabb H. A pathophysiologic role for T lymphocytes in murine acute cisplatin nephrotoxicity. J Am Soc Nephrol. 2006;17 (3):765–774. doi: 10.1681/ASN.2005010102. [DOI] [PubMed] [Google Scholar]
- 39.van Hennik MB, van der Vijgh WJ, Klein I, Elferink F, Vermorken JB, Winograd B, Pinedo HM. Comparative pharmacokinetics of cisplatin and three analogues in mice and humans. Cancer Res. 1987;47 (23):6297–6301. [PubMed] [Google Scholar]
- 40.Zamboni WC, Gervais AC, Egorin MJ, Schellens JH, Hamburger DR, Delauter BJ, Grim A, Zuhowski EG, Joseph E, Pluim D, Potter DM, Eiseman JL. Inter- and intratumoral disposition of platinum in solid tumors after administration of cisplatin. Clin Cancer Res. 2002;8 (9):2992–2999. [PubMed] [Google Scholar]
- 41.Kizu R, Higashi S, Kidani Y, Miyazaki M. Pharmacokinetics of (1R,2R-diaminocyclohexane)oxalatoplatinum(II) in comparison with cisplatin following a single intravenous injection in rabbits. Cancer Chemother Pharmacol. 1993;31 (6):475–480. doi: 10.1007/BF00685038. [DOI] [PubMed] [Google Scholar]
- 42.Hardie EM, Page RL, Williams PL, Fischer WD. Effect of time of cisplatin administration on its toxicity and pharmacokinetics in dogs. Am J Vet Res. 1991;52 (11):1821–1825. [PubMed] [Google Scholar]
- 43.Jacobs SS, Fox E, Dennie C, Morgan LB, McCully CL, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous oxaliplatin, cisplatin, and carboplatin in nonhuman primates. Clin Cancer Res. 2005;11 (4):1669–1674. doi: 10.1158/1078-0432.CCR-04-1807. [DOI] [PubMed] [Google Scholar]
- 44.Urien S, Lokiec F. Population pharmacokinetics of total and unbound plasma cisplatin in adult patients. Br J Clin Pharmacol. 2004;57 (6):756–763. doi: 10.1111/j.1365-2125.2004.02082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep Part 1. 1966;50 (4):219–244. [PubMed] [Google Scholar]
- 46.Sugiura Y, Kitoh J. The median and lateral substantia gelatinosa in the cervical cord of the musk shrew (Suncus murinus) and its synaptic composition. Anat Embryol (Berl) 1984;170 (1):21–28. doi: 10.1007/BF00319454. [DOI] [PubMed] [Google Scholar]