Abstract
Oral-facial-digital type VI syndrome (OFDVI) is a rare phenotype of Joubert syndrome (JS). Recently, C5orf42 was suggested as the major OFDVI gene, being mutated in 9 of 11 families (82 %). We sequenced C5orf42 in 313 JS probands and identified mutations in 28 (8.9 %), most with a phenotype of pure JS. Only 2 out of 17 OFDVI patients (11.7 %) were mutated. A comparison of mutated vs. non-mutated OFDVI patients showed that preaxial and mesoaxial polydactyly, hypothalamic hamartoma and other congenital defects may predict C5orf42 mutations, while tongue hamartomas are more common in negative patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s00439-014-1508-3) contains supplementary material, which is available to authorized users.
Oral-facial-digital type VI syndrome (OFDVI) is a rare phenotype in the spectrum of Joubert syndrome (JS) and is defined by the presence of the “molar tooth sign” (MTS) with at least one of these findings: (1) tongue hamartoma and/or additional lingual frenula and/or upper lip notch; (2) mesoaxial polydactyly; (3) hypothalamic hamartoma. Other oral-facial (e.g. cleft lip and palate) or digital (e.g. postaxial and preaxial polydactyly) abnormalities can also be present (Poretti et al. 2012).
Mutations in TMEM216 and in OFD1 have been reported in few OFDVI patients (Coene et al. 2009; Darmency-Stamboul et al. 2013; Valente et al. 2010). A recent study identified mutations in the C5orf42 gene in nine of 11 OFDVI (82 %) families (including four living children and eight fetuses), suggesting that C5orf42 could represent the major causative gene for OFDVI (Lopez et al. 2014).
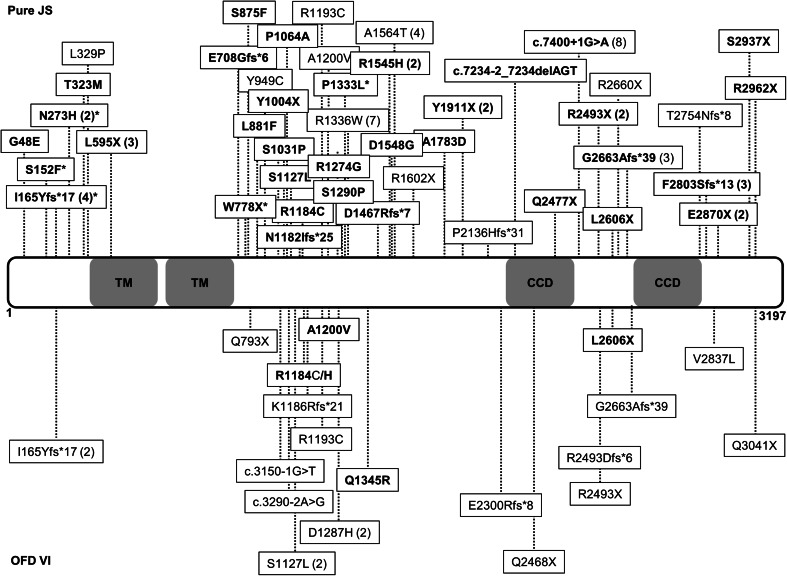
As part of a ciliopathy research project, we sequenced C5orf42 in 313 JS probands, and identified pathogenic mutations in 28 (8.9 %) (Fig. 1). Only two out of 17 OFDVI probands in our cohort (11.7 %) carried C5orf42 mutations, while one was mutated in OFD1. No mutations were detected in the remaining 14 (82.3 %) OFDVI patients in all tested genes (see Supplementary material online for methods, characterization of mutations and clinical features of mutated OFDVI patients).
Fig. 1.
Schematic representation of C5orf42 protein structure and distribution of all reported mutations. The two predicted transmembrane domains (TM, amino acids 592–612 and 631–651) and the two predicted coiled coil domains (CCD, amino acids 2,457–2,487 and 2,691–2,724) are shown. Mutations found in patients with pure Joubert syndrome and with OFDVI are presented in the upper and lower parts of the figure, respectively. Mutations identified in the present study are in bold. In brackets are the numbers of patients in whom each mutation has been identified. Asterisk indicates clinical data not available
To explain the striking discrepancy between our findings and those reported by Lopez et al., we compared clinical features in C5orf42 mutated (n = 14) vs. non-mutated (n = 17) OFDVI patients (Table 1). Preaxial and mesoaxial polydactyly, hypothalamic hamartomas and other congenital abnormalities were significantly more frequent in the mutated group, while tongue hamartomas or multiple lingual frenula occurred more commonly in non-mutated patients. Other oral-facial features, postaxial polydactyly and other brain abnormalities were equally represented in both groups. Despite the limited number of patients, these findings suggest that the current diagnostic criteria for OFDVI include two main phenotypic groups, one with preaxial and/or mesoaxial polydactyly and frequent additional congenital anomalies (for which C5orf42 is the major causative gene), and another with less severe presentation and prevalent oral-facial involvement, which genetic causes still remain to be identified.
Table 1.
Comparison of clinical features in C5orf42 mutated vs. non-mutated OFDVI patients
| Mutated | Non-mutated | p | |
|---|---|---|---|
| Any oral-facial feature | 7/12 (58 %) | 17/17 (100 %) | 0.006 |
| Tongue hamartomas/multiple lingual frenulaa | 6/12 (50 %) | 17/17 (100 %) | 0.002 |
| Other oral-facial featuresb | 4/12 (33 %) | 5/17 (29 %) | n.s. |
| Any polydactyly | 14/14 (100 %) | 13/17 (76 %) | n.s. |
| Mesoaxial polydactylya | 7/14 (50 %) | 1/17 (6 %) | 0.01 |
| Preaxial polydactyly | 14/14 (100 %) | 5/17 (29 %) | 0.0001 |
| Postaxial polydactyly | 9/14 (64 %) | 10/17 (59 %) | n.s. |
| Any CNS abnormality besides MTS | 8/14 (57 %) | 4/17 (24 %) | n.s. |
| Hypothalamic hamartomaa | 6/14 (43 %) | 1/17 (6 %) | 0.03 |
| Occipital encephalocele | 2/14 (14 %) | 1/17 (6 %) | n.s. |
| Other CNS abnormalitiesc | 4/14 (29 %) | 2/17 (12 %) | n.s. |
| Retinal/renal/hepatic involvement | 0/14 | 4/17 (24 %) | n.s. |
| Retinopathy (only living patients) | 0/2 | 3e/17 (18 %) | n.s. |
| Nephronophthisis (only living patients) | 0/2 | 2e/17 (12 %) | n.s. |
| Cystic dysplastic kidneys | 0/14 | 0/17 | n.s. |
| Congenital liver fibrosis | 0/14 | 0/17 | n.s. |
| Other congenital abnormalities outside the CNSd | 8/14 (57 %) | 1/17 (6 %) | 0.004 |
C5orf42 mutated patients include the 12 patients from 9 families reported by Lopez et al. (2014) and the two patients from the present paper; C5orf42 non-mutated patients (n = 17) are all from the present cohort, and include one patient mutated in OFD1 (see text) and 16 patients from 14 families. Statistical comparisons were made by Fisher’s exact test
aSufficient for diagnosis of OFDVI in association with the MTS
bCleft lip and/or palate, tooth abnormalities, lobulated tongue, short frenula
cPorencephaly, nodular heterotopia, polymicrogyria, corpus callosum abnormalities, hydrocephalus, arhinencephaly
dAbnormal ribs or long bones, cubitus valgus, heart or aortic defects, uterus septation, common mesentery, coloboma, microphthalmia, Hirschsprung disease, scoliosis
eIncludes two siblings
Twenty-seven C5orf42 mutated patients (from 23 families) in our study had pure JS (with retinopathy in one), while clinical data were unavailable in three. Considering all reported C5orf42 mutated patients (n = 58), over two-thirds showed a pure JS phenotype while only 24 % has OFDVI (Supplementary Table 1). Kidney or liver involvement was never noted, while polydactyly (mainly preaxial) was present in nearly half of mutated patients regardless of the phenotype. These findings delineate a specific C5orf42-related phenotype, and suggest a major role for this gene in limb development.
Overall, the identification of mutations in 28 of 313 JS probands makes C5orf42 a major contributor to the pathogenesis of this ciliopathy. How mutations in the same gene may cause pure JS or a much more severe oral-facial-digital syndrome remains an open question. Genotype–phenotype correlations seem to fail, since truncating and missense mutations affecting the entire length of the protein are detected in patients with either pure or OFDVI presentations (Fig. 1). As suggested for other ciliopathies, it is conceivable that additional, yet unidentified variants in distinct genes may act as genetic modifiers able to influence the penetrance and expression of oral-facial and digital features in patients bearing C5orf42 mutations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This work was partly supported by grants from the European Research Council (ERC Starting Grant 260888), the Telethon Foundation Italy (Grant GGP13146), and the Italian Ministry of Health (Ricerca Corrente 2014, Ricerca Finalizzata Malattie Rare 2008).
Contributor Information
Marta Romani, Email: m.romani@css-mendel.it.
Enza Maria Valente, Phone: +39 06 4416 0537, Email: e.valente@css-mendel.it.
References
- Coene KL, Roepman R, Doherty D, Afroze B, Kroes HY, Letteboer SJ, Ngu LH, Budny B, van Wijk E, Gorden NT, Azhimi M, Thauvin-Robinet C, Veltman JA, Boink M, Kleefstra T, Cremers FP, van Bokhoven H, de Brouwer AP. OFD1 is mutated in X-linked Joubert syndrome and interacts with LCA5-encoded lebercilin. Am J Hum Genet. 2009;85:465–481. doi: 10.1016/j.ajhg.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmency-Stamboul V, Burglen L, Lopez E, Mejean N, Dean J, Franco B, Rodriguez D, Lacombe D, Desguerres I, Cormier-Daire V, Doray B, Pasquier L, Gonzales M, Pastore M, Crenshaw ML, Huet F, Gigot N, Aral B, Callier P, Faivre L, Attie-Bitach T, Thauvin-Robinet C. Detailed clinical, genetic and neuroimaging characterization of OFD VI syndrome. Eur J Med Genet. 2013;56:301–308. doi: 10.1016/j.ejmg.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Lopez E, Thauvin-Robinet C, Reversade B, Khartoufi NE, Devisme L, Holder M, Ansart-Franquet H, Avila M, Lacombe D, Kleinfinger P, Kaori I, Takanashi JI, Le Merrer M, Martinovic J, Noel C, Shboul M, Ho L, Guven Y, Razavi F, Burglen L, Gigot N, Darmency-Stamboul V, Thevenon J, Aral B, Kayserili H, Huet F, Lyonnet S, Le Caignec C, Franco B, Riviere JB, Faivre L, Attie-Bitach T. C5orf42 is the major gene responsible for OFD syndrome type VI. Hum Genet. 2014;133:367–377. doi: 10.1007/s00439-013-1385-1. [DOI] [PubMed] [Google Scholar]
- Poretti A, Vitiello G, Hennekam RC, Arrigoni F, Bertini E, Borgatti R, Brancati F, D’Arrigo S, Faravelli F, Giordano L, Huisman TA, Iannicelli M, Kluger G, Kyllerman M, Landgren M, Lees MM, Pinelli L, Romaniello R, Scheer I, Schwarz CE, Spiegel R, Tibussek D, Valente EM, Boltshauser E. Delineation and diagnostic criteria of Oral-Facial-Digital Syndrome type VI. Orphanet J Rare Dis. 2012;7:4. doi: 10.1186/1750-1172-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, Adams M, Szymanska K, Mazzotta A, Lee JE, Tolentino JC, Swistun D, Salpietro CD, Fede C, Gabriel S, Russ C, Cibulskis K, Sougnez C, Hildebrandt F, Otto EA, Held S, Diplas BH, Davis EE, Mikula M, Strom CM, Ben-Zeev B, Lev D, Sagie TL, Michelson M, Yaron Y, Krause A, Boltshauser E, Elkhartoufi N, Roume J, Shalev S, Munnich A, Saunier S, Inglehearn C, Saad A, Alkindy A, Thomas S, Vekemans M, Dallapiccola B, Katsanis N, Johnson CA, Attie-Bitach T, Gleeson JG. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nat Genet. 2010;42:619–625. doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.