Abstract
The cellular and molecular basis of vertebrate touch reception remains least understood among the traditional five senses. Somatosensory afferents that innervate the skin encode distinct tactile qualities, such as flutter, slip and pressure. Gentle touch is thought to be transduced by somatosensory afferents whose tactile end organs selectively filter mechanical stimuli. These tactile end organs comprise afferent terminals in association with non-neuronal cell types such as Merkel cells, keratinocytes and Schwann cells. An open question is whether these non-neuronal cells serve primarily as passive mechanical filters or whether they actively participate in mechanosensory transduction. This question has been most extensively studied in Merkel cells, which are epidermal cells that complex with sensory afferents in regions of high tactile acuity such as fingertips, whisker follicles, and touch domes. Merkel cell-neurite complexes mediate slowly adapting type I (SAI) responses, which encode sustained pressure and represent object features with high fidelity. How Merkel cells contribute to unique SAI firing patterns has been debated for decades; however, three recent studies in rodent models provide some direct answers. First, whole-cell recordings demonstrate that Merkel cells are touch-sensitive cells with fast, mechanically activated currents that require Piezo2. Second, optogenetics and intact recordings show that Merkel cells mediate sustained SAI firing. Finally, loss-of-function studies in transgenic mouse models reveal that SAI afferents are also touch sensitive. Together, these studies identify molecular mechanisms of mechanotransduction in Merkel cells, reveal unexpected functions for these cells in touch and support a revised, two-receptor site model of mechanosensory transduction.
Keywords: Touch, Piezo2, mechanosensitive channels, mechanosensory cells, tactile
Touch receptors provide sensory inputs to inform the brain about objects in our environment. Touch sensation is vital for survival and, without it, even simple activities such as feeding, dressing or standing would be exceedingly difficult to accomplish. Like hearing, balance and proprioception, touch is initiated by mechanosensory receptor cells. These cells contain mechanotransduction channels, which convert physical stimuli into membrane potential changes, called receptor potentials, that trigger neuronal action potentials. These action potentials are transmitted by sensory afferents to the brain, where sensory percepts are formed. The ability to excite sensory afferents is a key feature that distinguishes mechanosensory receptor cells from myriad mammalian cell types that also express mechanically activated ion channels [7].
Surprisingly little is known about the molecular and cellular mechanisms that mediate touch reception in mammals. It is generally accepted that mechanotransduction occurs in the terminals of somatosensory afferents. Gentle-touch receptors terminate in specialized end organs comprising sensory afferent terminals and non-neuronal cells such as epidermal Merkel cells, keratinocytes and terminal Schwann cells. Non-neuronal cells have been viewed typically as indirect elements in transduction, providing mechanical filtering and/or trophic support to tactile afferents [27]. Recently, this view has been challenged by evidence that non-neuronal cells express sensory ion channels, presynaptic components and neurotransmitters, which might render them capable of exciting sensory afferents [16, 30, 42, 46].
Much of this evidence has derived from studies of epidermal Merkel cells, which are conserved vertebrate cells described in 1875 by Friedrich Sigmund Merkel [39]. In mammals, Merkel cells localize to areas of high tactile acuity, such as fingerpads, whisker follicles and touch domes. Most Merkel cells contact sensory afferents to form Merkel cell-neurite complexes in the basal epidermal layer. These complexes act as gentle-touch receptors that mediate slowly adapting type I (SAI) responses [24], which encode static pressure as well as object features such as edges, shapes or textures [28, 47, 37]. SAI responses are biphasic, with a high-frequency volley of action potentials during the dynamic (or moving) phase of stimulation and a sustained but irregular discharge during prolonged stimulation. Though it is well established that Merkel cell-neurite complexes are touch receptors [24, 62, 60], whether Merkel cells contribute to SAI responses through active or passive mechanisms has puzzled scientists for more than four decades.
Merkel, who called his eponymous cells tastzellen (touch cells), was the first to posit that they function in touch sensation. This model is supported by ultrastructural studies. Like sensory receptor cells of the inner ear and olfactory epithelium, Merkel cells bear microvilli, which are potential sites of sensory transduction [23]. Merkel cells also form synapse-like structures, marked by dense core vesicles, with sensory afferents [24]. Histochemical and molecular studies confirmed the presence of presynaptic markers and putative neurotransmitters in Merkel cells [16, 21, 43]; however, functional studies that tested the requirement for Merkel cells in touch reception have led to contradictory conclusions [12, 25, 29, 38, 40, 53].
Three models of sensory transduction in the Merkel cell-neurite complex, summarized below and in Fig. 1, have gained considerable attention (reviewed in [17–18]). This review discusses recent studies that directly test the predictions of these models.
Figure 1.
Prevailing models of mechanotransduction in the Merkel cell-neurite complex. I Merkel cells are mechanosensory cells that mediate transduction: 1. Activation of mechanosensitive channels depolarizes Merkel cells, resulting in 2. opening of voltage-activated calcium channel and calcium-induced calcium release in Merkel cells. 3. This stimulates release of unidentified neurotransmitters from Merkel cells to 5. trigger action potentials in SAI afferents. II Mechanosensory afferents transduce touch: SAI afferent terminals possess 4. mechanosensitive ion channels that directly depolarize sensory afferents to 5. trigger SAI spike trains. In this model, Merkel cells are not needed for mechanotransduction, but 6. play a passive or modulatory role in SAI responses. III Two-receptor-site model: Both Merkel cells and SAI afferents are mechanosensory cells that collaborate to produce biphasic SAI responses. Adapted from [36]
Model I. Like hair cells of the inner ear, Merkel cells could be secondary sensory cells that serve as sites of mechanotransduction. In this case, Merkel cells should transduce touch stimuli and release neurotransmitters to excite adjacent sensory neurons, which then generate action potentials. This model predicts that Merkel cells are intrinsically touch sensitive. If Merkel cells are sole sites of mechanotransduction, eliminating or silencing them will abolish touch-evoked SAI responses. Furthermore, depolarizing Merkel cells in the absence of touch should excite spike firing in SAI afferents.
Model II. Like other somatosensory afferents and olfactory neurons, SAI afferents could be primary sensory neurons [14]. In this scenario, afferent terminals would mediate mechanosensory transduction and Merkel cells could serve to either modulate or mechanically filter their responses. This model predicts that Merkel cells will not display fast, touch-activated currents. Moreover, SAI afferents should remain touch sensitive when Merkel cells are functionally silenced. Finally, depolarizing Merkel cells should not trigger SAI afferent discharges in the absence of mechanical stimulation.
Model III. Both Merkel cells and sensory afferents could transduce touch stimuli to synergistically produce the SAI response. This two-receptor-site model postulates that afferents function as rapidly adapting (RA) fibers to transduce dynamic stimuli and Merkel cells transduce static phase responses [13, 64]. This model predicts that Merkel cells will display fast, mechanosensitive currents and that functionally silencing them should produce rapidly activating (RA) responses in SAI afferents. Conversely, depolarizing Merkel cells should produce sustained discharges in SAI afferents.
Are Merkel cells intrinsically touch sensitive?
Previous studies have shown that Merkel cells can be activated by a variety of mechanical stimuli. Calcium imaging studies demonstrated that Merkel cells respond to hypotonic-evoked cell swelling [15, 54, 57] and fluid flow [6]. Similarly, membrane stretch activates sustained inward currents in dissociated Merkel cells [3]. Chan et al. [8] reported that displacement excited slow calcium elevations in Merkel cells; however, efforts by other groups to record displacement-evoked currents were unsuccessful [44, 63]. Thus, the relevance of Merkel-cell mechanosensitivity to touch reception has been unclear.
Recent studies by the Gu, Lumpkin and Patapoutian groups have re-examined this question by performing whole-cell recordings from Merkel cells in vitro and in situ [26, 36, 61]. The population of Merkel cells in epidermis is sparse, representing only ~0.1% of epidermal cells in mouse skin. Nonetheless, Merkel cells can be easily identified in Atoh1/nGFP transgenic mice, which express green fluorescent protein (GFP) driven by enhancer elements of the Atoh1 gene [32]. Atoh1 is a proneural transcription factor that is expressed in Merkel cells but not other skin cells [2]. Using Atoh1/nGFP transgenic mice, Merkel cells can be purified by fluorescence activated cell sorting and then cultured for electrophysiological recordings [48]. Under whole-cell voltage-clamp, dissociated mouse Merkel cells exhibited touch-activated inward currents in a stimulus-dependent manner [36, 61]. These mechanosensitive currents displayed sigmoidal stimulus-response curves and sub-millisecond activation, suggesting that they are mediated by fast, mechanically gated ion channels [36]. Recordings from Merkel cells in a semi-intact rat whisker follicle preparation revealed remarkably similar touch-activated currents with sub-millisecond latencies [26]. In all three studies, Merkel cell currents were similar to touch-evoked, RA currents observed in cultured mechanosensory neurons [11, 20, 22, 52]. Together, these data demonstrate that Merkel cells are 1) intrinsically mechanosensitive cells capable of transducing touch stimuli in the absence of surrounding keratinocytes or sensory neurons, and 2) mechanosensitive in situ, confirming that touch-activated responses are not an epiphenomenon of cell isolation.
At first glance, it seems counterintuitive that the Merkel cell’s rapidly inactivating mechanotransduction currents can stimulate slowly adapting responses in sensory afferents; however, several lines of evidence suggest that downstream mechanisms can prolong signalling. First, current-clamp recordings showed that Merkel-cell displacement produced sustained depolarization, despite RA mechanosensitive currents [61]. Second, computational modeling predicts that a rapidly adapting transduction current with a small steady-state component can account for SAI responses [31] and, in one study, Merkel-cell mechanotransduction channels displayed steady-state currents of ~20 pA [36]. Depolarizing current injections of this amplitude produced slow, calcium action potentials [26, 61] resembling those previously reported in Merkel cells [44, 63]. Thus, the Merkel cell’s voltage-activated calcium channels likely open downstream of mechanotransduction [16, 48, 63]. Consistent with this model, focal displacements excite prolonged calcium signalling throughout the Merkel cell’s soma, which probably reflect calcium entry and calcium-induced calcium release [15, 36]. In situ, whisker-follicle Merkel cells fired calcium action potentials in response to whisker movement or indirect stimulation [26]. Finally, we propose that inputs are summed across Merkel cells to produce robust SAI firing, because each SAI afferent typically innervates a cluster of Merkel cells (mean±SD; adult mouse: 16±6 Merkel cells per touch-dome afferent, N=15 [31]; human: 127±103 Merkel cells per touch dome [51]). How the responses from multiple Merkel cells are integrated into SAI firing patterns is an important question for future studies.
The current-clamp experiments described above confirm that Merkel cells are excitable, a feature that separates mechanosensory cells from non-sensory cells. These results are consistent with both Model I (that Merkel cells are mechanosensory) and Model III (two-receptor sites; Fig. 1).
Which mechanosensitive channels underlie touch-evoked responses in Merkel cells?
The biophysical properties of touch-activated currents in Merkel cells [26, 36] match those of mechanosensitive ion channels encoded by Piezo genes [9]. Mechanosensitive currents reversed at 1–8 mV in rat and mouse Merkel cells, suggesting that they are mediated by non-selective cation channels [26, 36]. Ionic permeability analysis confirmed that calcium-permeable cation channels carry Merkel-cell mechanotransduction currents [26]. Touch-activated currents were attenuated by Ruthenium Red and Gadolinium ions, which are commonly used blockers of mechanosensitive channels [9, 26, 36]]. Finally, inactivation time constants were estimated at 6–8 ms [26, 36], matching those of Piezo2-dependent currents in heterologous cell types [9].
Piezos are widely found throughout phylogeny, from protists to mammals, which have two Piezo genes [9, 10]. Piezo2 is expressed in somatosensory neurons and, in culture, siRNA-mediated Piezo2 silencing attenuates their touch-evoked RA currents [9]. Using a transgenic Piezo2-GFP reporter mouse, Woo et al. demonstrated that Piezo2 is expressed in Merkel cells but not other skin cells [61]. Quantitative real time PCR analysis confirmed that Piezo2 transcripts are enriched in Merkel cells compared with Piezo1 [36]. Rat Merkel cells also express Piezo2 [26], and Merkel-cell mechanotransduction currents in rat whisker follicles were reduced by shRNAs and an antibody raised against Piezo2 [26]. To test the effects of complete loss of Piezo2 function in Merkel cells, Woo et al. generated epidermal-specific Piezo2 knockout mice (Piezo2CKO, K14Cre;Piezo2Flox/Flox) [61]. In these mice, mechanically activated currents and membrane potential changes were completely abolished in cultured Merkel cells [61]. These results demonstrate that Piezo2 is required for Merkel-cell mechanosensitivity to touch stimuli.
Are SAI afferents touch sensitive in the absence of functionally intact Merkel cells?
To test whether Merkel cells are necessary for touch-evoked responses in SAI afferents in the intact skin, Maksimovic et al. [36] analyzed epidermal specific Atoh1 knockout mice (Atoh1CKO, K14Cre;Atoh1Flox/LacZ), which completely lack Merkel cells in developing and adult skin [41, 59]. Though Merkel cells are absent in these mice, touch domes are innervated by myelinated Aβ afferents [36]. To directly assess whether these afferents are mechanically sensitive in the absence of Merkel cells, single-unit recordings were made in a semi-intact preparation from touch-dome afferents, which were identified based on fluorescent FM1-43 uptake. In control genotypes, 5-s displacements of touch domes in intact skin produced prototypical SAI responses containing a high-frequency dynamic phase and an SA static phase. By contrast, touch dome afferents in Atoh1CKO mice showed reduced dynamic firing and intermediately adapting (IA) responses that ceased to fire during sustained touch. Thus, touch-dome afferents are capable of transducing touch stimuli without Merkel cells, which rules out Model I ( Fig. 1). Notably, touch-dome afferents lacking Merkel cells responded with markedly altered firing properties and were not capable of encoding sustained pressure. These findings are consistent with either Model II (that Merkel cells modulate mechanosensory responses) or Model III (two-receptor sites; Fig. 1).
Does Piezo2 in Merkel cells contribute to SAI responses? In a rat whisker preparation, shRNA-mediated Piezo2 silencing reduced, but did not abolish, compound action potentials recorded with suction electrodes [26]. Dynamic and static discharges were attenuated, suggesting that Piezo2 contributes to both phases of SA responses; however, interpretation of these results is complicated by the complex innervation of whisker follicles and the multiunit nature of the recordings. It is unclear to which extent mechanically evoked discharges reflect the contributions of Merkel-cell-associated afferents versus other fiber types that are also activated by whisker deflections.
In epidermal-specific Piezo2CKO mice, targeted single-unit recordings from touch-dome afferents showed IA responses with normal dynamic responses but static phases similar to those of Atoh1CKO touch-dome afferents [36, 61]. In strong support of two-receptor sites (Model III), these data demonstrate that mechanosensitive channels in sensory neurons can account for dynamic responses and that Merkel-cell mechanotransduction is essential for sustained responses to prolonged displacement (Fig. 1).
How do Merkel cells contribute to SAI responses?
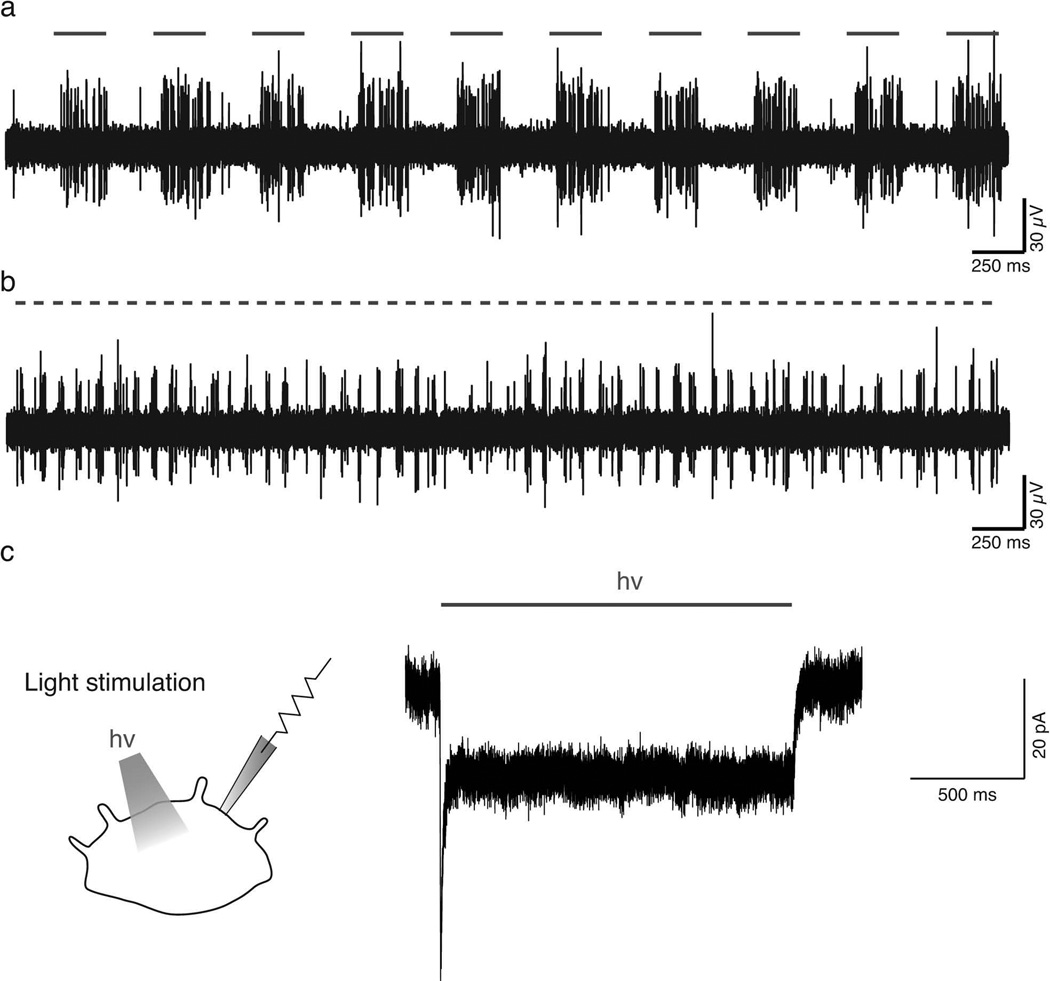
The intimate contact between Merkel cells and sensory terminals in the skin limits the ability to selectively displace Merkel cells without disturbing underlying afferents, which has complicated efforts to identify specific roles of Merkel cells in touch reception. The use of optogenetics overcomes this obstacle: selective optical control of genetically modified, light-sensitive Merkel cells allows for identification of activity patterns in sensory neurons that are triggered by Merkel-cell activation. Maksimovic et al. [36] used Cre-LoxP system in mice to drive expression of the light-gated cation channel, channelrhodopsin-2 (ChR2) [5] in Merkel cells but not in sensory afferents (CCKCre;Rosa26ChR2-tdTomato/+ and K14Cre;Rosa26ChR2-tdTomato/+). Light- and touch-evoked responses were measured from Aβ afferents using ex vivo skin-nerve recordings (Fig. 2a–b). Whole-cell recordings from ChR2-expressing Merkel cells in vitro confirmed that illumination elicited long-lasting inward currents (Fig. 2c). Light stimulation of ChR2-expressing Merkel cells activated sustained discharges in touch-dome afferents that mimicked static-phase firing of SAI responses. Conversely, optogenetic silencing of Merkel cells with archaerhodopsin-3 (ArchT), a light-sensitive, hyperpolarizing proton pump [19], reversibly inhibited SAI firing during the static phase of displacement. Together, these optogenetic experiments demonstrate that Merkel-cell depolarization is both necessary and sufficient to excite sustained discharges from SAI afferents in intact skin. Moreover, they provide the first direct demonstration that epidermal cells make functional, excitatory connections with sensory afferents. These findings are inconsistent with Model II and provide direct evidence to support Model III.
Figure 2.
Optogenetic stimulation of ChR2-expressing Merkel cells. In intact skin of transgenic mice (CCKCre/+;Rosa26ChR2-eYFP/+), blue-light stimulation drives fast responses in touch-dome afferents. Horizontal bars indicate blue light illumination. Illumination frequencies of 2 Hz (a; 250 ms light-on followed by 250 ms light-off) and 10 Hz (b; 50-ms light-on followed by 50 ms light-off) produce spike discharges in phase with light stimuli. Note that several touch domes were simultaneously illuminated during this multiunit recording. c A whole-cell voltage-clamp recording from a ChR2-expressing Merkel cell shows inward currents induced by light stimulation.
Two groups have attempted to interrogate the requirement for synaptic transmission between Merkel cells and sensory afferents by blocking voltage-activated calcium channels in intact recordings [26, 45]. This treatment suppresses touch-evoked responses; however, these intact recordings cannot rule out direct effects on calcium channels that might be located on sensory terminals.
Perspectives and open questions
The data discussed here indicate that Merkel cells are mechanosensory receptor cells that transduce mechanical stimuli and excite sensory afferents. Studies of mouse Merkel cells and touch-dome afferents demonstrate that both Merkel cells and sensory neurons act as mechanoreceptors, with Merkel cells mediating sustained firing to static touch. In the absence of Merkel cells or Piezo2-dependent mechanosensitivity of Merkel cells, sensory afferents exhibit reduced firing rates and IA properties. Piezo2, which is expressed in touch-dome afferents, is a leading candidate to initiate these responses in SAI afferents [34, 61]. Together, these findings lead to an updated version of the two receptor-site model by demonstrating that sensory afferents mediate IA rather than RA responses [36, 61].
This two-site model is consistent with observations in rat whisker Merkel cells [26, 34]. Blocking voltage-activated calcium channels attenuated, but did not abolish, compound action potentials in intact whisker recordings. Thus, additional studies are needed to determine whether whisker SAI afferents completely lack mechanosensitivity or exhibit transient response in the absence of Merkel-cell signaling.
Key questions are yet unanswered. Do Piezo2-dependent channels in Merkel cells require specialized subcellular machinery for efficient mechanical activation? Mechanosensitive channels may be distribute uniformly in the plasma membrane, or they might localize to microvilli, as they do in mechanosensory hair cells [33]. Do Merkel-cell mechanotransduction channels require accessory molecules, such as STOML3, for efficient gating [49]?
The nature of excitatory mechanisms that convey signals between Merkel cells and touch-dome afferents also remain to be identified. A plethora of potential neurotransmitters, including neuropeptides and classical, small-molecule neurotransmitters along with their molecular transporters, have been detected in Merkel cells of various species [35]. Release of the neuropeptide vasoactive intestinal peptide (VIP) has been reported from rat Merkel cells in vitro [4]. In general neuropeptides produce slow, long-lasting responses of receptive cells, on the order of seconds to minutes [58]. Light-induced SAI firing driven by ChR2-expressing Merkel cells can follow phasic optical stimulation on a timescale of tens to hundreds of milliseconds (Fig. 2); therefore, it seems unlikely that neuropeptides solely mediate these responses. Fast neurotransmitters such as serotonin or glutamate are likely involved, as proposed by previous studies [13, 16, 21, 43, 50, 55]. ATP is another candidate neurotransmitter; however, it also induces calcium responses in Merkel cells [6, 8], suggesting that ATP may potentiate Merkel cells to modulate neurotransmitter release [56]. The contributions of fast neurotransmitters, neuropeptides that might be co-released, and the identities of neurotransmitter receptors, are still unresolved (reviewed in [35]).
How do Merkel cells augment firing during dynamic stimulation? This is particularly important to address in high-acuity areas such as glabrous skin, where Merkel cell-neurite complexes are proposed to contribute to shape discrimination. In vertebrate and Drosophila auditory organs, sensory cells amplify mechanical inputs to tune responses for speed and sensitivity (reviewed in [1]). The observation that Merkel-cell knockout mice have reduced dynamic firing suggests that Merkel cells might play an analogous role in signal amplification. Alternatively, Merkel cells could perform non-sensory functions in development or skin mechanics that shape dynamic SAI responses. Further analysis of acute Merkel-cell silencing and biophysical studies in the intact skin are needed to understand the multifaceted roles of these fascinating epidermal cells in touch sensation.
Acknowledgements
We thank Ms. Blair Jenkins for assistance with figures and members of the Lumpkin lab for discussions. The authors are supported by National Institutes of Health grants R01AR051219 (to EAL) R01NS073119 (to EAL and Gregory J. Gerling), and fellowships to MN (Japan Society for the Promotion of Science Research Fellowships for Young Scientists 24-7585) and SM (F32NS080544).
References
- 1.Bechstedt S, Howard J. Hearing mechanics: a fly in your ear. Current biology : CB. 2008;18(18):R869–R870. doi: 10.1016/j.cub.2008.07.069. [DOI] [PubMed] [Google Scholar]
- 2.Ben-Arie N, Hassan BA, Bermingham NA, Malicki DM, Armstrong D, Matzuk M, Bellen HJ, Zoghbi HY. Functional conservation of atonal and Math1 in the CNS and PNS. Development. 2000;127(5):1039–1048. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- 3.Boulais N, Pennec JP, Lebonvallet N, Pereira U, Rougier N, Dorange G, Chesne C, Misery L. Rat Merkel cells are mechanoreceptors and osmoreceptors. PloS one. 2009;4(11):e7759. doi: 10.1371/journal.pone.0007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boulais N, Pereira U, Lebonvallet N, Gobin E, Dorange G, Rougier N, Chesne C, Misery L. Merkel cells as putative regulatory cells in skin disorders: an in vitro study. PloS one. 2009;4(8):e6528. doi: 10.1371/journal.pone.0006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8(9):1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 6.Cha M, Ling J, Xu GY, Gu JG. Shear mechanical force induces an increase of intracellular Ca2+ in cultured Merkel cells prepared from rat vibrissal hair follicles. Journal of neurophysiology. 2011;106(1):460–469. doi: 10.1152/jn.00274.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalfie M. Neurosensory mechanotransduction. Nature reviews Molecular cell biology. 2009;10(1):44–52. doi: 10.1038/nrm2595. [DOI] [PubMed] [Google Scholar]
- 8.Chan E, Yung WH, Baumann KI. Cytoplasmic Ca2+ concentrations in intact Merkel cells of an isolated, functioning rat sinus hair preparation. Experimental brain research. 1996;108(3):357–366. doi: 10.1007/BF00227259. [DOI] [PubMed] [Google Scholar]
- 9.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330(6000):55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M, Patapoutian A. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature. 2012;483(7388):176–181. doi: 10.1038/nature10812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delmas P, Hao J, Rodat-Despoix L. Molecular mechanisms of mechanotransduction in mammalian sensory neurons. Nature reviews Neuroscience. 2011;12(3):139–153. doi: 10.1038/nrn2993. [DOI] [PubMed] [Google Scholar]
- 12.Diamond J, Mills LR, Mearow KM. Evidence that the Merkel cell is not the transducer in the mechanosensory Merkel cell-neurite complex. Progress in brain research. 1988;74:51–56. doi: 10.1016/s0079-6123(08)62997-0. [DOI] [PubMed] [Google Scholar]
- 13.Fagan BM, Cahusac PM. Evidence for glutamate receptor mediated transmission at mechanoreceptors in the skin. Neuroreport. 2001;12(2):341–347. doi: 10.1097/00001756-200102120-00032. [DOI] [PubMed] [Google Scholar]
- 14.Gottschaldt KM, Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science. 1981;214(4517):183–186. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- 15.Haeberle H, Bryan LA, Vadakkan TJ, Dickinson ME, Lumpkin EA. Swelling-activated Ca2+ channels trigger Ca2+ signals in Merkel cells. PloS one. 2008;3(3):e1750. doi: 10.1371/journal.pone.0001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J, Lumpkin EA. Molecular profiling reveals synaptic release machinery in Merkel cells. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(40):14503–14508. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haeberle H, Lumpkin EA. Merkel Cells in Somatosensation. Chemosensory perception. 2008;1(2):110–118. doi: 10.1007/s12078-008-9012-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his "Merkel cell", morphology, development, and physiology: review and new results. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003;271(1):225–239. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 19.Han X, Chow BY, Zhou H, Klapoetke NC, Chuong A, Rajimehr R, Yang A, Baratta MV, Winkle J, Desimone R, Boyden ES. A high-light sensitivity optical neural silencer: development and application to optogenetic control of non-human primate cortex. Frontiers in systems neuroscience. 2011;5:18. doi: 10.3389/fnsys.2011.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(40):13384–13395. doi: 10.1523/JNEUROSCI.2926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitchcock IS, Genever PG, Cahusac PM. Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neuroscience letters. 2004;362(3):196–199. doi: 10.1016/j.neulet.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 22.Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. The Journal of physiology. 2006;577(Pt 3):815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iggo A, Findlater G. SENSORY RECEPTOR MECHANISMS. In: Hamann W, editor. Proceedings of the International Symposium on Sensory Receptor Mechanisms. Singapore: 8:. A. Iggo© 1984 World Scientific Publ. Co.; 1984. p. 117. [Google Scholar]
- 24.Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. The Journal of physiology. 1969;200(3):763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda I, Yamashita Y, Ono T, Ogawa H. Selective phototoxic destruction of rat Merkel cells abolishes responses of slowly adapting type I mechanoreceptor units. The Journal of physiology. 1994;479(Pt 2):247–256. doi: 10.1113/jphysiol.1994.sp020292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive abeta-afferent impulses. Cell. 2014;157(3):664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson KO. The roles and functions of cutaneous mechanoreceptors. Current opinion in neurobiology. 2001;11(4):455–461. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 28.Johnson KO, Lamb GD. Neural mechanisms of spatial tactile discrimination: neural patterns evoked by braille-like dot patterns in the monkey. The Journal of physiology. 1981;310:117–144. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kinkelin I, Stucky CL, Koltzenburg M. Postnatal loss of Merkel cells, but not of slowly adapting mechanoreceptors in mice lacking the neurotrophin receptor p75. The European journal of neuroscience. 1999;11(11):3963–3969. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 30.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(15):4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lesniak DR, Marshall KL, Wellnitz SA, Jenkins BA, Baba Y, Rasband MN, Gerling GJ, Lumpkin EA. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. eLife. 2014;3:e01488. doi: 10.7554/eLife.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lumpkin EA, Collisson T, Parab P, Omer-Abdalla A, Haeberle H, Chen P, Doetzlhofer A, White P, Groves A, Segil N, Johnson JE. Math1-driven GFP expression in the developing nervous system of transgenic mice. Gene expression patterns : GEP. 2003;3(4):389–395. doi: 10.1016/s1567-133x(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 33.Lumpkin EA, Hudspeth AJ. Detection of Ca2+ entry through mechanosensitive channels localizes the site of mechanoelectrical transduction in hair cells. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(22):10297–10301. doi: 10.1073/pnas.92.22.10297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma Q. Merkel cells are a touchy subject. Cell. 2014;157(3):531–533. doi: 10.1016/j.cell.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Maksimovic S, Baba Y, Lumpkin EA. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Annals of the New York Academy of Sciences. 2013;1279:13–21. doi: 10.1111/nyas.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014 doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maricich SM, Morrison KM, Mathes EL, Brewer BM. Rodents rely on Merkel cells for texture discrimination tasks. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(10):3296–3300. doi: 10.1523/JNEUROSCI.5307-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maricich SM, Wellnitz SA, Nelson AM, Lesniak DR, Gerling GJ, Lumpkin EA, Zoghbi HY. Merkel cells are essential for light-touch responses. Science. 2009;324(5934):1580–1582. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merkel F. Tastzellen und Tastkörperchen bei den Hausthieren und beim Menschen. Archiv f mikrosk Anat. 1875;11(1):636–652. [Google Scholar]
- 40.Mills LR, Diamond J. Merkel cells are not the mechanosensory transducers in the touch dome of the rat. Journal of neurocytology. 1995;24(2):117–134. doi: 10.1007/BF01181555. [DOI] [PubMed] [Google Scholar]
- 41.Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Developmental biology. 2009;336(1):76–83. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilius B, Biro T, Owsianik G. TRPV3: time to decipher a poorly understood family member! The Journal of physiology. 2014;592(Pt 2):295–304. doi: 10.1113/jphysiol.2013.255968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunzi MG, Pisarek A, Mugnaini E. Merkel cells, corpuscular nerve endings and free nerve endings in the mouse palatine mucosa express three subtypes of vesicular glutamate transporters. Journal of neurocytology. 2004;33(3):359–376. doi: 10.1023/B:NEUR.0000044196.45602.92. [DOI] [PubMed] [Google Scholar]
- 44.Nurse C, Cooper E. Electrophysiological Studies on Merkel Cells Isolated from Rat Vibrissal Mechanoreceptors. In: Hník P, Soukup T, Vejsada R, Zelená J, editors. Mechanoreceptors. Springer US: 1988. pp. 189–194. [Google Scholar]
- 45.Pacitti EG, Findlater GS. Calcium channel blockers and Merkel cells. Progress in brain research. 1988;74:37–42. doi: 10.1016/s0079-6123(08)62995-7. [DOI] [PubMed] [Google Scholar]
- 46.Pawson L, Prestia LT, Mahoney GK, Guclu B, Cox PJ, Pack AK. GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(9):2695–2705. doi: 10.1523/JNEUROSCI.5974-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phillips JR, Johnson KO. Neural mechanisms of scanned and stationary touch. The Journal of the Acoustical Society of America. 1985;77(1):220–224. doi: 10.1121/1.392262. [DOI] [PubMed] [Google Scholar]
- 48.Piskorowski R, Haeberle H, Panditrao MV, Lumpkin EA. Voltage-activated ion channels and Ca2+-induced Ca2+ release shape Ca2+ signaling in Merkel cells. Pflugers Archiv: European journal of physiology. 2008;457(1):197–209. doi: 10.1007/s00424-008-0496-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. Tuning Piezo ion channels to detect molecular-scale movements relevant for fine touch. Nature communications. 2014;5:3520. doi: 10.1038/ncomms4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Press D, Mutlu S, Guclu B. Evidence of fast serotonin transmission in frog slowly adapting type 1 responses. Somatosensory & motor research. 2010;27(4):174–185. doi: 10.3109/08990220.2010.516670. [DOI] [PubMed] [Google Scholar]
- 51.Reinisch CM, Tschachler E. The touch dome in human skin is supplied by different types of nerve fibers. Annals of neurology. 2005;58(1):88–95. doi: 10.1002/ana.20527. [DOI] [PubMed] [Google Scholar]
- 52.Rugiero F, Drew LJ, Wood JN. Kinetic properties of mechanically activated currents in spinal sensory neurons. The Journal of physiology. 2010;588(Pt 2):301–314. doi: 10.1113/jphysiol.2009.182360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Senok SS, Baumann KI, Halata Z. Selective phototoxic destruction of quinacrine-loaded Merkel cells is neither selective nor complete. Experimental brain research. 1996;110(3):325–334. doi: 10.1007/BF00229133. [DOI] [PubMed] [Google Scholar]
- 54.Soya M, Sato M, Sobhan U, Tsumura M, Ichinohe T, Tazaki M, Shibukawa Y. Plasma membrane stretch activates transient receptor potential vanilloid and ankyrin channels in Merkel cells from hamster buccal mucosa. Cell calcium. 2014;55(4):208–218. doi: 10.1016/j.ceca.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 55.Tachibana T, Endoh M, Fujiwara N, Nawa T. Receptors and transporter for serotonin in Merkel cell-nerve endings in the rat sinus hair follicle. An immunohistochemical study. Archives of histology and cytology. 2005;68(1):19–28. doi: 10.1679/aohc.68.19. [DOI] [PubMed] [Google Scholar]
- 56.Tachibana T, Nawa T. Recent progress in studies on Merkel cell biology. Anatomical science international. 2002;77(1):26–33. doi: 10.1046/j.0022-7722.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 57.Tazaki M, Suzuki T. Calcium inflow of hamster Merkel cells in response to hyposmotic stimulation indicate a stretch activated ion channel. Neuroscience letters. 1998;243(1-3):69–72. doi: 10.1016/s0304-3940(98)00066-4. [DOI] [PubMed] [Google Scholar]
- 58.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115. doi: 10.1016/j.neuron.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Keymeulen A, Mascre G, Youseff KK, Harel I, Michaux C, De Geest N, Szpalski C, Achouri Y, Bloch W, Hassan BA, Blanpain C. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. The Journal of cell biology. 2009;187(1):91–100. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wellnitz SA, Lesniak DR, Gerling GJ, Lumpkin EA. The regularity of sustained firing reveals two populations of slowly adapting touch receptors in mouse hairy skin. Journal of neurophysiology. 2010;103(6):3378–3388. doi: 10.1152/jn.00810.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014 doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woodbury CJ, Koerber HR. Central and peripheral anatomy of slowly adapting type I low-threshold mechanoreceptors innervating trunk skin of neonatal mice. The Journal of comparative neurology. 2007;505(5):547–561. doi: 10.1002/cne.21517. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita Y, Akaike N, Wakamori M, Ikeda I, Ogawa H. Voltage-dependent currents in isolated single Merkel cells of rats. The Journal of physiology. 1992;450:143–162. doi: 10.1113/jphysiol.1992.sp019120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamashita Y, Ogawa H. Slowly adapting cutaneous mechanoreceptor afferent units associated with Merkel cells in frogs and effects of direct currents. Somatosensory & motor research. 1991;8(1):87–95. doi: 10.3109/08990229109144732. [DOI] [PubMed] [Google Scholar]