Abstract
Rationale
Rnd3, a small Rho GTPase, is involved in the regulation of cell actin cytoskeleton dynamics, cell migration, and proliferation. The biological function of Rnd3 in the heart remains unexplored.
Objective
To define the functional role of the Rnd3 gene in the animal heart and investigate the associated molecular mechanism.
Methods and Results
By loss-of-function approaches, we discovered that Rnd3 is involved in calcium regulation in cardiomyocytes. Rnd3-null mice died at the embryonic stage with fetal arrhythmias. The deletion of Rnd3 resulted in severe Ca2+ leakage through destabilized ryanodine receptor type 2 (RyR2) Ca2+ release channels. We further found that downregulation of Rnd3 attenuated β2-adrenergic receptor (β2AR) lysosomal targeting and ubiquitination, which in turn resulted in the elevation of β2AR protein levels leading to the hyperactivation of protein kinase A (PKA) signaling. The PKA activation destabilized RyR2 channels. This irregular spontaneous Ca2+ release can be curtailed by PKA inhibitor treatment. Increases in the PKA activity along with elevated cyclic adenosine monophosphate (cAMP) levels were detected in Rnd3-null embryos, in neonatal rat cardiomyocytes, and non-cardiac cell lines with Rnd3 knockdown, suggesting a general mechanism for Rnd3-mediated PKA signaling activation. β2AR blocker treatment reduced arrhythmia and improved cardiac function.
Conclusion
Rnd3 is a novel factor involved in intracellular Ca2+ homeostasis regulation in the heart. Deficiency of the protein induces RyR2 dysfunction by a mechanism that attenuates Rnd3-mediated β2AR ubiquitination, which leads to the activation of PKA signaling. Increased PKA signaling in turn promotes RyR2 hyperphosphorylation, which contributes to arrhythmogenesis and heart failure.
Keywords: Calcium leakage, arrhythmogenesis, protein kinase A signaling, β2-adrenergic receptor, ubiquitination, calcium, arrhythmia, iodine receptor, Rho
INTRODUCTION
Rnd3, also known as RhoE, is one of the Rnd subsets of the Rho-family GTPases. It is generally believed that it functions as a repressor of Rho-associated coiled-coil protein kinase 1 (ROCK1).1-4 In contrast to the canonical mode of Rho-family proteins, members of the Rnd subfamily, including Rnd1, Rnd2, and Rnd3, bind but do not hydrolyze GTP. They are defective in GTPase activity, even in the presence of RhoGAPs.5-8 The study of the regulatory activation of ROCK1 demonstrated that Rnd3 inhibits RhoA-mediated ROCK1 signaling by specifically binding to ROCK1, but not ROCK2.1, 2 Rnd3 is the only endogenous ROCK1 antagonist discovered so far.4 The current knowledge about the biological functions of Rnd3 has been mainly achieved by in vitro cell culture studies and tissue screening assays including gain- and loss-of-function approaches. Overexpression of Rnd3 inhibited ROCK1-mediated biological effects including stress fiber formation, phosphorylation of myosin light chain phosphatase, and apoptosis. Reduced expression of Rnd3 potentiated ROCK1 activity.1, 2, 9. Rnd3 is also a substrate of ROCK1. The ROCK1-Rnd3 interaction mediates Rnd3 phosphorylation by ROCK1 at multiple sites within its N- and C-terminals, which stabilizes the Rnd3 protein, regulates Rnd3 cellular localization, and forms a negative feedback loop to ROCK1.10, 11 Emerging evidence suggests that Rnd3 might be involved in the regulation of the cell cycle,12 as well as cancer cell migration and invasion.13, 14 We recently uncovered a Rho kinase-independent function of Rnd3, and reported that genetic deletion of Rnd3 led to aqueductal stenosis in mouse brains through upregulation of Notch signaling.15 Two studies found that Rnd3 was indispensable in mouse neuron development,16, 17 suggesting Rnd3 is involved in much broader biological functions besides its inhibitory effect on Rho kinase. In this study, we provide evidence to reveal a function of Rnd3, in which Rnd3 regulates the β2AR-PKA signaling pathway. Deficiency of Rnd3 leads to global activation of PKA in vitro in cardiac and non-cardiac cells, and in vivo in animal hearts. The latter develops fetal arrhythmias through the destabilization of RyR2 calcium release channels.
Fetal or neonatal heart arrhythmias in humans are a common disorder. While the type and severity of congenital arrhythmias vary, some are life-threatening.18 Only limited genetic mutations leading to fetal arrhythmias have been identified.19 Continued identification of new genes/loci linked to fetal arrhythmias will expand our knowledge of the genetic components of the disease, and will have significant impacts on the disease diagnosis, prognosis, and treatment. Here, we present an animal model with fetal arrhythmias and reveal that the activation of the β2-adrenergic receptor-protein kinase A (β2ARPKA) signaling pathway contributes to the Rnd3 deficiency-mediated arrhythmic phenotype. In the mechanistic study, we provide evidence that the downregulation of Rnd3 is sufficient to initiate the activation of PKA signaling in vivo in animal hearts and in vitro in both cardiac and non-cardiac cells, suggesting a general mechanism for Rnd3-mediated PKA regulation. We further determine that Rnd3 is a regulator in the β2AR ubiquitination regulatory complex. Rnd3 regulates β2AR ubiquitination, mediated by the physical interaction between both proteins. The lack of Rnd3 prevents the ubiquitination of β2AR, resulting in the accumulation of β2AR protein. Excess β2AR promotes the activation of PKA signaling, which then contributes to the dysfunction of RyR2 calcium release channels. The β2AR antagonist treatment significantly reduced arrhythmia and improved cardiac contractility.
The pathological consequence of Rnd3 downregulation in the heart is unknown. By searching databases, we found one microarray screening study that showed a significant decrease in the Rnd3 mRNA levels in failing human myocardium (Profile GDS651/212724_at/RND3 in NCBI GEO profiles). The etiologic meaning of Rnd3 downregulation in human heart failure and arrhythmogenesis remains obscure. Clearly, our Rnd3-null mouse study provides a mechanistic base for the future investigation in humans.
METHODS
Methods and any associated references are provided in detail in the online version of the paper.
RESULTS
Genetic deletion of the Rnd3 gene in mice results in embryonic lethality with fetal heart arrhythmias
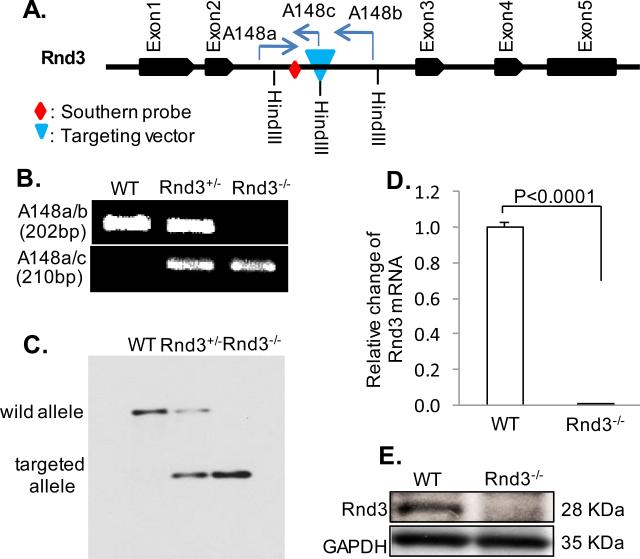
Rnd3 knockout mice were generated from a gene trap ES cell line. The targeting vector was inserted at Rnd3 intron 2 (Fig. 1A). The deletion of the Rnd3 gene was verified by PCR genotyping (Fig. 1B) and Southern blotting (Fig. 1C). q-PCR (Fig. 1D) and Western blot (Fig. 1E) assessments of the Rnd3 transcript and protein levels confirmed the Rnd3 knockout. No obvious morphological changes were observed in the mutant embryonic hearts (Online Fig. I). Genotyping results revealed that the majority of Rnd3-null mice died between E10.5 and E12.5 (Online Table I). To evaluate embryonic mouse cardiac functions in vivo, we performed echocardiographic analyses in female mouse wombs. Rnd3-null mice showed severe decreases in the cardiac ejection fraction and fractional shortening compared to wild-type control mice (Online Table II). Due to the technological challenges, we were not able to measure an individual mouse embryo electrocardiography (ECG), therefore, we assessed heart beats using echocardiography, and detected cardiac arrhythmias in E11 Rnd3-null mice (Online Video I and Video II).
Figure 1. Generation of Rnd3-Deficient Mice.
Targeting vector was inserted into Rnd3 intron 2 (A), and the insertion was verified by PCR (B) and Southern blot (C). Mouse genomic DNA (15 μg) was digested by HindIII and hybridized with a 5’ probe for Southern blot analysis. Interruption of Rnd3 gene expression was achieved and verified by q-PCR (D) and Western blot analysis (E). Statistical significance was determined by unpaired, two-tailed student's t test. Data are means ± s.d. A148a, A148b, and A148c were PCR primers used for genotyping. WT: wild-type.
Rnd3−/− cardiomyocytes display an abnormal intracellular Ca2+ release
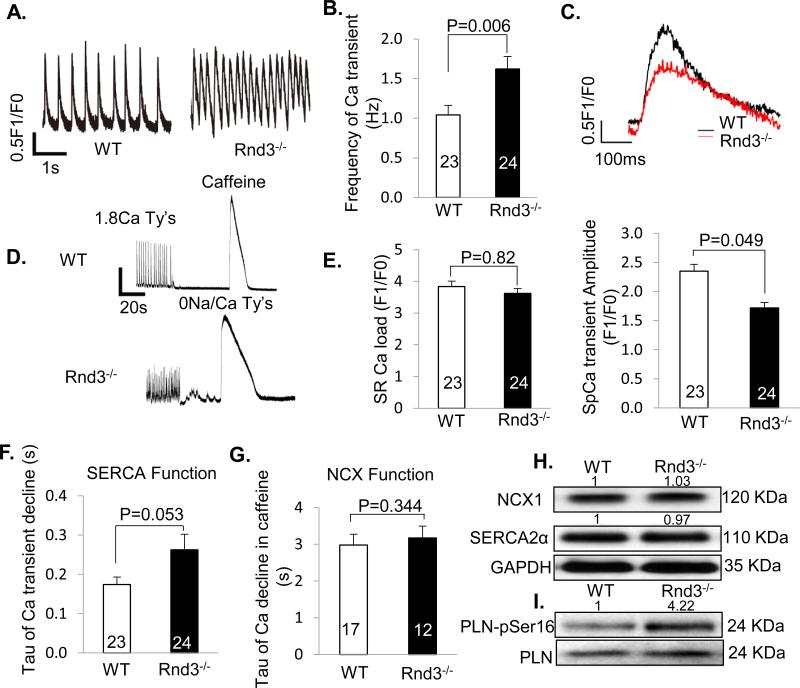
To explore a possible mechanism contributing to the arrhythmogenicity, we analyzed cardiomyocytes isolated from E10.5 embryonic hearts. We found that Rnd3−/− cardiomyocytes exhibited a significant increase in spontaneous rhythm (Fig. 2A) as shown by the increased frequency of Ca2+ oscillations (1.62 ± 0.16/Hz) compared to the WT (1.04 ± 0.12/Hz) (Fig. 2B). The amplitude of the spontaneous Ca2+ transient, however, was reduced by 27% in Rnd3−/− cardiomyocytes (1.72 ± 0.09 F1/F0) compared to WT myocytes (2.35 ± 0.12 F1/F0) (Fig. 2C upper and lower panels). Further experiments revealed obvious Ca2+ leakage occurring in Rnd3−/− cardiomyocytes under sodium- and calcium-free perfusate conditions (without the influences of Ca2+ pump and Na+/Ca2+ exchanger (NCX), (Fig. 2D). The cardiomyocyte sarcoplasmic reticulum (SR) Ca2+ load showed no significant differences between the two groups (Fig. 2E), suggesting normal or compensated sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and NCX functions. Measurements of the fluorescence decay times (Tau) for Ca2+ transient decline and Ca2+ decline in caffeine showed no statistically significant differences between the WT and the Rnd3 mutant hearts, indicating no impairment of the SERCA and NCX functions in the mutant heart (Fig. 2F and 2G). However, we observed a higher Tau value in SERCA function analysis (Fig. 2F), which could be due to the downregulation of the SERCA expression levels and/or a lower SERCA activity. To rule out these possibilities, NCX1 and SERCA2α protein levels, and phospholamban (PLN) phosphorylation at serine 16 in the animal hearts were assessed by immunoblot analyses. No changes in the NCX1 and SERCA2α protein levels were observed in the Rnd3-null heart compared to the WT heart (Fig. 2H). Hyperphosphorylation of PLN at serine 16 was detected in the Rnd3 mutant heart (Fig. 2I). This indicates that the mutant hearts compensate for the Ca2+ leakage by enhancing SERCA function, although this counter effect may not be sufficient to overcome the overall Ca2+ leakage shown by a prolonged Tau of Ca2+ transient decline (Fig. 2F). Together, the genetic deletion of Rnd3 resulted in an obvious Ca2+ leakage phenotype in mouse cardiomyocytes. This leak did not lead to a lower SR Ca2+ load due to the compensations by SERCA and NCX functions, therefore suggesting a RyR2 Ca2+ release channel dysfunction.
Figure 2. Abnormal Intracellular Ca2+ Release in Rnd3−/− Cardiomyocytes.
(A) Representative tracings of Ca2+ transients in WT and Rnd3−/− embryonic cardiomyocytes. (B) Rnd3−/− myocytes exhibited increased spontaneous Ca2+ transient activity. (C) Upper panel: representative single tracing of Ca2+ transient in WT and Rnd3−/− cardiomyocytes. Lower panel: Rnd3−/− cardiomyocytes exhibited decreased transient amplitudes. (D) Representative tracings of Ca2+ transients showed a noticeable Ca2+ leakage in Rnd3−/− cardiomyocytes under sodium- and calcium-free perfusate conditions followed by caffeine treatment. WT: wild-type. (E) No change of SR load in Rnd3−/− cardiomyocytes compared to the WT cells. (F) No compromised SERCA function was found in Rnd3−/− cardiomyocytes assessed by fluorescence decay time (tau). (G) The NCX function remained intact in Rnd3-null cardiomyocytes. (H) Immunoblotting analysis showed no difference in NCX1 and SERCA2α protein levels between the WT and Rnd3−/− hearts. (I) The hyperphosphorylation of phospholamban (PLN) at serine 16 was observed in Rnd3−/− hearts. The number in each column represents the number of cardiomyocytes analyzed from ten embryonic hearts in each group. At least 40 embryos were pooled and used for the immunoblotting analysis in each group. Statistical significance was determined by an unpaired, two-tailed student's t test. Data are means ± s.d. The number at the top of each immunoblot band (panel H and panel I) represents the average of densitometries from three experiments, normalized by GAPDH.
RyR2 Ca2+ release channels are destabilized in Rnd3−/− cardiomyocytes
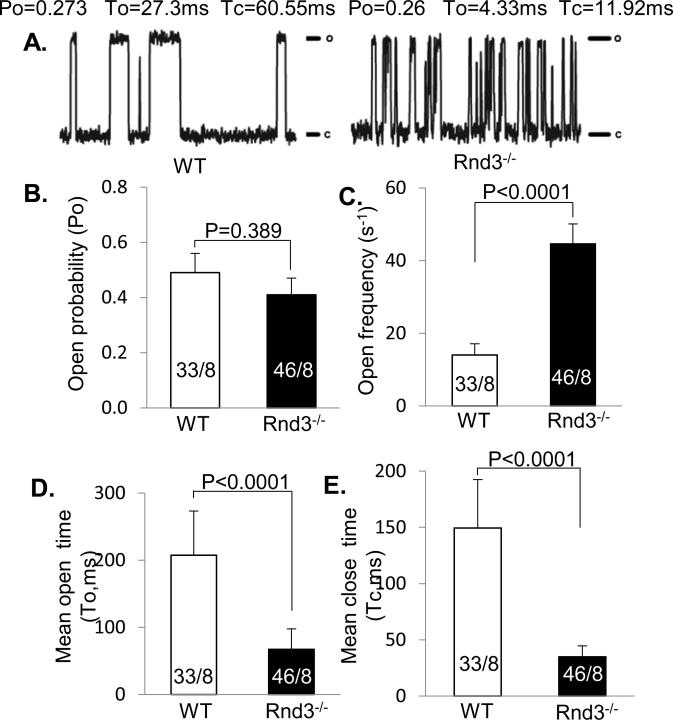
We then assessed RyR2 single channel function in planar lipid bilayers.20 While the average RyR2 basal opening probability was not statistically different between Rnd3−/− mice (0.41 ± 0.06) and WT mice (0.49 ± 0.07) (Fig. 3A and 3B), the frequency of the RyR2 opening was three times higher in Rnd3−/− mouse hearts (44.6 ± 5.5 s−1) compared to WT hearts (14.0 ± 3.1 s−1) (Fig. 3A and 3C). Both RyR2 channel open and close times were significantly shortened in Rnd3−/− mouse hearts (mean open time To= 67.1 ± 30.7 ms; mean close time Tc=34.9 ± 9.7 ms) (filled black bar in Fig. 3D and 3E, respectively) compared to WT (mean open time To=207.3 ± 66.0 ms; mean close time Tc=149.4 ± 43.1 ms) (empty bar in Fig. 3D and 3E, respectively). These data suggest that RyR2 channels are destabilized in Rnd3-null cardiomyocytes, contributing to abnormal intracellular Ca2+ release and an arrhythmogenic phenotype.
Figure 3. Single Channel Tracings Revealed Aberrant RyR2 Openings in Rnd3−/− Cardiomyocytes.
(A) Representative tracings of RyR2 channels from WT and Rnd3−/− mice, showing similar probability (Po) in Rnd3−/− mice (B). (C) Increased RyR2 open frequency (open event/s) along with decreased mean open time (To) (D) and closed time (Tc) (E) was observed in Rnd3−/− cardiomyocytes compared to WT. The numbers in each column indicate the total number of RyR2 channels recorded over a total number of mouse embryos. c: channel closed state; o: channel open state. WT: wild-type. Statistical significance was determined by unpaired, two-tailed student's t test. Data are means ± s.d.
Rnd3 knockdown leads to elevated PKA activity and hyperphosphorylation of RyR2
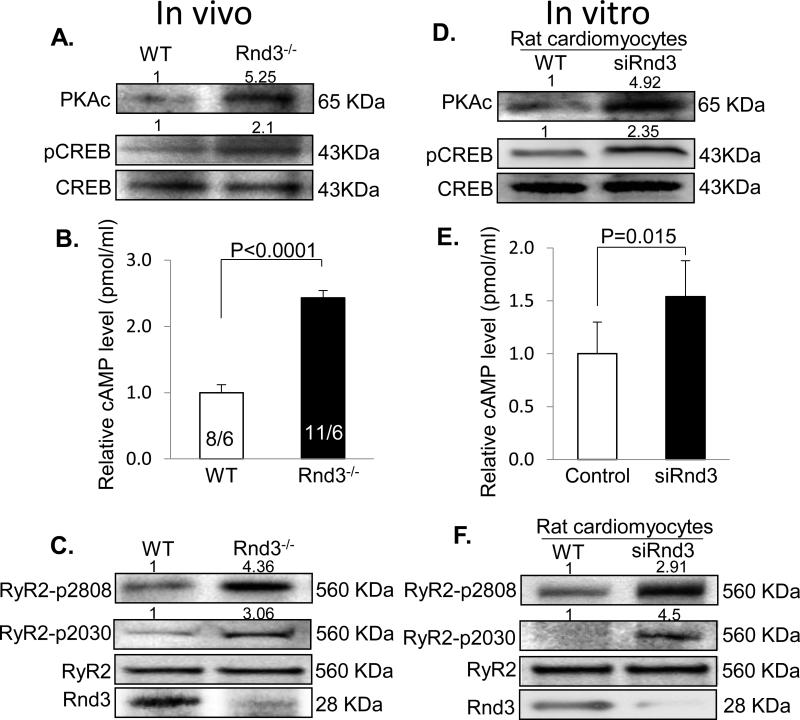
Next, we explored the molecular mechanism of Rnd3 deficiency-mediated RyR2 destabilization. RyR2 Ca2+ release channels, tetramer proteins, are critical for calcium handling and are tightly regulated. We, as well as others, have demonstrated that RyR2 channel hyperphosphorylation by PKA is closely associated with Ca2+ dysregulation including SR Ca2+ leakage, which contributes to cardiac arrhythmias and heart failure.21-24 We therefore assessed the protein levels of the active PKA isoform (PKAc) and the phosphorylation status of cyclic AMP-responsive element-binding protein (CREB), a known PKA substrate. The immunoblot data displayed remarkable increases in the PKAc protein levels and the hyperphosphorylation of CREB in the Rnd3 mutant heart (Fig. 4A). The elevated PKA activation in the Rnd3 mutant heart was also confirmed by the observation of an increase in PLN phosphorylation at serine 16, a major target of PKA in the early immunoblot of this study (Fig. 2I). In parallel with the assessment of PKA activity, the cAMP levels were measured in mouse embryos. More than a 2-fold increase in the cAMP concentration was detected in Rnd3−/− E10.5 embryos compared to WT embryos (Fig. 4B). Meanwhile, obvious increases in RyR2 phosphorylation levels at serines 2808 and 2030 were observed in Rnd3-null hearts with no changes in the RyR2 total protein expression levels (Fig. 4C). Thus, several lines of evidence in vivo indicate that Rnd3 deficiency results in activation of PKA signaling, which leads to RyR2 channel hyperphosphorylation.
Figure 4. Rnd3 Knockdown Led to Elevated PKA Activity and Hyperphosphorylation of RyR2 in Vivo and in Vitro.
(A) The active protein PKA isoform (PKAc) was detected by an immunoblot and an obvious increase in PKAc was found along with hyperphosphorylated CREB (pCREB) in Rnd3 mutant hearts. (B) More than a 2-fold increase in the cAMP level assessed by the ELISA assay was observed in E10.5 mutant embryos compared to WT embryos. The data were pooled from eight embryos out of six female mice (8/6) for the WT group and eleven embryos out of six female mice (11/6) for the Rnd3−/− group. (C) Western blot analysis displayed significant increases in RyR2 phosphorylation at serine 2808 and serine 2030 in Rnd3 mutant hearts. The same results were achieved in the neonatal rat cardiomyocytes treated by the siRNA specific for Rnd3 (D-F). The cAMP data from rat cardiomyocytes were pooled from three repeated experiments in each group with triplicate analyses for each time. At least 40 embryos were pooled and used for the immunoblotting analysis in the Rnd3-null heart experiment. cAMP: cyclic adenosine monophosphate. WT: wild-type. Statistical significance was determined by an unpaired, two-tailed student's t test. Data are means ± s.d. The number at the top of each immunoblot band represents the average of densitometries from three experiments, normalized by CREB for pCREB and GAPDH for PKAc in panel A and D, and by RyR2 in panel C and F.
To demonstrate that the Rnd3-mediated change in the PKA activity was not due to any secondary or compensated effects by the animal in vivo, we knocked down Rnd3 in neonatal rat cardiomyocytes in vitro using the specific siRNA for Rnd3. Increases in the levels of PKAc protein, CREB hyperphosphorylation, and intracellular cAMP concentrations paralleled the knockdown of Rnd3 expression in the same cohort of rat cardiomyocytes (Fig. 4D and 4E). Noticeable increases in RyR2 phosphorylation at serines 2808 and 2030 were detected in siRNA-treated rat cardiomyocytes as well (Fig. 4F). These data are consistent with the in vivo results from the Rnd3 mutant mice.
Since PKA signaling is essential for a broad array of biological activities, we examined the Rnd3 deficiency-mediated PKA activation in non-cardiac cells as well. In accordance with our cardiomyocyte results, significant increases in the PKA activity and CREB hyperphosphorylation were observed in mouse embryonic fibroblasts (MEF) isolated from Rnd3−/− mice (Online Fig. IIA), and HEK293T epithelial cells (Online Fig. IIB), and C2C12 myoblasts (Online Fig. IIC) treated with the siRNA for Rnd3 knockdown. The higher cAMP levels were also found in the Rnd3 downregulated groups compared with the controls (Online Fig. IID), suggesting a general mechanism of PKA activation regulated by Rnd3. We demonstrated the increased cAMP levels along with the elevated PKA activities both in vivo in the Rnd3-null mouse hearts and embryos, and in vitro in the Rnd3 knockdown cardiac and non-cardiac cells. These data suggest that the downregulation of Rnd3 is sufficient to initiate PKA signaling activation that leads to RyR2 channel hyperphosphorylation.
PKA activation is involved in the Rnd3 deficiency-mediated Ca2+ leakage
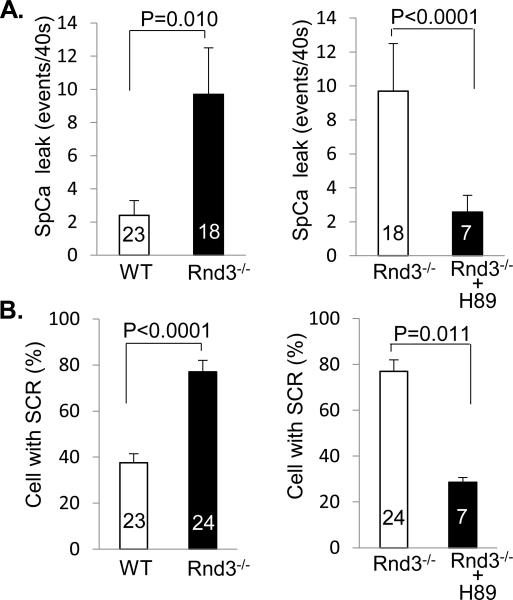
We have demonstrated the activation of PKA induced by Rnd3 downregulation. Now we would like to know if Rnd3 deficiency-mediated Ca2+ leakage can be blocked by the inhibition of PKA activity. We treated cardiomyocytes with a PKA inhibitor, H89. Consistent with the previous data in this study, we observed four times higher spontaneous Ca2+ leakage events (SCR) in Rnd3−/− cardiomyocytes compared to WT cells (Fig. 5A). However, the Ca2+ leakage was blocked by the H89 treatment (Fig. 5A). The percentage of the mutant cardiomyocyte cells with SCR was double that of the WT cardiomyocytes (Fig. 5B). After H89 treatment, this high percentage returned to the level of the WT cardiomyocytes (Fig. 5B). This result suggests that PKA activation is involved in the Rnd3 deficiency-mediated Ca2+ leakage.
Figure 5. Rnd3 Deficiency-Mediated Ca2+ Leakage was Blocked by PKA Inhibitor Treatment.
Assessments of Ca2+ leakage by quantification of spontaneous Ca2+ leakage events (A) and the cells with SCR (B). The leaky phenotype was blocked by PKA inhibitor H89 treatment. SCR: spontaneous Ca2+ release. The number in each column represents the number of cardiomyocytes analyzed. We used ten embryonic hearts for the WT group, ten embryonic hearts for the Rnd3−/− group, and three embryonic hearts for the Rnd3−/−+H89 group. WT: wild-type. Statistical significance was determined by an unpaired, two-tailed student's t test. Data are means s.d.
In the assessment of PKA activation, we also evaluated RyR2 phosphorylation at serine 2814, a CaMK phosphorylation site. The enhancement of the phosphorylation was detected in the Rnd3−/− heart (Online Fig. IIIA). Treatment of the mutant cardiomyocytes with CaMK inhibitor KN93 partially attenuated the Ca2+ leakage (Online Fig. IIIB). However, the inhibitory effect on the Ca2+ leakage by KN93 was moderate compared to the blockage by H89 treatment (Fig. 5). Therefore, we focused on PKA activation in this study.
Adult cardiomyocytes with Rnd3 haploinsufficiency (Rnd3+/−) also exhibit an abnormal intracellular Ca2+ release and the Ca2+ leakage is attenuated by PKA inhibitor treatment
To investigate if the calcium leakage occurred in the adult cardiomyocytes as well, we conducted the same cohort of experiments in the cardiomyocytes isolated from adult Rnd3+/− mouse hearts. The spontaneous calcium transient incidence in Rnd3+/− cells was five times higher than that in the wild-type cells (Online Fig. IVA), and significantly higher calcium transient frequencies were also detected in the mutant cells compared to the controls (Online Fig. IVB). These abnormal intracellular calcium releases were attenuated by H89 treatment (Online Fig. IVA and IVB). The calcium leakage in Rnd3 haploinsufficient cardiomyocytes was further augmented by isoproterenol (ISO) treatment (Online Fig. IVC and IVD). Consistent with the embryonic cardiomyocytes, the calcium leakage did not lead to a lower SR Ca2+ load (Online Fig. IVE and IVF). The representative tracings of Ca2+ transients were presented in Online Fig. IIIG. Overall, the results indicate that Rnd3 downregulation leads to calcium leakage in both embryonic and adult mouse cardiomyocytes, and the leakage phenotype is attenuated by PKA inhibitor treatment.
Adult Rnd3 haploinsufficient mice displayed ventricular arrhythmias
Since Rnd3-null mice are embryonically lethal, we evaluated the electrophysiology of adult Rnd3 heterozygous mice. Nine mice (5 Rnd3+/− and 4 WT) underwent the electrophysiology study protocol. All mice exhibited sinus rhythm before pacing studies started (Online Fig. VA). Baseline programmed electrical stimulation evoked ventricular arrhythmias in 40% (2/5) of Rnd3+/− mice and none (0/4) in the WT mice (using either ventricular extrastimulus or burst pacing) (Online Fig. VB and VE). After intraperitoneal injection of isoproterenol (3 mg/kg) and caffeine (120 mg/kg), the average heart rate increased by 16% in both groups (16 ± 6% in WT mice and 16 ± 9% in Rnd3+/− mice; P=0.73) (Online Fig. VC). Ventricular arrhythmias were induced in 80% (4/5) of the Rnd3+/− mice versus 0% (0/4) of the WT mice (P=0.048) by ventricular burst pacing after isoproterenol/caffeine injection (Online Fig. VD and VE). The remaining Rnd3+/− mouse died following ventricular burst pacing at 40 msec. The ECG rhythm of this mouse revealed sinus bradycardia followed by AV block and asystole. Thus, haploinsufficiency for Rnd3 creates a substrate for ventricular arrhythmias that can be induced by cardiac pacing in particular after beta-adrenergic stimulation.
Rnd3 deficiency results in the elevation of β2AR protein levels through the ubiquitination mechanism
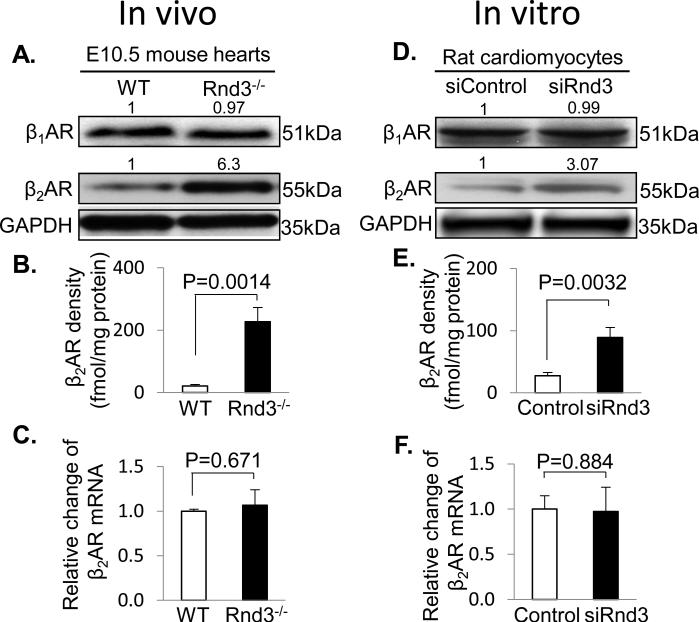
Finally, we explored the molecular mechanism of the Rnd3 deficiency-mediated PKA activation and found an increase in the β2AR, but not β1AR, protein levels in the Rnd3-null mouse heart (Fig. 6A). Since the assessment of β2AR protein expression levels is critical, we validated the specificity of the β2AR antibody by probing for β2AR expression in the β1AR/β2AR double-knockout and the β2AR single knockout mouse hearts, respectively (Online Fig. VI). To further verify and quantify the β2AR protein increase detected by the immunoblot analysis, the 125I-iodocyanopindolol binding assay, a more stringent radioligand method, was used. We observed that the membrane-associated β2AR was 10 times higher in Rnd3−/− hearts (227.4 fmol/mg) compared to the wild-type control hearts (21.36 fmol/mg) (Fig. 6B). Interestingly, we did not detect a correlated increase at the β2AR mRNA levels in the same cohort of the mutant hearts (Fig. 6C). To rule out any possible secondary or compensated effects leading to this increase in β2AR protein levels by the animal in vivo, we conducted the same set of experiments in vitro in the neonatal rat cardiomyocytes treated with the siRNA specific for Rnd3. Again, upregulated β2AR protein levels were detected by both immunoblot analyses (Fig. 6D) and 125I-iodocyanopindolol binding assay (Fig. 6E), but the β1AR protein levels remained the same. The disconnection of the increase in β2AR protein levels without an increase in its transcript levels was also observed (Fig. 6F). Finally, we forced expression of Rnd3 and found a downregulation of β2AR protein levels (Online Fig. VII).
Figure 6. Rnd3 Deficiency Resulted in the Elevation of β2AR Protein Levels.
(A) Immunoblotting analyses showed no obvious difference in β1AR protein levels between the WT and Rnd3-null hearts, while there was a significant increase in β2AR protein levels. (B) The increase in β2AR protein levels in Rnd3 mutant hearts was verified and quantified by a radioligand binding assay. (C) q-PCR assessments of β2AR mRNA levels in the WT and Rnd3-null hearts. The same observations were detected in rat neonatal cardiomyocytes transfected with siRnd3 to knockdown Rnd3: panel (D) for the immunoblot analysis, panel (E) for the radioligand binding assay, and panel (F) for the q-PCR assay. At least 40 embryo hearts were pooled and used for the immunoblotting, radioligand binding and q-PCR assays. WT: wild-type. Ctrl: control. β1AR: β1-adrenergic receptor. β2AR: β2-adrenergic receptor. The number at the top of each immunoblot band in panel A and D represents the average of densitometries from three experiments, normalized by GAPDH. Statistical significance was determined by unpaired, two-tailed student's t test. Data are means ± s.d.
In the previous experiments, we demonstrated that Rnd3 deficiency-mediated PKA activation was a general mechanism that happened in both cardiac and non-cardiac cells. Therefore, we wanted to know if the β2AR protein upregulation occurs without a change in its transcript levels in non-cardiac cells with Rnd3 downregulation. The associated assessments of the β2AR protein and mRNA levels in the non-cardiac MEF cells isolated from Rnd3−/− mice (Online Fig. VIIIA and VIIIB, respectively), and the HEK293T cells (Online Fig. VIIIC and VIIID, respectively), and myoblast C2C12 cells (Online Fig. VIIIE and VIIIF, respectively) with Rnd3 knocked down by siRNA were performed. Again, noticeable increase in the β2AR protein but not mRNA levels were found. These data strongly suggest that β2AR is under post-translational regulation through Rnd3.
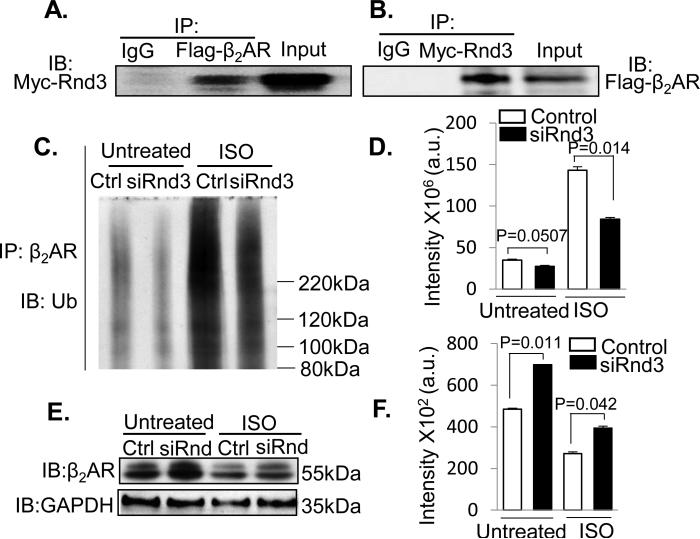
Since ubiquitination is a critical post-translational regulatory mechanism for β2AR, we investigated whether Rnd3 plays a role in β2AR ubiquitination. First, we determined the interaction of Rnd3 and β2AR ex vivo by co-transfection of Rnd3 and β2AR in HEK293T cells followed by mutual coimmunoprecipitations as shown in Fig. 7A and 7B. Then, the levels of β2AR ubiquitination were assessed by immunoprecipitation of β2AR followed by anti-ubiquitin immunoblotting analysis under both baseline and isoproterenol (ISO) treatment conditions. As shown in Fig. 7C (in the untreated group), the amount of ubiquinated species of β2AR was significantly reduced by the knockdown of Rnd3 with about a 22% decline in the normalized amount of ubiquitinated β2AR compared to the control without ISO treatment (Fig. 7D in the untreated group). Consistent with the baseline result, a 41% reduction in the normalized amount of ubiquitinated β2AR in the Rnd3 knockdown group was observed relative to the control (Fig. 7C in ISO group and 7D in ISO group) while the total amount of β2AR ubiquitination was increased by the ISO treatment as expected. In parallel with the reductions in β2AR ubiquitination, higher expression levels of β2AR protein were once again detected in the Rnd3 knockdown groups under both baseline (untreated) and ISO treated conditions by cycloheximide chase analysis (Fig. 7E and 7F).
Figure 7. Rnd3 Physically Interacted with β2AR and Rnd3 Deficiency Attenuated β2AR Ubiquitination Resulting in the Elevation of β2AR Protein Levels.
(A-B) Co-immunoprecipitation (IP) pull-downs were conducted followed by immunoblotting analyses (IB). The blots displayed the interaction of Rnd3 and β2AR proteins. (C) A representative immunoblotting analysis for ubiquitin showed the decline in the ubiquitination level of β2AR in the Rnd3 knockdown (siRnd3) group (Untreated). The same result was observed as well in the two ISO treated (for 10 min) groups while the overall ubiquitination levels were increased as expected. (D) The results of panel C were quantified from three repeated experiments. (E) The β2AR protein levels from the whole cell lysates for the experiments presented in panel C were assessed by immunoblotting analysis. Along with the reductions of β2AR ubiquitination in the Rnd3 knockdown groups, noticeable increases in the β2AR protein levels were observed under both untreated and ISO-treated conditions. (F) The results of panel E were quantified. WT: wild-type. Ctrl: control. a.u.: arbitrary unit. β2AR: β2-adrenergic receptor. ISO: isoproterenol. Statistical significance was determined by unpaired, two-tailed student's t test. Data are means ±s.d.
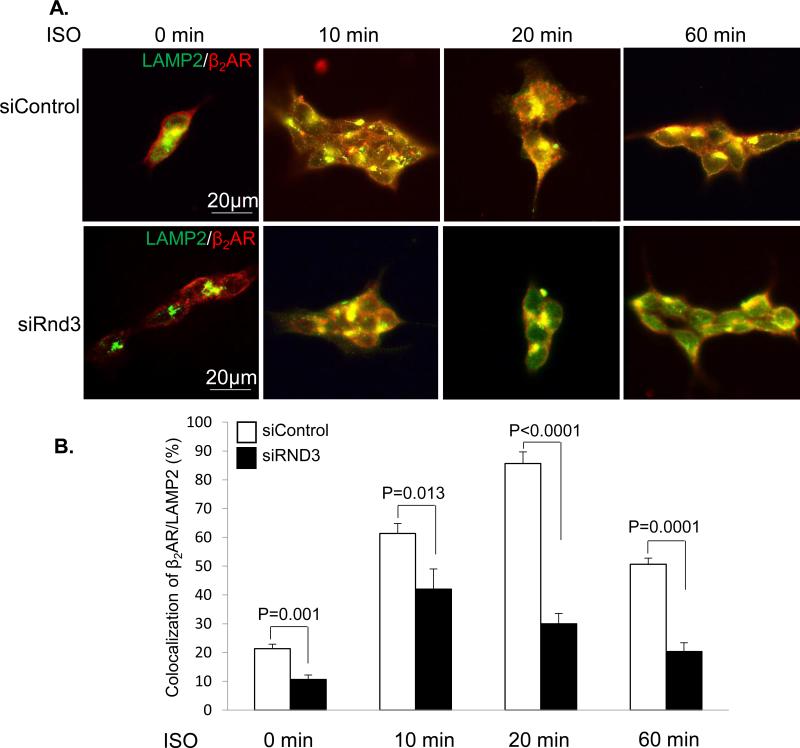
β2AR lysosomal targeting has been shown as an important mechanism for ubiquinated β2AR degradation 25. We conducted isoproterenol-stimulated β2AR lysosomal trafficking experiments to monitor β2AR receptor dynamic subcellular localization in a flag-β2AR stable expression cell line (293Tflag-β2AR cells). Immunostaining for LAMP2 (lysosome-associated membrane protein 2), a lysosome marker, and β2AR was performed followed by fluorescent microscopy analysis. The receptor internalization into lysosomes and endocytic vesicles was visualized (Fig. 8A) and quantified by the colocalization of β2AR with LAMP2 (Fig. 8B). In the control group without isoproterenol stimulation, β2AR was evenly distributed across the cell membrane with a basal level of β2AR within lysosomes. However, Rnd3 deficiency significantly reduced β2AR receptor internalization into lysosomes (10.7%) compared to the control group (21.3%), and this attenuated lysosomal trafficking of β2AR was still detected 10, 20, and 60 minutes after isoproterenol stimulation. The data indicate that Rnd3 is a critical factor for the integrity of lysosome-mediated β2AR regulation. The result is consistent with the regulatory role of Rnd3 in β2AR degradation observed early in this study.
Figure 8. β2AR Lysosome Trafficking was Attenuated by Rnd3 Knockdown.
(A) Visualization of dynamic changes of β2AR (Red) internalization into lysosomes with isoproterenol (ISO) treatments. LAMP2 (green), a protein marker of lysosomes. (B) Quantifications of the colocalization of β2AR with LAMP2. The data represent a summary of three independent experiments. Statistical significance was determined by unpaired, two-tailed student's t test. Data are means ± s.d.
Regulation of post-translational modification-mediated βAR degradation is complicated and involves multiple biological processes and factors. Although it is not the focus of the study, we still examined the expression levels of β-arrestin, an adaptor protein of E3 ligase for β2AR ubiquitination, and G-protein coupled receptor kinase 2 (Grk2) in the mutant heart since both factors are involved in β2AR ubiquitination. No significant differences of the two protein expression levels were detected between the Rnd3-null heart and the wild-type heart (Online Fig. IX).
We then assessed hydroxylation regulation of β2AR receptor and sumoylation modification of arrestin 3 and caveolin-3 in Rnd3 deficient mouse hearts, since hydroxylation and sumoylation are involved in β2AR receptor post-translational modification degradation.26-28 The former is through Egl-9 family hypoxia-inducible factor 3 (EGLN3), a member of the EGLN family of prolyl hydroxylases, also called prolyl hydroxylase domain-containing protein 3 (PHD3), which catalyzes hydroxylation of β2AR and facilitates the receptor ubiquitination by von Hippel-Lindau E3 ubiquitin ligase complex (pVHL).26 In the sumoylation modification mechanism, arrestin 3 and caveolin-3 sumoylation enhanced β2AR internalization and endocytosis.27, 28 We evaluated the amount of EGLN3 and pVHL binding to β2AR by immunoprecipitation followed by immunoblot specific for EGLN3 and pVHL in mouse hearts. As displayed in Online Fig. XA, we did not find significant differences in EGLN3 and pVHL proteins bound to β2AR among wild-type, Rnd3+/−, and Rnd3−/− animal hearts. Meanwhile, the immunoprecipitation from the same cohort of mouse hearts showed no obvious changes of arrestin 3 and caveolin-3 sumoylation modifications (Online Fig. XB), indicating a minimal role of hydroxylation and sumoylation in Rnd3-mediated β2AR regulation.
Finally, we conducted a mouse rescue experiment using ICI118,551, a β2AR antagonist. The drug significantly improved Rnd3−/− embryo mouse cardiac contractility (Online Table II) and reduced arrhythmia. We only detected arrhythmia in 1 out of 16 mutant embryos.
Together with the previous results, we provide the evidence that Rnd3 is a factor involved in β2AR ubiquitination and RyR2 stabilization regulation. Rnd3 deficiency attenuates β2AR lysosomal trafficking and ubiquitination, resulting in the prolonged activation of PKA signaling. The latter targets RyR2 channels and leads to channel dysfunction, which eventually contributes to arrhythmias and heart failure in Rnd3-null embryonic hearts. The β2AR antagonist treatment reduced arrhythmia and improved cardiac function.
DISCUSSION
The destabilization of RyR2 calcium release channels is responsible for many heart diseases, including cardiac arrhythmias and heart failure. Phosphorylation of RyR2 has been demonstrated as an important modification for its functional integrity. In patients with RyR2 channel mutations, the RyR2 channels exhibit “leaky” defects under PKA phosphorylation conditions, a situation that mimics sympathetic activation during exercise.29, 30 The PKA-mediated destabilization of the RyR2 channels was demonstrated in several mouse models as well.21-23, 31-33 Overexpression of PKA catalytic domain in mouse heart tissues was sufficient to lead to dilated cardiomyopathy and sudden cardiac death.22 Genetic inhibition of PKA-mediated RyR2 phosphorylation attenuated RyR2 leakage,23 and prevented heart failure induced by chronic stimulation of β-adrenergic signaling.34 We would like to indicate that whether PKA-mediated RyR2 phosphorylation at serine 2808 (S2808) directly leads to RyR2 dysfunction remains controversial.35 Several studies found that genetic alteration of RyR2 channels at S2808, a situation that was suggested to mimic the PKA hyperphosphorylation, showed no different phenotypes in the genetically altered mice compared to the control wild-type mice after stress.36-38 This disparity suggests that the PKA-mediated regulation of RyR2 function might be comprehensive and involve multiple factors, various hyperphosphorylation sites, and different biochemical modifications of RyR2 channels.39, 40 One of the strategies for future study would be to crossbreed the Rnd3-null mice with RyR2-S2808A mice, a phosphorylation-resistant mouse strain, to see if the mouse phenotype can be rescued.
The biological function of RyR2 in E-C coupling in fetal hearts has been investigated in many studies. It is not doubted that there is a functional maturation progression in RyR2-mediated SR calcium release in embryonic cardiomyocytes during development. Early studies suggested a minimal role of RyR2 in E-C coupling in embryonic cardiomyocytes.41, 42 However, these results have been challenged by recent studies.43-45 More recent studies demonstrated that the major source of calcium transients is from SR through RyR2 in fetal hearts. While the definitive role of RyR2 in embryonic cardiomyocytes still may be far from the conclusion, it is gradually believed that RyR2-mediated SR calcium release already functions early in the embryonic heart, which is important for normal fetal heart function and development.43-45 Consistent with these studies, we demonstrated the regulatory role of Rnd3 in an embryonic heart, and we also showed that Rnd3 haploinsufficiency is sufficient to induce the calcium leakage in adult cardiomyocytes, indicating a limited effect of cardiac development on Rnd3-mediated calcium regulation. Furthermore, we demonstrated that the adult Rnd3 haploinsufficient mice were hypersensitive to arrhythmogenic stimuli and displayed severe ventricular arrhythmias after cardiac pacing, particularly with beta-adrenergic stimulation. We also recently found that Rnd3 haploinsufficient mice were predisposed to hemodynamic stress and developed heart failure after pressure overload.46
Agonist binding to G protein-coupled receptors leads to cAMP-dependent PKA activation. Broad arrays of biological and pathological effects are regulated through the activation of this signaling pathway. In this study, we detected a significant increase in PKA activity in both cardiac and non-cardiac cells with Rnd3 downregulation, suggesting a general mechanism of Rnd3-mediated PKA regulation. We further revealed that the elimination of Rnd3 expression resulted in the increase of β2AR protein, but there was no increase in the mRNA levels. β2AR lysosomal targeting and ubiquitination attenuation contributed to this elevation of β2AR protein levels. The molecular mechanism of β2AR receptor degradation through ubiquitination and the associated ubiquitin protein ligase (E3) adapter β-arrestin1/2 was first elucidated by Robert J. Lefkowitz.47 The process is regulated by Grk2 phosphorylation that facilitates β-arrestin binding to the receptor resulting in degradation and/or internalization (desensitization) of the receptor.48-50 Clinically, the elevated Grk2 protein levels are detected in lymphocytes from patients that had a myocardial infarction, and are suggested to be inversely associated with patient cardiac functions.51 To investigate if Grk2 and β-arrestin were involved in Rnd3-mediated β2AR receptor degradation, we assessed the expression levels of the two proteins and did not detect the changes of Grk2 and arrestin expression levels in the Rnd3-null heart. Emerging evidence shows that the process of β2AR receptor trafficking and degradation are dynamic and complex. Additional new factors have been continually identified in the signalosome complex.25, 52-54 We report that Rnd3 is another regulator in the β2AR ubiquitination regulatory complex. The lack of Rnd3 prevents the ubiquitination of β2AR, despite the normal expression levels of β-arrestin1/2 and Grk2, resulting in the accumulation of β2AR protein, which promotes the activation of PKA signaling. Finally, we demonstrated that application of β2AR blocker can rescue the mouse phenotype by reserving cardiac contractility and reducing cardiac arrhythmia.
It is important to realize that there is a limitation in this study. It is unclear whether Rnd3 is a constitutively associated factor in the multimeric complex or just a “hit and run” regulatory protein during β2AR receptor trafficking and degradation. Further sets of experiments are absolutely necessary. The answer will provide a further mechanism for our understanding of β2AR dynamic regulation and calcium release regulation.
Rnd3 has also been shown to play an essential role in the Notch signaling pathway by facilitating NICD protein degradation through ubiquitin proteasome system (UPS) in mouse brains in our recent study.15 Given the fact that there are more than 600 putative E3 ligases in humans,55 our future study will investigate whether Rnd3 directly binds to an E3 ligase functioning as an ancillary protein, or interacts with arrestin family members.
In summary, there are five findings in this study. First, the Rnd3 gene is indispensable for mouse development. Rnd3-null mice are embryonically lethal with cardiac arrhythmias. Second, our data reveal that Rnd3 is essential for the normal function of RyR2 Ca2+ release channels in both the embryonic and adult mouse heart. Genetic deletion of Rnd3 results in Ca2+ leakage through RyR2 channel destabilization that in turn prompts arrhythmias. Third, we validate that hyperphosphorylation of RyR2 by PKA contributes to Rnd3 deficiency-mediated RyR2 dysfunction in mouse hearts. Fourth, we demonstrate that the downregulation of Rnd3 is sufficient to initiate activation of PKA signaling in vivo in animal hearts and in vitro in both cardiac- and non-cardiac cells, suggesting a general mechanism of Rnd3-mediated PKA activation. Finally, we discover the molecular mechanism of Rnd3-mediated PKA activation and determine that Rnd3 is a regulator in the β2AR ubiquitination regulatory complex. The lack of Rnd3 prevents the ubiquitination of β2AR, resulting in the accumulation of β2AR protein, which promotes the activation of PKA signaling (Online Fig. XI). Given the fundamental roles of the RyR2 channels in cell signaling, arrhythmogenicity, heart failure, and the βAR-PKA signaling pathway, the present findings have both basic and clinical significances and provide a potential new target for pharmacological manipulations.
Supplementary Material
Novelty and Significance.
What Is Known?
Small G protein Rnd3 functions as Rho kinase inhibitor involved in cell migration, proliferation, and cytoskeleton dynamics.
It plays an essential role in central nerve system development.
It regulates cell cycling, cancer cell migration and invasion.
What New Information Does This Article Contribute?
Rnd3 participates in intracellular Ca2+ homeostasis regulation in the mouse heart.
Rnd3 deficiency results in Ca2+ leakage through RyR2 channel destabilization that in turn prompts cardiac arrhythmias and heart failure in mice.
The associated molecular mechanism is that lack of Rnd3 prevents the ubiquitination of β2AR, resulting in the accumulation of β2AR protein, which promotes the activation of PKA signaling. Increased PKA signaling in turn promotes RyR2 hyperphosphorylation, which contributes to arrhythmogenesis. Application of β2AR blocker reduces animal arrhythmia and improves heart function.
Understanding the molecular mechanisms of dynamic regulations of β2AR and RyR2 channels is critical for the treatment of cardiac arrhythmias and heart failure. The current findings add new information about Rnd3 as a novel regulator in the β2AR ubiquitination, RyR2 channel stability and mouse embryonic development, and provide a potential new target for pharmacological manipulations in cardiac arrhythmia and heart failure treatment.
ACKNOWLEDGEMENTS
We thank Dr. Donald M. Bers for his critical reviews of the paper. We are grateful to Dr. Vladimir N. Potaman, Mrs. Kelsey Andrade, and Mrs. Alexis M. Boggs for editorial assistance to the manuscript. We thank Dr. Robert J. Lefkowitz for making the β2-adrenergic receptor vector available at Addgene for this study. This work was supported by the NIH-NHLBI R01HL113640 (X.A.); the NIH-NHLBI R01HL089598, R01HL091947, and the Foundation Leducq 08CVD01 (X.H.T.W.); the AHA Postdoctoral Fellowship 13POST17260043 (X.Y.); the AHA Grant-in-Aid 0855030F, the NIH-NHLBI R01HL102314, R01HL123953, R21HL094844 and K02HL098956 (J.C.).
SOURCES OF FUNDING
This work was supported by the NIH-NHLBI R01HL113640 (X.A.), the NIH-NHLBI R01HL089598, R01HL091947, and the ‘Alliance for Calmodulin Kinase Signaling in Heart Disease’ (08CVD01) grant from the Foundation Leducq (X.H.T.W.), the AHA Grant-in-Aid (0855030F), the NIH-NHLBI R01HL102314, R21HL094844 and K02HL098956 (J.C.).
Nonstandard Abbreviations and Acronyms
- Rnd3
Rho family GTPase 3
- RyR2
ryanodine receptor 2
- PKA
protein kinase A
- cAMP
cyclic adenosine monophosphate
- β2AR
adrenergic receptor, beta 2
- ROCK1
Rho-associated coiled-coil protein kinase 1
- SR
sarcoplasmic reticulum
- NCX
Na+/Ca2+ exchanger
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- PLN
phospholamban
- SCR
spontaneous Ca2+ release
- CREB
cyclic AMP responsive element-binding protein
- MEF
mouse embryonic fibroblast
- ISO
isoproterenol
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Riento K, Guasch RM, Garg R, Jin B, Ridley AJ. Rhoe binds to rock i and inhibits downstream signaling. Mol Cell Biol. 2003;23:4219–4229. doi: 10.1128/MCB.23.12.4219-4229.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. Rhoe is a pro-survival p53 target gene that inhibits rock i-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–2472. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Wennerberg K, Forget MA, Ellerbroek SM, Arthur WT, Burridge K, Settleman J, Der CJ, Hansen SH. Rnd proteins function as rhoa antagonists by activating p190 rhogap. Curr Biol. 2003;13:1106–1115. doi: 10.1016/s0960-9822(03)00418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riento K, Villalonga P, Garg R, Ridley A. Function and regulation of rhoe. Biochem Soc Trans. 2005;33:649–651. doi: 10.1042/BST0330649. [DOI] [PubMed] [Google Scholar]
- 5.Foster R, Hu KQ, Lu Y, Nolan KM, Thissen J, Settleman J. Identification of a novel human rho protein with unusual properties: Gtpase deficiency and in vivo farnesylation. Mol Cell Biol. 1996;16:2689–2699. doi: 10.1128/mcb.16.6.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobes CD, Lauritzen I, Mattei MG, Paris S, Hall A, Chardin P. A new member of the rho family, rnd1, promotes disassembly of actin filament structures and loss of cell adhesion. J Cell Biol. 1998;141:187–197. doi: 10.1083/jcb.141.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guasch RM, Scambler P, Jones GE, Ridley AJ. Rhoe regulates actin cytoskeleton organization and cell migration. Mol Cell Biol. 1998;18:4761–4771. doi: 10.1128/mcb.18.8.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiegen D, Blumenstein L, Stege P, Vetter IR, Ahmadian MR. Crystal structure of rnd3/rhoe: Functional implications. FEBS Lett. 2002;525:100–104. doi: 10.1016/s0014-5793(02)03094-6. [DOI] [PubMed] [Google Scholar]
- 9.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 10.Riento K, Totty N, Villalonga P, Garg R, Guasch R, Ridley AJ. Rhoe function is regulated by rock i-mediated phosphorylation. Embo J. 2005;24:1170–1180. doi: 10.1038/sj.emboj.7600612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Komander D, Garg R, Wan PT, Ridley AJ, Barford D. Mechanism of multi-site phosphorylation from a rock-i:Rhoe complex structure. Embo J. 2008;27:3175–3185. doi: 10.1038/emboj.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villalonga P, Guasch RM, Riento K, Ridley AJ. Rhoe inhibits cell cycle progression and ras-induced transformation. Mol Cell Biol. 2004;24:7829–7840. doi: 10.1128/MCB.24.18.7829-7840.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein RM, Aplin AE. Rnd3 regulation of the actin cytoskeleton promotes melanoma migration and invasive outgrowth in three dimensions. Cancer Res. 2009;69:2224–2233. doi: 10.1158/0008-5472.CAN-08-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by ddr1 and the cell polarity regulators par3 and par6. Nature cell biology. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin X, Liu B, Yang X, Yue X, Diao L, Wang J, Chang J. Genetic deletion of rnd3 results in aqueductal stenosis leading to hydrocephalus through up-regulation of notch signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1219995110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pacary E, Heng J, Azzarelli R, Riou P, Castro D, Lebel-Potter M, Parras C, Bell DM, Ridley AJ, Parsons M, Guillemot F. Proneural transcription factors regulate different steps of cortical neuron migration through rnd-mediated inhibition of rhoa signaling. Neuron. 2011;69:1069–1084. doi: 10.1016/j.neuron.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mocholi E, Ballester-Lurbe B, Arque G, Poch E, Peris B, Guerri C, Dierssen M, Guasch RM, Terrado J, Perez-Roger I. Rhoe deficiency produces postnatal lethality, profound motor deficits and neurodevelopmental delay in mice. PLoS One. 2011;6:e19236. doi: 10.1371/journal.pone.0019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Killen SAS, Fish FA. Fetal and neonatal arrhythmias. NeoReviews. 2008;9:e242–e252. [Google Scholar]
- 19.Vignati G. Pediatric arrhythmias: Which are the news? Journal of cardiovascular medicine. 2007;8:62–66. doi: 10.2459/01.JCM.0000247438.12817.9e. [DOI] [PubMed] [Google Scholar]
- 20.van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase ii promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. Pka phosphorylation dissociates fkbp12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 22.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circulation research. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 23.Sarma S, Li N, van Oort RJ, Reynolds C, Skapura DG, Wehrens XH. Genetic inhibition of pka phosphorylation of ryr2 prevents dystrophic cardiomyopathy. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13165–13170. doi: 10.1073/pnas.1004509107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimoto S, J OU, Kawai M, Hoshina T, Kusakari Y, Komukai K, Sasaki H, Hongo K, Kurihara S. Protein kinase a-dependent phosphorylation of ryanodine receptors increases ca2+ leak in mouse heart. Biochem Biophys Res Commun. 2009;390:87–92. doi: 10.1016/j.bbrc.2009.09.071. [DOI] [PubMed] [Google Scholar]
- 25.Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting, and degradation of the beta2-adrenergic receptor. The Journal of biological chemistry. 2008;283:22166–22176. doi: 10.1074/jbc.M709668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie L, Xiao K, Whalen EJ, Forrester MT, Freeman RS, Fong G, Gygi SP, Lefkowitz RJ, Stamler JS. Oxygen-regulated beta(2)-adrenergic receptor hydroxylation by egln3 and ubiquitylation by pvhl. Science signaling. 2009;2:ra33. doi: 10.1126/scisignal.2000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuhs SR, Insel PA. Caveolin-3 undergoes sumoylation by the sumo e3 ligase piasy: Sumoylation affects g-protein-coupled receptor desensitization. J Biol Chem. 2011;286:14830–14841. doi: 10.1074/jbc.M110.214270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wyatt D, Malik R, Vesecky AC, Marchese A. Small ubiquitin-like modifier modification of arrestin-3 regulates receptor trafficking. J Biol Chem. 2011;286:3884–3893. doi: 10.1074/jbc.M110.152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 30.Meli AC, Refaat MM, Dura M, Reiken S, Wronska A, Wojciak J, Carroll J, Scheinman MM, Marks AR. A novel ryanodine receptor mutation linked to sudden death increases sensitivity to cytosolic calcium. Circ Res. 2011;109:281–290. doi: 10.1161/CIRCRESAHA.111.244970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ullrich ND, Valdivia HH, Niggli E. Pka phosphorylation of cardiac ryanodine receptor modulates sr luminal ca2+ sensitivity. J Mol Cell Cardiol. 2012;53:33–42. doi: 10.1016/j.yjmcc.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR. Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. The Journal of clinical investigation. 2010;120:4375–4387. doi: 10.1172/JCI37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Kohno M, Matsuzaki M. Fkbp12.6-mediated stabilization of calcium-release channel (ryanodine receptor) as a novel therapeutic strategy against heart failure. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X, Szeto C, Gao E, Tang M, Jin J, Fu Q, Makarewich C, Ai X, Li Y, Tang A, Wang J, Gao H, Wang F, Ge XJ, Kunapuli SP, Zhou L, Zeng C, Xiang KY, Chen X. Cardiotoxic and cardioprotective features of chronic beta-adrenergic signaling. Circulation research. 2013;112:498–509. doi: 10.1161/CIRCRESAHA.112.273896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bers DM. Ryanodine receptor s2808 phosphorylation in heart failure: Smoking gun or red herring. Circulation research. 2012;110:796–799. doi: 10.1161/CIRCRESAHA.112.265579. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H, Makarewich CA, Kubo H, Wang W, Duran JM, Li Y, Berretta RM, Koch WJ, Chen X, Gao E, Valdivia HH, Houser SR. Hyperphosphorylation of the cardiac ryanodine receptor at serine 2808 is not involved in cardiac dysfunction after myocardial infarction. Circulation research. 2012;110:831–840. doi: 10.1161/CIRCRESAHA.111.255158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase a phosphorylation site in the cardiac ryanodine receptor. Circulation research. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 38.MacDonnell SM, Garcia-Rivas G, Scherman JA, Kubo H, Chen X, Valdivia H, Houser SR. Adrenergic regulation of cardiac contractility does not involve phosphorylation of the cardiac ryanodine receptor at serine 2808. Circulation research. 2008;102:e65–72. doi: 10.1161/CIRCRESAHA.108.174722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of camkiidelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10274–10279. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marx SO, Marks AR. Dysfunctional ryanodine receptors in the heart: New insights into complex cardiovascular diseases. J Mol Cell Cardiol. 2013;58:225–231. doi: 10.1016/j.yjmcc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takeshima H, Komazaki S, Hirose K, Nishi M, Noda T, Iino M. Embryonic lethality and abnormal cardiac myocytes in mice lacking ryanodine receptor type 2. The EMBO journal. 1998;17:3309–3316. doi: 10.1093/emboj/17.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Klitzner TS. Maturational changes in excitation-contraction coupling in mammalian myocardium. Journal of the American College of Cardiology. 1991;17:218–225. doi: 10.1016/0735-1097(91)90730-w. [DOI] [PubMed] [Google Scholar]
- 43.Moorman AF, Schumacher CA, de Boer PA, Hagoort J, Bezstarosti K, van den Hoff MJ, Wagenaar GT, Lamers JM, Wuytack F, Christoffels VM, Fiolet JW. Presence of functional sarcoplasmic reticulum in the developing heart and its confinement to chamber myocardium. Developmental biology. 2000;223:279–290. doi: 10.1006/dbio.2000.9752. [DOI] [PubMed] [Google Scholar]
- 44.Seki S, Nagashima M, Yamada Y, Tsutsuura M, Kobayashi T, Namiki A, Tohse N. Fetal and postnatal development of ca2+ transients and ca2+ sparks in rat cardiomyocytes. Cardiovascular research. 2003;58:535–548. doi: 10.1016/s0008-6363(03)00255-4. [DOI] [PubMed] [Google Scholar]
- 45.Fu JD, Li J, Tweedie D, Yu HM, Chen L, Wang R, Riordon DR, Brugh SA, Wang SQ, Boheler KR, Yang HT. Crucial role of the sarcoplasmic reticulum in the developmental regulation of ca2+ transients and contraction in cardiomyocytes derived from embryonic stem cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2006;20:181–183. doi: 10.1096/fj.05-4501fje. [DOI] [PubMed] [Google Scholar]
- 46.Yue X, Yang X, Lin X, Yang T, Yi X, Dai Y, Guo J, Li T, Shi J, Wei L, Fan GC, Chen C, Chang J. Rnd3 haploinsufficient mice are predisposed to hemodynamic stress and develop apoptotic cardiomyopathy with heart failure. Cell Death Dis. 2014;5:e1284. doi: 10.1038/cddis.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta 2-adrenergic receptor and beta-arrestin. Science. 2001;294:1307–1313. doi: 10.1126/science.1063866. [DOI] [PubMed] [Google Scholar]
- 48.Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, Gao E, Chuprun JK, Wang Y, Talan M, Dorn GW, 2nd, Lakatta EG, Koch WJ, Feldman AM, Xiao RP. Gi-biased beta2ar signaling links grk2 upregulation to heart failure. Circulation research. 2012;110:265–274. doi: 10.1161/CIRCRESAHA.111.253260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hata JA, Williams ML, Schroder JN, Lima B, Keys JR, Blaxall BC, Petrofski JA, Jakoi A, Milano CA, Koch WJ. Lymphocyte levels of grk2 (betaark1) mirror changes in the lvad-supported failing human heart: Lower grk2 associated with improved beta-adrenergic signaling after mechanical unloading. Journal of cardiac failure. 2006;12:360–368. doi: 10.1016/j.cardfail.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 50.Drake MT, Violin JD, Whalen EJ, Wisler JW, Shenoy SK, Lefkowitz RJ. Beta-arrestin-biased agonism at the beta2-adrenergic receptor. J Biol Chem. 2008;283:5669–5676. doi: 10.1074/jbc.M708118200. [DOI] [PubMed] [Google Scholar]
- 51.Santulli G, Campanile A, Spinelli L, Assante di Panzillo E, Ciccarelli M, Trimarco B, Iaccarino G. G protein-coupled receptor kinase 2 in patients with acute myocardial infarction. Am J Cardiol. 2011;107:1125–1130. doi: 10.1016/j.amjcard.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 52.Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases usp33 and usp20 coordinate beta2 adrenergic receptor recycling and resensitization. The EMBO journal. 2009;28:1684–1696. doi: 10.1038/emboj.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han SO, Xiao K, Kim J, Wu JH, Wisler JW, Nakamura N, Freedman NJ, Shenoy SK. March2 promotes endocytosis and lysosomal sorting of carvedilol-bound beta2-adrenergic receptors. The Journal of cell biology. 2012;199:817–830. doi: 10.1083/jcb.201208192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nabhan JF, Pan H, Lu Q. Arrestin domain-containing protein 3 recruits the nedd4 e3 ligase to mediate ubiquitination of the beta2-adrenergic receptor. EMBO Rep. 2010;11:605–611. doi: 10.1038/embor.2010.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li W, Bengtson MH, Ulbrich A, Matsuda A, Reddy VA, Orth A, Chanda SK, Batalov S, Joazeiro CA. Genome-wide and functional annotation of human e3 ubiquitin ligases identifies mulan, a mitochondrial e3 that regulates the organelle's dynamics and signaling. PLoS One. 2008;3:e1487. doi: 10.1371/journal.pone.0001487. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.