Abstract
Here, we explore the evolution and development of skin-associated adipose tissue with the goal of establishing nomenclature for this tissue. Underlying the reticular dermis, a thick layer of adipocytes exists that encases mature hair follicles in rodents and humans. The association of lipid-filled cells with the skin is found in many invertebrate and vertebrate species. Historically, this layer of adipocytes has been termed subcutaneous adipose, hypodermis, and subcutis. Recent data has revealed a common precursor for dermal fibroblasts and intradermal adipocytes during development. Furthermore, the development of adipocytes in the skin is independent from that of subcutaneous adipose tissue development. Finally, the role of adipocytes has been shown to be relevant for epidermal homeostasis during hair follicle regeneration and wound healing. Thus, we propose a refined nomenclature for the cells and adipose tissue underlying the reticular dermis as intradermal adipocytes and dermal white adipose tissue, respectively.
Keywords: skin, dermis, adipocytes, intradermal, dermal adipose tissue
Scope
White adipose tissue (WAT) develops in distinct regions of the body called depots, which are thought to influence disease when enlarged during obesity. The largest adipose tissue depots are abdominal subcutaneous white adipose tissue (SWAT) under the skin and visceral white adipose tissue (VWAT) that surrounds internal organs. Much attention has focused on the role of SWAT and VWAT adipocytes due to the link between visceral adiposity and insulin resistance (1, 2). However, adipose tissue exists within other locations including non-abdominal subcutaneous depots and adipocytes associated with the skin’s dermis. In this comment, we discuss the organization of dermal white adipose tissue (DWAT) in different species and its developmental origins with the goal of redefining nomenclature for DWAT as a distinct adipocyte depot.
Skin and adipocytes: Together from the beginning
White adipose tissue (WAT) develops in distinct locations in many vertebrate species and is composed of unilocular adipocytes that store energy in the form of fatty acids that can be released and broken down into ATP. WAT also performs endocrine functions that are involved in food intake, glucose homeostasis, lipid metabolism, inflammation and angiogenesis and is thought to provide thermal insulation and mechanical cushioning of the body.
Multicellular organisms evolved the ability to store energy in a specific tissue with the development of specialized tissues. While prokaryotes and single cell eukaryotes only store lipids within intracellular organelles, multicellular organisms developed specialized lipid-storing cells (3). Interestingly, lipid-containing cells exist in most organisms within or adjacent to the outer surface or epidermis. Invertebrates such as C. elegans accumulate lipids in skin-like epidermal cells (4, 5). Primitive vertebrates such as lampreys, jawless vertebrates, have lipid-storing cells in specialized depots underneath the skin (6).
Within many vertebrate species including pigs, dolphins, rats, humans and mice, distinct adipocyte depots exist with several layers of adipocytes being adjacent to the epidermis. Traditionally, all skin-associated WAT has been considered one depot termed the hypodermis or subcutis. However, each adipose layer has distinct morphologies and physiological characteristics (7). In rodents, the WAT depot that exists directly below the reticular dermis in the skin is clearly separated from the subcutaneous WAT depot by a striated muscle, the panniculus carnosus (Figure 1). While many other mammals including humans lack the panniculus carnosus, histologically and anatomically distinct layers of adipose tissue exist under the reticular dermis in several species including pigs and humans (Figure 1).
Figure 1.
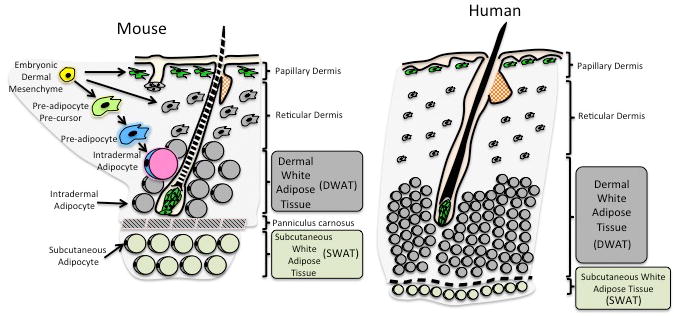
Defining the development and anatomical location of Dermal White Adipose Tissue (DWAT) and Subcutaneous White Adipose Tissue (SWAT) in mammalian skin. The embryonic dermis (yellow) gives rise to DWAT that consists of the intradermal pre-adipocyte and intradermal adipocytes populations in adult mouse skin. DWAT is morphologically and developmentally distinct from SWAT, which is located beneath the panniculus carnosus (white adipocytes). Human skin does not have a detectable panniculus carnosus, however, there are functional and morphological distinctions between DWAT (grey) and SWAT (white).
In pigs, three layers of subcutaneous adipose tissue exist each separated by a distinct layer of fascia (8). The outer and middle layers of subcutaneous porcine adipose tissue are characterized by distinct fatty acid composition (9, 10), cellularity, and enzymatic activity (8). In humans, several studies suggest that two layers of adipose tissue under the reticular dermis exist that are histologically and anatomically distinct (11-14). MRI studies have shown that women with cellulite have a thicker deep layer of subcutaneous adipose tissue compared to those without cellulite (15). Furthermore, the most superficial adipocyte layer in human skin is morphologically and metabolically distinct compared to the deeper adipocyte layer (13, 16). Thus, the organization of skin associated adipose tissue of pigs and humans suggests that, like rodents, distinct subcutaneous and dermal adipose tissue depots exist.
Developmental origin of skin-associated adipocytes
The distinction between subcutaneous and skin associated adipocytes in rodents is established during development. Each adipose depot has distinct developmental timing and gene expression patterns (17). Indeed, in rats and pigs, mature adipocytes in the skin’s dermis form postnatally after hair follicle morphogenesis (18). It has been shown, in mice that resident immature adipocytes are present by embryonic day 14 and form independently from the subcutaneous adipocyte depot ex vivo and in vivo (19). These studies provide evidence that resident skin adipocytes are established during development.
Recent lineage tracing studies using several mouse models expressing Cre-recombinase in distinct fibroblast populations revealed that during development, the dermal mesenchyme is established by a common precursor that generates fibroblasts and adipocytes. However, postnatally, fibroblast lineages maintained cells of the upper dermis and did not contribute to adipocyte regeneration (21). It has also been shown that skin-resident adipocyte precursor cells exist in the dermis in mice and can regenerate adipocytes in the skin during the hair cycle and following wounding (22, 23). Thus, skin-associated adipocytes are established in the dermal mesenchyme alongside fibroblast lineages in a manner that is distinct from the development of subcutaneous adipocytes. While inhibition of adipogenesis can influence fibroblast biology (23, 25), whether dermal fibroblasts or intradermal adipocytes share a common precursor postnatally as in muscle (26, 27) will be an interesting area of future investigation.
Redefining the nomenclature for skin-associated adipocytes
In the literature, the terminology used to describe fat/adipose tissue in rodent skin has been inconsistent, and often ultimately misleading. It is most commonly referred to as hypodermis, subcutaneous fat, or skin fat, although adipose tissue or adipose layer is also used as an alternative to fat. A few authors with knowledge of the detailed anatomy of adipose tissue depots have pointed to existence of a separate dermal adipose layer in rodents (24, 28), while other groups have recognized this distinction in an experimental context (22, 29). Nevertheless for many skin-related studies, including those investigating relationships between hair follicles and adipose tissues (20, 30, 31), the term “subcutaneous” has been used excusably but nevertheless erroneously to refer to adipose tissue that we now know derives from the dermis. We propose that that the term intradermal adipocytes (22, 24)(Figure 1) is the best way to describe these cells, as it accurately reflects their immediate developmental origin and anatomical location. Similarly, we suggest that dermal white adipose tissue (DWAT) is the best description for the adipose compartment in the dermis underlying the reticular dermis (19). In keeping with the terminology of visceral (VWAT) and subcutaneous (SWAT), DWAT defines a unique adipocyte population in the skin that contains many cell types but is primarily composed of adipocytes.
The importance of this nomenclature is not only pertinent to skin biology, but is also important for identifying novel aspects of adipocyte biology more globally. Genetically modified or abnormal mice have been used to model virtually all aspects of adipocyte biology and adipose related pathologies. Knowledge that adipocytes derived from the dermis represent a separate population could retrospectively change the perspective on many existing studies. Where investigators refer to the subcutaneous fat/adipocyte layer without providing a precise description or visual evidence of what this describes anatomically, it is impossible to tell whether they mean the true subcutaneous adipose tissue (ie below the panniculus carnosus in mice), the dermal adipose tissue above, or both. This renders interpretation of some work extremely problematic. This being said, there now appears to be broader recognition of the key distinction between dermal and subcutaneous adipose tissue (32).
Acknowledgments
We thank members of the Horsley laboratory for critically reading the manuscript. R.D. and F.W. are funded by the Wellcome Trust, Medical Research Council and the European Union. C.A.B.J. is supported by Medical Research Council (MRC) Grant G1000846 and Diabetes UK. C.M.C. is supported by NIAMS RO1 AR42177 and AR60306. V.H. is a Pew Scholar in Biomedical Research and is funded by the NIH (AR060295) and the Connecticut Department of Public Health (12SCBYALE01).
References
- 1.Björntorp P. Abdominal obesity and risk. Clin Exp Hypertens A. 1990;12(5):783–794. doi: 10.3109/10641969009073499. [DOI] [PubMed] [Google Scholar]
- 2.Kissebah AH, et al. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab. 1982;54(2):254–260. doi: 10.1210/jcem-54-2-254. [DOI] [PubMed] [Google Scholar]
- 3.Birsoy K, Festuccia WT, Laplante M. A comparative perspective on lipid storage in animals. J Cell Sci. 2013;126(Pt 7):1541–1552. doi: 10.1242/jcs.104992. [DOI] [PubMed] [Google Scholar]
- 4.Mullaney BC, Ashrafi K. C. elegans fat storage and metabolic regulation. Biochim Biophys Acta. 2009;1791(6):474–478. doi: 10.1016/j.bbalip.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adamo L, et al. Biomechanical forces promote embryonic haematopoiesis. Nature. 2009;459(7250):1131–1135. doi: 10.1038/nature08073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lethbridge RC, Potter IC. Quantitative studies on the skin of the paired species of lampreys, Lampetra fluviatilis (L.) and Lampetra planeri (BLOCH) Journal of Morphology. 1980;164(1):39–46. doi: 10.1002/jmor.1051640104. [DOI] [PubMed] [Google Scholar]
- 7.Hausman GJ. Histochemistry of connective tissue cells in subcutaneous adipose tissue of normal and decapitated pig fetuses. Acta Anat (Basel) 1984;118(3):147–152. doi: 10.1159/000145835. [DOI] [PubMed] [Google Scholar]
- 8.Anderson DB, Kauffman RG, Kastenschmidt LL. Lipogenic enzyme activities and cellularity of porcine adipose tissue from various anatomical locations. J Lipid Res. 1972;13(5):593–599. [PubMed] [Google Scholar]
- 9.Dean HK, Hilditch TP. The body fats of the pig: The influence of body temperature on the composition of depot fats. Biochem J. 1933;27(6):1950–1956. doi: 10.1042/bj0271950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sink JD, Watkins JL, Ziegler JH, Miller RC. Analysis of Fat Deposition in Swine by Gas-Liquid Chromatography. Journal of Animal Science. 1964;23(1):121–125. [Google Scholar]
- 11.Smith SR, et al. Contributions of total body fat, abdominal subcutaneous adipose tissue. Metabolism. 2001;50(4):425–435. doi: 10.1053/meta.2001.21693. [DOI] [PubMed] [Google Scholar]
- 12.Miyazaki M, Kim YC, Gray-Keller MP, Attie AD, Ntambi JM. The biosynthesis of hepatic cholesterol esters and triglycerides is impaired in mice with a disruption of the gene for stearoyl-CoA desaturase 1. J Biol Chem. 2000;275(39):30132–30138. doi: 10.1074/jbc.M005488200. [DOI] [PubMed] [Google Scholar]
- 13.Walker GE, et al. Deep subcutaneous adipose tissue: a distinct abdominal adipose depot. Obesity (Silver Spring) 2007;15(8):1933–1943. doi: 10.1038/oby.2007.231. [DOI] [PubMed] [Google Scholar]
- 14.Sbarbati A, et al. Subcutaneous adipose tissue classification. Eur J Histochem. 2010;54(4):e48. doi: 10.4081/ejh.2010.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Querleux B, Cornillon C, Jolivet O, Bittoun J. Anatomy and physiology of subcutaneous adipose tissue by in vivo magnetic resonance imaging and spectroscopy: Relationships with sex and presence of cellulite. Skin Research and Technology. 2002;8(2):118–124. doi: 10.1034/j.1600-0846.2002.00331.x. [DOI] [PubMed] [Google Scholar]
- 16.Gasperoni C, Salgarello M. Rationale of subdermal superficial liposuction related to the anatomy of subcutaneous fat and the superficial fascial system. Aesthetic Plast Surg. 1995;19(1):13–20. doi: 10.1007/BF00209305. [DOI] [PubMed] [Google Scholar]
- 17.Gesta S, Tseng Y-H, Kahn CR. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell. 2007;131(2):242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Hausman GJ, Campion DR, Richardson RL, Martin RJ. Adipocyte development in the rat hypodermis. Am J Anat. 1981;161(1):85–100. doi: 10.1002/aja.1001610107. [DOI] [PubMed] [Google Scholar]
- 19.Wojciechowicz K, Gledhill K, Ambler CA, Manning CB, Jahoda CA. Development of the mouse dermal adipose layer occurs independently of subcutaneous adipose tissue and is marked by restricted early expression of FABP4. PLoS One. 2013;8(3):e59811. doi: 10.1371/journal.pone.0059811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wojciechowicz K, Markiewicz E, Jahoda CA. C/EBPalpha identifies differentiating preadipocytes around hair follicles in foetal and neonatal rat and mouse skin. Exp Dermatol. 2008;17(8):675–680. doi: 10.1111/j.1600-0625.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- 21.Driskell RR, et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Festa E, et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146(5):761–771. doi: 10.1016/j.cell.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140(7):1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt B, Horsley V. Unravelling hair follicle-adipocyte communication. Exp Dermatol. 2012;21(11):827–830. doi: 10.1111/exd.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohgo S, et al. Bleomycin inhibits adipogenesis and accelerates fibrosis in the subcutaneous adipose layer through TGF-β1. Exp Dermatol. 2013;22(11):769–771. doi: 10.1111/exd.12256. [DOI] [PubMed] [Google Scholar]
- 26.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12(2):153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12(2):143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 28.Cinti S. The adipose organ. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):9–15. doi: 10.1016/j.plefa.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 29.van Genderen C, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994;8(22):2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- 30.Plikus M, et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature. 2008;451(7176):340–344. doi: 10.1038/nature06457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klein J, et al. What are subcutaneous adipocytes really good for? Exp Dermatol. 2007;16(1):45–70. doi: 10.1111/j.1600-0625.2006.00519_1.x. [DOI] [PubMed] [Google Scholar]
- 32.Youbin Wang, Zheng Qi, Xiaojun Wang. Dermis Reconstruction and Dermis Fat Graft Through an Intraoral Incision: A New Method to Correct the Furrowed Philtral Column Deformity in Lesser-Form Cleft Lip. 2013 doi: 10.1597/12-076. http://dx.doi.org/10.1597/12-076. [DOI] [PubMed]


