Abstract
Mutations of the transmembrane channel-like 1 (TMC1) gene can cause dominant and recessive forms of deafness in humans and mice. TMC1 is one of eight mammalian TMC genes of unknown function. The multi-pass transmembrane topologic structure of the proteins they encode suggests roles as a receptor, transporter, channel or pump. Tmc1 and the closely related Tmc2 gene are expressed in neurosensory hair cells of the auditory and vestibular end organs of the mouse inner ear. Recent studies have demonstrated that Tmc1 and Tmc2 are specifically required for mechanoelectrical transduction in hair cells. The exact role of these proteins in mechanoelectrical transduction is unknown. TMC1 and TMC2 are viable candidates for the mechanoelectrical transduction channel of hair cells, whose component molecules have eluded identification for over 30 years. We expect that studies of TMC proteins will yield insights into molecular components and mechanisms of mechanosensation in auditory and vestibular hair cells, as well as in other tissues and organs.
Keywords: channel, deafness, hair cell, hearing, mechanoelectrical, mechanosensory, mechanotransduction, TMC1, TMC2, transduction
Introduction
Approximately one in 1000 children is born with permanent sensorineural hearing loss (SNHL), and an additional one in 1000 develops permanent SNHL by the age of 9 years [6]. More than one half of all cases of hearing loss with a prelingual onset (prior to the normal development of oral speech) are thought to have a hereditary basis [28]. Most cases of hereditary hearing loss are nonsyndromic, in which the hearing loss is not associated with other abnormalities. The majority of nonsyndromic hereditary hearing loss is inherited as a recessive trait (DFNB), approximately 20% is dominant (DFNA), and 1 to 3% is X-linked (DFNX) [25]. There is one report of a family segregating SNHL linked to a complex rearrangement of the Y chromosome (DFNY1) [34]. In general, DFNB phenotypes are severe to profound hearing loss with a congenital or prelingual onset, while DFNA phenotypes show progressive hearing loss with a postlingual onset, usually beginning in the third decade of life or later [9]. Sensorineural hearing loss has a high degree of genetic heterogeneity, with more than 70 genes identified that cause nonsyndromic hearing loss (http://hereditaryhearingloss.org accessed on July 10, 2014)”.
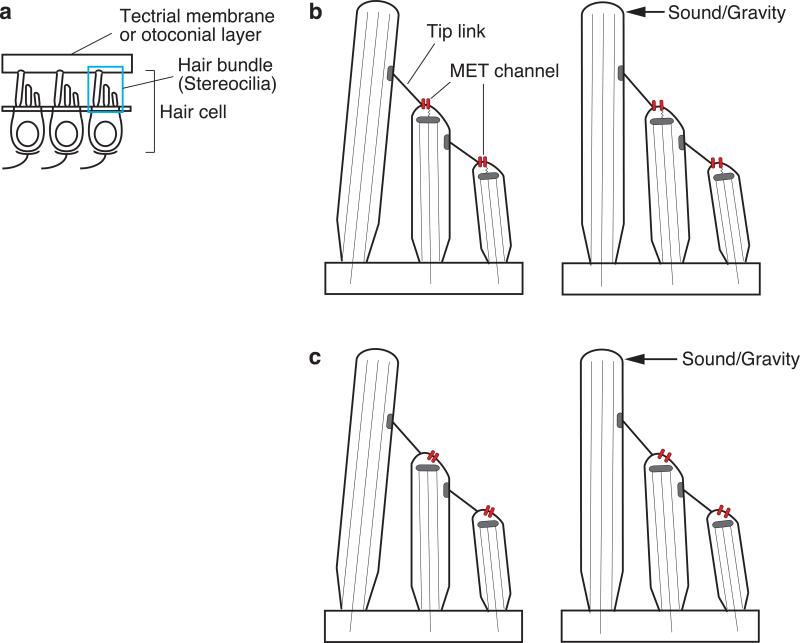
Inner ear hair cells are the sensory receptors for hearing and balance in vertebrates (Fig. 1). The hair cells convert mechanical stimuli of sound, gravity or head accelerations into electrical signals that are transmitted to the central nervous system via the auditory or vestibular nerves. This process, known as mechanoelectrical transduction, is mediated via a mechanically sensitive hair bundle on the apical surface of each hair cell (HC). The hair bundle is composed of hundreds of modified microvilli, or stereocilia, arranged like a staircase with rows of increasing height (Fig. 1). The tip of each shorter stereocilium is connected to the lateral surface of an adjacent taller stereocilium by a narrow extracellular filament called a tip link. The upper portion of a mature tip link is composed of cadherin-23 and the lower portion is composed of protocadherin-15 [14,1]. Both of these proteins have many different splice isoforms [1,22]. The isoforms thought to comprise the tip link contain a long extracellular N-terminus containing multiple cadherin domains, a single transmembrane domain, and a shorter cytoplasmic domain [14].
Fig. 1.
Hair cell mechanoelectrical transduction. a A hair bundle on the apical surface of each hair cell is composed of hundreds of stereocilia that are arranged like a staircase with rows of increasing height. b The tip of each shorter stereocilium is connected to the lateral surface of an adjacent taller stereocilium by a narrow extracellular filament called a tip link. Mechanically gated ion channels, called mechanoelectrical transduction (MET) channels, are located at or near the tips of shorter stereocilia. The MET channel is thought to be opened by: b tension on the tip link, or c lateral tension of the plasma membrane elicited by tension on the tip link.
Electrophysiological recordings have demonstrated the existence of mechanically gated ion channels, called mechanotransduction, mechanotransducer, or mechanoelectrical transduction (MET) channels, at or near the tips of stereocilia [11,12]. The molecular identity of this channel has been the subject of intense interest. Calcium imaging of hair cells has demonstrated that functional MET occurs at or near the tips of shorter stereocilia [2], implying that MET channels are located at or near the insertion of tip links at the tips of shorter stereocilia. How the tip link opens the MET channel is unknown, but there are two broadly described models: the tethered-channel model (Fig. 1b) and the lateral-tension model (Fig. 1c) [8]. These models are based upon the observation that the open probability of the MET channel is increased by deflection of the hair bundle toward the tallest row of stereocilia, whereas the open probability is decreased by deflection in the opposite direction. In the tethered-channel model, the MET channel is directly gated by tension on the tip link. In the lateral-tension model, the MET channels are opened by lateral tension of the membrane that is stretched by tension on the tip link.
TMC1 mutations and deafness in humans
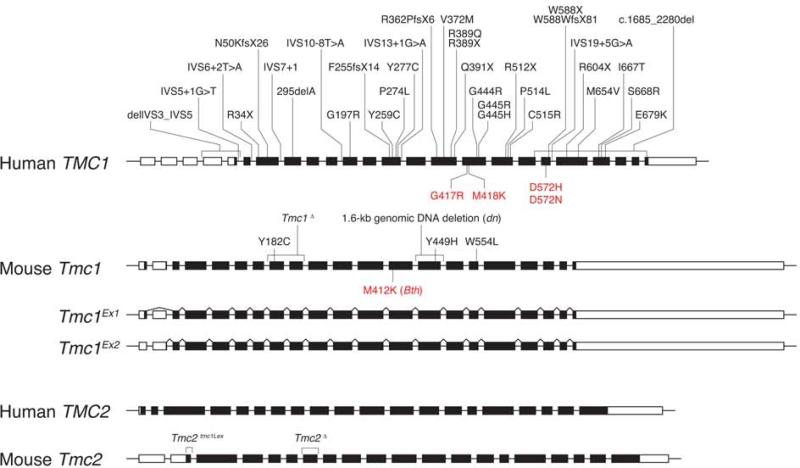
Transmembrane channel-like 1 (TMC1) was identified as the gene underlying both dominant and recessive forms of nonsyndromic SNHL at the DFNA36 and DFNB7/11 loci, respectively [19,33]. TMC1 is located on chromosome 9q13 and contains 24 exons, including four exons encoding sequence upstream of a methionine start codon in exon 5 (Fig. 2) [19]. TMC1 and mouse Tmc1 mRNA expression were detected in human fetal cochleae and mouse inner ears, respectively [19]. To date, 35 recessive mutations have been identified in TMC1 in reports from around the globe. The recessive mutations include genomic deletions, missense substitutions, and frameshift, nonsense, and splice site mutations. The predicted effects of most of these mutations indicate that they act via a loss-of-function mechanism. In contrast, dominant DFNA36 mutations are all missense amino acid substitutions that must act via a dominant-negative or gain-of-function mechanism since heterozygous carriers of recessive mutations are haploinsufficient for TMC1 but do not have hearing loss. Four mutations, p.D572N, p.D572H, p.G417R and p.M418K have been reported at the DFNA36 locus (Fig. 2).
Fig. 2.
TMC1 and TMC2 mutations in humans and mice. Thirty-five recessive mutations (black font) and four dominant mutations (red font) have been identified in human TMC1. Similarly, recessive (black) and semidominant alleles (red font) of mouse Tmc1 cause hearing loss in the deafness (dn) and Beethoven (Bth) mutant mouse strains, respectively. Tmc1dn encodes an in-frame deletion of TMC1 that may retain some function even though it cannot rescue MET at the tips of stereocilia. Tmc2tmc1Lex is predicted to encode a TMC2 protein truncated by 57 amino acids at its N-terminus. Mouse Tmc1 transcripts include two isoforms called Tmc1Ex1 and Tmc1Ex2.
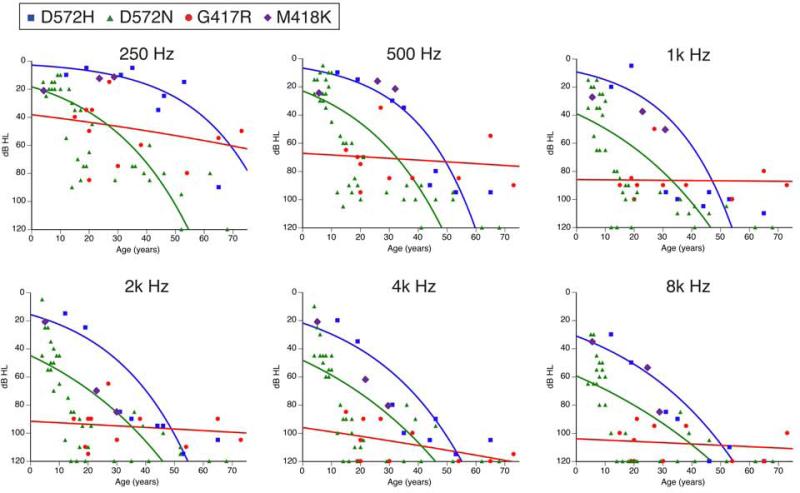
DFNA36 hearing loss is postlingual and progressive [24,18,35], although the rates of progression vary. The audioprofiles of reported DFNA36 families are shown in Fig. 3. p.D572H carriers and p.D572N carriers show similar rates of progression of hearing loss, even though the p.D572N carriers show a greater mean severity of hearing loss at all ages and at all frequencies in comparison to p.D572H carriers. On the other hand, p.G417R carriers show more severe hearing loss in comparison to p.D572N carriers and p.D572H carriers up to the fourth and fifth decades of life, while the rate of deterioration of hearing thresholds in p.G417R carriers is much slower than that in p.D572N carriers or p.D572H carriers. As a result, p.G417R carriers still have residual hearing in their seventh and eighth decades of life. Cochlear implants are reported to successfully rehabilitate hearing in p.D572N carriers and p.D572H carriers [24,18]. Although there is currently no therapy to prevent deterioration of DFNA36 hearing loss, the delayed onset of hearing loss of DFNA36 provides a potential therapeutic window to maintain the residual hearing.
Fig. 3.
Hearing loss caused by dominant mutations of TMC1. Pure-tone air conduction thresholds for the right ears of p.D572H carriers and the better-hearing ears of p.D572N, p.G417R or p.M418K carriers are plotted against age for each stimulus test frequency.
DFNB7/11 hearing loss is typically congenital and severe to profound [19]. The only published exception to this generalization is the phenotype co-segregating with homozygosity for a mutation, c.1763+3A>G, affecting the splice donor site of intron 19 of TMC1, in a Dutch family [5]. Initial audiograms of three affected family members at the ages of 6, 12 and 13 years showed hearing loss affecting only the high frequencies. The hearing loss progressed rapidly to severe to profound levels by the third decade of life [5], resembling the progression associated with the p.D572N and p.D572H mutations at the DFNA36 locus. A plausible explanation for the residual hearing is that c.1763+3A>G does not abolish normal splicing of TMC1 mRNA; some normal TMC1 protein is translated and sufficient for hearing at an early age.
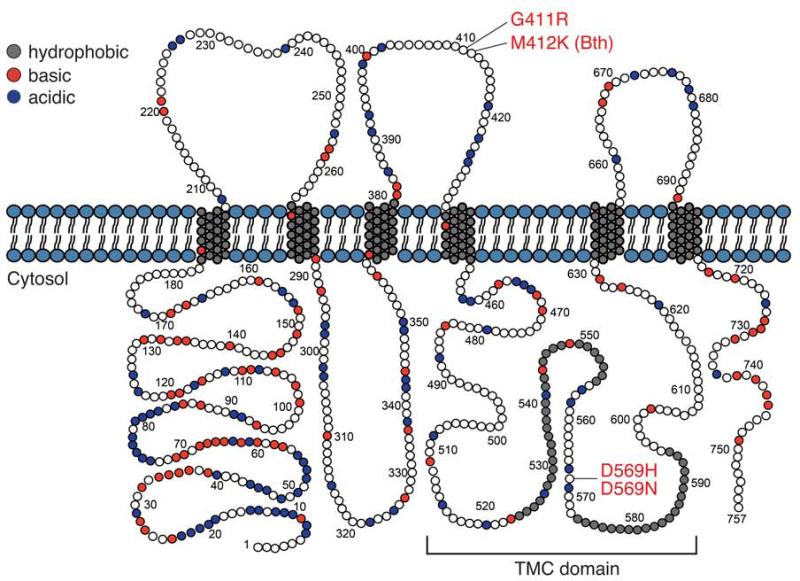
TMC1 is predicted to encode an 87-kDa polypeptide with six transmembrane domains and cytosolic N- and C-termini. This predicted topologic structure resembles that of Shaker K+ and TRP channels [19,21] (Fig. 4), suggesting that TMC1 may function as a receptor, pump, transporter, channel, or modifiers of such proteins [19,21,20,15]. This topologic organization of TMC1 has been experimentally confirmed for mouse TMC1 expressed in heterologous systems [21]. In silico hydropathy analysis detects two adjacent hydrophobic regions that do not span the membrane, raising the speculative possibility that they could form a pore-loop if TMC1 indeed functions as an ion channel [21]. These two adjacent hydrophobic regions are located in the TMC domain, which is the most highly conserved region among all of the known TMC homologs, including Drosophila melanogaster and Caenorhabditis elegans. The TMC domain is a 120-amino-acid domain encompassing a cytoplasmic loop and the adjacent fifth transmembrane domain [20]. Other than these conserved regions, there were no significant sequence or domain similarities to other proteins. However, both human and mouse TMC1 and TMC2 have a peculiar motif of alternating clusters of charged amino acids in their N-termini [19].
Fig. 4.
Mouse TMC1. Tmc1 is predicted to encode an 87-kDa polypeptide with six transmembrane domains and cytosolic N- and C-termini. The TMC domain, a 120-amino-acid domain encompassing a cytoplasmic loop and the adjacent fifth transmembrane domain, is the most highly conserved region among the known TMC homologs. Both human and mouse TMC1 and TMC2 have a motif of alternating clusters of charged amino acids in their N-termini. The positions of the human DFNA36 mutations are numbered according to their orthologous locations in mouse TMC1 and indicated in red. P.M412K in Beethoven (Bth) mutant mice is an identical substitution in an orthologous position as the human p.M418K.
TMC1 mutant mouse models of deafness
Recessive and semidominant alleles of Tmc1 cause hearing loss in the deafness (dn) and Beethoven (Bth) mouse lines, respectively. The dn mice were originally reported as the first mouse model of human nonsyndromic deafness since they did not exhibit the typical abnormal motor vestibular behaviors suggestive of peripheral vestibular dysfunction in mice [32]. In mice, ataxic gait, running in circles, head-bobbing, -shaking or –arching are all common manifestations of a severe peripheral vestibular deficit. Since the vast majority of humans with nonsyndromic SNHL do not exhibit these overt signs of vestibular dysfunction, the dn mouse, lacking the abnormal motor vestibular behavior, was considered to be an important model for human SNHL. A series of physiologic and histologic studies implicated the hair cell as the primary site of pathology in dn mice [32]. Tmc1dn/dn mice have no detectable cochlear microphonic or action potentials at any age, in spite of an intact resting endocochlear potential [32]. Tmc1dn encodes a 1.6-kb deletion encompassing the 171-bp exon 14 and the adjacent regions of introns 13 and 14 of Tmc1: IVS13_IVS14del1.6kb (Fig. 2) [19]. Tmc1dn thus encodes an mRNA with an in-frame deletion of exon 14, which encodes a portion of the highly conserved TMC domain of TMC1. It is unknown if the mutant protein is expressed or if it might possess residual activity or dominant-negative effects at the molecular level.
Tmc1Bth mice arose in an ENU (N-ethyl-N-nitrosourea) mutagenesis program [33]. Tmc1Bth/+ mice initially have a slight hearing loss that rapidly progresses to profound deafness, while Tmc1Bth/ Bth mice are profoundly deaf at the age of 3 weeks, when fully mature hearing can first be measured [33,30]. Neither Tmc1Bth/Bth nor Tmc1Bth/+ mice exhibit abnormal motor vestibular behavior. Tmc1Bth encodes a missense mutation that is predicted to substitute a lysine for a methionine residue (p.M412K) (Fig. 2) [33]. p.M412K is an identical substitution in an orthologous position as the human p.M418K identified in a Chinese family [36] It is also adjacent to the orthologous amino acid residue affected by the p.G417R mutation identified in humans with DFNA36. This clustering of dominant mutations is likely to reflect functional significance of these amino acid residues in TMC1 (Fig. 4).
A targeted deletion allele of Tmc1 (Tmc1Δ) was generated in order to minimize the potential problem of functional residual TMC1 activity or potential dominant-negative effects associated with studies of Tmc1dn. In Tmc1Δ, the exons encoding the predicted first transmembrane domain of Tmc1 are replaced with a gene trap construct fused to a lacZ reporter gene (Fig. 2) [13]. Even if Tmc1Δ mRNA were spliced around the gene trap, the deleted exons would result in a frameshift, premature termination of translation, and a truncated TMC1 protein. Tmc1Δ/Δ mice have profound hearing loss and no overtly abnormal vestibular behavior [13].
TMC2 in humans and mice
TMC2 on human chromosome 20 (Fig. 2) is another member of the TMC gene family that includes eight paralogs (TMC1 to TMC8) in mammals [20,15]. TMC2 and Tmc2 mRNA expression were also detected in human fetal cochleae and mouse inner ears, respectively [19]. TMC2 shows the highest sequence similarity and phylogenetic relationship to TMC1 among the TMC family members. However, TMC2 mutations have never been reported for hearing loss or vestibular disorders in humans.
A targeted deletion allele of Tmc2 (Tmc2Δ) was generated using the same strategy for generating Tmc1Δ mice [13]. The exon (7) encoding the first transmembrane domain of Tmc2 was replaced with a gene trap construct fused to a lacZ reporter gene (Fig. 2). Tmc2Δ/Δ mice appeared to be phenotypically normal: they have neither hearing loss nor overtly abnormal vestibular behaviors.
Tmc2tmc1Lex encodes a deletion of exon 2, leaving exon 1 and a potential alternative start codon intact, thus preserving the long open reading frame in Tmc2tmc1Lex (https://mmrrc.ucdavis.edu/doc/di_032627PCR_Protocol.pdf, accessed on February 20, 2014) (Fig. 2) [16,17]. The predicted transcript of Tmc2tmc1Lex is analogous to a naturally occurring splice isoform of Tmc1 (Tmc1Ex1); in both transcripts lacking exon 2, translation may potentially initiate at a start codon in exon 1 at the beginning of the long open reading frame. The Tmc1Ex1 isoform is sufficient to rescue Tmc1 function in vitro [13] and in vivo (unpublished observation). Since there is no TMC2 protein or Tmc2 mRNA expression data for Tmc2tmc1Lex, its functional activity, or lack thereof, remains unknown.
TMC1 and TMC2 expression in the inner ear
Tmc1 and Tmc2 mRNA transcripts are present in the inner-ear tissues of postnatal mice. In situ hybridization analysis revealed that both Tmc1 and Tmc2 mRNAs are expressed in hair cells of the early postnatal mouse cochlear and vestibular organs [13,19]. Quantitative RT-PCR analysis of mouse inner ear tissues revealed the early postnatal onset and dynamic spatiotemporal expression of Tmc1 and Tmc2. In the cochlea, Tmc1 and Tmc2 mRNA levels show a rapid increase within five days after birth. The increase of Tmc2 mRNA immediately precedes the acquisition of mechanotransduction in the cochlea [23], with the onset of Tmc1 mRNA expression occurring a few days later. Tmc2 mRNA expression is transient in the cochlea, while Tmc1 expression was sustained up to the last measured time point (P22).
In hair cells of the utricle, one of the two vestibular end organs that detect linear acceleration of the head, Tmc1 expression is first detected at approximately E18. A rise in Tmc2 expression begins between E16 and E17, preceding the acquisition of mechanotransduction by 6-12 hours [7]. Both Tmc1 and Tmc2 mRNA expression levels remain elevated through P21.
The data reveal a developmental switch from Tmc2 to Tmc1 expression in cochlear hair cells, but not in vestibular hair cells. This might reflect a more ancient function for Tmc2 since the peripheral vestibular system is thought to be the evolutionary precursor of the peripheral auditory system. The developmental switch to Tmc1 expression in mature cochlear hair cells may be a molecular recapitulation of phylogeny in which Tmc1 evolved to meet specialized molecular and cellular requirements of auditory hair cells.
The expression pattern of Tmc2 in mature auditory and vestibular hair cells suggests a mechanism to explain the lack of an abnormal motor vestibular phenotype in deaf humans and mice that carry TMC1 and Tmc1 mutations, respectively. Tmc2 is expressed and available to compensate for the loss of Tmc1 in mature vestibular hair cells, but not in mature cochlear hair cells.
Clues and hurdles to identifying TMC function
The localization of TMC1 (and TMC2) proteins in situ is critical to understand their function in hair cells. However, the immunolocalization of TMC proteins in native tissue has been hampered by a lack of antibodies that specifically bind to TMC proteins in situ [13]. Mutai et al. [29] reported that an antibody to chicken TMC2 (GgTmc2) binds to the plasma membranes of cochlear hair cells and supporting cells, but a lack of negative controls precluded a definitive localization. The difficulty in generating specific antibodies may reflect an inherent lack of immunogenicity of TMC proteins, epitope masking, low expression levels of the proteins in situ, or a combination of these factors.
Another major experimental hurdle has been the inability to express any of the mammalian TMC genes in a heterologous expression system in which the proteins traffic to the plasma membrane [13]. This has prevented the application of electrophysiology or other methods to test the hypothesis that TMC1 or other TMC proteins can function as channels, transporters, pumps or receptors. The heterologous mammalian TMC proteins are retained in intracellular compartments where they lead to cell degeneration.
The heterologous expression of a Caenorhabditis elegans homolog of Tmc1 in Chinese hamster ovary (CHO-K1) cells was recently reported [4]. This induced a sodium-sensitive conductance at the plasma membrane that was inhibited by a nonspecific blocker, GdCl3, of nonspecific cation channels. The conclusion that this conductance is directly attributable to tmc-1 itself awaits confirmation of plasma membrane localization and loss of the induced conductance with a mutated tmc-1 control.
The first physiologic study to characterize the function of mammalian Tmc1 was reported by Marcotti et al. [26]. They observed intact MET currents and reduced K+ currents in the cochlear hair cells of Tmc1dn/dn and Tmc1Bth/Bth mice at P8 and P15. They concluded that Tmc1 is not necessary for mechanotransduction but is required for maturation of cochlear hair cells.
TMC1 and TMC2 are required for mechanoelectrical transduction
Tmc1 mutant mice show deafness without overtly abnormal vestibular behavior, while Tmc2 mutant mice show normal hearing and balance at the behavioral level. In contrast, early postnatal Tmc1Δ/Δ;Tmc2Δ/Δ pups lack the ability to right themselves from a supine position [13]. They subsequently develop the classic abnormal vestibular behaviors such as head-bobbing and –arching, ataxic gait, and circling. This observation was the first evidence of a genetic interaction between Tmc1 and Tmc2 in hair cells.
In mice segregating Tmc1Δ and Tmc2Δ, normal hearing, as measured by auditory brainstem response thresholds, requires only the presence of a single wild-type allele of Tmc1. In contrast, an abnormal vestibular phenotype, detected by a reduction of the acceleration gain of the vestibuloocular reflex, shows digenic inheritance [13]. Tmc1Δ/Δ;Tmc2Δ/Δ mutant mice have no detectable auditory brainstem responses or vestibulo-ocular reflexes. The mean acceleration gain is correlated with the number of wild type alleles of Tmc1 and Tmc2. Mice that carry any wild type alleles of either Tmc1 or Tmc2 have detectable vestibulo-ocular reflexes and do not exhibit abnormal motor vestibular behaviors. This genetic interaction of Tmc1 and Tmc2 may reflect functional redundancy at the molecular level.
A genetic interaction of Tmc1 and Tmc2 was also revealed at the level of hair cell MET: early postnatal Tmc1Δ/Δ;Tmc2Δ/Δ hair cells have no detectable MET currents. This was true for hair cells isolated from either auditory or vestibular organs. Unlike many other mouse mutants that affect hair cell MET, the loss of MET in Tmc1Δ/Δ;Tmc2Δ/Δ hair cells was not a secondary result of disrupted hair bundle development or structure. The loss of MET was unlikely to be caused by dominant-negative effects of mutant Tmc1Δ or Tmc2Δ translation products since heterozygous Tmc1Δ or Tmc2Δ mice have normal auditory brainstem responses, vestibule-ocular reflexes, and hair cell MET. Furthermore, exogenously expressed TMC1 or TMC2 rescued MET currents in Tmc1Δ/Δ;Tmc2Δ/Δ HCs [13]. These results indicated that expression of either TMC1 or TMC2 is necessary for MET in early postnatal mouse hair cells.
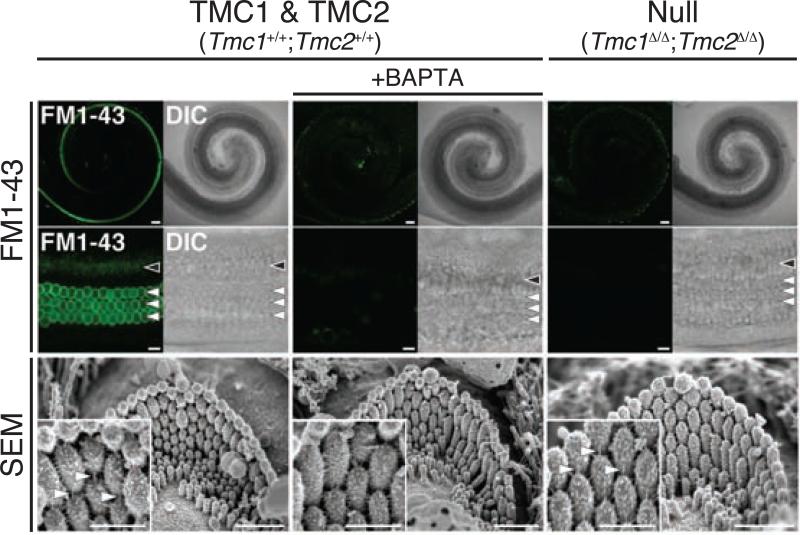
This conclusion was corroborated with two additional assays for functional MET channels in hair cells. These assays utilized two molecules known to permeate the hair cell MET channel: the fluorescent styryl dye FM1-43 (Fig. 5), and the aminoglycoside gentamicin (Fig. 6). Neither FM1-43 nor gentamicin was able to enter Tmc1Δ/Δ;Tmc2Δ/Δ hair cells, whereas the presence of either a wild type Tmc1 or a Tmc2 allele was able to restore uptake [13]. Taken together, these studies provide multiple lines of evidence that the presence of either TMC1 or TMC2 is required for MET in early postnatal hair cells. Possible roles for TMC1 and TMC2 include: (1) assembly or trafficking of the hair cell MET channel; (2) accessory or associated protein of the MET channel; (3) assembly or structure of a larger MET complex; or (4) pore-forming subunit of the MET channel.
Fig. 5.
TMC proteins are required for hair cell uptake of FM1-43. Tmc1Δ/Δ;Tmc2Δ/Δ hair cells have intact hair bundles and tip links, but do not take up FM1-43, indicating a lack of functional mechanoelectrical transduction channels. Reprinted from Kawashima et al. (2011)
Fig. 6.
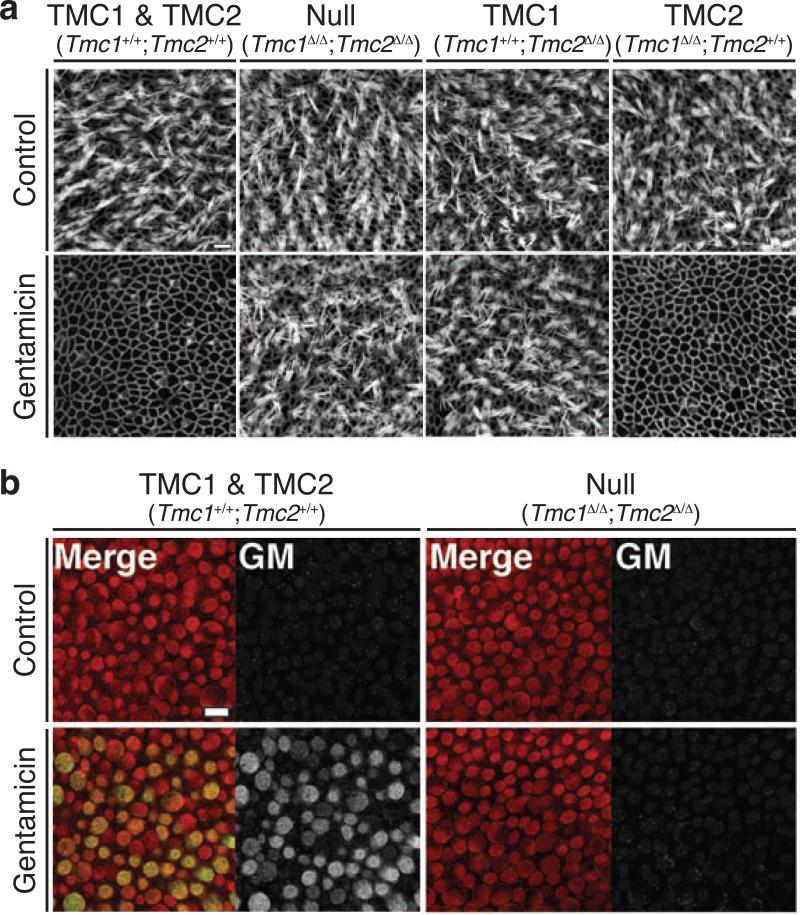
TMC proteins are required for hair cell uptake of gentamicin. Gentamicin permeates functional MET channels and leads to hair cell degeneration. a After incubation with gentamicin, hair bundles on wild-type hair cells were completely eliminated. The hair bundles of cells with a wild type Tmc2 allele were almost completely eliminated, while hair bundles lacking wild type Tmc2 alleles were mostly preserved. b Robust immunoreactivity of gentamicin was detected within wild-type hair cells, while no immunoreactivity of gentamicin was detected within hair cells lacking wild type alleles of Tmc1 and Tmc2. This indicates that hair cell preservation was due to a lack of gentamicin permeation into hair cells. Reprinted from Kawashima et al. (2011)
The hypothesis that TMC1 and TMC2 are components of the MET complex predicts that they would be located at or near the tips of stereocilia. As an alternative to immunolocalization of endogenous TMC proteins, exogenous TMC proteins tagged with AcGFP were expressed in explanted mouse cochlear and vestibular cultures [13]. TMC2::AcGFP was detected on both cochlear and vestibular hair bundles, in which the fluorescent signal increased toward the tips of stereocilia. Expression of TMC1::AcGFP was much harder to detect due to hair cell degeneration, but it was detected near the tips of stereocilia in experiments in which fluorescence could be observed in intact hair bundles [13]. The data demonstrated that exogenous fluorophore-tagged TMC1 and TMC2 can be localized to the tips of stereocilia, but definitive localization data is still needed.
Are TMC1 and TMC2 components of mechanoelectrical transduction channels?
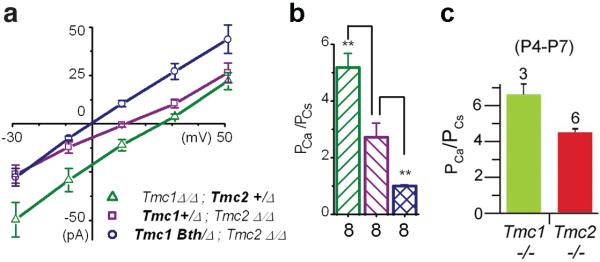
In addition to genetic evidence and hair cell localization data, two additional lines of evidence are required to formally test the hypothesis that a candidate protein comprises the MET channel of hair cells. First, mutations of the pore region should affect the core biophysical properties (e.g., ionic selectivity and conductance) associated with the pore. Pan et al. [31] and Kim and Fettiplace [17] took the first steps toward probing MET channel properties associated with different Tmc1 and Tmc2 alleles (Fig. 7). Calcium ionic permeability and whole-cell currents depend on Tmc genotype: these properties varied according to whether the hair cell was Tmc1- or Tmc2-deficient (Fig. 7b-c). Pan et al. took these studies a step further by employing a novel stimulation paradigm to investigate single-channel properties of inner HCs (Fig. 7a-b). Hair cells that expressed Tmc2, but not Tmc1, had larger single-channel currents with higher Ca2+ permeability than HCs that expressed Tmc1 but not Tmc2. Remarkably, hair cells that expressed only Tmc1Bth, but no wild-type Tmc1 or Tmc2, had smaller single-channel conductance and lower Ca2+ permeability [31]. These results indicate that TMC1 and TMC2 can affect the core properties of hair cell MET channels. However, it remains unknown whether the Tmc1Bth mutation causes a direct effect on the pore of the MET channel or an indirect effect. It is possible the effects are indirectly mediated by a functional, if not physical, interaction with the pore-forming residues of the channel.
Fig. 7.
Inner hair cells that express wild type Tmc1, Tmc2 or mutant Tmc1Bth. have distinct calcium permeability. a Mean current-voltage relationships for mechanotransduction assayed from P5-P7 inner hair cells of the genotypes indicated in the legend. Eight cells of each genotype were measured with 100 mM external Ca++ and 145 mM internal Cs+ as the charge carriers. Reversal potential was measured as the X-intercept. b The Goldman-Hodgkin-Katz equation was used with the reversal potential data from panel a to estimate the calcium permeability ratio relative to cesium. Panels A and B were reprinted from Pan et al. (2013). c Calcium permeability ratios measured in mouse inner hair cells. Reprinted from Kim and Fettiplace (2013). Although the stimulation methods, recording solutions and mouse strains differed, the permeability ratios were qualitatively similar to those of Pan et al.
The role of TMC1 and TMC2 in hair cell MET has been called into question by reports of a novel hair cell current elicited by reverse deflection (toward the shortest stereocilia) with an oscillating fluid jet [16, 27]. Detection of this current in wild-type cells required prior treatment of the hair bundle with a Ca2+ chelator that disrupts tip links and MET at the tips of stereocilia. The novel current was inhibited with several nonspecific inhibitors of the MET channel, and did not saturate with increasing stimulus intensity. Kim et al. [16] observed this novel current in wild-type as well as double homozygous Tmc1dn;Tmc2tmc1Lex mutant hair cells and concluded that neither TMC1 nor TMC2 could be components of the MET channel. However, Marcotti et al. [27] described an anomalous mechanosensitive response in cochlear hair cells from wild-type mice, Cdh23 and Myo7a mutant mice – which lack conventional MET currents – and found that the anomalous MET currents had several biophysical properties that were significantly different from conventional MET. Beurg et al. [3] also described pharmacological differences between conventional MET and anomalous MET. Given these differences, it is unclear whether the anomalous MET currents are carried by the same pore-forming subunits as conventional MET currents.
Importantly, Kim et al. [16] observed the absence of conventional MET in the double homozygous Tmc1dn;Tmc2tmc1Lex mutant hair cells consistent with data from Tmc1Δ/Δ;Tmc2Δ/Δ hair cells [13, 31]. Thus, it remains possible that TMC1 and TMC2 are components of conventional MET channels and that the novel current could be mediated by other TMC proteins or unrelated ion channels. Conclusive testing of the hypothesis that TMC1 and TMC2 are components of the MET channel will require other experimental approaches.
Studies of Tmc orthologs in Caenorhabditis elegans have further implicated TMC proteins as ion channel subunits. Genetic deletion of the homolog tmc-1 results in loss of salt-evoked neuronal activity and behavioral avoidance of high concentrations of NaCl [4]. tmc-1 expressed in CHO cells generated a predominantly cationic conductance activated by high extracellular sodium [4]. The demonstration of channel activity for any mammalian TMC protein would be an important step toward identifying the function(s) of this novel gene family. Elucidation of the molecular function(s) of TMC proteins may reveal the molecular basis of a variety of unexplained mechanosensory phenomena in human health and disease.
Future prospects
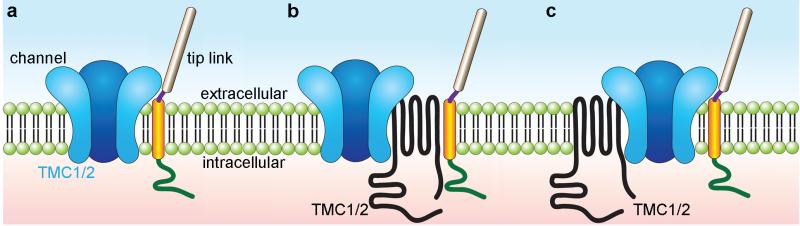
Further tests are required to test the hypothesis that TMC1 and TMC2 are components of the mechanotransduction complex. Definitive localization data that show TMC proteins at the tips of stereocilia would support a role for TMCs in mechanotransduction. Identification of a pore domain or amino acid residues that line the pore would support the channel hypothesis. While existing data are consistent with the channel hypothesis, they are also consistent with other models in which TMC1 and TMC2 are components of the transduction channel complex, but not part of the permeation pathway itself. Fig. 8 presents three possible models. First, TMC1 and TMC2 may be pore-forming subunits (Fig. 8a). Second, they may serve as linker proteins that are necessary to transmit tension to the channel from tip links (Fig. 8b). Third, they may be essential transduction complex components that function to anchor MET channel subunits to the cytoskeleton (Fig. 8c). In a fourth scenario, TMC1 and TMC2 may function as essential components of the channel by forming a vestibule at the mouth of the pore [31]. The location of the p.M412K mutation in the extracelluar S3-S4 linker is consistent with this configuration [10]. These models are not mutually exclusive, and there are certainly other possibilities.
Fig. 8.
Schematic models of possible TMC1 and TMC2 functions. a TMC1 and TMC2 may function as pore-forming subunits of the mechanotransduction channel, either in homomeric assemblies of TMC1 or TMC2 subunits or in heteromeric assemblies with other TMCs or other pore-forming subunits. b TMC1 and TMC2 may function as essential linker proteins that convey tension from the tip link to the transduction channel. In this scenario, TMC1 and TMC2 are not pore-forming subunits but are part of the transduction complex, close enough to affect permeation properties, perhaps stabilizing pore-forming subunits. c TMC1 and TMC2 may serve to anchor transduction channels to the cytoskeleton. In this scenario, loss of TMC1 and TMC2 would render the channel insensitive to tension applied perpendicular to the membrane. As with scenario b, TMC1 and TMC2 must be close enough to the channel to affect permeation properties in this model. Other models are also possible.
The identification of Tmc1 and Tmc2 and implication of their protein products in mechanosensory processes has provided a long-awaited and much-needed molecular entry into mechanosensation in mammals. We expect future studies of TMC proteins will help elucidate molecular and cellular underpinnings of mechanosensation in other organs and tissues. Manipulation of these molecules and pathways could be used to prevent or treat a variety of conditions that are potentially due to alterations of mechanosensation. The range of tissue expression of TMC proteins suggest these conditions could include disorders of hearing, balance, somatosensation, blood pressure, and immunity.
Acknowledgments
Kiyoto Kurima and Andrew Griffith are supported by NIH intramural research fund Z01-DC000060-13. Bifeng Pan and Jeffrey R. Holt are supported by NIH/NIDCD grant #RO1DC013521. Yoshiyuki Kawashima is supported by JSPS KAKENHI grant #26462551.
Footnotes
Conflict of interest
Kiyoto Kurima and Andrew J. Griffith hold the following U.S. patents: 7,166,433 (Transductin-2 and Applications to Hereditary Deafness), 7,192,705 (Transductin-1 and Applications to Hereditary Deafness), and 7,659,115 (Nucleic Acid Encoding Human Transductin-1 Polypeptide).
References
- 1.Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Griffith AJ, Frolenkov GI, Belyantseva IA, Richardson GP, Friedman TB. The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci. 2006;26(26):7022–7034. doi: 10.1523/JNEUROSCI.1163-06.2006. doi:10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beurg M, Fettiplace R, Nam JH, Ricci AJ. Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci. 2009;12(5):553–558. doi: 10.1038/nn.2295. doi:10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beurg M, Kim KX, Fettiplace R. Conductance and block of hair-cell mechanotransducer channels in transmembrane channel-like protein mutants. J Gen Physiol. 2014;144(1):55–69. doi: 10.1085/jgp.201411173. doi:10.1085/jgp.201411173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzigeorgiou M, Bang S, Hwang SW, Schafer WR. tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature. 2013;494(7435):95–99. doi: 10.1038/nature11845. doi:10.1038/nature11845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Heer A-MR, Collin RWJ, Huygen PLM, Schraders M, Oostrik J, Rouwette M, Kunst HPM, Kremer H, Cremers CWRJ. Progressive Sensorineural Hearing Loss and Normal Vestibular Function in a Dutch DFNB7/11 Family with a Novel Mutation in TMC1. Audiol Neurotol. 2011;16:93–105. doi: 10.1159/000313282. doi:10.1159/000313282. [DOI] [PubMed] [Google Scholar]
- 6.Fortnum HM, Summerfield AQ, Marshall DH, Davis AC, Bamford JM. Prevalence of permanent childhood hearing impairment in the United Kingdom and implications for universal neonatal hearing screening: questionnaire based ascertainment study. Bmj. 2001;323(7312):536–540. doi: 10.1136/bmj.323.7312.536. doi: http://dx.doi.org/10.1136/bmj.323.7312.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geleoc GS, Holt JR. Developmental acquisition of sensory transduction in hair cells of the mouse inner ear. Nat Neurosci. 2003;6(10):1019–1020. doi: 10.1038/nn1120. doi:10.1038/nn1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillespie PG, Muller U. Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell. 2009;139(1):33–44. doi: 10.1016/j.cell.2009.09.010. doi:10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffith AJ, Friedman TB. Autosomal and X-Linked Auditory Disorders. Springer; New York: 2002. [Google Scholar]
- 10.Holt JR, Pan B, Koussa MA, Asai Y. TMC function in hair cell transduction. Hear Res. doi: 10.1016/j.heares.2014.01.001. In press doi:10.1016/j.heares.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hudspeth AJ. Extracellular current flow and the site of transduction by vertebrate hair cells. J Neurosci. 1982;2(1):1–10. doi: 10.1523/JNEUROSCI.02-01-00001.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaramillo F, Hudspeth AJ. Localization of the hair cell's transduction channels at the hair bundle's top by iontophoretic application of a channel blocker. Neuron. 1991;7(3):409–420. doi: 10.1016/0896-6273(91)90293-9. doi:0896-6273(91)90293-9. [DOI] [PubMed] [Google Scholar]
- 13.Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, Griffith AJ. Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. The Journal of clinical investigation. 2011;121(12):4796–4809. doi: 10.1172/JCI60405. doi:10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, Kachar B. Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature. 2007;449(7158):87–91. doi: 10.1038/nature06091. doi:10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- 15.Keresztes G, Mutai H, Heller S. TMC and EVER genes belong to a larger novel family, the TMC gene family encoding transmembrane proteins. BMC Genomics. 2003;4(1):24. doi: 10.1186/1471-2164-4-24. doi:10.1186/1471-2164-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim KX, Beurg M, Hackney CM, Furness DN, Mahendrasingam S, Fettiplace R. The role of transmembrane channel-like proteins in the operation of hair cell mechanotransducer channels. The Journal of general physiology. 2013 doi: 10.1085/jgp.201311068. doi:10.1085/jgp.201311068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim KX, Fettiplace R. Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. The Journal of general physiology. 2013;141(1):141–148. doi: 10.1085/jgp.201210913. doi:10.1085/jgp.201210913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitajiri S, Makishima T, Friedman TB, Griffith AJ. A novel mutation at the DFNA36 hearing loss locus reveals a critical function and potential genotype-phenotype correlation for amino acid-572 of TMC1. Clin Genet. 2007;71(2):148–152. doi: 10.1111/j.1399-0004.2007.00739.x. doi:10.1111/j.1399-0004.2007.00739.x. [DOI] [PubMed] [Google Scholar]
- 19.Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, Ghosh M, Menon PS, Deshmukh D, Oddoux C, Ostrer H, Khan S, Deininger PL, Hampton LL, Sullivan SL, Battey JF, Jr., Keats BJ, Wilcox ER, Friedman TB, Griffith AJ. Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet. 2002;30(3):277–284. doi: 10.1038/ng842. doi:10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- 20.Kurima K, Yang Y, Sorber K, Griffith AJ. Characterization of the transmembrane channel-like (TMC) gene family: functional clues from hearing loss and epidermodysplasia verruciformis. Genomics. 2003;82(3):300–308. doi: 10.1016/s0888-7543(03)00154-x. doi:10.1016/S0888-7543(03)00154-X. [DOI] [PubMed] [Google Scholar]
- 21.Labay V, Weichert RM, Makishima T, Griffith AJ. Topology of Transmembrane Channel-like Gene 1 Protein. Biochemistry. 2010;49:8592–8598. doi: 10.1021/bi1004377. doi:10.1021/bi1004377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagziel A, Ahmed ZM, Schultz JM, Morell RJ, Belyantseva IA, Friedman TB. Spatiotemporal pattern and isoforms of cadherin 23 in wild type and waltzer mice during inner ear hair cell development. Dev Biol. 2005;280(2):295–306. doi: 10.1016/j.ydbio.2005.01.015. doi:10.1016/j.ydbio.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 23.Lelli A, Asai Y, Forge A, Holt JR, Geleoc GS. Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol. 2009;101(6):2961–2973. doi: 10.1152/jn.00136.2009. doi:10.1152/jn.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makishima T, Kurima K, Brewer CC, Griffith AJ. Early onset and rapid progression of dominant nonsyndromic DFNA36 hearing loss. Otol Neurotol. 2004;25(5):714–719. doi: 10.1097/00129492-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet. 1993;46(5):486–491. doi: 10.1002/ajmg.1320460504. doi:10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- 26.Marcotti W, Erven A, Johnson SL, Steel KP, Kros CJ. Tmc1 is necessary for normal functional maturation and survival of inner and outer hair cells in the mouse cochlea. J Physiol. 2006;574(Pt 3):677–698. doi: 10.1113/jphysiol.2005.095661. doi:10.1113/jphysiol.2005.095661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcotti W, Corns LF, Desmonds T, Kirkwood NK, Richardson GP, Kros CJ. Transduction without tip links in cochlear hair cells is mediated by ion channels with permeation properties distinct from those of the mechano-electrical transducer channel. J Neurosci. 2014;34(16):5505–5514. doi: 10.1523/JNEUROSCI.4086-13.2014. doi:10.1523/JNEUROSCI.4086-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354(20):2151–2164. doi: 10.1056/NEJMra050700. doi:10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 29.Mutai H, Mann S, Heller S. Identification of chicken transmembrane channel-like (TMC) genes: expression analysis in the cochlea. Neuroscience. 2005;132(4):1115–1122. doi: 10.1016/j.neuroscience.2005.01.046. doi:10.1016/j.neuroscience.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi Y, Kurima K, Makishima T, de Angelis MH, Fuchs H, Frolenkov G, Kitamura K, Griffith AJ. Multiple quantitative trait loci modify cochlear hair cell degeneration in the Beethoven (Tmc1Bth) mouse model of progressive hearing loss DFNA36. Genetics. 2006;173(4):2111–2119. doi: 10.1534/genetics.106.057372. doi:10.1534/genetics.106.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR. TMC1 and TMC2 Are Components of the Mechanotransduction Channel in Hair Cells of the Mammalian Inner Ear. Neuron. 2013;79(3):504–515. doi: 10.1016/j.neuron.2013.06.019. doi:10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steel KP, Bock GR. The nature of inherited deafness in deafness mice. Nature. 1980;288(5787):159–161. doi: 10.1038/288159a0. [DOI] [PubMed] [Google Scholar]
- 33.Vreugde S, Erven A, Kros CJ, Marcotti W, Fuchs H, Kurima K, Wilcox ER, Friedman TB, Griffith AJ, Balling R, Hrabe De Angelis M, Avraham KB, Steel KP. Beethoven, a mouse model for dominant, progressive hearing loss DFNA36. Nat Genet. 2002;30(3):257–258. doi: 10.1038/ng848. doi:10.1038/ng848. [DOI] [PubMed] [Google Scholar]
- 34.Wang Q, Xue Y, Zhang Y, Long Q, Asan, Yang F, Turner DJ, Fitzgerald T, Ng BL, Zhao Y, Chen Y, Liu Q, Yang W, Han D, Quail MA, Swerdlow H, Burton J, Fahey C, Ning Z, Hurles ME, Carter NP, Yang H, Tyler-Smith C. Genetic basis of Y-linked hearing impairment. Am J Hum Genet. 2013;92(2):301–306. doi: 10.1016/j.ajhg.2012.12.015. doi:10.1016/j.ajhg.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang T, Kahrizi K, Bazazzadeghan N, Meyer N, Najmabadi H, Smith RJ. A novel mutation adjacent to the Bth mouse mutation in the TMC1 gene makes this mouse an excellent model of human deafness at the DFNA36 locus. Clin Genet. 2010;77(4):395–398. doi: 10.1111/j.1399-0004.2009.01338.x. doi:10.1111/j.1399-0004.2009.01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao Y, Wang D, Zong L, Zhao F, Guan L, Zhang P, Shi W, Lan L, Wang H, Li Q, Han B, Yang L, Jin X, Wang J, Wang J, Wang Q. A novel DFNA36 mutation in TMC1 orthologous to the Beethoven (Bth) mouse associated with autosomal dominant hearing loss in a Chinese family. PLoS One. 2014;9(5):e97064. doi: 10.1371/journal.pone.0097064. doi:10.1371/journal.pone.0097064. [DOI] [PMC free article] [PubMed] [Google Scholar]