Abstract
Steroid hormone receptors are widely and heterogeneously expressed in the brain, and are regulated by age and gonadal hormones. Our goal was to quantify effects of aging, long-term estradiol (E2) treatment, and their interactions, on expression of G protein-coupled estrogen receptor (GPER), estrogen receptor α (ERα) and progesterone receptor (PR) immunoreactivity in two hypothalamic regions, the arcuate (ARC) and the periventricular area (PERI) of rhesus monkeys as a model of menopause and hormone replacement. Ovariectomized (OVX) rhesus macaques were young (~11 years) or aged (~25 years), given oil (vehicle) or E2 every 3 weeks for 2 years. Immunohistochemistry and stereologic analysis of ERα, PR, and GPER was performed. More effects were detected for GPER than the other two receptors. Specifically, GPER cell density in the ARC and PERI, and the percent of GPER-immunoreactive cells in the PERI, were greater in aged than in young monkeys. In addition, we mapped the qualitative distribution of GPER in the monkey hypothalamus and nearby regions. For ERα, E2 treated monkeys tended to have higher cell density than vehicle monkeys in the ARC. The percent of PR density in the PERI tended to be higher in E2 than vehicle monkeys of both ages. This study shows that the aged hypothalamus maintains expression of hormone receptors with age, and that long-term cyclic E2 treatment has few effects on their expression, although GPER was affected more than ERα or PR. This result is surprising in light of evidence for E2 regulation of the receptors studied here, and differences may be due to the selected regions, long-term nature of E2 treatment, among other possibilities.
Reproductive aging in females is highly divergent among mammalian species. Menopause is limited to those few species that menstruate (humans, great apes, and some non-human primates); it is a natural transition to reproductive senescence associated with decreased levels of the sex steroid hormones estradiol (E2) and progesterone (P4) (Trévoux et al., ‘86; Burger et al., 2002). Estrogens and progestins are not only critical for reproduction, but also play significant roles in the normal functioning of brain networks, cardiovascular systems, bone maintenance, and many others (Baulieu and Robel, ‘90; Inoue, 2002; McEwen, 2002). In women, the menopausal decline in circulating hormones is often accompanied by symptoms that have a dramatic negative impact on quality of life such as mood alterations, sleep disruptions, increased risk of osteoporosis and more. There are many available treatments for mitigation of menopausal symptoms, the most common being hormone replacement therapy (HRT) containing estrogens, or estrogens in combination with progestins. The risks versus benefits of health outcomes are highly controversial (Herrinton and Weiss, ‘93; Fitzpatrick et al., 2000; Rossouw et al., 2002; Canonico et al., 2008; Talboom et al., 2008; Prentice et al., 2009; Terauchi et al., 2012; Manson et al., 2013), with differential results due in part to variations in hormone formulations and timing/duration of hormone treatment relative to the menopausal transition.
Non-human primates undergo many similar neurobiological (functional and cellular) and physical (e.g., osteoporosis, metabolic) alterations with menopause as in women (Hao et al., 2003, 2007; Rapp et al., 2003; Maffucci and Gore, 2006). Furthermore, mammalian species that do not menstruate may also undergo a loss of reproductive capacity with aging, often very differently from primates due to those species’ unique reproductive properties such as strong seasonal breeding period, estrous cycles or induced ovulation (as opposed to spontaneous ovulation and reproductive cycles), and other reproductive traits (Maffucci and Gore, 2006; Kermath and Gore, 2012). Although there may be variability, a conserved property across species is that reproductive senescence involves the three levels of the hypothalamic-pituitary-gonadal (HPG) axis. Declines in hypothalamic function precede ovarian failure in rodents and primates, although the ovary may play a more primary role in women (Wise, ‘84; Richardson et al., ‘87; Gougeon et al., ‘94; Gore et al., 2000; Gill et al., 2002a,b; Weiss et al., 2004; Downs and Wise, 2009). While age-related changes in positive and negative feedback on gonadotropin-releasing hormone (GnRH) and gonadotropin release clearly occur in rodents, the evidence is less clear for both non-human and human primates (Van Look et al., ‘77; Wise and Ratner, ‘80; Gore et al., 2000; Tsai et al., 2004; Hall, 2007; Downs and Wise, 2009; Rance, 2009; Shaw et al., 2011). The neurons that synthesize GnRH are modulated by ovarian hormonal feedback both directly and indirectly via steroid hormone receptors, including G protein-coupled estrogen receptor (GPER), estrogen receptor α (ERα), and progesterone receptor (PR), among others (Van Look et al., ‘77; Liu and Yen, ‘83; Sullivan et al., ‘95; Terasawa, ‘95; Skinner et al., ‘98; Wilson et al., 2002; Dorling et al., 2003; Petersen et al., 2003; Glidewell-Kenney et al., 2007). An important research gap is whether, and how, hormone feedback on the hypothalamus may change with aging, and on which cells these effects are mediated. The mechanism for these changes is also unclear, although it may involve age-related change in expression or function of the steroid hormone receptors that mediate steroid hormone effects [reviewed in Chakraborty and Gore (2004)].
In the current study, we addressed this question in female monkeys as a translational model for the neurobiology of menopause in women (Gilardi et al., ‘97; Kaplan, 2004). Rhesus monkeys have 28-day menstrual cycles and undergo natural reproductive senescent changes that mirror the human menopausal transition, albeit much later in life (Krey et al., ‘75; Gilardi et al., ‘97; Archer, 2004; Gore et al., 2004). We focused our work on two sub-regions of the hypothalamus involved in HPG function in primates, the arcuate nucleus (ARC) and the periventricular region (PERI), which are integral to reproduction, growth, thermoregulation and metabolism (Wiegand and Terasawa, ‘82; Hofman, ‘97; Downs and Wise, 2009; Castellanoa et al., 2010; Mittelman-Smith et al., 2012). These hypothalamic areas also express high levels of steroid hormone receptors and are important targets of E2 feedback in the regulation of the HPG axis (Bethea et al., ‘96; Skinner et al., ‘98; Blurton-Jones et al., ‘99; Mills et al., 2002; Petersen et al., 2003; Rapp et al., 2003; Tsai et al., 2004; Michael et al., 2005). To determine whether there are age-related changes in steroid hormone receptors, and altered responses of these receptors to E2 feedback, we quantified the density and percentage of cells that express GPER, ERα, and PR in the ARC and PERI of young and aged macaques that were ovariectomized (OVX) and given E2 or vehicle treatment for 2 years. Because relatively little is known about the distribution of GPER neurons in the adult brain, we also mapped their localization across the hypothalamus.
MATERIALS AND METHODS
Animals
Adult female rhesus macaques (Macaca mulatta) from the California National Primate Research Center at the University of California at Davis were used. These animals were part of a larger Program Project Grant collaboration on “Estrogen and the Aging Brain,” and first described in (Hao et al., 2003). Table S1 shows animal demographics, age distributions, and age at euthanasia, with 21 young adults (mean age 11 ± 4.4 years) and 15 aged adults (mean age 24.9 ± 2.2 years). They were singly housed to enable collection of daily urine samples, with water available ad libitum, monkey chow provided in excess of nutritional needs, and regularly supplemented with fresh fruit. Candidate monkeys, classified as either pre- or perimenopausal by their reproductive history, had not been used in any invasive or pharmacological analyses before this study. They underwent behavioral assessment of learning and memory for companion studies (Hao et al., 2003, 2006, 2007; Rapp et al., 2003; Wang et al., 2010). All experimental procedures were approved by Institutional Animal Care and Use Committees at the University of California-Davis and the Mount Sinai School of Medicine and conformed to National Institutes of Health guidelines.
Ovariectomy and Estradiol Replacement
All animals were bilaterally ovariectomized (OVX) and randomly assigned to vehicle or estradiol treatment groups. The post-OVX recovery period was ~3 months to ensure that all animals had a consistent estrogen-free baseline prior to experimentation, as verified by urinary estradiol assays. After this 3-month recovery, treatment was initiated, with an intramuscular injection given every 3 weeks. Treatments were estradiol cypionate (E2C; 100 μg/ml in sterile peanut oil, Pharmacia, Peapack, NJ) in 13 young (YE) and 7 aged (AE) monkeys, or vehicle (peanut oil) in 8 young (YV) and 8 aged (AV) monkeys. Some additional animals (not included in Table S1) were excluded after the study started due to illnesses, sudden death or incomplete OVX. The efficacy of E2C injections was previously demonstrated by a rapid rise in circulating E2 that peaked within 24 h, at levels comparable with the preovulatory surge in intact female monkeys and similar to women (Oriowo et al., ‘80; Rahimy et al., ‘99; Shideler et al., 2003; Hao et al., 2007). Treatment duration was 2–3 years with all injections coded and administered in a blind fashion.
Perfusion and Tissue Processing
Twenty-four hours after the last E2C or vehicle injection, animals were deeply anesthetized and perfused as per (Hao et al., 2003). Fixative was 1% paraformaldehyde (PFA) for 1 min, then 4% PFA for 12 min in phosphate buffered saline (PBS) with post-fixation for 6 hr in 4% PFA. At the time of euthanasia, at 24 hr post-E2 monkeys were expected to be in the negative feedback phase of steroid feedback onto the HPG axis (Oriowo et al., ‘80; Terasawa, ‘95; Rahimy et al., ‘99). Group assignment was validated at perfusion, resulting in the exclusion of two vehicle animals that had residual ovarian tissues. After perfusion, the hypothalamus was dissected, and shipped in PBS to the University of Texas at Austin. Other tissues were allocated to other investigators and collaborators. Hypothalamic tissues used for the current study were coronally sectioned with a vibrating microtome (VT 1000s: Leica Instruments, Nussloch, Germany) at 40 μm, cryoprotected, and stored at −20°C for subsequent immunohistochemistry and light microscopy studies.
Immunohistochemistry
Immunohistochemistry was performed for each of three receptor proteins, using a 1:10 series sections through the ARC and PERI regions of each monkey, when available. Some of the hypothalamic blocks were damaged during dissection or sectioning, therefore, not all of the animals were represented for all three of the receptor analyses. On average, five and seven sections were used per monkey for the ARC and PERI, respectively. Final n’s represent monkey numbers (not number of tissue sections) and are shown in group data graphs; they represent the maximum number of monkeys for each antibody for which a complete series could be collected. Attrition was due primarily to poor tissue quality, and was randomly distributed across groups. The same protocol was used to quantify each of the hormone receptors, with the exception of primary antibody concentration. All steps were carried out at room temperature with constant agitation, unless otherwise indicated. PBS washes were performed prior to all steps, with the exception of the primary antibody. The tissue was treated to quench endogenous peroxidase activity for 20 min (30% of 3:1 methanol: 3% H2O2 in PBS) and to block non-specific binding for 1 hr (10% normal goat serum (NGS), S-1000, Vector Laboratories, Burlingame, CA, USA; 2% bovine serum albumin, A9085, Sigma-Aldrich, St. Louis, MO, USA; 0.5% triton X, in PBS) prior to incubating in one of the polyclonal primary antibodies for 48 hr (anti-GPER [1:4,000] a gift from Dr Edward Filardo, anti-ERα [1:10,000] Santa Cruz Biotechnology (Santa Cruz, CA, USA), cat# HC-20 sc-543, and anti-PR [1:20 from ready-to-use pre-diluted stock] MyBioSource (San Diego, CA, USA), cat# MBS300415). All antibodies were raised in rabbit and directed against the C-terminus of the corresponding human hormone receptor. Tissues were then incubated in biotinylated goat anti-rabbit secondary antibody for 1 hr (1:600; BA-1000; Vector Laboratories, in 5% NGS) and avidin-biotin-peroxidase complex for 1 hr (PK 6200; Vector Laboratories). Target was visualized with a 3,3′-diaminobenzidine (DAB)/peroxidase reaction (SK-4100; Vector Laboratories) for GPER and ERα, and nickel-DAB for PR, on ice. Sections were mounted on glass slides, dried overnight, and counterstained with methyl green (0.5% in 0.1 M Na acetate buffer pH 4.2, MP Biomedicals LLC, Solon, OH, USA), a nucleic acid stain (Kurnick, ‘50), to visualize the nuclei of all cells before affixing the cover glass with DPX (44581; Fluka, Steinheim, Germany). The GPER antibody, described by Filardo et al., has previously been validated via peptide block in rat and western blot in both rat and monkey (Filardo et al., 2000, 2007; Noel et al., 2009; Hammond et al., 2011; Kenealy et al., 2011a). Prior validation of the ERα antibody was carried out via Western blot in human (Long and Nephew, 2006; Gorosito et al., 2008) and peptide block in monkey (Wang et al., 2010). Although we could not perform preadsorption controls or Western blots of the PR antibody (the vendor does not disclose the antigen sequence, and the antibody is not compatible with western blots), this antibody was previously utilized in the California mouse, Peromyscus californicus (Fuxjager et al., 2010), and that study showed nuclear labeling very similar to that found in our macaque hypothalamic tissues. Further, the PR antibody cellular localization (nuclear) and distribution in the nervous system herein matches that of other PR antibodies as published in monkey and rats (Bethea and Fahrenbach, ‘92; Bethea et al., ‘96; Quadros and Wagner, 2008; Quadros et al., 2008; Furuta et al., 2010). All IHC runs contained negative-control sections that excluded the primary antibody and had no immunoreactivity.
Stereological Analysis
StereoInvestigator software (MicroBrightField Bioscience, Williston, VT, USA) and an Olympus BX-61 microscope were used for quantification. The optical fractionator method was used (Gundersen and Jensen, ‘87; West et al., ‘91; Glaser and Wilson, ‘98), with parameters appropriate to achieve coefficients of error (CEs) no greater than 0.12 (Gunderson m = 1). Abbreviations and terminology used throughout were taken from a combination of three atlases and modified to reflect most current usage (Bleier, ‘84; Paxinos et al., ‘90, Brainmaps.org). Counter-stained cells were used to identify the borders of the ARC and PERI according to neuroanatomical landmarks found in two atlases of the rhesus macaque brain (Bleier, ‘84; Paxinos et al., ‘90). The boundaries of the ARC were the median eminence (ventral), 3rd ventricle (medial), ventromedial hypothalamus and medial preoptic area (lateral and dorsal), and the anterior hypothalamic area and paraventricular nucleus PVN; (dorsal). The PERI was caudal to the ventral diagonal band; medial to the medial preoptic nucleus and anterior hypothalamic area; lateral to the 3rd ventricle; and ventral to the PVN. Note that our PERI area is a composite from the atlases and is slightly more extensive based on methyl green density. Immunoreactive (IR) cells were counted at the following magnification: GPER 40×, ERα 100×, PR 100×, and methyl green labeled cells at 40×. Cell density (population estimate of IR cells/volume sampled μm3) is reported, rather than total population, due to an uneven number of sections per region between animals due to tissue damage/loss. The percentage of IR cells was further calculated (population estimate of IR cells/population estimate of all cells), in the respective region, for each individual. Additionally, we used the Nucleator method in conjunction with optical fractionator to measure the cross-sectional area of GPER cell bodies (six rays, centered in the middle of nucleus at the widest part of each cell) (Gundersen et al., ‘88; Korbo and Andersen, ‘95). The percent of cells that fell within size bins was found for each animal and the average of the group is presented. Animals were excluded only if there were fewer than three sections containing the corresponding region. All slides were labeled with a random code and were quantified blind to group.
Statistical Analysis
All analysis was done using R statistical packages (Fox and Weisberg, 2011; R Development Core Team, 2012). Due to small sample sizes, high intra-group variability, and non-normal data distribution (analysis as determined by the Shapiro–Wilks normality and Levene’s equality of variance tests), we used non-parametric tests for analysis. Kruskal–Wallis (P) was used to determine significant main effects [P ≤ 0.05], followed by a post-hoc test using pairwise Wilcoxon (W) with a Benjamini and Hochberg false discovery rate correction for multiple comparisons (Wilcoxon, ‘45; Kruskal and Wallis, ‘52; Shapiro and Wilk, ‘65; Hochberg and Benjamini, ‘90; Benjamini and Hochberg, ‘95). Correlations between all variables were calculated using the Pearson coefficient, also followed by a Benjamini and Hochberg correction. Outliers were determined with the Grubbs-test, using a cut off of 2.5 × the standard deviation from mean of all animals, for each receptor and within each region, regardless of group. Only significant [P ≤ 0.05] and non-significant trends [0.05 < P ≤ 0.10] are reported here. We opted to present trends because of the low power and the variability of monkeys. Table S2 lists the P-value results of all statistical tests.
RESULTS
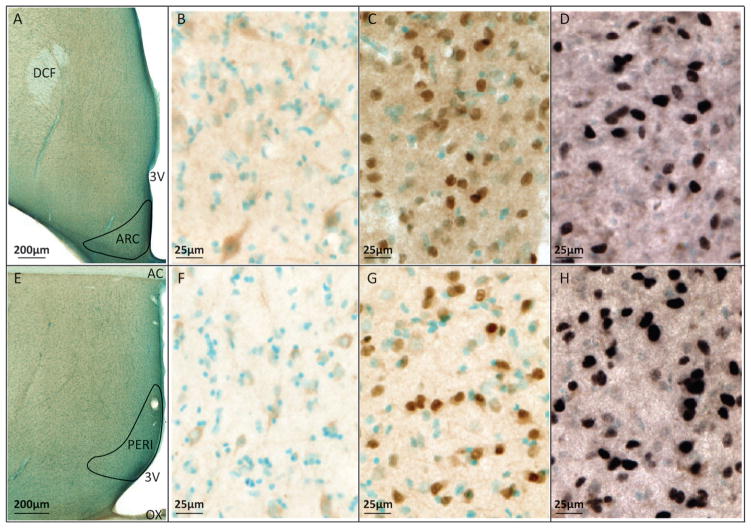
Both ERα and PR were densely expressed in both the ARC and PERI of young and aged female rhesus macaques, while GPER was less dense. Examples of the boundaries of the ARC and PERI, and immunolabeling of each receptor in the two regions of a representative monkey, are provided in Figure 1.
Figure 1.
Micrographs showing expression of GPER, ERα and PR in the ARC (top) and PERI (bottom). (A,E) Low magnification of the ARC and PERI regions used for stereologic analysis. Scale bars = 200 μm. Boundaries were determined using distribution of counterstained cells and the anatomical landmarks: dorsal column of the fornix (DCF), third ventricle (3V), anterior commissure (AC), and optic chiasm (OX). The remaining representative micrographs were obtained from consecutive sections of monkey #18261 (AV group). (B,F) GPER; (C,G) ERα; (D,H) PR, are shown in the ARC and PERI, respectively. Counterstained nuclei are blue-green. Scale bars = 25 μm (B–D, F–H). Images were modified in Photoshop with slight contrast adjustments to allow for clearer visualization. However, because stereologic counting was done on the microscope using the original slides, photographic adjustments had no influence on the actual quantification of data.
GPER
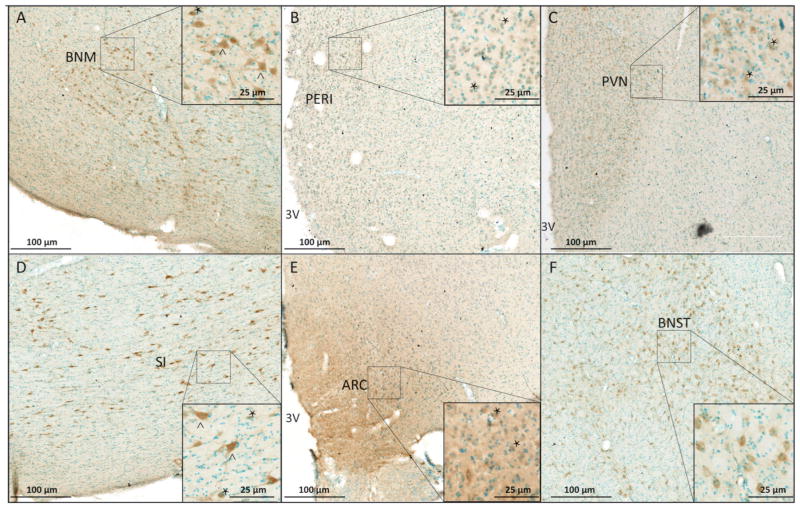
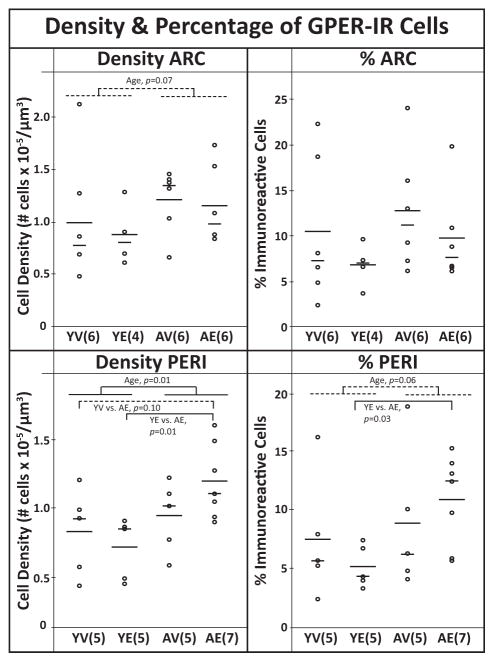
GPER immunoreactivity was observed on cell processes and in perikarya (Fig. 2) and was widely dispersed throughout the hypothalamus and surrounding regions. It is noteworthy that cellular labeling was relatively light in the ARC and PERI (Figs. 1 and 2), as well as the PVN (another hypothalamic region) compared to other non-hypothalamic regions with darker labeling, shown for basal nucleus of Meyert (BNM), bed nucleus of the stria terminalis (BNST), and the substantia innominata (SI; Fig. 2). The qualitative distribution and density of cells is shown in Table 1 and high-resolution images are available in Figure S1. Stereological counting of GPER-IR cells in the PERI demonstrated significant main effect of age, with aged animals having a greater density (P = 0.01) and a trend for a higher percentage of GPER (P = 0.06) regardless of treatment (Fig. 3). Post-hoc analysis revealed that AE monkeys had significantly higher density and % GPER-IR cells than YE monkeys (W = 0.01, W = 0.03, respectively). There was also a trend of AE animals having a greater density of GPER-IR than YV (W = 0.10). In the ARC, there was a trend for an age effect, with older animals having greater GPER-IR density than young (P = 0.07).
Figure 2.
Representative micrographs of distribution and cellular morphologies of GPER-IR cells in the hypothalamus and surrounding areas. The distribution was similar across all monkeys, and GPER immunostained cells are shown from two representative monkeys (#28603 and #29136). Micrographs are shown for: (A) basal nucleus of Meynert (BNM); (B) periventricular region (PERI); (C) paraventricular nucleus (PVN); (D) substantia innominata (SI); (E) arcuate nucleus (ARC) and (F) bed nucleus of the stria terminalis (BNST). Some representative large cells (>150 μm2) are indicated by ^ and small cells (<150 μm2) by ^. Counterstained cells have blue nuclei. Scale bars = 100 μm for low magnification images and 25 μm for the insets.
Table 1.
Distribution and characterization of GPER immunoreactivity throughout the hypothalamus and surrounding regions.
| Abbreviation | Nucleus | Density of GPER | Type of label (axons, lg/sm cell) |
|---|---|---|---|
| AcN | Nucleus of anterior commissure | +/++ | Small to large cell bodies, some processes |
| AHA | Anterior hypothalamic area | −−/+ | Processes with some small cell bodies |
| ARC | Arcuate nucleus | +++ | Processes with small to large cell bodies |
| BNM | Basal nucleus of Meynert | ++ | Large cell bodies and processes |
| BNST | Bed nucleus of stria terminalis | −/+ | Small to large cell bodies and some processes |
| Ca | Caudate nucleus | −/++ | Small and medium cell bodies |
| CP | Cerebral peduncle | −− | Few large cell bodies |
| DA | Dorsal hypothalamic area | + | Medium to very large cell bodies |
| DBh | Nucleus of diagonal band of Broca, horizontal portion | ++ | Small to large cell bodies and processes |
| DBv | Nucleus of diagonal band of Broca, vertical portion | − | Small cell bodies |
| DP | dorsal premammillary nucleus | −/+ | Very small to large cell bodies |
| FF | Fields of Forel | −− | Some medium cell bodies |
| Gp | Globus pallidus | −/+ | Large cell bodies and processes |
| HAA | Anterior hypothalamic area | +/++ | Very small, small, and medium cell bodies |
| HDM | Dorsomedial nucleus of hypothalamus | −/+ | Some small and medium cell bodies |
| HLA | Lateral hypothalamic area | − | Small, medium, and large cell bodies |
| HLmc | Magnocellular nucleus of lateral hypothalamus | +/++ | Medium to very large cell bodies |
| IC | Internal capsule | −− | Large cell bodies |
| LSv | Lateral septal nucleus, ventral portion | ++ | Very small cell bodies |
| ME | Median eminence | ++ | Axons, some large cell bodies |
| MPN | Medial preoptic nucleus | ++ | Small and medium cell bodies |
| MPO | Median preoptic nucleus | − | Small and medium cell bodies |
| MS | Medial septal nucleus | + | Small and medium cell bodies |
| OLT | Olfactory tubercle | +++ | Small cell bodies and processes |
| OX | Optic chiasm | − | Processes with few large cell bodies |
| PA | Posterior hypothalamic area | −/+ | Small to large cell bodies and processes |
| PERI | Periventricular region | +/++ | Very small to medium cell bodies and processes |
| PVN | Paraventricular nucleus | ++ | Very small to large cell bodies and processes |
| Pf | Perifornical nucleus | +/++ | Small to very large cell bodies with some processes |
| PM | Premammillary nucleus | +/++ | Small cell bodies and processes |
| POA | Preoptic area | −/+ | Small and medium cell bodies |
| SCN | Suprachiasmatic nucleus | +/++ | Very small and small cell bodies and processes |
| SI | Substantia innominata | +/++ | Medium to very large cell bodies and processes |
| SM | Stria medullaris of thalamus | +/++ | Very small and small cell bodies and processes |
| SON | Supraoptic nucleus | +++ | Medium to large cell bodies and processes |
| STr | Stria terminalis | + | Small cell bodies and processes |
| Sv | Subventricular nucleus | ++ | Medium cell bodies with processes |
| TCA | Area of tuber cinereum | −/+ | Processes with some small and medium cell bodies |
| Th | Thalamus | +/++ | Medium to large cell bodies and processes |
| TM | Tubero mammillary area | − | Processes with small cell bodies |
| TU | Lateral tuberal nucleus | + | Very small to large cell bodies and processes |
| VMH | Ventromedial nucleus of hypothalamus | + | Very small to medium cell bodies |
| VPa | Ventral pallidum | −/+ | Medium and large cell bodies, some processes |
| ZI | Zona incerta | −/+ | Medium cell bodies |
| 3V | Third ventricle | θ | None |
| AC | Anterior commissure | θ | None |
| DCF | Descending columns of the fornix | θ | None |
| LV; OT | Lateral ventricle; optic tract | θ | None; None |
θ, none; −−, very sparse to none; −, sparse; +, moderate; ++, dense; +++, very dense.
GPER distribution and cellular morphologies in the hypothalamus and surrounding areas. Table provides a list and qualitative description of GPER immunolabeling in specific regions, and a list of abbreviations.
Abbreviations and terminology were taken from a combination of 3 atlases and modified to reflect most current usage (Bleier, ‘84; Paxinos et al., ‘90, Brainmaps.org).
Figure 3.
The density and percentage of immunoreactive GPER cells in the ARC and PERI. Each graph includes the individual data points (circles), mean (long horizontal line), and median (short horizontal line). In the PERI, aged animals had significantly higher GPER density than young. Post hoc analyses showed AE monkeys > YE and a trend of AE > YV. There was also a trend (P < 0.1) of aged animals having a greater % immunolabeled cells than young (shown by the dotted lines), driven by AE > YE. In the ARC there was a trend for more GPER cells in aged compared to young animals, regardless of treatment. Sample sizes are shown in parentheses.
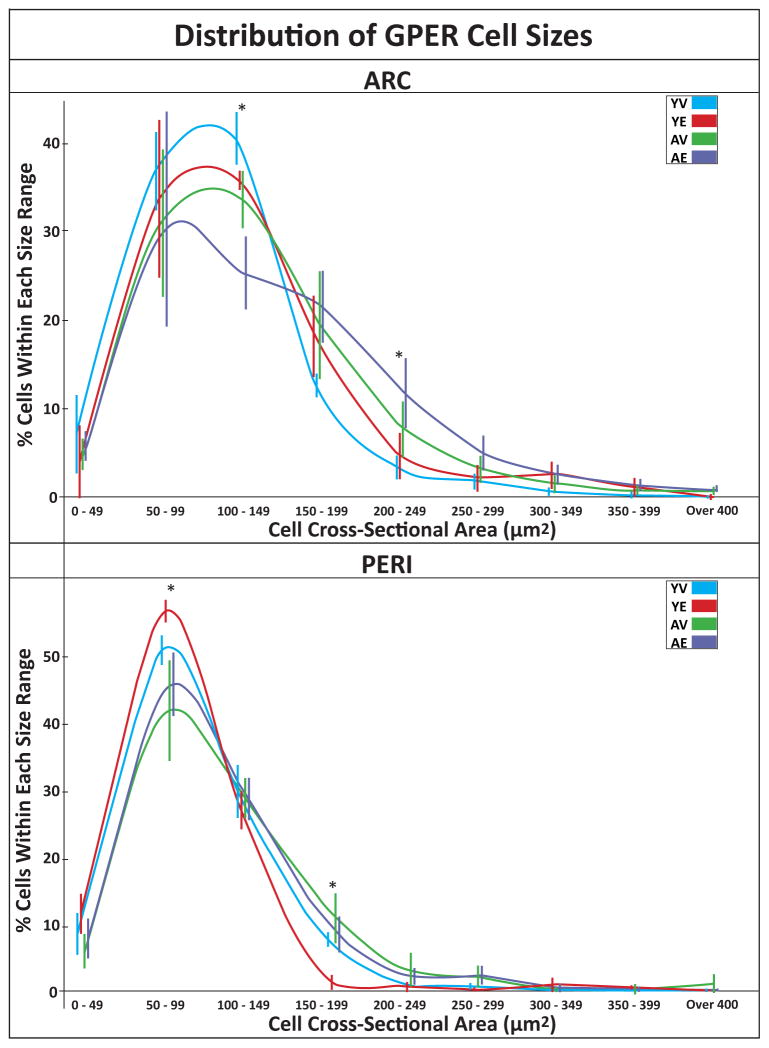
During the analysis of GPER we noticed that many of the young animals had very small GPER-IR cells (defined as <150 μm2), while aged monkeys had fewer such cells. We quantified cell cross-sectional area using the Nucleator tool in StereoInvestigator. Figure 4 shows the distribution of GPER size, divided into 50 μm2 bins, for both regions. In the ARC, aged animals tended to have fewer cells between 100 and 149 μm2, with YE > AE and YE > AV (P = 0.07, W = 0.08, W = 0.09, respectively) and aged monkeys tended to have more cells between 200 and 249 μm2 than the young, regardless of treatment (P = 0.09). In the PERI, young animals tended to have more cells between 50 and 99 μm2 than aged (P = 0.07), post-hoc analysis showed that this was driven by YE having a greater percentage than AE or AV (P = 0.07, P = 0.07, respectively). Additionally, within cells between 150–199 μm2, there was a trend for an effect of treatment and group (P = 0.09 and P = 0.06, respectively). This was driven by YE having fewer cells of this size than YV, AV, or AE (W = 0.02, W = 0.03, W = 0.07, respectively). Finally, in the PERI, it was noteworthy that the YV group had no cells between 350 and 400 μm2, and only 1 cell over 400 μm2.
Figure 4.
Size of GPER cell bodies in ARC and PERI. The size of GPER cells was determined using the StereoInvestigator Nucleator feature. Data shown are for the percentage of cells that fell within 50 μm2 size bins (mean ±SEM). The lines and corresponding error bars are color coded with YV in blue, YE in red, AV in green and AE in purple. The error bars are offset to allow better visualization. In the ARC, there was a trend of young monkeys having more small cells between 100 and 149 μm2 than the aged animals (P =0.07), with YV <AE (P =0.09), and YE <AE (P =0.08). There was also a trend of aged monkeys having more large cells between 200 and 250 μm2. In the PERI, there was a trend of young monkeys having more small cells between 50 and 99 μm2 than the aged animals (P =0.07), with YV <AE (P =0.07), and YE <AE (P =0.07). There was also a trend of vehicle monkeys having more large cells between 200 and 250 μm2 than treated animals, due to YE <YV (P =0.02), YE <AV (P =0.03), and YE <AE (P =0.07). YV monkeys had no cells between 350 and 400 μm2, and only 1 cell over 400 μm2 in the PERI. *P ≤0.10.
ERα
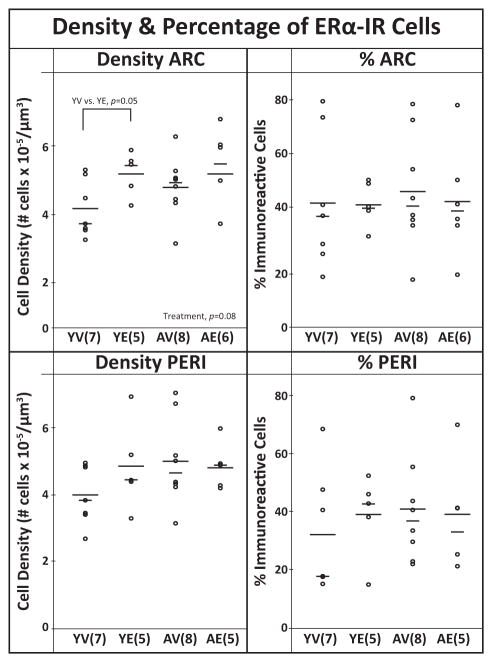
ERα immunoreactivity was predominantly detected in cell nuclei, but some cytoplasmic and/or membrane labeling was observed in the ARC and PERI (Fig. 1). In the ARC, there was a trend for a treatment effect (P = 0.08) driven by YE having greater ERα-IR cell density than YV (W = 0.05; Fig. 5). No significant effects of aging, treatment, or interactions, were found for the density or percent of ERα-IR cells in the PERI (Fig. 5).
Figure 5.
The density and percentage of immunoreactive ERα cells in the ARC and PERI. Each graph includes the individual data points (circles), mean (long horizontal line), and median (short horizontal line). In the ARC, there was a trend of higher density in E2 treated animals compared to vehicle (P =0.08), driven by YE >YV (P =0.05). The percent of ERα-IR cells were not affected by age or treatment. Sample sizes are shown in parentheses.
PR
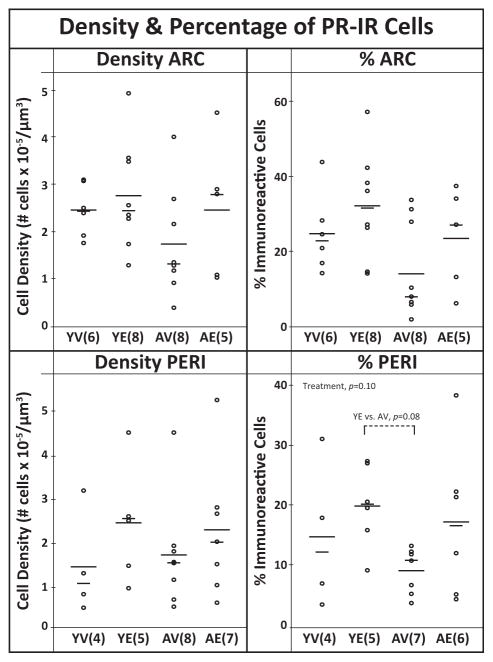
PR was almost exclusively nuclear in cellular localization (Fig. 1). There was a trend for a treatment effect in the PERI with YE having a higher percentage of PR-IR cells than AV (P = 0.10, W = 0.08), but no effects on the density (Fig. 6). In the ARC, no effects of age, treatment or interaction were found in the density or percent of PR-IR.
Figure 6.
The density and percentage of immunoreactive PR cells in the ARC and PERI. Each graph includes the individual data points (circles), mean (long horizontal line), and median (short horizontal line). The density of PR-IR cells was not affected by age or treatment. In the PERI there was a trend for a treatment effect on % cells (P =0.10), with E2 > vehicle. Sample sizes are shown in parentheses.
Total Cell Density in ARC and PERI
There were no significant effects of aging, treatment, or interaction in the total number of cells in either the ARC or PERI (see Table S2 for statistical results).
Correlation Network
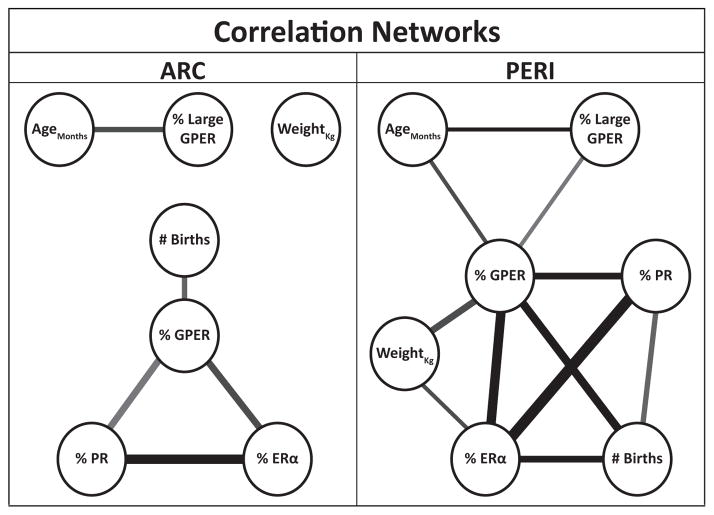
Figure 7 depicts the relationship between the percent of GPER, ERα and PR-IR cells, percent large GPER (>150 μm2), body weight, age and number of live births (see Supplemental Table S1). Supplemental Table S2 contains all of the Pearson’s correlation coefficients (r) and corresponding significance levels (P). We excluded cell density and total cells # because they were highly correlated with percent immunoreactive cells and inclusion of all three measures occluded the relationships with the demographic data. Only correlations with P ≤ 0.10 were included. The lines indicate the significance level (shade) and correlation coefficient (thickness).
Figure 7.
Correlation network of animal characteristics and % immunoreactive cells. Thickness and shade of lines indicate the strength of correlation and significance level, respectively. All resulting r and P values for networks are available in Supplemental Table S2. Cell density, % and total cells # were highly correlated therefore we included only % IR cells for simplicity sake. In both regions, GPER cells formed a hub with the nuclear receptors, # births, and (in the PERI) other characteristics of the animals.
DISCUSSION
This study investigated the effects of aging, E2 treatment, and their interactions on the expression of hormone receptors in two hypothalamic regions of female rhesus macaques. Our results extend previous work in several ways. First, the evolutionary conservation of the neurobiology of reproduction, especially similarities in menstrual cycles and hypothalamic circuitry between monkeys and humans, makes macaques an important translational model. Second, the experimental model of long-term, cyclic E2 given over at least a 2-year period, more closely mimics the therapeutic estrogen treatments used in many perimenopausal women. Third, we focused on ARC and PERI, because of their role in hormone feedback and other neuroendocrine functions (Bethea et al., ‘96; Skinner et al., ‘98; Blurton-Jones et al., ‘99; Mills et al., 2002; Petersen et al., 2003; Rapp et al., 2003; Tsai et al., 2004; Michael et al., 2005). To our knowledge, studies assessing the expression of steroid hormone receptors in a model of aging and long-term E2 treatment have not been performed in these regions in monkeys. Finally, research on the neurobiological expression of GPER is a relatively new area, with limited data on its expression in the adult and aging hypothalamus. Overall, our results were surprising in that E2 and aging had limited effects on the numbers of ERα or PR-IR cells in the ARC or PERI of female macaques. By contrast, the GPER system in the PERI, and to a lesser extent the ARC, had higher expression in aging monkeys, as well as an interaction of age and E2. Additionally, we identified differences in populations of GPER neurons, with aged animals having a larger proportion of large GPER-IR cells, and fewer smaller cells, than young animals. Finally, we are the first to describe the distribution of GPER throughout the rhesus macaque hypothalamus and adjacent areas. An interpretation of these data is provided below.
GPER
GPER is a membrane ER that mediates rapid actions of E2 (Filardo et al., 2002; Noel et al., 2009; Kenealy et al., 2011a,b). GPER is becoming increasingly well-established as involved in metabolic, cardiovascular, and immune physiology (Wang et al., 2008; Prossnitz and Barton, 2011; Sharma and Prossnitz, 2011; Filardo and Thomas, 2012). While expressed in the hypothalamus of monkeys, rats, mice and hamsters (Brailoiu et al., 2007; Canonaco et al., 2008; Hazell et al., 2009), to our knowledge a functional role for GPER in HPG regulation has not yet been identified. In fact, the GPER knockout mouse, to our knowledge, has no reproductive phenotype (Brailoiu et al., 2007; Prossnitz and Barton, 2011). Nevertheless, in monkeys, GPER is expressed in cultured fetal and adult GnRH neurons and mediates rapid GnRH release (Noel et al., 2009; Kenealy et al., 2011a,b). This is consistent with the possibility that GPER plays a role in the E2-mediated regulation of the HPG axis. While expressed in hypothalamus (Brailoiu et al., 2007; Canonaco et al., 2008; Hazell et al., 2009; Noel et al., 2009), to our knowledge, quantitative measures of GPER hypothalamic expression and its regulation by estradiol and aging have not been reported in any species prior to our study. Of the three receptors studied herein, GPER was the only one to have robust changes in its expression and morphology with aging and E2 treatment in both nuclei examined, though we cannot speculate as to the physiological role these changes play. Nevertheless, two observations were potentially most important. First, GPER-IR cell density in the PERI was highest in the AE monkeys. Second, we discovered that young monkeys have fewer large and more small GPER cells than aged monkeys, raising the possibility that GPER cells undergo hypertrophy with age in the two brain regions examined. It is unknown whether the larger GPER cells represent a novel population of neurons in aging monkeys, or if a subpopulation of GPER neurons undergoes hypertrophy with aging. We favor the latter explanation in light of reports that other neuronal subpopulations in the medial and caudal ARC of humans undergo age-related hypertrophy (Rance et al., ‘90; Abel and Rance, ‘99; Rometo and Rance, 2008; Rance, 2009). In our current study, the large GPER cells were predominantly (87%) found in the same area of the ARC. Some of these same neurons that undergo hypertrophy are integral to the regulation of the HPG axis, including those that express kisspeptin, prodynorphin, and neurokinin B. Additionally, to our knowledge, there are no reports of age-related hypertrophy in the PERI region in any animal. Future work should investigate whether these cells also express GPER.
ERα
We chose to examine ERα due to its critical role in reproduction, as demonstrated by the infertility of ERα-knockout mice (Lubahn et al., ‘93). The only significant finding was that YE had greater ERα density than the YV group in the ARC, an effect not found in the aging monkeys. Previous work in rodents has been mixed, with evidence that E2 treatment can increase, a decrease or have no effect on ERα mRNA or protein, with differences potentially attributable to species, hypothalamic region, and the nature of E2 treatment (Lauber et al., ‘91; Shughrue et al., ‘92; Blaustein, ‘93; Olster, ‘98; Chakraborty et al., 2003). An age-related increase of ERα-IR cell numbers in rats was found in the anteroventral periventricular (AVPV), but not the MPN or ARC, with no effect of short-term E2 treatment observed in any age group (Chakraborty et al., 2003). Interactions of E2 and age have also been reported in these and other studies (Funabashi et al., 2000; Chakraborty et al., 2003). For example, Funabashi et al. (2000) found that in young rats, 4 days of E2 had no effect on ERα mRNA in the preoptic area (POA) and reduced expression in the ARC and ventromedial hypothalamus (VMH). By contrast, middle-aged animals had decreased ERα mRNA with E2 in the POA only, and old animals showed no effect of E2 treatment (Funabashi et al., 2000). Aged rats had a decline in ERα mRNA in the periventricular preoptic nuclei, but not in the medial preoptic nucleus or VMN. Differences in results are likely due to species as well as duration of E2 treatments.
There are limited data on changes to ERα with aging or E2 in nonhuman primates. In young adult monkeys, Bethea et al. showed no effect of E2 on ERα mRNA or protein in the ARC, PVN, VMN or pituitary (Bethea et al., ‘96). In companion studies performed on prefrontal cortex and hippocampus of the same monkeys used in our current study, no changes in ERα-IR cells were found (Adams et al., 2002; Wang et al., 2010). It is therefore not entirely surprising that we found few differences in ERα-IR cell density, and no change in the percentage of these cells in the PERI and ARC, with age or E2 treatment.
We were unable to perform immunohistochemistry on ERβ in this study due to the absence of a reliable antibody to detect this protein in perfused brains (Snyder et al., 2010). However, there are data showing that ERβ is co-expressed in GnRH neurons, and mediates rapid GnRH responses to estrogen treatment in rats (Hrabovszky et al., 2001; Abrahám et al., 2003). In addition, ERβ mRNA is expressed in several hypothalamic regions, including paraventricular nucleus, preoptic area, and ventromedial nucleus, in the rhesus macaque brain (Pau et al., ‘98; Gundlah et al., 2000). Therefore, this is an important area for future studies when a validated ERβ antibody becomes available.
PR
The hypothalamic expression of PR, a member of the nuclear hormone receptor family, is integral to normal reproduction. Female PR knockout mice have substantial impairments, including deficits in sexual behavior that indicate that PR in the brain is necessary for normal reproductive functionality (Lydon et al., ‘96). Although E2-mediated up-regulation of PR is well documented, it is age, sex, brain region and species dependent (Blaustein and Turcotte, ‘89; Bethea and Fahrenbach, ‘92; Olster, ‘98; Mills et al., 2002; Quadros and Wagner, 2008; Rometo and Rance, 2008). Here, we saw no significant effects of age or long-term cyclic E2, though there was a trend for higher % PR cells in E2 compared to vehicle monkeys of both ages. The small magnitude of these results may be explained by the low power of this study. In fact, there is considerable variation in expression of PR (and other receptors) across individuals. Considering the variability in life history in monkeys up to 25 years of age (see Supplemental Table S1), this is to be expected, and is translationally relevant to humans.
The literature on PR expression in the hypothalamus of rats is predominantly based on short-term E2 treatment regimens. Similar to what we observed, other groups showed that young monkeys responded to short-term (28 days) constant E2 treatment with increases in PR-IR cell numbers and mRNA in some (VMN and ARC), but not all (PVN) hypothalamic regions (Bethea and Fahrenbach, ‘92; Bethea et al., ‘96). Another study showed that in the hypothalamus of rats, there is a disconnect between mRNA and protein induction by short-term (4 day) E2 treatment: Furuta et al. (2010) demonstrated that the increase in PR-IR is attenuated with age, while Funabashi et al. (2000) showed that aged rats maintained a robust PR mRNA response to E2. Similar to our results in the ARC, young female rats showed no increase in PR-IR in their VMN after an acute injection of E2, while the older females responded with increased PR-immunoreactivity (Quadros et al., 2008). By contrast to our results, there was also a consistent PR induction in the medial preoptic nuclei of rats at both ages (Quadros et al., 2008). Taken together, there is a complex relationship among age, duration of E2 treatment and PR expression, in many species. Ongoing work in our laboratory utilizing different E2 regimens and comparing chronic to cyclic E2 may shed further light on this question. Regardless of future results, current data reported here suggest little change in ARC and PERI PR-IR cells with age and E2 treatment in our monkey model.
Correlation Networks
Analyses of relationships among the different variables revealed that GPER immunoreactivity (both % and density; data shown in Figure 7 are for % GPER) is a hub in both ARC and PERI. In support of our cell size data, in the PERI age was positively correlated with percent of cells >150 μm2. Other interesting points are that ERα and PR were highly correlated, both with each other as well as with GPER. In both regions, reproductive parity was highly correlated with expression of all three receptors in the PERI, but only with GPER in ARC. Though interesting, correlation results must be interpreted carefully as they do not necessarily indicate causation.
IMPLICATIONS AND CONCLUSIONS
Our study demonstrates that the aged primate hypothalamus retains the ability to express steroid hormone receptors at levels comparable to young adults. There were also few effects of long-term E2 on ERα and PR cell % or density. Additionally, there were no changes in the total number of cells in either region, which is consistent with findings in other populations of macaques (Roberts et al., 2012). Differences between our model and published data using shorter-term E2 treatment suggest that changes previously observed in aged animals in the expression of ERα caused by HRT may be relatively transient, and that the nonhuman primate brain eventually returns to the pre-treatment baseline. Novel data on GPER indicate that there are differences between YE and AE monkeys, with the AE group having the highest numbers of GPER-IR cells in the PERI. It is not known, however, how density of populations of hypothalamic cells may play out as functional changes in responsiveness to steroid hormones. Furthermore, our observation of age-related GPER-IR cell hypertrophy is consistent with other hypertrophic neuronal populations in humans and monkeys including kisspeptin and neurokinin B (Ule et al., ‘83; Rance et al., ‘90; Rometo and Rance, 2008; Rance, 2009). In nonhuman primates, these data suggest that, following long-term cyclic E2, ERα and PR in the PERI and ARC probably do not contribute significantly to an age-related decline in hormone sensitivity. Additionally, GPER may play a significant role in reproductive aging, either as a contributing or compensatory factor, something that requires future experimentation to resolve its physiological role.
Supplementary Material
Acknowledgments
We are grateful to Dr. Jeffrey Roberts and Heather McKay at the California National Primate Research Center for expert care of the animals. Dr. Weiling Yin and Megan Noel assisted with preparation and sectioning of hypothalamic tissues. Christina X. Tran, Sateria A. Lozano-Delaney, and Syed S. Zafar were very helpful with data collection and organization. Dr. Hans Hofmann and Dr. Theresa Jones provided important advice on the analysis of correlations and stereological results.
Grant sponsor: NIH; grant number: PO1 AG16765.
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s website.
LITERATURE CITED
- Abel TYW, Rance NE. Proopiomelanocortin gene expression is decreased in the infundibular nucleus of postmenopausal women. Brain Res Mol Brain Res. 1999;69:202–208. doi: 10.1016/s0169-328x(99)00111-4. [DOI] [PubMed] [Google Scholar]
- Abrahám IM, Han SK, Todman MG, Korach KS, Herbison AE. Estrogen receptor beta mediates rapid estrogen actions on gonadotropin-releasing hormone neurons in vivo. J Neurosci. 2003;23:5771–5777. doi: 10.1523/JNEUROSCI.23-13-05771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Fink SE, Shah RA, Janssen WGM, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DF. Role of the nonhuman primate for research related to women’s health. ILAR J. 2004;45:212–219. doi: 10.1093/ilar.45.2.212. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bethea CL, Fahrenbach WH. Immunocytochemical localization of progestin receptors in monkey hypothalamus: effect of estrogen and progestin. Endocrinology. 1992;130:895–905. doi: 10.1210/endo.130.2.1733733. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Brown NA, Kohama SG. Steroid regulation of estrogen and progestin receptor messenger ribonucleic acid in monkey hypothalamus and pituitary. Endocrinology. 1996;137:4372–4383. doi: 10.1210/endo.137.10.8828498. [DOI] [PubMed] [Google Scholar]
- Blaustein JD. Estrogen receptor immunoreactivity in rat brain: rapid effects of estradiol injection. Endocrinology. 1993;132:1218–1224. doi: 10.1210/endo.132.3.7679973. [DOI] [PubMed] [Google Scholar]
- Blaustein JD, Turcotte JC. Estradiol-induced progestin receptor immunoreactivity is found only in estrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology. 1989;49:454–461. doi: 10.1159/000125152. [DOI] [PubMed] [Google Scholar]
- Bleier R. Hypothalamus of the rhesus monkey: a cytoarchitec-tonic atlas. Madison, WI: University of Wisconsin Press; 1984. [Google Scholar]
- Blurton-Jones MM, Roberts JA, Tuszynski MH. Estrogen receptor immunoreactivity in the adult primate brain: neuronal distribution and association with p75, trkA, and choline acetyltransferase. J Comp Neurol. 1999;405:529–542. [PubMed] [Google Scholar]
- Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193:311–321. doi: 10.1677/JOE-07-0017. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley EC, Robertson DM, Dennerstein L. Hormonal changes in the menopause transition. Recent Prog Horm Res. 2002;57:257–275. doi: 10.1210/rp.57.1.257. [DOI] [PubMed] [Google Scholar]
- Canonaco M, Giusi G, Madeo A, Facciolo RM, Lappano R, Canonaco A, Maggiolini M. A sexually dimorphic distribution pattern of the novel estrogen receptor G-protein-coupled receptor 30 in some brain areas of the hamster. J Endocrinol. 2008;196:131–138. doi: 10.1677/JOE-07-0392. [DOI] [PubMed] [Google Scholar]
- Canonico M, Plu-Bureau G, Lowe GDO, Scarabin P-Y. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. Br Med J. 2008;336:1227–1231. doi: 10.1136/bmj.39555.441944.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanoa JM, Bentsend AH, Mikkelsend JD, Tena-Sempere M. Kisspeptins: bridging energy homeostasis and reproduction. Brain Res. 2010;1364:129–138. doi: 10.1016/j.brainres.2010.08.057. [DOI] [PubMed] [Google Scholar]
- Chakraborty TR, Gore AC. Aging-related changes in ovarian hormones, their receptors, and neuroendocrine function. Exp Biol Med. 2004;229:977–987. doi: 10.1177/153537020422901001. [DOI] [PubMed] [Google Scholar]
- Chakraborty T, Hof P, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ERα) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–421. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- Dorling AA, Todman MG, Korach KS, Herbison AE. Critical role for estrogen receptor alpha in negative feedback regulation of gonadotropin-releasing hormone mRNA expression in the female mouse. Neuroendocrinology. 2003;78:204–209. doi: 10.1159/000073703. [DOI] [PubMed] [Google Scholar]
- Downs JL, Wise PM. The role of the brain in female reproductive aging. Mol Cell Endocrinol. 2009;299:32–38. doi: 10.1016/j.mce.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153:2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KL, Frackelton AR. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16:70–84. doi: 10.1210/mend.16.1.0758. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn J, Pang Y, Graeber C, Shaw S, Dong J, Thomas P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology. 2007;148:3236–3245. doi: 10.1210/en.2006-1605. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick LA, Pace C, Wiita B. Comparison of regimens containing oral micronized progesterone or medroxyprogesterone acetate on quality of life in postmenopausal women: a cross-sectional survey. J Womens Health Gend Based Med. 2000;9:381–387. doi: 10.1089/15246090050020691. [DOI] [PubMed] [Google Scholar]
- Fox J, Weisberg S. An {R} companion to applied regression. Thousand Oaks, CA: Sage Publishers; 2011. [Google Scholar]
- Funabashi T, Kleopoulos SP, Brooks PJ, Kimura F, Pfaff DW, Shinohara K, Mobbs CV. Changes in estrogenic regulation of estrogen receptor a mRNA and progesterone receptor mRNA in the female rat hypothalamus during aging: an in situ hybridization study. Neurosci Res. 2000;38:85–92. doi: 10.1016/s0168-0102(00)00150-4. [DOI] [PubMed] [Google Scholar]
- Furuta M, Fukushima A, Chiba S, Sano A, Akema T, Kimura F, Funabashi T. Progesterone receptor immunoreactivity in the brains of ovariectomized aged rats. Neuroreport. 2010;21:777–781. doi: 10.1097/WNR.0b013e32833c5b6f. [DOI] [PubMed] [Google Scholar]
- Fuxjager MJ, Forbes-Lorman RM, Coss DJ, Auger CJ, Auger AP, Marler CA. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA. 2010;107:12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi KV, Shideler SE, Valverde CR, Roberts JA, Lasley BL. Characterization of the onset of menopause in the rhesus macaque. Biol Reprod. 1997;57:335–340. doi: 10.1095/biolreprod57.2.335. [DOI] [PubMed] [Google Scholar]
- Gill S, Lavoie HB, Bo-Abbas Y, Hall JE. Negative feedback effects of gonadal steroids are preserved with aging in postmenopausal women. J Clin Endocrinol Metab. 2002a;87:2297–2302. doi: 10.1210/jcem.87.5.8510. [DOI] [PubMed] [Google Scholar]
- Gill S, Sharpless JL, Rado K, Hall JE. Evidence that GnRH decreases with gonadal steroid feedback but increases with age in postmenopausal women. J Clin Endocrinol Metab. 2002b;87:2290–2296. doi: 10.1210/jcem.87.5.8508. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Wilson PD. The coefficient of error of optical fractionator population size estimates: a computer simulation comparing three estimators. J Microsc. 1998;192:163–171. doi: 10.1046/j.1365-2818.1998.00417.x. [DOI] [PubMed] [Google Scholar]
- Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL. Nonclassical estrogen receptor alpha signaling mediates negative feedback in the female mouse reproductive axis. Proc Natl Acad Sci USA. 2007;104:8173–8177. doi: 10.1073/pnas.0611514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Oung T, Yung S, Flagg RA, Woller MJ. Neuroendocrine mechanisms for reproductive senescence in the rat: gonadotropin-releasing hormone neurons. Endocrinology. 2000;13:315–323. doi: 10.1385/ENDO:13:3:315. [DOI] [PubMed] [Google Scholar]
- Gore AC, Windsor-Engnell BM, Terasawa E. Menopausal increases in pulsatile gonadotropin-releasing hormone release in a nonhuman primate (Macaca mulatta) Endocrinology. 2004;145:4653–4659. doi: 10.1210/en.2004-0379. [DOI] [PubMed] [Google Scholar]
- Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Gougeon A, Ecochard R, Thalabard JC. Age-related changes of the population of human ovarian follicles: increase in the disappearance rate of non-growing and early-growing follicles in aging women. Biol Reprod. 1994;50:653–663. doi: 10.1095/biolreprod50.3.653. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakken-Berg B, Sørensen AFB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sample intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344–351. doi: 10.1055/s-2007-984740. [DOI] [PubMed] [Google Scholar]
- Hammond R, Nelson D, Gibbs RB. GPR30 co-localizes with cholinergic neurons in the basal forebrain and enhances potassium-stimulated acetylcholine release in the hippocampus. Psychoneur-oendocrinology. 2011;36:182–192. doi: 10.1016/j.psyneuen.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Janssen WG, Tang Y, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen increases the number of spinophilin-immunoreactive spines in the hippocampus of young and aged female rhesus monkeys. J Comp Neurol. 2003;465:540–550. doi: 10.1002/cne.10837. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffier AE, Leffier SR, Janssen WGM, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WGM, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci USA. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell G, Yao S, Roper J, Prossnitz E, O’Carroll A, Lolait S. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrinton LJ, Weiss NS. Postmenopausal unopposed estrogens characteristics of use in relation to the risk of endometrial carcinoma. Ann Epidemiol. 1993;3:308–318. doi: 10.1016/1047-2797(93)90035-3. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Benjamini Y. More powerful procedures for multiple significance testing. Stat Med. 1990;9:811–818. doi: 10.1002/sim.4780090710. [DOI] [PubMed] [Google Scholar]
- Hofman M. Lifespan changes in the human hypothalamus. Exp Gerontol. 1997;32:559–575. doi: 10.1016/s0531-5565(96)00162-3. [DOI] [PubMed] [Google Scholar]
- Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immuno-reactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142:3261–3264. doi: 10.1210/endo.142.7.8176. [DOI] [PubMed] [Google Scholar]
- Inoue T. Progesterone production and actions in the human central nervous system and neurogenic tumors. J Clin Endocrinol Metab. 2002;87:5325–5331. doi: 10.1210/jc.2002-012096. [DOI] [PubMed] [Google Scholar]
- Kaplan JR. Modeling women’s health with nonhuman primates and other animals. ILAR J. 2004;45:83–88. doi: 10.1093/ilar.45.2.83. [DOI] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Ronnekleiv OK, Terasawa E. STX, a novel nonsteroidal estrogenic compound, induces rapid action in primate GnRH neuronal calcium dynamics and peptide release. Endocrinology. 2011a;152:3182–3191. doi: 10.1210/en.2011-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenealy BP, Keen KL, Terasawa E. Rapid action of estradiol in primate GnRH neurons: the role of estrogen receptor alpha and estrogen receptor beta. Steroids. 2011b;76:861–866. doi: 10.1016/j.steroids.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kermath BA, Gore AC. Neuroendocrine control of the natural transition to reproductive senescence. Neuroendocrinology. 2012;96:1–12. doi: 10.1159/000335994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korbo L, Andersen BB. The distributions of Purkinje cell perikaryon and nuclear volume in human and rat cerebellum with the nucleator method. Neuroscience. 1995;69:151–158. doi: 10.1016/0306-4522(95)00223-6. [DOI] [PubMed] [Google Scholar]
- Krey LC, Butler WR, Knobil E. Surgical disconnection of the medial basal hypothalamus and pituitary function in the rhesus monkey. I Gonadotropin secretion. ILAR J. 1975;96:1073–1087. doi: 10.1210/endo-96-5-1073. [DOI] [PubMed] [Google Scholar]
- Kruskal W, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:582–621. [Google Scholar]
- Kurnick NB. Methyl green-pyronin; basis of selective staining of nucleic acids. J Gen Physiol. 1950;33:243–264. doi: 10.1085/jgp.33.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129:3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- Liu JH, Yen SS. Induction of midcycle gonadotropin surge by ovarian steroids in women: a critical evaluation. J Clin Endocrinol Metab. 1983;57:797–802. doi: 10.1210/jcem-57-4-797. [DOI] [PubMed] [Google Scholar]
- Long X, Nephew KP. Fulvestrant (ICI 182,780)-dependent interacting proteins mediate immobilization and degradation of estrogen receptor-alpha. J Biol Chem. 2006;281:9607–9615. doi: 10.1074/jbc.M510809200. [DOI] [PubMed] [Google Scholar]
- Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Conneely OM, O’Malley BW. Reproductive phenotypes of the progesterone receptor null mutant mouse. J Steroid Biochem Mol Biol. 1996;56:67–77. doi: 10.1016/0960-0760(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Maffucci JA, Gore AC. Age-related changes in hormones and their receptors in animal models of female reproductive senescence. In: Conn PM, editor. Handbook of models for human aging. New York, NY: Academic Press/Elsevier; 2006. pp. 533–552. [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O’Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- Michael RP, Clancy AN, Zumpe D. Mating activates estrogen receptor-containing neurons in the female monkey brain. Physiol Behav. 2005;85:404–413. doi: 10.1016/j.physbeh.2005.04.026. [DOI] [PubMed] [Google Scholar]
- Mills RH, Romeo HE, Lu JKH, Micevych PE. Site-specific decrease of progesterone receptor mRNA expression in the hypothalamus of middle-aged persistently estrus rats. Brain Res. 2002;955:200–206. doi: 10.1016/s0006-8993(02)03440-6. [DOI] [PubMed] [Google Scholar]
- Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109:19846–19851. doi: 10.1073/pnas.1211517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E. Involvement of G protein-coupled receptor 30 (GPR30) in rapid action of estrogen in primate LHRH neurons. Mol Endocrinol. 2009;23:349–359. doi: 10.1210/me.2008-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olster DH. Lordosis-enhancing medial preoptic area lesions do not alter hypothalamic estrogen receptor- or progestin receptor-immunoreactivity in prepubertal female guinea pigs. Brain Res. 1998;790:254–263. doi: 10.1016/s0006-8993(98)00068-7. [DOI] [PubMed] [Google Scholar]
- Oriowo MA, Landgren BM, Stenstrom B, Diczfalusy E. A comparison of the pharmacokinetic properties of three estradiol esters. Contraception. 1980;21:415–424. doi: 10.1016/s0010-7824(80)80018-7. [DOI] [PubMed] [Google Scholar]
- Pau CY, Pau KY, Spies HG. Putative estrogen receptor beta and alpha mRNA expression in male and female rhesus macaques. Mol Cell Endocrinol. 1998;146:59–68. doi: 10.1016/s0303-7207(98)00197-x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Huang X-F, Toga A. The rhesus monkey brain in stereotaxic coordinates. San Diego, CA: Academic Press; 1990. [Google Scholar]
- Petersen SL, Ottem EN, Carpenter CD. Direct and indirect regulation of gonadotropin-releasing hormone neurons by estradiol. Biol Reprod. 2003;69:1771–1778. doi: 10.1095/biolreprod.103.019745. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Manson JE, Langer RD, Anderson GL, Pettinger M, Jackson RD, Johnson KC, Kuller LH, Lane DS, Wactawski-Wende J, Brzyski R, Allison M, Ockene J, Sarto G, Rossouw JE. Benefits and risks of postmenopausal hormone therapy when it is initiated soon after menopause. Am J Epidemiol. 2009;170:12–23. doi: 10.1093/aje/kwp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7:715–726. doi: 10.1038/nrendo.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology. 2008;149:3054–3061. doi: 10.1210/en.2007-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadros PS, Schlueter LJ, Wagner CK. Distribution of progesterone receptor immunoreactivity in the midbrain and hindbrain of postnatal rats. Dev Neurobiol. 2008;68:1378–1390. doi: 10.1002/dneu.20664. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: 2012. [Google Scholar]
- Rahimy MH, Ryan KK, Hopkins NK. Lunelle monthly contraceptive injection (medroxyprogesterone acetate and estradiol cypionate injectable suspension): steady-state pharmokinetics of MPA and E2 in surgically sterile women. Contraception. 1999;60:209–214. doi: 10.1016/s0010-7824(99)00086-4. [DOI] [PubMed] [Google Scholar]
- Rance NE. Menopause and the human hypothalamus: evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides. 2009;30:111–122. doi: 10.1016/j.peptides.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance NE, McMullen NT, Smiaek JE, Price DL, Young WS., III Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab. 1990;71:79–85. doi: 10.1210/jcem-71-1-79. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SJ, Senikas V, Nelson JF. Follicular depletion during the menopausal transition: evidence for accelerated loss and ultimate exhaustion. J Clin Endocrinol Metab. 1987;65:1231–1237. doi: 10.1210/jcem-65-6-1231. [DOI] [PubMed] [Google Scholar]
- Roberts DE, Killiany RJ, Rosene DL. Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. J Comp Neurol. 2012;520:1181–1197. doi: 10.1002/cne.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol. 2008;20:1376–1381. doi: 10.1111/j.1365-2826.2008.01796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Shapiro SS, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- Sharma G, Prossnitz ER. Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology. 2011;152:3030–3039. doi: 10.1210/en.2011-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw ND, Srouji SS, Histed N, Hall JE. Differential effects of aging on estrogen negative and positive feedback. Am J Physiol Endocrinol Metab. 2011;301:351–355. doi: 10.1152/ajpendo.00150.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shideler SE, Gee NA, Chen J, Laughlin LS, Rapp PR, Morrison JH, Roberst JA, Moran FM, Lasley BL. Contribution of ovarian steroid production to urinary estrone conjugate concentrations in Macaca mulatta. Am J Primatol. 2003;61:111–121. doi: 10.1002/ajp.10114. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- Skinner DC, Evans NP, Delaleu B, Goodman RL, Bouchard P, Caraty A. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc Natl Acad Sci USA. 1998;95:10978–10983. doi: 10.1073/pnas.95.18.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder MA, Smejkalova T, Forlano PM, Woolley CS. Multiple ER(beta) antisera label in ER(beta) knockout and null mouse tissues. J Neurosci Methods. 2010;188:226–234. doi: 10.1016/j.jneumeth.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Witkin JW, Ferin M, Silverman AJ. Gonadotropin-releasing hormone neurons in the rhesus macaque are not immunoreactive for the estrogen receptor. Brain Res. 1995;685:198–200. doi: 10.1016/0006-8993(95)00352-q. [DOI] [PubMed] [Google Scholar]
- Talboom JS, Williams BJ, Baxley ER, West SG, Bimonte-Nelson HA. Higher levels of estradiol replacement correlate with better spatial memory in surgically menopausal young and middle-aged rats. Neurobiol Learn Mem. 2008;90:155–163. doi: 10.1016/j.nlm.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasawa E. Control of luteinizing hormone-releasing hormone pulse generation in nonhuman primates. Cell Mol Neurobiol. 1995;15:141–164. doi: 10.1007/BF02069563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terauchi M, Honjo H, Mizunuma H, Aso T. Effects of oral estradiol and levonorgestrel on cardiovascular risk markers in postmenopausal women. Arch Gynecol Obstet. 2012;285:1647–1656. doi: 10.1007/s00404-012-2222-9. [DOI] [PubMed] [Google Scholar]
- Trévoux R, De Brux J, Castanier M, Nahoul K, Soule JP, Scholler R. Endometrium and plasma hormone profile in the peri-menopause and post-menopause. Maturitas. 1986;8:309–326. doi: 10.1016/0378-5122(86)90039-3. [DOI] [PubMed] [Google Scholar]
- Tsai H-W, LaPolt PS, Olcott AP, Lu JKH. Temporal changes occur in the neuroendocrine control of gonadotropin secretion in aging female rats: role of progesterone. Biol Reprod. 2004;71:845–852. doi: 10.1095/biolreprod.104.029090. [DOI] [PubMed] [Google Scholar]
- Ule G, Schwechheimer K, Tschahargane C. Morphological feedback effect on neurons of the nucl. arcuatus (sive infundibularis) and nucl. subventricularis hypothalami due to gonadal atrophy. Virchows Arch A Pathol Anat Histopathol. 1983;400:297–308. doi: 10.1007/BF00612191. [DOI] [PubMed] [Google Scholar]
- Van Look PF, Lothian H, Hunter WM, Michie EA, Baird DT. Hypothalamic–pituitary–ovarian function in perimenopausal women. Clin Endocrinol (Oxf) 1977;7:13–31. doi: 10.1111/j.1365-2265.1977.tb02936.x. [DOI] [PubMed] [Google Scholar]
- Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22:636–648. doi: 10.1210/me.2007-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AC, Hara Y, Janssen WG, Rapp PR, Morrison JH. Synaptic estrogen receptor-alpha levels in prefrontal cortex in female rhesus monkeys and their correlation with cognitive performance. J Neurosci. 2010;30:12770–12776. doi: 10.1523/JNEUROSCI.3192-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G, Skurnick JH, Goldsmith LT, Santoro NF, Park SJ. Menopause and hypothalamic-pituitary sensitivity to estrogen. JAMA. 2004;292:2991–2996. doi: 10.1001/jama.292.24.2991. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E. Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology. 1982;34:395–404. doi: 10.1159/000123335. [DOI] [PubMed] [Google Scholar]
- Wilcoxon F. Individual comparisons by ranking methods. Biometrics. 1945;1:80–83. [Google Scholar]
- Wilson ME, Rosewell KL, Kashon ML, Shughrue PJ, Merchenthaler I, Wise PM. Age differentially influences estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) gene expression in specific regions of the rat brain. Mech Ageing Dev. 2002;123:593–601. doi: 10.1016/s0047-6374(01)00406-7. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estradiol-induced daily luteinizing hormone and prolactin surges in young and middle-aged rats: correlations with age-related changes in pituitary responsiveness and catecholamine turnover rates in microdissected brain areas. Endocrine. 1984;115:801–809. doi: 10.1210/endo-115-2-801. [DOI] [PubMed] [Google Scholar]
- Wise PM, Ratner A. Effect of ovariectomy on plasma LH, FSH, estradiol and progesterone and medial basal hypothalamic LHRH concentrations in old and young rats. Neuroendocrinology. 1980;30:15–19. doi: 10.1159/000122968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.