Abstract
Alternative splicing plays an important role in proteasome diversity and gene expression regulation in eukaryotic cells. Hdm2, the human homologue of mdm2 (murine double minute oncogene 2), is known to be an oncogene as its role in suppression of p53. Hdm2 alternative splicing, occurs in both tumor and normal tissues, is believed to be a response of cells for cellular stress, and thus modulate p53 activity. Therefore, understanding the regulation of hdm2 splicing is critical in elucidating the mechanisms of tumor development and progression. In this study, we determined the effect of ultraviolet B light (UVB) on alternative splicing of hdm2. Our data indicated that UVB (50 mJ/cm2) alone is not a good inducer of alternative splicing of hdm2. The less effectiveness could be due to the induction of ROS and p53 by UVB because removing ROS by L-NAC (10 mM) in p53 null cells could lead to alternative splicing of hdm2 upon UVB irradiation.
INTRODUCTION
The murine double minute oncogene 2 (mdm2) gene, which codes for mdm2 protein, was originally cloned from the spontaneously transformed mouse cell line 3T3 (1). Mdm2 overexpression promotes transformation of primary mouse fibroblasts as well as tumor formation in nude mice (2). Hdm2, the human homologue of the mdm2 gene, encodes a 90-kDa protein with N-terminal p53-binding domain, as well as a central acidic domain, a zinc-binding motif, and a C-terminal Ring finger motif (3). The binding of hdm2 to p53 induces the suppression and degradation of p53, as well as export p53 from the nucleus, thus hdm2 function as a negative regulator of p53 (4–6). On the other hand, p53 can also bind to hdm2 to increase its stability, thus function as a negative feedback loop for self-regulation (7,8). There are more than 40 mdm2 variants being characterized, though most of them has unknown functions (9,10). Compared to normal cells, cancer cells usually have higher levels of alternatively spliced mdm2, especially transcripts lacking p53-binding domains (11–14). In human lung cancer cells, as previous research shown, in addition to full-length hdm2, three alternatively spliced forms of hdm2 also exist. All three alternatively spliced hdm2 forms lack most of the p53-binding domain, thus cannot bind and interact with p53 (15). Moreover, alternatively spliced hdm2 can also bind to the full-length hdm2 and interfere its interaction with p53, which in term leads to the increase of p53 protein level and activity as well (10,15). Previous studies indicate that genotoxic stimuli, such as ultraviolet C light (UVC), induce alternative splicing of hdm2 and expression of hdm2alt1 in non-small cell lung carcinomas (16,17). Since UVC is not a physiological wavelength we receive from sunlight, the effect of UVB on alternative splicing of hdm2 is determined in this study. Our results indicate that UVB-induced alternative splicing of hdm2 is dependent on ROS formation and p53 status of the irradiated cells.
MATERIAL AND METHODS
Cell culture
H1299 (p53-null) human lung cancer cells were grown in Dulbecco’s Minimal Essential Medium (Cellgro). A549 (p53-wt) human lung cancer cells were grown in F-12K medium (Cellgro). Both media were supplemented with 10% fetal bovine serum and penicillin/streptomycin. The cells were incubated at 37°C with 5% CO2.
DNA Transfection
H1299 cells were transiently transfected with p53-EGFP-N1 vector using lipofectamine 2000 (Invitrogen) following manufacture’s instruction manual. H1299 cells (3×105) were seeded into 6-well plate and incubated over night. A transfection mixture was made by mixing and incubating 6 µL lipofectamine 2000 with 3 µg plasmid in 200 µL DMEM medium for 20 min. The transfection mixture was then added directly onto cells containing 2 mL culture medium and incubated for 24 h before the cells were exposed to UVB or UVC radiation.
UV Radiation
Both UVB and UVC were generated from a Bench XX-Series UV Lamp (UVP Inc.). The intensities of UVB and UVC were calibrated by a UVX digital radiometer (UVP Inc.) after the lamps warmed up for 5 min. For UVB, two 15-watt UVB tubes (UVP Inc.) were equipped. The cells were UVB-irradiated with 50 mJ/cm2 at a dose rate of 3.8 mW/cm2. For UVC, one 15-watt UVC tube (UVP Inc.) was equipped. The cells were UVC-irradiated with 3 mJ/cm2 at a dose rate of 0.3 mW/cm2. Medium was removed before exposing cells to UVR and fresh medium was added to the culture plates with or without drugs after UVR. Cells were continue incubating at 37°C with 5% CO2 until further analysis.
Drug treatment
The cells were pretreated with L-NAC (10 mM, Sigma) for 1 h and then irradiated with UVB or UVC as indicated. After radiation, the cells were continuously incubated with L-NAC (10 mM) until harvesting.
RNA isolation and reverse transcriptase PCR
Total RNA was isolated from cells using Trizol (Invitrogen) according to manufacturer’s protocol. Briefly, the cells on a 35 mm dish were lysed with 1 mL Trizol. The lysates were transferred to a 1.5 mL centrifuge tube followed by adding 0.2 mL chloroform. RNA at the aqueous phase was then removed and precipitated using 0.5 mL 100% isopropanol. The RNA pellet was then washed twice by 75% ethanol and dissolved in RNase-free water. The first strand cDNA was reverse-transcribed from 1 µg RNA using SuperScript III Reverse Transcriptase (Invitrogen) following manufacturer’s protocol. Briefly, in a 20 µL reaction system, 1 µg total mRNA was mixed with 1 µL of 50 µM oligo(dT)20 and 1 µL 10 mM dNTP, and heated to 65°C for 5 min followed by incubation on ice for 2 min. Then 5X first-strand buffer and 0.1 M DTT and 1 µL of Superscript III RT were added with 5 min incubation at 25 °C followed by 60 min 50 °C and 15 min 75°C heating.
Nested PCR
1 µL cDNA was used as template for the nested PCR using GoTaq Green Master Mix (Promega). The primers for the first and second round were as following:
First round: 5’-CTGGGGAGTCTTGAGGGACC-3’ and 5’-CAGGTTGTCTAAATTCCTAG.
Second round: 5’-CGCGAAAACCCCGGATGGTG-3’ and 5’-CTCTTATAGACAGGTCAACTAG.
The PCR reaction for both rounds were performed on Mastercycle Gradient (Eppendorf) starting with incubation at 94°C for 2 min followed by 25 cycles of 94°C at 30 s, 55°C at 30 s and 72°C at 1 min, and then incubated at 72°C for 10 min for elongation.
Western blot analysis
Nonidet P-40 (NP-40) lysis buffer (2% NP-40, 80 mM NaCl, 100 mM Tris-HCl pH 8.0, 0.1% SDS) with proteinase inhibitor mixture (Complete™, Roche Molecular Biochemicals) was used to lyse cells. Cell lysate was incubated on ice for 15 min and then centrifuged at 14,000 rpm at 4°C for 15 min. Protein concentration was measured by Protein DC Assay kit (Bio-Rad Laboratories). Equal amounts of protein were subjected on SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then blocked in 5% milk in Tris buffered saline plus Tween 20 (TBST) for 45 min and probed with anti-p53 (SC-126 Santa Cruz), or anti-β-actin (A1978, sigma) at 4°C overnight. After washing with TBST, the membrane was incubated with corresponding HRP-conjugated anti-rabbit or anti-mouse antibody for 45 min at room temperature. Membrane was then washed 5 min in TBST for three times followed by 5 min wash in TBS for two times, and developed using West Pico Supersignal chemiluminescent substrate (Pierce).
RESULTS
Standardization of hdm2ALT detection
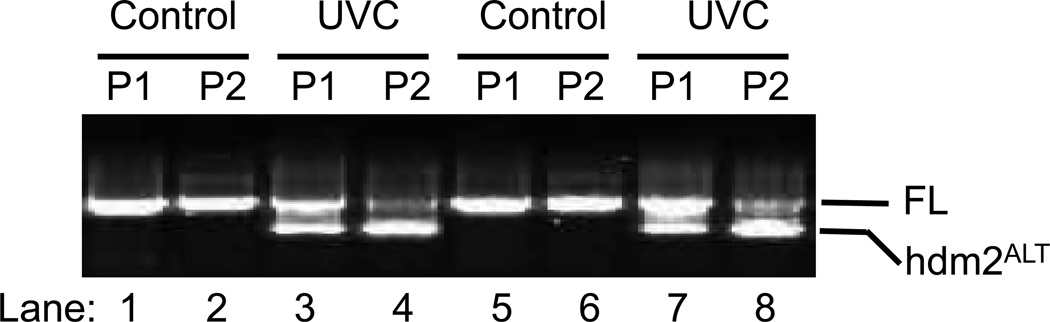
To detect the hdm2alt, we first standardized nested PCR and electrophoresis using the H1299 cell line and UVC radiation according to previous literatures (14,15). Yet, by blasting the primers with Homo sapiens mdm2 mRNA sequence (NM_002392.4) of hdm2, we found that there was a missing 10-nucleotide gap (underlined) for the previously reported forward primer (5’- CGCGAAAACCCCGGATGGTGAGGA GCAGGCAAATGTGCA-3’) of the second round PCR. We therefore re-designed the primers and did a comparison with the original primers. Our data indicated that while control samples showed no difference, the new primer had a better selectivity in detection of the hdm2alt1 after the cells were UVC irradiated (Fig. 1. Lane 3 vs. 4; 7 vs. 8). Our results indicate that our newly designed primer is more specific for detecting the alternatively spliced hdm2 and thus it will be used for all the following studies.
Fig. 1.
The modification of mdm2ALT detection by nested PCR. Total RNA of control H1299 cells and UVC radiated (3 J/m2) H1299 cells (24 h) were extracted using Trizon and converted to cDNA by Reverse transcriptase PCR. Full length and alternatively splicing mdm2 were detected by nested PCR followed by electrophoresis using the same primers for first round PCR, but different primers for second round PCR, P1 stands for original primers while P2 stands for modified PCR.
Free radicals inhibits UVB-induced alterative splicing of hdm2
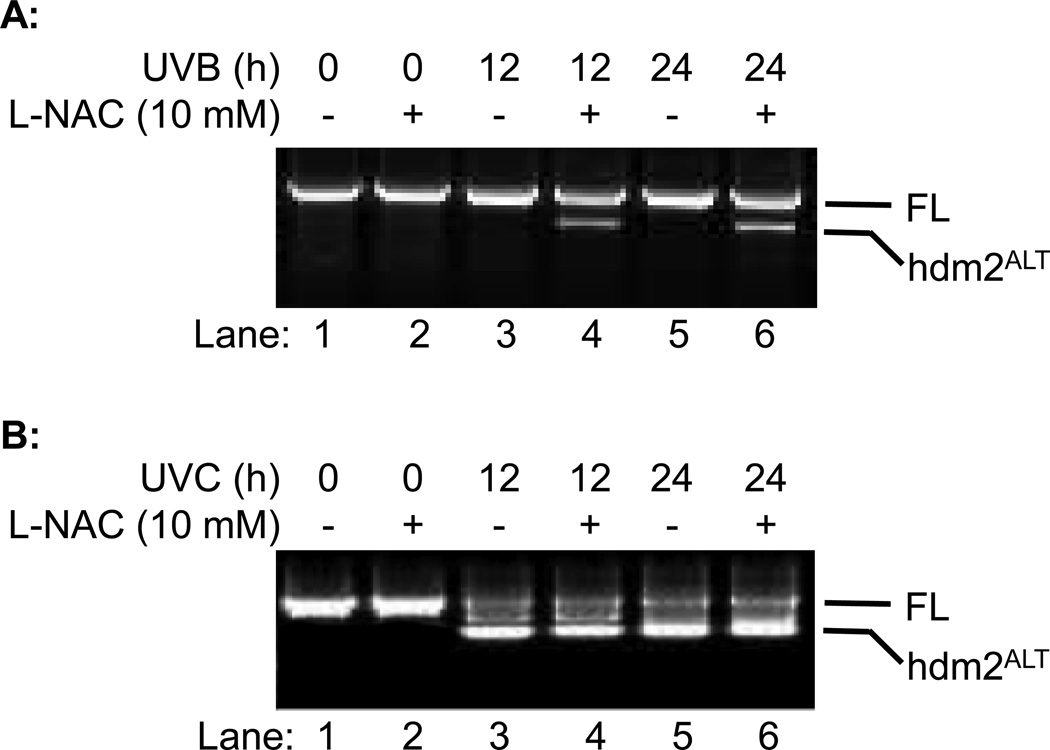
UVB radiation can cause DNA damage and induced production of free radicals in skin (18–23). Previous studies suggest that genotoxicity caused by IR and UVC will induce alternative splicing of hdm2 in H1299 cells (16,17,24). In this study, we determined whether UVB could also induce alternative splicing of hdm2 and whether free radicals contribute to it. L-NAC, a potent free radical scavenger, was used to remove free radicals and UVC-induce hdm2ALT was used as control. Our data showed that UVB radiation alone did not induce the alternative splicing of hdm2 in H1299 cells while UVC did (Fig. 2A, Lanes 3 and 5 vs. 2B, Lanes 3 and 5). However, treating cell with L-NAC increased hdm2 splicing upon UVB radiation at both 12 and 24 h (Fig. 2A, Lanes 4 and 6 vs. 3 and 5); yet had no significant effect on UVC-induced hdm2ALT formation (Fig. 2B, Lanes 4 and 6 vs. 3 and 5). These results suggest that while genotoxicity induce hdm2ALT formation, ROS elevation may inhibit alternative splicing of hdm2.
Fig. 2.
The effect of L-NAC on hdm2 splicing on H1299 cells. H1299 cells were exposed to (A) UVB (50 mJ/cm2) or (B) UVC (3 J/m2) radiation with or without L-NAC treatment. Cells were collected 12 or 24 h post UV radiation and hdm2 splicing were analyzed by nested PCR.
The role of p53 in regulation of hdm2 alternative splicing
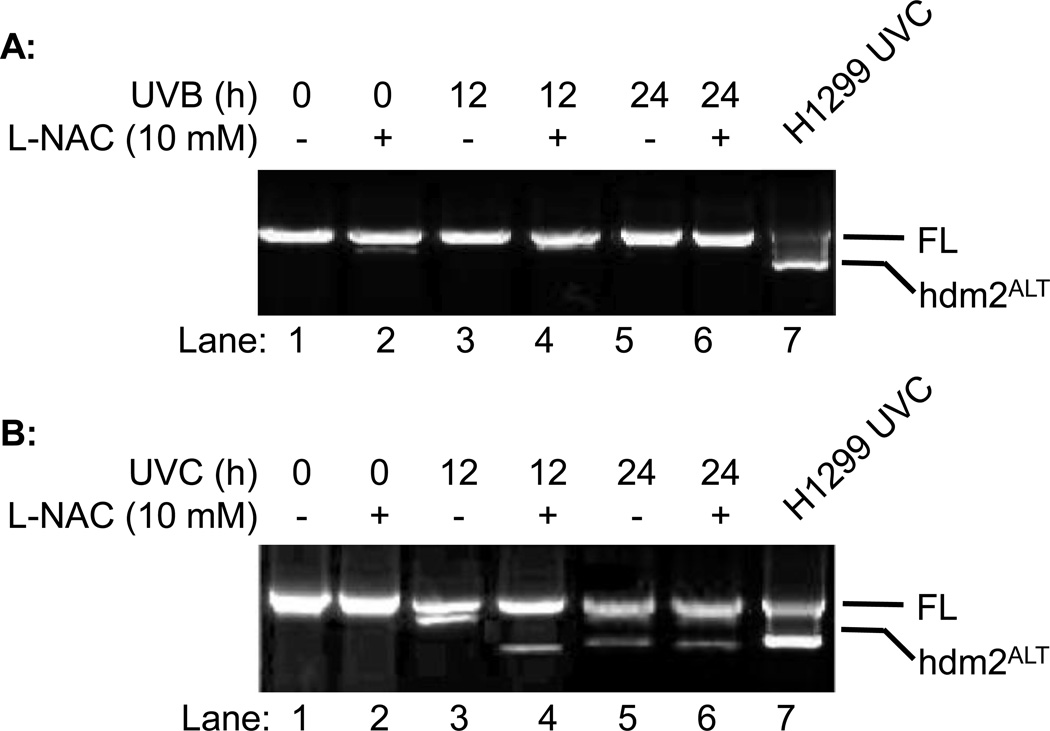
H1299 does not express p53, which is known to be involved in regulation of ROS-induced mRNA splicing machinery (25,26). To determine whether p53 plays a role in regulation of alternative splicing of hdm2, we determined alternative splicing of hdm2 in p53 positive A549 lung carcinoma cells after UVB and/or L-NAC treatment. Unlike H1299 cells, our data showed that L-NAC promoted alternative splicing of hdm2 upon UVB irradiation was suppressed in A549 cells (Fig. 3A) compared to H1299 cells. Meanwhile, the UVC-induced hdm2ALT expression was also inhibited (Fig. 3B, Lane 3) or reduced in A549 cells in the presence or absence of L-NAC (Fig. 3B, Lanes 4–6). These results suggested that p53 might negatively regulate the alternative splicing of hdm2 after UVB or UVC radiation.
Fig. 3.
The effect of L-NAC on hdm2 splicing on A549 cells. A549 cells were exposed to (A) UVB (50 mJ/cm2) or (B) UVC (3 J/m2) radiation with or without L-NAC treatment. Cells were collected 12 or 24 h post UV radiation and hdm2 splicing were analyzed by nested PCR.
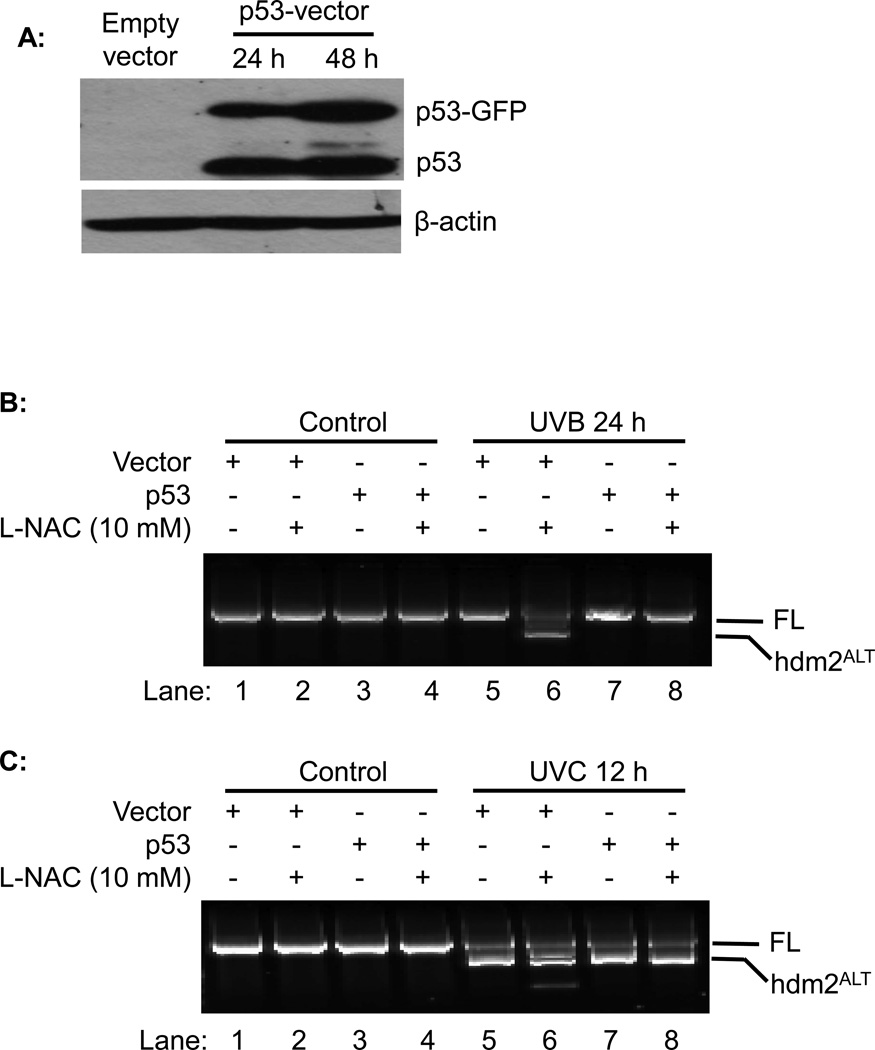
To further investigate the role of p53 in regulation the alternative splicing of hdm2, we transiently transfected GFP-tagged p53 vector into H1299 cells. Western blot analysis indicated that p53 and p53-GFP were highly overexpressed in H1299 cells at 24 h and 48 h post transfection (Fig 4A). The cells were UVB or UVC irradiated at 24 h post-transfect in the presence of absence of L-NAC. Our data showed that L-NAC promoted alternative splicing of hdm2 upon UVB irradiation was totally abolished by overexpression of p53 (Fig. 4A, Lane 8 vs. 6). On the other hand, the overexpression of p53 in H1299 cells had no effect on UVC induced hdm2 splicing with or without L-NAC treatment (Fig 4C). These results confirmed that p53 negatively regulates the L-NAC promoted alternative splicing of hdm2 upon UVB radiation.
Fig. 4.
The effect of p53 on hdm2 splicing. H1299 cells were transfected with p53 expression vector, and followed by UV radiation with or without L-NAC treatment. (A) p53 over expression were analyzed by western blot. (B) WT and p53 over expressed H1299 cells were exposed to UVB radiation with or without L-NAC treatment. (C) WT and p53 over expressed H1299 cells were exposed to UVC radiation with or without L-NAC treatment.
DISCUSSION
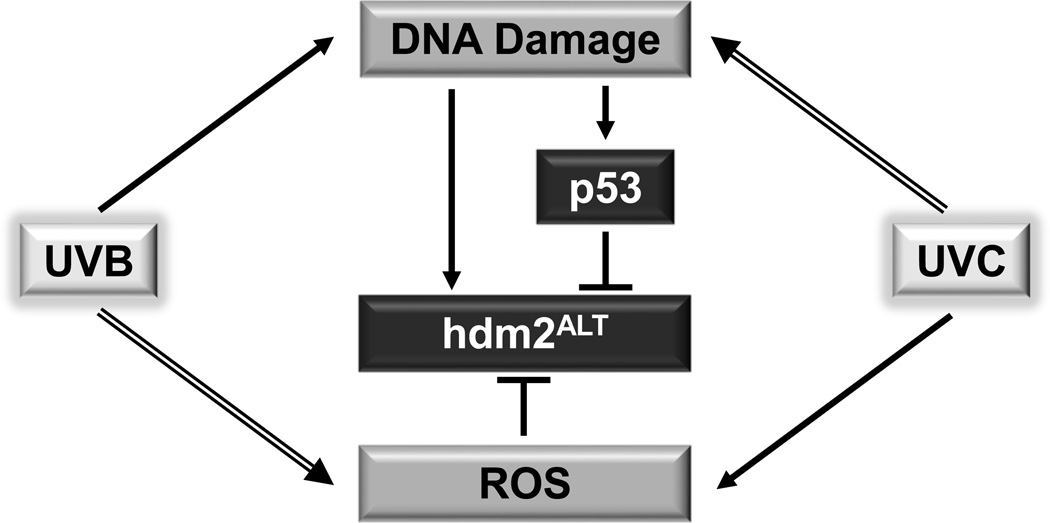
Alternative splicing of hdm2 mRNA plays an important role in regulation of p53 activity and cell apoptosis (5,27,28). UVB can cause DNA damage and induce ROS production; and both of them have been shown to be involved in regulation of mRNA alternative splicing (26,29–31). However, the effect of UVB on alternative splicing of hdm2 has never been studied. The role of p53 and ROS in regulation of hdm2ALT formation upon UVB irradiation is not known. In this study, we demonstrated that UVB (50 mJ/cm2) is not a good inducer of hdm2ALT as UVC does (Fig. 2). This could be due to that UVC is stronger than UVB in induction of genotoxicity (32,33), which was suggested to cause alternative splicing of hdm2 (16). Interestingly, our data showed that UVB could induce hdm2ALT formation in the presence of L-NAC, which had no significant effect on UVC-induced alternative splicing of hdm2 (Fig. 2). This result suggest that ROS generated by UVB radiation may serve as an inhibitor of the alternative splicing of hdm2. This assumption is supported by the factor that UVC is weaker than UVB in inducing ROS formation (34–36). Besides ROS, we furthermore demonstrated that p53 was also negatively regulated the alternative splicing of hdm2 upon UVB radiation (Figs. 3–4). This result contradicts with previous report indicating that hydroxyl peroxide (H2O2)-induced hdm2ALT formation is p53 dependent (37). The controversial results could be due to that different splicing mechanisms might be involved in genotoxicity-induced and H2O2-induced hdm2ALT formation. In summary, based on our results, we propose a model (Fig. 5) that UVB or UVC induced DNA damage promotes alternative splicing of hdm2. Meanwhile ROS elevation or p53 activation induced by UVB or UVC can inhibit alternative splicing of hdm2. Because UVB is a weak inducer of DNA damager but a stronger inducer of ROS than UVC is, UVB alone does not induce the alternative splicing of hdm2 as UVC does due to the cancelation of the three effects. Our findings will provide guidance for using ROS scavenger as therapeutics to protect skin from UVB cause damage and skin cancer.
Fig. 5.
Model for ROS and p53 regulated alternative splicing of hdm2 upon UVB and UVB irradiation.
Acknowledgements
This work was supported by National Institutes of Health RO1 CA86926 (to S.W.) and Molecular and Cellular Biology Program, Ohio University (to L.T.).
Footnotes
This paper is part of the Special Issue commemorating the 65th birthday of Dr. Craig A. Elmets
REFERENCES
- 1.Fakharzadeh SS, Trusko SP, George DL. Tumorigenic potential associated with enhanced expression of a gene that is amplified in a mouse tumor cell line. EMBO J. 1991;10:1565–1569. doi: 10.1002/j.1460-2075.1991.tb07676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lundgren K, Montes de Oca Luna R, McNeill YB, Emerick EP, Spencer B, Barfield CR, Lozano G, Rosenberg MP, Finlay CA. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997;11:714–725. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- 3.Marine JC, Jochemsen AG. Mdmx as an essential regulator of p53 activity. Biochem Biophys Res Commun. 2005;331:750–760. doi: 10.1016/j.bbrc.2005.03.151. [DOI] [PubMed] [Google Scholar]
- 4.Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol. 2000;2:563–568. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 5.Vargas DA, Takahashi S, Ronai Z. Mdm2: A regulator of cell growth and death. Adv Cancer Res. 2003;89:1–34. doi: 10.1016/s0065-230x(03)01001-7. [DOI] [PubMed] [Google Scholar]
- 6.Jeyaraj S, O'Brien DM, Chandler DS. MDM2 and MDM4 splicing: an integral part of the cancer spliceome. Front Biosci (Landmark Ed) 2009;14:2647–2656. doi: 10.2741/3402. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Bayle JH, Olson D, Levine AJ. The p53-mdm-2 autoregulatory feedback loop. Genes Dev. 1993;7:1126–1132. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]
- 8.Kulikov R, Winter M, Blattner C. Binding of p53 to the central domain of Mdm2 is regulated by phosphorylation. J Biol Chem. 2006;281:28575–28583. doi: 10.1074/jbc.M513311200. [DOI] [PubMed] [Google Scholar]
- 9.Okoro DR, Rosso M, Bargonetti J. Splicing up mdm2 for cancer proteome diversity. Genes Cancer. 2012;3:311–319. doi: 10.1177/1947601912455323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartel F, Taubert H, Harris LC. Alternative and aberrant splicing of MDM2 mRNA in human cancer. Cancer Cell. 2002;2:9–15. doi: 10.1016/s1535-6108(02)00091-0. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto R, Tada M, Nozaki M, Zhang CL, Sawamura Y, Abe H. Short alternative splice transcripts of the mdm2 oncogene correlate to malignancy in human astrocytic neoplasms. Cancer Res. 1998;58:609–613. [PubMed] [Google Scholar]
- 12.Liang H, Atkins H, Abdel-Fattah R, Jones SN, Lunec J. Genomic organisation of the human MDM2 oncogene and relationship to its alternatively spliced mRNAs. Gene. 2004;338:217–223. doi: 10.1016/j.gene.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 13.Lukas J, Gao DQ, Keshmeshian M, Wen WH, Tsao-Wei D, Rosenberg S, Press MF. Alternative and aberrant messenger RNA splicing of the mdm2 oncogene in invasive breast cancer. Cancer Res. 2001;61:3212–3219. [PubMed] [Google Scholar]
- 14.Sigalas I, Calvert AH, Anderson JJ, Neal DE, Lunec J. Alternatively spliced mdm2 transcripts with loss of p53 binding domain sequences: transforming ability and frequent detection in human cancer. Nat Med. 1996;2:912–917. doi: 10.1038/nm0896-912. [DOI] [PubMed] [Google Scholar]
- 15.Evans SC, Viswanathan M, Grier JD, Narayana M, El-Naggar AK, Lozano G. An alternatively spliced HDM2 product increases p53 activity by inhibiting HDM2. Oncogene. 2001;20:4041–4049. doi: 10.1038/sj.onc.1204533. [DOI] [PubMed] [Google Scholar]
- 16.Chandler DS, Singh RK, Caldwell LC, Bitler JL, Lozano G. Genotoxic stress induces coordinately regulated alternative splicing of the p53 modulators MDM2 and MDM4. Cancer Res. 2006;66:9502–9508. doi: 10.1158/0008-5472.CAN-05-4271. [DOI] [PubMed] [Google Scholar]
- 17.Dias CS, Liu Y, Yau A, Westrick L, Evans SC. Regulation of hdm2 by stress-induced hdm2alt1 in tumor and nontumorigenic cell lines correlating with p53 stability. Cancer Res. 2006;66:9467–9473. doi: 10.1158/0008-5472.CAN-05-3013. [DOI] [PubMed] [Google Scholar]
- 18.Liu W, Wu S. Differential roles of nitric oxide synthases in regulation of ultraviolet B light-induced apoptosis. Nitric Oxide. 2010;23:199–205. doi: 10.1016/j.niox.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu S, Wang L, Jacoby AM, Jasinski K, Kubant R, Malinski T. Ultraviolet B light-induced nitric oxide/peroxynitrite imbalance in keratinocytes--implications for apoptosis and necrosis. Photochem Photobiol. 2010;86:389–396. doi: 10.1111/j.1751-1097.2009.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hideg E, Jansen MA, Strid A. UV-B exposure, ROS, and stress: inseparable companions or loosely linked associates? Trends Plant Sci. 2013;18:107–115. doi: 10.1016/j.tplants.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Sinha RP, Hader DP. UV-induced DNA damage and repair: a review. Photochem Photobiol Sci. 2002;1:225–236. doi: 10.1039/b201230h. [DOI] [PubMed] [Google Scholar]
- 22.Budden T, Bowden NA. The Role of Altered Nucleotide Excision Repair and UVB-Induced DNA Damage in Melanomagenesis. Int J Mol Sci. 2013;14:1132–1151. doi: 10.3390/ijms14011132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ming M, Feng L, Shea CR, Soltani K, Zhao B, Han W, Smart RC, Trempus CS, He YY. PTEN positively regulates UVB-induced DNA damage repair. Cancer Res. 2011;71:5287–5295. doi: 10.1158/0008-5472.CAN-10-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh RK, Tapia-Santos A, Bebee TW, Chandler DS. Conserved sequences in the final intron of MDM2 are essential for the regulation of alternative splicing of MDM2 in response to stress. Exp Cell Res. 2009;315:3419–3432. doi: 10.1016/j.yexcr.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Allende-Vega N, Dayal S, Agarwala U, Sparks A, Bourdon JC, Saville MK. p53 is activated in response to disruption of the pre-mRNA splicing machinery. Oncogene. 2013;32:1–14. doi: 10.1038/onc.2012.38. [DOI] [PubMed] [Google Scholar]
- 26.Disher K, Skandalis A. Evidence of the modulation of mRNA splicing fidelity in humans by oxidative stress and p53. Genome. 2007;50:946–953. doi: 10.1139/g07-074. [DOI] [PubMed] [Google Scholar]
- 27.Lenos K, Jochemsen AG. Functions of MDMX in the modulation of the p53-response. J Biomed Biotechnol. 2011;2011:876173. doi: 10.1155/2011/876173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perry ME, Mendrysa SM, Saucedo LJ, Tannous P, Holubar M. p76(MDM2) inhibits the ability of p90(MDM2) to destabilize p53. J Biol Chem. 2000;275:5733–5738. doi: 10.1074/jbc.275.8.5733. [DOI] [PubMed] [Google Scholar]
- 29.Munoz MJ, Perez Santangelo MS, Paronetto MP, de la Mata M, Pelisch F, Boireau S, Glover-Cutter K, Ben-Dov C, Blaustein M, Lozano JJ, Bird G, Bentley D, Bertrand E, Kornblihtt AR. DNA damage regulates alternative splicing through inhibition of RNA polymerase II elongation. Cell. 2009;137:708–720. doi: 10.1016/j.cell.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Lenzken SC, Loffreda A, Barabino SM. RNA Splicing: A New Player in the DNA Damage Response. Int J Cell Biol. 2013;2013:153634. doi: 10.1155/2013/153634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cote GJ, Zhu W, Thomas A, Martin E, Murad F, Sharina IG. Hydrogen peroxide alters splicing of soluble guanylyl cyclase and selectively modulates expression of splicing regulators in human cancer cells. PLoS One. 2012;7:e41099. doi: 10.1371/journal.pone.0041099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uehara F, Miwa S, Tome Y, Hiroshima Y, Yano S, Makoyamamoto, Efimova E, Matsumoto Y, Maehara H, Bouvet M, Kanaya F, Hoffman RM. Comparison of UVB and UVC Effects on the DNA Damage-Response Protein 53BP1 in Human Pancreatic Cancer. J Cell Biochem. 2014 doi: 10.1002/jcb.24837. [DOI] [PubMed] [Google Scholar]
- 33.Rahman I, Karim A, Idrees M, Khan MI. Cellular and genomic toxicity produced by UV light in Chinese hamster ovary cells. Pak J Pharm Sci. 2014;27:295–301. [PubMed] [Google Scholar]
- 34.Svobodova A, Walterova D, Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 35.Joshi PC. Ultraviolet radiation-induced photodegradation and 1O2, O2-. production by riboflavin, lumichrome and lumiflavin. Indian J Biochem Biophys. 1989;26:186–189. [PubMed] [Google Scholar]
- 36.Zhang X, Rosenstein BS, Wang Y, Lebwohl M, Wei H. Identification of possible reactive oxygen species involved in ultraviolet radiation-induced oxidative DNA damage. Free Radic Biol Med. 1997;23:980–985. doi: 10.1016/s0891-5849(97)00126-3. [DOI] [PubMed] [Google Scholar]
- 37.Pikkarainen S, Kennedy RA, Marshall AK, Tham el L, Lay K, Kriz TA, Handa BS, Clerk A, Sugden PH. Regulation of expression of the rat orthologue of mouse double minute 2 (MDM2) by H(2)O(2)-induced oxidative stress in neonatal rat cardiac myocytes. J Biol Chem. 2009;284:27195–27210. doi: 10.1074/jbc.M109.037887. [DOI] [PMC free article] [PubMed] [Google Scholar]