Abstract
Rationale
YAP, the nuclear effector of Hippo signaling, regulates cellular growth and survival in multiple organs, including the heart, by interacting with TEAD sequence specific DNA-binding proteins. Recent studies showed that YAP stimulates cardiomyocyte proliferation and survival. However, the direct transcriptional targets through which YAP exerts its effects are poorly defined.
Objective
To identify direct YAP targets that mediate its’ mitogenic and anti-apoptotic effects in the heart.
Methods and Results
We identified direct YAP targets by combining differential gene expression analysis in YAP gain- and loss-of-function with genome-wide identification of YAP bound loci using chromatin immunoprecipitation and high throughput sequencing. This screen identified Pik3cb, encoding p110β, a catalytic subunit of phosphoinositol-3-kinase (PI3K), as a candidate YAP effector that promotes cardiomyocyte proliferation and survival. YAP and TEAD occupied a conserved enhancer within the first intron of Pik3cb, and this enhancer drove YAP-dependent reporter gene expression. Yap gain- and loss-of-function studies indicated that YAP is necessary and sufficient to activate the PI3K-Akt pathway. Like Yap, Pik3cb gain-of-function stimulated cardiomyocyte proliferation, and Pik3cb knockdown dampened YAP mitogenic activity. Reciprocally, impaired heart function in Yap loss-of-function was significantly rescued by AAV-mediated Pik3cb expression.
Conclusion
: Pik3cb is a crucial direct target of YAP, through which the YAP activates PI3K-AKTpathway and regulates cardiomyocyte proliferation and survival.
Keywords: Hippo, Yap, Pik3cb, PI3 kinase, Akt, heart failure, cardiomyocyte proliferation, AAV, regeneration
INTRODUCTION
Adult mammalian cardiomyocytes largely exit from the cell cycle, thereby limiting the innate regenerative capacity of the mature heart.1 As a result, there are currently no treatments for heart disease that reverse cardiomyocyte loss, a root cause for many cases of heart failure.
Recently, the transcriptional co-activator YAP was found to be essential for fetal cardiomyocyte proliferation, and activated YAP drove adult cardiomyocyte proliferation2-5. YAP is the nuclear effector of the Hippo kinase cascade, a recently defined pathway that regulates cell proliferation and survival to establish organ size.6 Hippo pathway kinases MST1/2 and LATS1/2 phosphorylate YAP, retarding its transcriptional activity by promoting its nuclear export. Hippo kinase inactivation enhanced YAP activity and stimulated fetal and adult cardiomyocyte proliferation.7, 8
YAP regulates transcription of its direct target genes by binding to sequence-specific DNA binding proteins, with TEAD1-4 (TEA domain family members 1-4) being key transcriptional partners.6 However, few direct YAP targets that are essential for its growth promoting activity are known. The phosphoinositide 3-kinase (PI3K)-AKT pathway was reported to be activated downstream of YAP,4 although the link between these pathways was not determined. The PI3K-AKT pathway promotes cardiomyocyte proliferation, survival, and physiological hypertrophy4, 5. Class IA PI3Ks are composed of a p85 regulatory subunit and a p110 catalytic subunit. The p110 catalytic subunit is encoded by three different genes, Pik3ca, Pik3cb, and Pik3cd, with most cells expressing Pik3ca and Pik3cb9. Early lethality of Pik3cb null embryos indicates that Pik3ca and Pik3cb isoforms are not fully redundant.10 However, most work has focused on Pik3ca and much less is known about Pik3cb.
In this study, through an unbiased whole genome screen we identified Pik3cb as a direct YAP target that links YAP to PI3K-AKT activation. Our data indicate that Pik3cb activation is sufficient to drive adult cardiomyocyte proliferation and is necessary for the mitogenic activity of YAP.
METHODS
Additional detailed materials and methods are provided in the online supplement.
Animal experiments
All animal procedures were approved by the Institutional Animal Care and Use Committee. Echocardiography and myocardial infarction (MI) surgery were performed blinded to genotype and treatment group.
Cell culture
We isolated ventricular cardiomyocytes (NRVMs) from 4-day-old rat hearts2. HL1 cells were obtained from William Claycomb and cultured as described11.
Other procedures
Hearts were fixed, embedded, cryosectioned, and immunostained as described.12 Antibodies are listed in Online Table I.
HL1 cells overexpressing 3FLAG-YAP[S127A] were used for ChIP-seq as described11. HiSeq 2000 (Illumina) sequencing data were used to identify binding peaks (Online Table II). ChIP-qPCR was performed using antibodies and primers listed in Online Tables I and III.
Gene expression was measured by qRTPCR using primers listed in Online Table III or by microarray (Mouse Gene ST 2.0 array, Affymetrix) using RNA collected from E12.5 mouse heart.
Data are available at GEO (accession number GSE57719) or the Cardiovascular Development Consortium server at https://b2b.hci.utah.edu/gnomex/.
Statistics
Values are expressed as mean ± SEM. To test for statistical significance, we used Student's t-test (two groups) or ANOVA with the Tukey HSD post-hoc test (more than two groups). Tests were performed using JMP10.0 (SAS).
RESULTS
YAP directly activates Pik3cb expression through TEAD
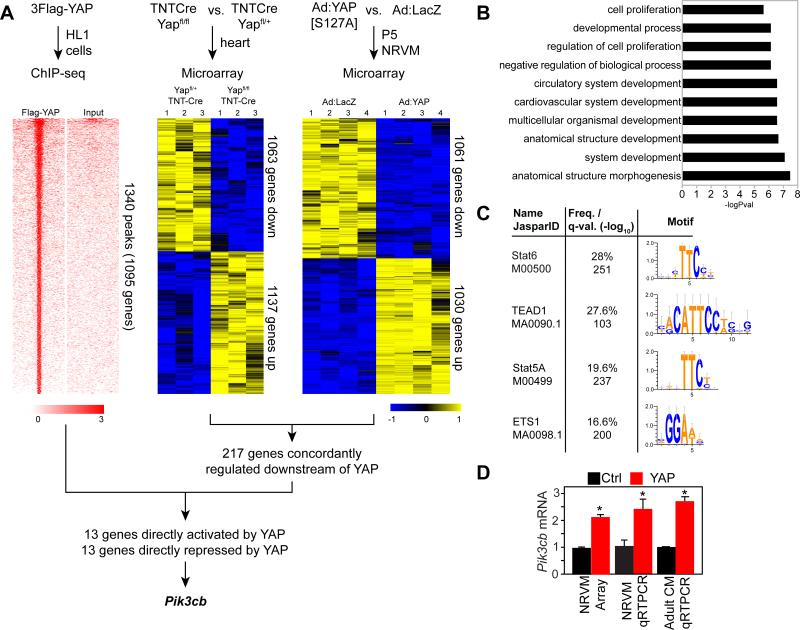
YAP plays crucial roles in regulating cardiomyocyte proliferation and survival2-5, 7, 8, but the direct targets of YAP that mediate its effects are largely unknown. To identify candidate genes that are directly regulated by YAP and that mediate its proliferative activity, we overexpressed activated, FLAG-tagged YAP (3xFlag-YAP[S127A], in which the inhibitory Hippo phosphorylation site serine 127 is mutated to alanine) in HL1 cardiomyocyte-like cells. Chromatin immunoprecipitation (ChIP) and high throughput sequencing (ChIP-seq) identified 1340 YAP-bound chromatin regions (Fig. 1A; Online Table II). YAP bound to genes enriched for functional terms including cardiovascular system development, regulation of cell proliferation, and cell proliferation (Fig. 1B). Motif discovery using these YAP-bound regions yielded the consensus TEAD motif (P=9.7E-47), confirming the predominant interaction of YAP with TEAD and providing validation for the ChIP-seq dataset. Scanning the YAP-bound regions for known transcription factor binding motifs also identified the TEAD motif, as well as the STAT and ETS motifs, which share sequence similarity to the TEAD motif (Fig. 1C).
Figure 1. Genome-wide screen to identify genes directly regulated by YAP in cardiomyocytes.
A. Strategy to identify genes directly bound by YAP and differentially expressed in YAP gain- and loss-of-function. ChIP-seq was performed using Flag-tagged YAP in HL1 cells. Tag heatmap shows signal intensity in ChIP and input sample in a 4 kb window centered on YAP peaks. Differential gene expression in YAP loss-of-function and gain-of-function were determined by microarray analysis of E12.5 hearts with cardiomyocyte-restricted YAP knockout, and NRVMs that overexpressed activated YAP. Heatmap shows the row-scaled expression z-score. B. Gene Ontology term analysis of YAP- bound genes. C. Motif analysis of the top 1000 YAP bound regions. D.YAP overexpression induced upregulation of Pik3cb. *, P<0.05.
We used microarray gene expression profiling to identify genes downstream of YAP. We compared E12.5 mouse hearts with TNTCre-mediated YAP inactivation in cardiomyocytes (YAPfl/fl::TNTCre) to Yapfl/+::TNTCre littermate controls (Fig. 1A). We also compared adenovirus-mediated YAP[S127A] overexpression in neonatal rat ventricular cardiomyocytes to adenoviral LACZ expression (Fig. 1A), which we reported previously2. In the murine loss-of-function dataset, we identified 2200 differentially expressed genes (1137 and 1063 up- and down-regulated in knockout, respectively; p<0.05; absolute log2 fold-change>0.2; n=3; Online Table III). In the neonatal rat gain-of-function dataset, there were 2091 differentially expressed genes (1030 and 1061 up- and down-regulated in Yap overexpression, respectively; p<0.05; absolute log2 fold-change>0.5; n=4; see ref. 2). There were 217 genes with concordant regulation by YAP in both datasets (Online Table IV). These 217 genes were enriched for functional terms encompassing heart development (P=0.00045).
The intersection of genes associated with YAP-bound chromatin regions and concordantly regulated downstream of YAP in both differential expression datasets contained 26 genes, with 13 activated by YAP, and 13 repressed by YAP (Online Table IV). Given that YAP was previously reported to activate the PI3K-AKT pathway though uncertain mechanisms,4 we were interested to find Pi3kcb among the candidates for direct YAP activation. Pik3cb is a little studied isoform that encodes the phosphoinositol-3-kinase (PI3K) catalytic subunit (also referred to as p110β), a key kinase that regulates cell growth and metabolic activity13, 14. Like YAP,2 PIK3CB proteins levels decline in the heart with increasing post-natal age (Online Fig. I). qRT-PCR of NRVMs confirmed that Ad:YAP[S127A] activated expression of Pik3cb compared to Ad:LacZ (Fig. 1D).
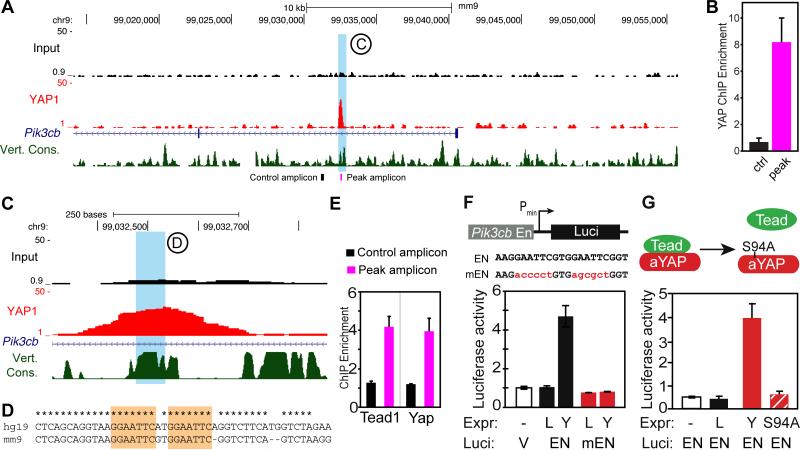
The HL1 ChIP-seq data revealed a YAP-bound sequence residing in the first intron of Pik3cb (Fig. 2A). We validated YAP binding to the identified sequence by ChIP-qPCR, using a pair of primers spanning the YAP bound sequence and a control pair recognizing a sequence 1.3 kb away (Fig. 2B). This YAP-bound sequence contained an evolutionarily conserved sequence (AGGAATTCGTGGAATT) containing two repeats of a motif that is similar to the TEAD, STAT, and ETS motifs (Fig. 2C-D). ChIP-qPCR to confirmed YAP and TEAD occupancy of this Pik3cb region but not the adjacent control region in neonatal heart (Fig. 2E). While YAP-TEAD interaction is well described, YAP has not been reported to interact with STAT or ETS. Co-IP experiments showed no detectable interaction between YAP and STAT3, STAT5a, STAT6, or ETS1 (Online Fig. II–A-C). These data suggest that YAP activates the Pik3cb enhancer via TEAD in cardiomyocytes.
Figure 2. YAP binds and activates an enhancer of Pik3cb.
A. Genome browser view showing YAP ChIP-seq signal around Pik3cb. HL1 cells overexpressing FLAG-tagged YAP were processed for FLAG ChIP-seq. Blue shaded area highlights a YAP peak in the first intron on Pik3cb. B. ChIP-qPCR measurement of FLAG-YAP occupancy of the peak region and an adjacent control region. Locations of amplicons are indicated by the black and purple labels in the bottom of panel (A). C-D. Magnified view of the YAP-occupied region of Pik3cb, also showing vertebrate conservation. The sequence of the region in blue is shown in (D). * indicates identity between mouse and human genomes. Orange shading indicates two conserved binding motifs. E. ChIP-qPCR measurement of TEAD1 and YAP occupancy of the peak region and adjacent control region in 8-day-old mouse heart. Amplicons indicated by lack and purple labels in the bottom of panel (A). F. A 552 bp region encompassing the YAP ChIP-seq peak (En) was cloned into a minimal promoter-luciferase construct. A mutant version (mEN) contained the base substitutions in the TEAD motif indicated in red. NRVMs were transfected with YAP (Y) or LacZ (L) expression constructs and wild-type (En) or mutant (mEN) Pik3cb enhancer-luciferase reporter constructs. Luciferase activity was normalized to an internal transfection control. G. NRVMs were transfected with the Pik3cb intronic enhancer-luciferase reporter construct (EN) and either YAP (Y), YAP[S94A] (YS94A), or LacZ (L). Luciferase activity was normalized to an internal transfection control.
To measure the transcriptional activity of the YAP-bound region of Pik3cb, which we refer to as the Pik3cb enhancer, we cloned a 552 bp genomic DNA fragment containing the conserved element and potential TEAD binding sites into a minimal promoter luciferase reporter construct. Co-transfection with Yap in NRVMs showed that Yap stimulates activity of the enhancer by ~5-fold. Yap stimulation was abrogated by mutation of the core conserved element (Fig. 2F). YAP[S94A], deficient in TEAD interaction,15 failed to activate the Pik3cb enhancer (Fig. 2G), indicating that YAP stimulates Pik3cb expression through TEAD. On the other hand, S3I-201, a Stat3 inhibitor which prevents Stat3 binding DNA and suppresses Stat3 dependent transcription16, did not affect YAP activation of the Pik3cb enhancer or YAP-induced cardiomyocyte proliferation (Online Fig. II-D,E).
Together, these data indicate that YAP binds to an evolutionarily conserved motif in the first intron of Pik3cb through TEAD to upregulate Pik3cb expression in cardiomyocytes.
Yap is sufficient and required to upregulate Pik3cb /Pik3ca and activate PI3K-Akt pathway in vivo
Having shown that YAP directly binds and activates the Pik3cb enhancer, we next asked if YAP is necessary and sufficient to stimulate Pik3cb expression in vivo and thereby activate the PI3K-Akt pathway. To overexpress YAP in cardiomyocytes in vivo, we used adeno-associated virus serotype 9 (AAV9), a safe, efficient and cardiotropic vector for in vivo gene transfer17, 18 . We used a cardiomyocyte-specific chicken troponin T promoter (cTNT) to further enhance cardiac selectivity. We validated the cardiomyocyte specificity of this gene transfer system by analyzing the recombination pattern of AAV9:cTNT-iCre, in which the cTNT promoter drives expression of mammalian codon-optimized Cre recombinase. We systemically administered AAV9:cTNT-iCre by subcutaneous injection into 1-2 day old mouse pups harboring the Rosa26fsTRAP allele, in which Cre recombination activates GFP expression. Seven days after virus administration, most TNNI3 positive cardiomyocytes expressed GFP, but TNNI3 negative non-cardiomyocytes did not (Online Fig. III-A). These data show that AAV9-cTNT drives cardiomyocyte-selective expression within the heart.
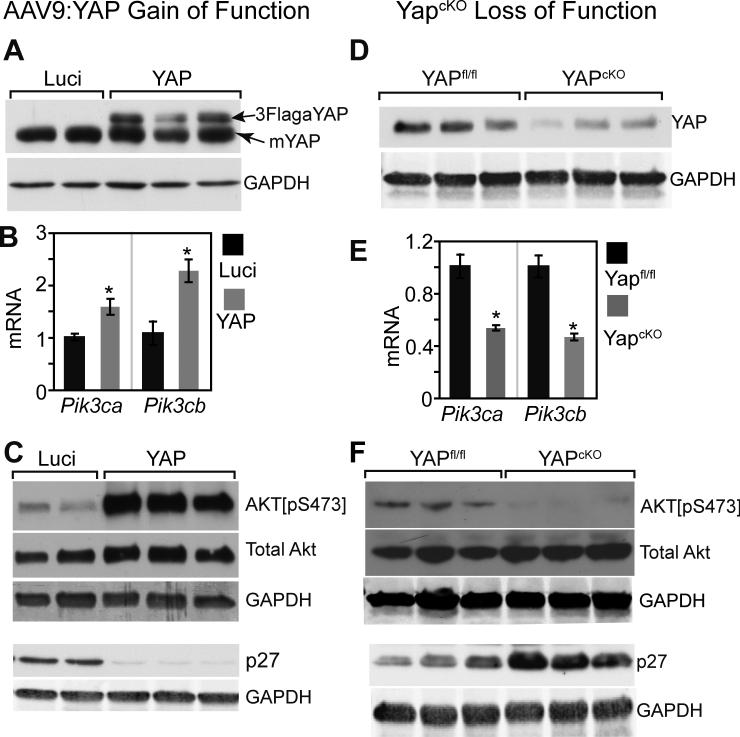
We then replaced the iCre gene with FLAG-tagged, activated YAP (3Flag-YAP[S127A]) to generate AAV9:cTNT-Yap virus (AAV9:Yap). As a negative control, we generated AAV9:cTNT-Luciferase (AAV9:Luci). AAV9:Yap and AAV9:Luci were injected into 1-2 day old pups. 7 days later, the expression of exogenous 3Flag-YAP[S127A] was clearly detected by western blot (Fig. 3A). Expression of activated YAP caused more than two-fold upregulation of Pik3cb (Fig. 3B). Pik3ca was also upregulated, although to a lesser degree compared to Pik3cb (Fig. 3B).
Figure 3. YAP is sufficient and required for activating the PI3K-AKT pathway.
A. AAV9:YAP-mediated overexpression of activated YAP. AAV9:YAP was subcutaneously injected into P3 pups. 7 days later, hearts were collected for western blotting. AAV9:Luci served as a negative control. B. qRTPCR measurement of Pik3cb and Pik3ca mRNA level in hearts of mice treated with AAV9:YAP or AAV9:Luci. *, P<0.05. n=4. C. Western blot assessment of AKT pathway activation state in hearts of AAV9:YAP or AAV9:luci-treated mice. Primary antibodies were directed against AKT[pS473] (activated AKT), total AKT, GAPDH (internal loading control), or p27. D. YAP protein level in hearts from 4-week-old YapcKO mice and their littermate controls (Yapfl/fl). E. qRT-PCR measurement of Pik3cb and Pik3ca mRNA level in YapcKO and Yapfl/fl heart. *, P<0.05, n=3. F. Western blot assessment of AKT pathway activation state in YapcKO and Yapfl/fl heart.
Xin et al. previously reported that YAP activation in cultured neonatal rat ventricular cardiomyocytes increased the levels of activated AKT (Akt[p-S473]) without changing total AKT protein level4. YAP was linked to AKT activation in this system by upregulation of IGF1R. In mitotic tissue (skin) and cultured cells, YAP was also shown to activate the PI3K-Akt pathway by suppressing expression of PTEN19, an inhibitor of PI3K-Akt signaling. We found that YAP activation in cardiomyocytes in vivo increased AKT activation without changing total AKT level (Fig. 3C). YAP significantly upregulated IGF1R by ~1.5-fold, but did not alter PTEN level (Online Fig. III-B,C).
We next asked if YAP is required for normal expression of Pik3cb and Pik3ca in the heart. We generated Yapfl/fl::Myh6-Cre mice (YAPcKO), in which cardiomyocyte-specific Myh6-Cre inactivates a conditional YAPflox allele. We confirmed efficient YAP inactivation by western blotting, which showed marked downregulation of cardiac YAP protein (Fig. 3D). In YapcKO hearts, Pik3cb and Pik3ca mRNA were both significantly reduced (Fig.3E). Moreover, phosphorylated but not total Akt was reduced in YapcKO mice heart, indicating that Yap is required for maintenance of the normal level of Akt activation (Fig. 3F).
The cell cycle inhibitor p27 (CDKN1B) is a direct target of Akt, and Akt-mediated p27 phosophorylation leads to p27 degradation20. We therefore measured p27 protein level as a downstream readout of Akt activation. In AAV9:YAP-treated hearts, p27 protein was downregulated (Fig. 3C), while in YapcKO hearts, p27 protein was upregulated (Fig. 3F). These data further confirm that Akt activity is governed by YAP activity in cardiomyocytes.
Collectively, both gain- and loss-of-function data indicate that YAP promotes Pik3cb and Pik3ca upregulation and stimulates PI3K-Akt pathway activation in vivo.
Pik3cb overexpression activated AKT and induced cardiomyocyte proliferation
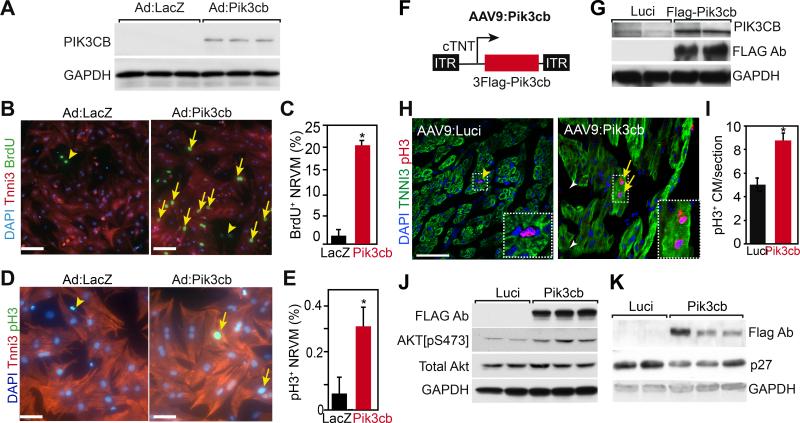
To determine if Pik3cb is sufficient to activate Akt and drive cardiomyocyte proliferation, we generated Ad:Pik3cb, an adenovirus that expresses Pik3cb. We validated over-expression of PIK3CB protein in NRVMs by Ad:Pik3cb (Fig. 4A). We then treated NRVMs with Ad:Pik3cb and measured the cardiomyocyte proliferation rate using two independent markers, phosphorylated histone H3 (pH3), an M phase marker, and BrdU uptake, an S phase marker. Pik3cb overexpression significantly increased the fraction of cardiomyocytes positive for BrdU and pH3 (Fig.4B-E). These data indicate that Pik3cb is sufficient to stimulate proliferation of cultured neonatal cardiomyocytes in vitro.
Figure 4. Pik3cb overexpression increased cardiomyocyte proliferation.
A. Adenovirus-mediated Pik3cb overexpression in cultured NRVMs isolated on postnatal day 4 (P4). PIK3CB protein was detected by immunoblotting. B-E. Effect of Pik3cb overexpression on P4 NRVM proliferation in vitro was measured by BrdU incorporation rate (B, C) and phosphohistone H3 positive (pH3+) cardiomyocyte frequency (D,E). Bar = 50 μm. Arrows indicate BrdU and pH3 positive cardiomyocytes. Arrowheads indicate non-myocytes. *, P<0.05. n=3. F. Schematic of the AAV9:Pik3cb construct. 3Flag epitope-tagged Pik3cb was expressed from the cardiac troponin T promoter. G. AAV9:Luci and AAV9:Pik3cb were injected subcutaneously into P2 neonatal mice. 8 days later, hearts extract western blots were probed with FLAG, PIK3CB, or GAPDH antibodies. H, I. Effect of Pik3cb overexpression on neonatal cardiomyocyte proliferation in vivo. AAV9:Luci or AAV9:Pik3cb were administered subcutaneously to P2 neonatal mice. Hearts were analyzed by immunofluorescent staining at P9. Arrows and arrowheads indicate pH3+ cardiomyocytes and non-myocytes, respectively. Boxed area is enlarged in inset. Bar = 50 μm. *, P<0.05. n=4. J,K. Immunoblot analysis of P9 heart lysates. Mice were treated with AAV:Luci or AAV9:Pik3cb at P2.
To extend these data to an in vivo context, we generated AAV9:cTNT-3Flag-Pik3cb (AAV9:Pik3cb) (Fig. 4F) and delivered it or control AAV9:Luci systemically to 1-2 day old neonatal mice. Seven days after virus administration (8-9 days of age), we collected hearts for immunoblotting and histological studies. We confirmed expression of Flag-tagged human PIK3CB protein in the heart (Fig. 4G). To determine the effect of Pik3cb gain-of-function on cardiomyocyte proliferation, we measured the fraction of cardiomyocytes that were positive for pH3. Compared with AAV9:Luci, AAV9:Pik3cb significantly increased the pH3+ CM fraction (Fig. 4H-I), suggesting that PIK3CB is sufficient to increase neonatal cardiomyocyte proliferation in vivo.
We next addressed the ability of Pik3cb to stimulate adult cardiomyocyte proliferation in the context of disease. Myocardial infarction (MI) was induced in two month-old CFW mice by coronary artery ligation (Online Fig. IV-A). The freshly infarcted myocardium was treated with AAV9:Luci or AAV9:Pik3cb. 4 days after MI, mice were treated with one dose of EdU to label dividing cardiomyocytes. 5 days after MI, hearts were collected for analysis. In the border zone, AAV9:Pik3cb treatment resulted in higher cardiomyocyte EdU labeling index than AAV:Luci treatment (Online Fig. IV-B,C). Cardiomyocyte apoptosis was also significantly reduced in AAV9:Pik3cb treatment compared to control (Online Fig. IV-D,E). These data show that Pik3cb promotes cardiomyocyte cell cycle activity and enhances cardiomyocyte survival after MI.
One mechanism by which a treatment might increase cardiomyocyte proliferation is by promoting dedifferentiation. To assess whether Pik3cb stimulates cardiomyocyte dedifferentiation, we treated P2 neonatal hearts with AAV9-Luci or AAV9-Pik3cb. We then analyzed expression of myocardial differentiation markers at P9. We detected no change in expression of Myh6, Myh7, or Nkx2-5 (Online Fig. IV-F), suggesting that Pik3cb does not promote cardiomyocyte dedifferentiation.
We next examined the effect of Pik3cb gain-of-function on downstream Akt signaling. Interestingly, Pik3cb overexpression upregulated Pik3ca (Online Fig. IV-G), suggesting that YAP indirectly upregulates Pik3ca through its direct effect on Pik3cb. AAV9:Pik3cb-treated hearts showed higher level of activated Akt (Akt[pS473]) than AAV9:Luci-treated hearts, while the level of total Akt was comparable between groups (Fig. 4J). Furthermore, the protein level of p27 was decreased in AAV9:Pik3cb-treated hearts (Fig. 4K; P<0.05; quantification in Online Fig. IV-H). These data demonstrate that overexpression of PIK3CB activates Akt and increases cardiomyocyte proliferation in vivo.
Pik3cb is required for YAP-stimulated Akt activation and cardiomyocyte proliferation
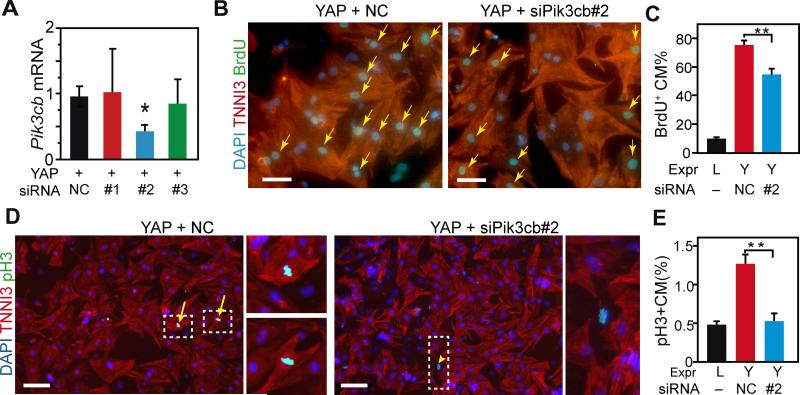
Because YAP directly regulated Pik3cb, which is sufficient to increase cardiomyocyte proliferation, we hypothesized that Pik3cb is required for YAP-induced Akt activation and cardiomyocyte proliferation. To analyze the requirement of Pik3cb downstream of YAP in cultured NRVMs, we synthesized three siRNAs and measured their reduction of YAP-stimulated Pik3cb expression. siPik3cb#2 reduced Pik3cb expression to 42% of control values, while the other two siRNAs were not effective (Fig. 5A). Then we stimulated NRVMs with activated YAP and treated them with siPik3cb#2 or control siRNA. siPik3cb#2 partially blocked the YAP-induced increase in cardiomyocyte proliferation markers EdU+ and pH3+ (Fig. 5B-E). These data indicate that Pik3cb is necessary for YAP to fully induce cardiomyocyte proliferation in vitro.
Figure 5. Yap stimulation of NRVM proliferation requires Pik3cb.
A. Validation of siRNAs directed against Pik3cb. NRVMs were transfected with the indicated siRNAs. After 2 days, Pik3cb mRNA was measured by qRT-PCR. NC, negative control. B-E. Effect of Pik3cb knockdown on YAP-stimulated NRVM proliferation. P4 NRVMs were treated with control (NC) or Pik3cb siRNA and adenovirus expressing LacZ (L) or YAP (Y). Proliferation was measured 1 day later by BrdU uptake (B,C) or pH3 staining (D,E). Dashed rectangles indicate areas enlarged in insets. Arrows and arrowheads indicate positive cardiomyocytes and non-myocytes, respectively. Bar = 50 μm (B) or 100 μm (D). *, P<0.05. n=3.
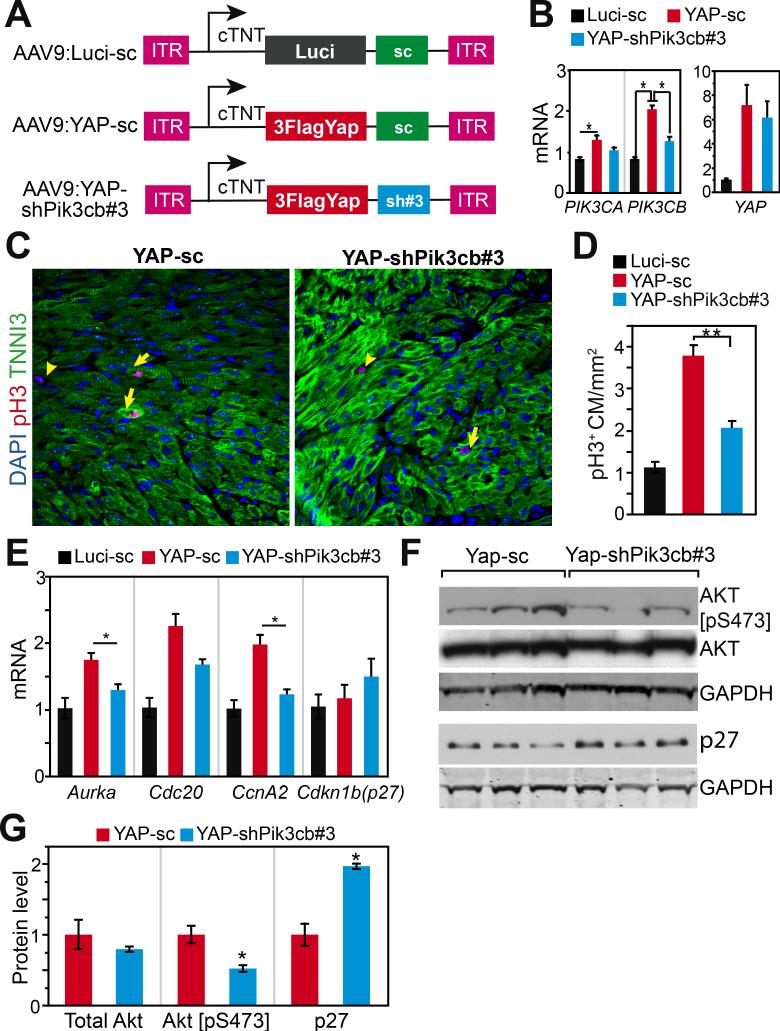
To study the requirement of Pik3cb downstream of YAP in cardiomyocytes in vivo, we followed a previously validated strategy21 and embedded an shRNA directed against Pik3cb into the 3’ UTR of AAV9:YAP to simultaneously overexpress Yap and knock down Pik3cb in the mouse heart. We tested four different shRNAs, and shPik3cb#3 yielded the greatest Pik3cb knockdown (Online Fig. V-A). We then cloned this shRNA, or a scrambled negative control shRNA, downstream of YAP or luciferase to yield AAV9:YAP-shPik3cb, AAV9:YAP-sc, and AAV9:Luci-sc (Fig. 6A). AAV9:YAP-shPik3cb was designed to overexpress activated YAP and simultaneously knock down Pik3cb, whereas AAV9:YAP-sc was designed to overexpress activated YAP without perturbing Pik3cb expression.
Figure 6. Yap stimulation of mouse cardiomyocyte proliferation requires Pik3cb in vivo.
A. Schematic view of AAV plasmids. shRNA effective for Pik3cb knockdown (shPik3cb#3) was cloned into AAV ITR plasmid downstream of YAP[S127A] (human) to simultaneously express YAP and knockdown Pik3cb. Control constructs contained Luci (luciferase) or scrambled control shRNA (sc). B. qRT-PCR measurement of Pik3cb, Pik3ca and Yap mRNA levels from P9 mouse heart. AAV was injected into mice at P2. Primers that amplified both mouse and human Yap were used for measuring Yap expression level. C, D. pH3 immunofluorescence staining of heart sections. AAV was injected into 2-dayold mouse pups and hearts were analyzed at P9. E. qRT-PCR measurement of cell cycle genes from P9 hearts after AAV treatment at P2. F, G. Effect of Pik3cb knockdown in the presence of activated YAP on activated Akt and p27 protein levels. B, D, E, n=4. *, P<0.05.
Compared with AAV9:Luci-sc control virus, both AAV9:YAP-shPik3cb and AAV9:YAP-sc strongly upregulated Yap to comparable degrees (~6.5-fold upregulation). Consistent with our earlier data, AAV9:YAP-sc upregulated Pik3cb by 2.5-fold and Pik3ca by 1.4-fold compared to AAV9:Luci-sc control (Fig. 6B). AAV9:YAP-sc robustly stimulated cardiomyocyte proliferation in the neonatal mouse heart, as reflected by pH3 staining (Fig. 6C,D). In comparison, AAV9:YAP-shPik3cb upregulation of Pik3cb was significantly attenuated. Reduced Pik3cb expression corresponded to less induction of cardiomyocyte proliferation by AAV9:YAP-shPik3cb (Fig. 6C,D). These changes were not associated with altered expression of Myh6, Myh7, or Nkx2-5, suggesting they were not due to altered cardiomyocyte differentiation (Online Fig. V-B). These data indicate that Pik3cb is required for full stimulation of cardiomyocyte proliferation by YAP.
We previously showed that several cell cycle genes including aurora A kinase (Aurka), cell division cycle 20 (Cdc20), and cyclin A2 (Ccna2) are upregulated by YAP2. Consistent with these data, AAV9:YAP-sc upregulated these genes compared to AAV9:Luci-sc control (Fig. 6E). Reduced Pik3cb expression by AAV9:YAP-shPik3cb interfered with Aurka and Ccna2 upregulation by YAP (Fig. 6E). Average Cdc20 expression also decreased, although this did not reach statistical significance. Meanwhile, Cdkn1b (p27) mRNA level was not significantly affected, in keeping with its regulation by its post-transcriptional regulation by PI3K-AKT20. These results demonstrate that YAP regulation of the expression of a subset of cell cycle genes is dependent upon upregulation of Pik3cb.
Because YAP is sufficient to increase Pik3cb expression and activate AKT (Fig. 2), and AKT is a downstream target of PIK3CB, we hypothesized that PIK3CB is required for YAP to activate AKT. To test this hypothesis, we measured activated and total AKT, as well as p27 by western blotting of hearts transduced with AAV9:YAP-sc, or AAV9:YAP-shPik3cb (Fig. 6F-G). Total AKT levels were comparable between groups. YAP-sc elevated activated AKT, and this stimulation was dampened substantially by shPik3cb. Although the p27 mRNA level did not change between AAV9:YAP-sc and AAV9:YAP-shPik3cb groups (Fig. 6E), the protein level of p27 was increased by shPIK3cb (Fig. 6F), consistent with destabilization of p27 protein by activated AKT.
These data indicate that YAP stimulates AKT at least in part through upregulation of Pik3cb. Pik3cb upregulation improved YapcKO heart function.
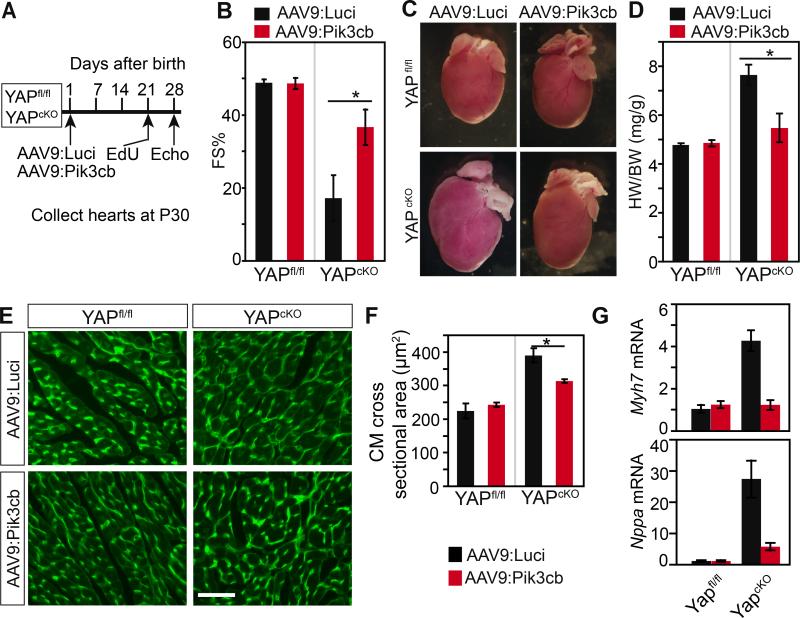
Cardiac-specific depletion of Yap caused pathological hypertrophy and heart failure3, 5. Since YAP is required to activate Pik3cb and to maintain PI3K-AKT activity, we hypothesized that Pik3cb overexpression would restore function of YapcKO hearts. We tested this hypothesis by treating 1 day old YapcKO or Yapfl/fl pups with either AAV9:Pik3cb or AAV9:Luci (Fig. 7A). At 3 weeks of age, we administered one dose of EdU to label the cardiomyocytes traversing S phase of the cell cycle. At 4 weeks of age, we measured cardiac function by echocardiography, then we collected the hearts for analysis. Treatment of control (Yapfl/fl) mice with AAV9:Pik3cb did not significantly affect heart function compared to AAV9:Luci, indicating that Pik3cb overexpression is well tolerated (Fig. 7B). YAPcKO hearts had severe systolic dysfunction that was partially rescued by AAV9:Pik3cb (Fig. 7B). Due to technical difficulties, we were not able to quantitate the fraction of cardiomyocytes transduced by AAV9:Pik3cb. It is possible that the incomplete rescue was due to incomplete cardiomyocyte transduction, or to Pik3cb-independent YAP activities. Nevertheless, our data indicate that decreased Pik3cb and PI3K-AKT signaling is an important contributor to cardiac dysfunction in YAP loss of function.
Figure 7. AAV9:Pik3cb mitigated heart dysfunction in YapcKO mice.
A. Experimental design. AAV9:Luci or AAV9:Pik3cb were administered to YapcKO or control (Yapfl/fl) mice at P1. Echocardiography and heart collection were performed at P30. B. Effect of Pik3cb overexpression on heart dysfunction of YapcKO mice. Fractional shortening (FS) was measured by echocardiography. *, P<0.05. n=4. C,D. Effect of Pik3cb overexpression on cardiomegaly of YapcKO mice. *, P<0.05. n=4. E,F. Effect of Pik3cb overexpression on YapcKO cardiomyocyte size. Wheat germ agglutinin (WGA) stained heart cross-sectional area. Bar=50 μm. *, P<0.05. n=3. G. Effect of Pik3cb overexpression on expression of hypertrophic marker genes Nppa and Myh7 in YapcKO heart.
Next we considered the effect of Pik3cb overexpression on cardiomegaly observed in YAPcKO. AAV9:Pik3cb treatment of control (Yapfl/fl) mice did not significantly affect heart size compared to AAV9:Luci (Fig. 7C,D), indicating that Pik3cb overexpression does not cause cardiac hypertrophy. YAPcKO hearts showed significant cardiomegaly, likely secondary to cardiac dilation in heart failure. Cardiomegaly was attenuated by AAV9:Pik3cb but not AAV9:Luci (Fig. 7C,D), consistent with improvement of ventricular function (Fig. 7B). We further examined the effect of Pik3cb overexpression on cardiac hypertrophy caused by YAP depletion in the heart at the level of cardiomyocyte size. AAV9:Pik3cb treatment of control (Yapfl/fl) mice did not significantly alter cardiomyocyte cross-sectional area, compared to AAV9:Luci treatment (Fig. 7E,F). Cardiomyocyte cross-sectional area was significantly greater in YAPcKO heart. Pik3cb overexpression by AAV9:Pik3cb significantly blunted this cardiomyocyte hypertrophy, compared to AAV9:Luci (Fig. 7E,F).
Cardiac hypertrophy is often associated with upregulation of the genes Nppa and Myh7. We assessed the effect of AAV9:Pik3cb on the expression of these hypertrophic marker genes in YapcKO heart. AAV9:Pik3cb markedly downregulated Nppa and Myh7 compared to AAV9:Luci (Fig. 7G), consistent with attenuation of cardiomyocyte hypertrophy. These data indicate that AAV9:Pik3cb partially mitigated hypertrophic marker gene activation caused by cardiac YAP depletion.
Together these data demonstrate that Pik3cb overexpression attenuates cardiac dysfunction and pathological cardiac hypertrophy of YapcKO mice.
AAV9:Pik3cb increased cardiomyocyte proliferation and decreased cardiomyocyte apoptosis in YapcKO mice
We investigated potential mechanisms through which AAV9:Pik3cb rescued dysfunction and hypertrophy of YapcKO hearts. YapcKO mice were reported to have more cardiomyocyte apoptosis3, and their cardiomyocyte regenerative capacity was impaired5. Because the PI3K-AKT pathway promotes both cell proliferation and survival22, we hypothesized that Pik3cb overexpression ameliorates the YapcKO phenotype by increasing cardiomyocyte proliferation, decreasing apoptosis, or both.
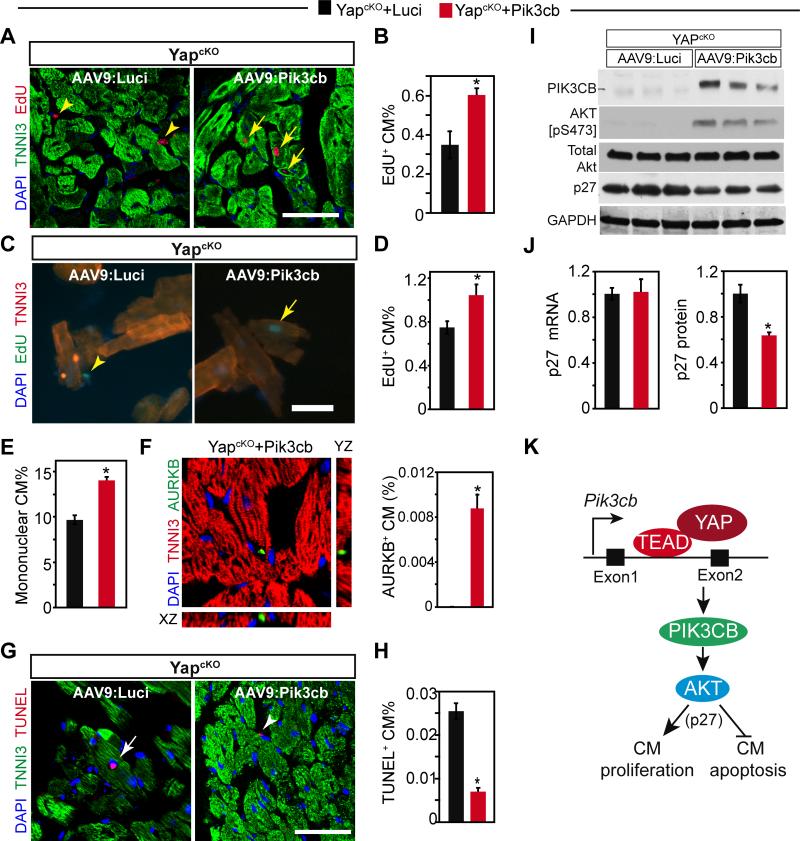
To investigate the effect on cardiomyocyte proliferation, we measured the fraction of cardiomyocytes that incorporated EdU, administered at 3 weeks of age (experimental timeline shown in Fig. 7A). Staining of tissue sections showed that cardiomyocyte EdU uptake was higher in YapcKO mice treated with AAV9:Pik3cb, compared to those treated with AAV9:Luci (Fig. 8A,B). We further confirmed this finding by staining dissociated cardiomyocytes (Fig. 8C,D), which obviates potential artifacts that can occur in tissue sections. Moreover, AAV9:Pik3cb increased the fraction of mononuclear cardiomyocytes (Fig. 8E), which contains the proliferative cardiomyocyte subset.23, and it increased the frequency that we observed cardiomyocytes in cytokinesis, as marked by staining with Aurora B kinase (Fig. 8F).23 Together, these data demonstrated that Pik3cb stimulated cardiomyocyte proliferation in YAP-deficient hearts.
Figure 8. AAV9:Pik3cb increased cardiomyocyte proliferation and decreased cardiomyocyte apoptosis in the absence of YAP.
YapcKO mice were treated with AAV9:Pik3cb or AAV9:Luci as indicated in Fig. 7A. A-F. Effect of Pik3cb overexpression on depressed cardiomyocyte proliferation seen in YapcKO heart. Cardiomyocyte proliferation was measured by uptake of EdU (A-D) 4 weeks after birth. A-B. EdU staining and quantification. EdU was administered once at P21. C-D. EdU quantification with dissociated cardiomyocytes. EdU was administered at P21 and P24. Arrows and arrowheads indicate EdU positive cardiomyocytes and non-myocytes, respectively. E. Percentage of mononuclear cardiomyocytes. F. Measurement of cytokinesis. Aurora kinaseB antibody was used to detect cardiomyocytes undergoing cytokinesis. G-H. Effect of Pik3cb overexpression on cardiomyocyte apoptosis in YapcKO heart, as measured by TUNEL assay. Arrows and arrowheads indicate TUNEL+ cardiomyocytes and nonmyocytes, respectively. I. Effect of Pik3cb overexpression on AKT activation and p27 expression in YapcKO heart. Lysates collected from one-month old mouse hearts were analyzed by western blotting. J. Effect of Pik3cb overexpression on p27 mRNA and protein levels in YapcKO heart. K. YAP-TEAD stimulated Pik3cb transcription through an enhancer in the first intron. Pik3cb activates AKT, which suppresses cardiomyocyte proliferation and increases cardiomyocyte apoptosis, in part through increased p27 protein. Bar = 50 μm. *, P<0.05. n=3.
To study the effect of Pik3cb overexpression on apoptosis in YAP-deficient cardiomyocytes, we measured the fraction of cardiomyocytes positive for TUNEL-staining, a marker of apoptosis. TUNEL+ cardiomyocytes were less frequent in YapcKO, treated with AAV9:Pik3cb compared AAV9:Luci, indicating that Pik3cb rescues cardiomyocyte apoptosis caused by YAP-deficiency (Fig. 8G,H).
Yap activation increased the expression of both Pik3ca and Pik3cb (Fig. 3B), and Pik3cb was sufficient to induce the expression of Pik3ca in wild type mouse heart (Online Fig. IV-G). These data suggest that Pik3cb functions downstream of Yap to regulate Pik3ca expression, AKT activation, and p27 levels. Indeed, Pik3cb overexpression in the absence of YAP rescued AKT activation without changing total AKT level (Fig. 8I). AKT activation corresponded with decreased p27 protein but not mRNA (Fig. 8I-J).
Together, our data support a model in which YAP directly activates Pik3cb expression through TEAD binding to an enhancer in the first Pik3cb intron. PIK3CB subsequently promotes expression of Pik3ca and activation of AKT, which regulates cardiomyocyte apoptosis and proliferation, in part through p27 (Fig. 8K).
DISCUSSION
Emerging studies have revealed the critical role of Hippo-YAP signaling in heart development, growth, and homeostasis.2-5, 7, 8. One major pathway through which Hippo-YAP signaling regulates cardiomyocyte growth and survival is the PI3K-AKT signaling axis3, 4. This pathway has well established, pleiotropic effects on cardiomyocyte proliferation, growth, survival, and function24. However, the mechanistic link between YAP and the PI3K-AKT pathway was not previously known. Our genome-wide screen for directly activated YAP target genes showed that YAP, through its transcriptional partner TEAD, directly activates Pik3cb expression via an enhancer in the first intron of Pik3cb. Our functional analyses demonstrate that YAP requires Pik3cb to promote cardiomyocyte proliferation and activate the AKT pathway. Together these findings establish Pik3cb as a regulator of cardiac growth, serving as a direct link between Hippo-YAP and PI3K-AKT signaling pathways.
In mammals, most cells express both Pik3ca and Pik3cb, isoforms of the p110 catalytic subunit of Class IA PI3K. These isoforms each have unique functions, as germline inactivation of either Pik3ca or Pik3cb caused embryonic lethality before E10.510, 25. Compared to Pik3ca, relatively less is known about Pik3cb and how it differs from Pik3ca. Interestingly, Pik3cb is unique among PI3-kinases in signaling downstream of both receptor tyrosine kinases and G-protein coupled receptors26. Adult, cardiac-specific inactivation of Pik3cb did not cause a baseline cardiac phenotype, while similar inactivation of Pik3ca caused cardiac dysfunction27. These data showing that Pik3cb is dispensable for adult heart homeostasis are compatible with our observations, as it is likely that Pik3cb regulates physiological heart growth and stress responses downstream of YAP. Indeed, both YAP and PIK3CB protein levels in the heart decline with postnatal age, and our data point to a role of Pik3cb in promoting heart growth downstream of Yap. Additional studies will be required to interrogate further the roles of Pik3cb in heart development, stress responses, and regeneration.
Our work demonstrates that YAP functions directly upstream of Pi3kcb to enhance its expression. However, Pik3cb expression is likely regulated by other factors as well, as its expression was reduced but not completely abrogated by YAP knockout. Recently, it was reported that growth factors such as EGF signal through receptor tyrosine kinases to PI3K, which then promotes disassembly of the Hippo kinase complex, relieving its inhibition of YAP transcriptional activity28. Combined, these studies suggest reciprocal, mutually stimulatory cross-talk between YAP and PI3K that establish a feed forward regulatory circuit, in which Yap increases the expression of the PI3K subunit Pik3cb, and PI3K stimulates YAP activity.
YapcKO mice had heart dysfunction3, 5. Several targets of Yap have been identified, such as Ctgf, Birc3 and Birc5, but none have been shown to be essential for the YapcKO phenotype. By activating Pik3cb in 1-day old YapcKO pups, we largely restored heart function of YapcKO mice, indicating that Pik3cb is a major target of Yap and is important for preserving heart function. This result differs from the observation that Pik3cb is dispensible for normal heart homeostasis27. We reasoned that the different experimental contexts (normal adult heart versus failing juvenile heart) likely account for the different results. YAP is normally downregulated in the mature adult heart2, and it is possible that YAP and Pik3cb play more vital roles during heart development and postnatal growth. Furthermore, the YapcKO mouse has systolic dysfunction at birth that progresses over ~3 months to death. Thus, the YapcKO represents a stressed heart, which may have a different requirement for Pik3cb than a normal heart.
The heart failure phenotype of YapcKO is likely multifactorial. Consistent with published data, we found less proliferation and more apoptosis in the YapcKO mice3, indicating that the heart dysfunction of YapcKO mice is at least partially due to cumulative effect of cardiomyocyte insufficiency. Pik3cb rescued YapcKO heart function and our data indicate that this is through both mitogenic and pro-survival activities, in keeping with known roles of PI3K-AKT signaling.The cell cycle inhibitor p27, which is normally down-regulated at the protein level by AKT phosphorylation-triggered degradation20, was upregulated in YapcKO. P27 heterozygous inactivation enhanced cardiomyocyte proliferation29, suggesting that its aberrant expression in YapcKO heart contributes to reduced cardiomyocyte proliferation seen in these mutants (Fig.8E). At present, we are unable to determine the relative contribution of Pik3cb's proproliferative versus pro-survival effects to its overall beneficial activity.
Other cardiomyocyte functions are also likely to be regulated by YAP. For instance, TEAD transcription factors are implicated in regulating sarcomere gene expression through its recognition sequence, known as the MCAT motif30, suggesting a role for YAP in regulation of sarcomere assembly and function. Consistent with this idea, YAP-bound genes in our ChIP-seq data were more highly enriched for functional terms related to cardiovascular system development than terms related to cell proliferation. YAP has also been reported to regulate cell metabolism, a function that intersects with a well-described function of PI3K-AKT signaling. A subset of these YAP activites are likely to be independent of PIK3CB. This, in combination with incomplete transduction of all cardiomyocytes by AAV9:Pik3cb, likely accounts for incomplete Pik3cb rescue of YapcKO hearts.
In summary, we identified Pik3cb as a crucial direct target of YAP that links Hippo-YAP and PI3KAKT signaling pathways (Fig. 8K). YAP, through its transcriptional partner TEAD, increases Pik3cb expression, which further activates AKT. Pik3cb activation downstream of YAP promotes cardiomyocyte proliferation and survival.
Supplementary Material
Novelty and Significance.
What Is Known?
Loss of cardiomyocytes is associated with increased mortality and morbidity.
There is no effective means to replace the lost cardiomyocytes.
The transcriptional co-activator YAP is essential for heart growth and for normal adult heart systolic function. Inactivation of YAP causes lethal dilated cardiomyopathy with reduced cardiomyocyte proliferation and increased apoptosis.
YAP activation increases cardiomyocyte proliferation, albeit modestly.
The direct targets of Yap that convey its mitotic signal have not been defined.
YAP is known to activate the phosphoinositide 3-kinase (PI3K)-AKT pathway, a key regulator of cell proliferation and survival. However, the molecular link is not known.
What New Information Does This Article Contribute?
Through an unbiased, genome-wide screen, we found that YAP directly activates expression of Pik3cb through a conserved enhancer within its first intron. Pik3cb encodes a less-studied isoform of the catalytic subunit of PI3K.
Pik3cb promotes cardiomyocte proliferation and survival by stimulating Pik3ca expression and AKT activation.
YAP-induced cardiomyocyte proliferation requires upregulation of Pik3cb.
Heart failure resulting from inactivation of YAP was mitigated by activation of Pik3cb. Forced expression of Pik3cb normalized cardiomyocyte proliferation and survival in the absence of YAP, pointing out the key role of Pik3cb and the PI3K-AKT pathway in the physiological function of YAP.
This study focused on delineating the mechanism by which YAP regulates cardiomyocyte proliferation and survival. By employing unbiased whole genome profiling methods and AAV-mediated gain- and loss-of-function studies, we confirmed that Pik3cb is a major downstream target of YAP, being both sufficient and necessary for YAP activation of AKT. These data show that Pik3cb is a crucial link between Hippo-YAP and PI3K-AKT pathways.
ACKNOWLEDGEMENTS
We thank Jianming Jiang for sharing with us the protocol of making AAV-shRNA.
SOURCES OF FUNDING
Z.L. was supported by an AHA postdoctoral fellowship. W.T.P. was supported by NIH HL095712, HL100401, and U01HL098166, by an AHA Established Investigator Award, and by charitable contributions from Gail Federici Smith, Karen Carpenter, Edward Marram, and Dr. and Mrs. Edwin A. Boger.
Nonstandard Abbreviations and Acronyms
- YAP
Yes-associated protein
- MST1/2
Macrophage Stimulating 1/2
- LATS1/2
Large tumor suppressor kinase 1
- TEAD1-4
TEA-domain family members 1-4
- PI3K
phosphatidylinositol-4,5-bisphosphate 3-kinase
- AKT
protein kinase B
- MI
myocardial infarction
- ChIP-seq
Chromatin immunoprecipitation followed by high throughput sequencing
- ChIP-qPCR
Chromatin immunoprecipitation followed by quantitative PCR
- STAT
Signal transducers and activators of transcription ETS v-ets avian erythroblastosis virus E26 oncogene homolog
- p27
cyclin-dependent kinase inhibitor 1B
- NRVM
neonatal rat ventricular cardiomyocyte
- EdU
5-ethynyl-2 ´-deoxyuridine
- BrdU
bromodeoxyuridine
- cTNT
cardiac troponin T
- AAV9
adeno-associated virus serotype 9 AAV9:cTNT-3Flag-Pik3cb (AAV9:Pik3cb) AAV9 with cTNT promoter expressing Pik3cb with 3 FLAG epitope tags
- AAV9:cTNT-Luci
(AAV9:Luci) AAV9 with cTNT promoter expressing luciferase
- AAV9:YAP-shPik3cb
AAV9 with cTNT promoter expressing YAP[S127A] and a Pik3cb-directed shRNA
- AAV9:YAP-sc
AAV9 with cTNT promoter expressing YAP[S127A] and a scrambled shRNA
- AAV9:Luci-sc
AAV9 with cTNT promoter expressing luciferase and a scrambled shRNA
- YAPcKO
cardiac specific YAP knockout (Myh6-Cre::Yapfl/fl)
Footnotes
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Zhou B, Lin Z, Pu WT. Mammalian Myocardial Regeneration. In: Hill JA, Olson E, editors. Muscle: Fundamental Biology and Mechanisms of Disease. Vol. 1252. Elsevier; pp. 555–69. [Google Scholar]
- 2.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, Pu WT. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–9. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, Sadoshima J. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–88. doi: 10.1074/jbc.M112.436311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, Richardson JA, Sadek HA, Bassel-Duby R, Olson EN. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–44. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hong W, Guan K-L. The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol. 2012;23:785–93. doi: 10.1016/j.semcdb.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–90. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 10.Bi L, Okabe I, Bernard DJ, Nussbaum RL. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm Genome. 2002;13:169–72. doi: 10.1007/BF02684023. [DOI] [PubMed] [Google Scholar]
- 11.He A, Kong SW, Ma Q, Pu WT. Co-occupancy by multiple cardiac transcription factors identifies transcriptional enhancers active in heart. Proc Natl Acad Sci U S A. 2011;108:5632–7. doi: 10.1073/pnas.1016959108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, Pu WT. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–13. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–9. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciraolo E, Iezzi M, Marone R, Marengo S, Curcio C, Costa C, Azzolino O, Gonella C, Rubinetto C, Wu H, Dastru W, Martin EL, Silengo L, Altruda F, Turco E, Lanzetti L, Musiani P, Ruckle T, Rommel C, Backer JM, Forni G, Wymann MP, Hirsch E. Phosphoinositide 3-kinase p110beta activity: key role in metabolism and mammary gland cancer but not development. Sci Signal. 2008;1:ra3. doi: 10.1126/scisignal.1161577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiquee K, Zhang S, Guida WC, Blaskovich MA, Greedy B, Lawrence HR, Yip ML, Jove R, McLaughlin MM, Lawrence NJ, Sebti SM, Turkson J. Selective chemical probe inhibitor of Stat3, identified through structure-based virtual screening, induces antitumor activity. Proc Natl Acad Sci U S A. 2007;104:7391–6. doi: 10.1073/pnas.0609757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaspar BK, Roth DM, Lai NC, Drumm JD, Erickson DA, McKirnan MD, Hammond HK. Myocardial gene transfer and long-term expression following intracoronary delivery of adeno-associated virus. J Gene Med. 2005;7:316–24. doi: 10.1002/jgm.665. [DOI] [PubMed] [Google Scholar]
- 18.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 19.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, Basnet H, Mahadevan N, Fitamant J, Bardeesy N, Camargo FD, Guan KL. YAP mediates crosstalk between the Hippo and PI(3)K-TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–9. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nacusi LP, Sheaff RJ. Akt1 sequentially phosphorylates p27kip1 within a conserved but non-canonical region. Cell Div. 2006;1:11. doi: 10.1186/1747-1028-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang J, Wakimoto H, Seidman JG, Seidman CE. Allele-specific silencing of mutant Myh6 transcripts in mice suppresses hypertrophic cardiomyopathy. Science. 2013;342:111–4. doi: 10.1126/science.1236921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill KM, Kalifa S, Das JR, Bhatti T, Gay M, Williams D, Taliferro-Smith L, De Marzo AM. The role of PI 3-kinase p110beta in AKT signally, cell survival, and proliferation in human prostate cancer cells. Prostate. 2010;70:755–64. doi: 10.1002/pros.21108. [DOI] [PubMed] [Google Scholar]
- 23.Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–70. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 24.Aoyagi T, Matsui T. Phosphoinositide-3 kinase signaling in cardiac hypertrophy and heart failure. Curr Pharm Des. 2011;17:1818–24. doi: 10.2174/138161211796390976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bi L, Okabe I, Bernard DJ, Wynshaw-Boris A, Nussbaum RL. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110alpha subunit of phosphoinositide 3-kinase. J Biol Chem. 1999;274:10963–8. doi: 10.1074/jbc.274.16.10963. [DOI] [PubMed] [Google Scholar]
- 26.Dbouk HA, Backer JM. Novel approaches to inhibitor design for the p110β phosphoinositide 3-kinase. Trends Pharmacol Sci. 2013;34:149–53. doi: 10.1016/j.tips.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Z, Jiang YP, Wang W, Xu XH, Mathias RT, Entcheva E, Ballou LM, Cohen IS, Lin RZ. Loss of cardiac phosphoinositide 3-kinase p110 alpha results in contractile dysfunction. Circulation. 2009;120:318–25. doi: 10.1161/CIRCULATIONAHA.109.873380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–74. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poolman RA, Li JM, Durand B, Brooks G. Altered expression of cell cycle proteins and prolonged duration of cardiac myocyte hyperplasia in p27KIP1 knockout mice. Circ Res. 1999;85:117–27. doi: 10.1161/01.res.85.2.117. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida T. MCAT elements and the TEF-1 family of transcription factors in muscle development and disease. Arterioscler Thromb Vasc Biol. 2008;28:8–17. doi: 10.1161/ATVBAHA.107.155788. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.