Abstract
Background
History and severity of atopic dermatitis (AD) are risk factors for peanut allergy. Recent evidence suggests that children can become sensitized to food allergens through an impaired skin barrier. Household peanut consumption, which correlates strongly with peanut protein levels in household dust, is a risk factor for peanut allergy.
Objective
We sought to assess whether environmental peanut exposure (EPE) is a risk for peanut sensitization and allergy and whether markers of an impaired skin barrier modify this risk.
Methods
Peanut protein in household dust (in micrograms per gram) was assessed in highly atopic children (age, 3-15 months) recruited to the Consortium of Food Allergy Research Observational Study. History and severity of AD, peanut sensitization, and likely allergy (peanut-specific IgE, ≥5 kUA/mL) were assessed at recruitment into the Consortium of Food Allergy Research study.
Results
There was an exposure-response relationship between peanut protein levels in household dust and peanut skin prick test (SPT) sensitization and likely allergy. In the final multivariate model an increase in 4 log2 EPE units increased the odds of peanut SPT sensitization (1.71-fold; 95% CI, 1.13- to 2.59-fold; P = .01) and likely peanut allergy (PA; 2.10-fold; 95% CI, 1.20- to 3.67-fold; P < .01). The effect of EPE on peanut SPT sensitization was augmented in children with a history of AD (OR, 1.97; 95% CI, 1.26-3.09; P < .01) and augmented even further in children with a history of severe AD (OR, 2.41; 95% CI, 1.30-4.47; P < .01); the effect of EPE on PA was also augmented in children with a history of AD (OR, 2.34; 95% CI, 1.31-4.18; P < .01).
Conclusion
Exposure to peanut antigen in dust through an impaired skin barrier in atopically inflamed skin is a plausible route for peanut SPT sensitization and PA.
Key words: Atopic dermatitis, peanut sensitization, peanut allergy, environmental peanut exposure, dust
Abbreviations used: AD, Atopic dermatitis; CoFAR, Consortium of Food Allergy Research; EPE, Environmental peanut exposure; FLG, Filaggrin; IQR, Interquartile range; LLQ, Lower limit of quantitation; LR, Logistic regression; OR, Odds ratio; PA, Peanut allergy; sIgE, Specific IgE; SPT, Skin prick test
Skin barrier dysfunction plays an important role in the development of atopic dermatitis (AD),1,2 and AD is often cited as the first step in the allergic march.3,4 There is a clear association between early-onset AD and food allergy5,6 and a growing body of evidence that epicutaneous exposure to peanut through an impaired skin barrier increases the risk of peanut sensitization and clinically confirmed peanut allergy.7-9 Among children with peanut allergy with AD in the Avon Longitudinal Study of Parents and Children, 90% had been exposed to creams containing Arachis (peanut) oil in the first 6 months of life.6 In BALB/c mice epicutaneous peanut exposure has been shown to induce a potent allergic TH2-type response and anaphylaxis after a single oral antigen challenge7-9; however, in these studies this was only achieved if skin stripping, leading to skin barrier impairment and inflammation, was performed before antigen application. In flaky tail mice that carry a mutation within the murine flg gene, topical application of ovalbumin leads to a cellular infiltrate and antigen-specific antibody response, even without skin stripping.10
We have shown that early exposure to peanut antigen in household dust is a risk factor for the development of peanut sensitization and clinically confirmed peanut allergy in children who carry a filaggrin (FLG) null mutation in the Manchester Asthma and Allergy Study cohort.11 In another study environmental exposure to peanut measured indirectly based on household peanut consumption was associated with peanut allergy, particularly when compared with atopic children.12 Peanut protein in household dust was not objectively quantified in this study; however, other studies have measured peanut allergens in dust,13,14 and we have shown that peanut allergen levels in dust from the infant's bed and play area correlate with household peanut consumption and stimulate an allergic response in effector cells of patients with peanut allergy.15
We hypothesized that an impaired skin barrier in children with AD or FLG null mutations would modify the effect of environmental peanut exposure (EPE), as defined by peanut protein in household dust (in micrograms per gram), on peanut sensitization and allergy. If proved, this hypothesis would support the notion that a primary mode leading to the development of peanut sensitization and allergy occurs through presentation of environmental peanut antigen through an impaired skin barrier to underlying antigen-presenting cells. The purpose of this study was to assess whether early EPE increases the risk of peanut sensitization and allergy in young atopic children.
Methods
Participants were from the National Institutes of Health–sponsored Consortium of Food Allergy Research (CoFAR). The design and methodology are described elsewhere.16 In brief, 512 children less than 15 months of age were recruited with a convincing clinical history of cow's milk allergy, egg allergy, or both and a positive skin prick test (SPT) response to cow's milk, egg, or both, respectively, or with moderate-to-severe AD with a positive SPT response to cow's milk, egg, or both but without known peanut allergy. Study procedures were reviewed and approved by a National Institute of Allergy and Infectious Diseases Data Safety Monitoring Board and by local institutional review boards, and written signed informed consent was obtained. The analyses included 359 (70.1%) of 512 participants who provided enough dust to analyze approximately 10 mg for peanut protein.
SPTs were performed with the GreerPick (Greer Laboratories, Lenoir, NC) on the infant's back. Results were obtained after 15 minutes, and the average mean wheal diameter (after subtraction of the saline negative control) was recorded. Children with peanut SPT responses of 3 mm or greater were described as peanut SPT sensitized, and children with peanut SPT responses of less than 3 mm were described as not sensitized. Children with serum specific IgE (sIgE) to peanut (ImmunoCAP system; Thermo Fisher Scientific, Uppsala, Sweden) of 0.35 kUA/mL or greater were described as peanut sIgE sensitized. Children with serum sIgE levels to peanut of 5 kUA/mL or greater were described as having a serologic diagnosis of likely peanut allergy (PA); this was postulated as in previous studies, 70% to 90% of 5- to 7-year-old children had positive diagnostic peanut challenge results with this level of peanut sIgE.17-19 Children were defined as not peanut allergic if they had a history of tolerating eating peanut (regardless of sensitization status) or if they were not sensitized to peanut, even if there was no history of peanut ingestion. Peanut-sensitized children (peanut SPT response ≥3 mm or peanut sIgE level of between 0.35 and 5 kUA/mL) without a history of peanut ingestion were excluded from the PA analysis because they did not undergo a peanut challenge at baseline and thus could not be defined as having peanut allergy or peanut tolerance. Of 359 subjects with available living room dust, 150 (41.8%) children had no history of ingestion of peanut and peanut SPT responses of 3 mm or greater or sIgE levels of 0.35 kUA/mL or greater and thus were excluded from the PA analysis. Of the remaining children, 89 (42.6%) of 209 were considered to have a serologic diagnosis of PA because of a peanut sIgE level of 5 kUA/mL or greater. There were 120 children considered not to have peanut allergy who either reported peanut consumption without a reaction (n = 20/209 [9.6%]) or who were not sensitized to peanut (n = 100/209 [47.8%]).
FLG genotyping was performed with genomic DNA extracted from blood. The FLG null mutations R501X, 2282del4, S3247X, and R2447X were assessed with a TaqMan-based allelic discrimination assay (Applied Biosystems, Life Technologies, Cheshire, United Kingdom) by using previously described probes and primers.20,21 History of AD and maximum severity of AD were graded by (1) extent of disease (by “rule of 9”), (2) course of disease (by history), and (3) intensity of disease (disturbance of night's sleep by itching), each on a 3-point scale, as previously described.22 The rule of 9 is used to calculate the area of the body's skin affected for SCORAD score assessment, where the head and neck amount to 9%, the upper limbs amount to 9% each, the lower limbs amount to 18% each, the anterior trunk amounts to 18%, the back amounts to 18%, and the genitals amount to 1%.23
EPE was quantified from dust collected at baseline from the family's living room floor. Families were asked to avoid vacuuming their living room floors for 3 days before obtaining dust. Participants were provided with a DUSTREAM adaptor and collector (Indoor Biotechnologies, Warminster, United Kingdom), a nylon collection filter, a disposable template, and instructions for vacuuming. The living room floor was vacuumed for 2 minutes within a 1-m2 surface area. Dust samples were sieved, and fine dust was extracted in a proportional volume of extraction solution.24 Peanut protein in dust was determined by using the Veratox polyclonal ELISA against whole peanut protein (Neogen, Lansing, Mich), which has been validated for sensitivity, specificity, and reliability in measuring peanut protein in food25,26 and dust.24 The lower limit of quantification (LLQ) of the assay was defined as 100 ng/mL whole peanut (25 ng/mL peanut protein), and samples of less than this value were defined as LLQ/2 (12.5 ng/mL peanut protein, which equated to between 1.05 and 1.23 μg/g depending on the weight of dust obtained).27 There were 16 (4.5%) of 359 living room dust samples with peanut protein levels of less than the LLQ. Results were converted from nanograms per milliliter into micrograms of peanut protein per gram of dust. Participant information was kept blind from the researcher performing the ELISA dust analyses. Dust samples were also obtained from the infant's bed dust; details are described in the Methods section in this article's Online Repository at www.jacionline.org.
Statistical analysis
Data were entered into SPSS (SPSS 19.0; SPSS, Chicago, Ill) and STATA (STATA/IC 12.1; StataCorp, College Station, Tex) spreadsheets for analysis. Associations between demographic, clinical, and household factors and peanut SPT sensitization and PA were assessed by using a logistic regression (LR) model for children with available dust for analysis. Peanut protein levels in dust (micrograms per gram) underwent log2 transformation to normalize data. EPE spanned approximately 12 log2 scales (1.05-3761.68 μg/g), and therefore we showed the effect of 4 log2 unit increases in EPE on peanut SPT sensitization and PA. In a stepwise process all factors with a trend toward an association with peanut SPT sensitization or PA on univariate analysis (P < .15) were included in the multivariate model, and then only those covariates with a P value of less than .05 were included in the final multivariate model. The same covariates were included in the multivariate analysis for all children, children with a history of AD, and children with a history of severe AD. We assessed EPE as a continuous variable and as quartiles by dividing the span of continuous EPE into 4 equal groups. Visual graphs were inspected, and the linearity of the logit (p/[1−p]) and log2 continuous peanut protein level was reasonable for both peanut SPT sensitization and PA on univariate analysis. Overlapping 95% CIs of odds ratios (ORs) among EPE quartiles supported the linearity of the exposure-response relationship between log2 EPE and the logit of Prob (peanut SPT sensitization = positive) and Prob (PA = positive). Therefore we used continuous EPE as the optimum representation of the primary exposure variable throughout the article.
The effect of EPE on peanut sensitization or PA was assessed in a univariate and multivariate LR model in all children and subgroups of children without a history of AD, with a history of AD, or with a history of severe AD. We subsequently included an interaction term with EPE and a history of AD (vs no AD) or history of severe AD (vs no AD). To establish the relationship between EPE during the child's early life and maternal peanut consumption in pregnancy, peanut protein levels in living room dust (in micrograms per gram) were compared in homes in which mothers either avoided or consumed peanut during pregnancy by using the Mann-Whitney U test. Statistical significance was assessed at a P value of less than .05.
Results
EPE is associated with peanut SPT sensitization and PA
ORs (95% CIs) of factors possibly associated with peanut SPT sensitization or PA (peanut sIgE, ≥5 kUA/mL) are displayed in Tables I (univariate LR analysis), II, and III (multivariate LR analyses). There was a significant association between a 4-unit log2 increase in EPE and peanut SPT sensitization both on univariate analysis (n = 359; OR, 1.52; 95% CI, 1.08-2.14; P = .01) and multivariate LR analysis (n = 292; OR, 1.71; 95% CI, 1.13-2.59; P = .01), adjusting for parental report of hay fever ever in the child, egg SPT wheal diameter (in millimeters), and maternal peanut consumption during pregnancy and breast-feeding (which were also associated with peanut SPT sensitization at P < .05). There was a trend toward an association between EPE and PA on univariate analysis (n = 209; OR, 1.46; 95% CI, 0.92-2.29; P = .11) and a significant association on multivariate LR analysis (n = 209; OR, 2.10; 95% CI, 1.20-3.67; P < .01), adjusting for ethnicity, egg SPT wheal diameter, and cow's milk SPT wheal diameter (which were also associated with PA at P < .05). The relationship between peanut protein in the infants' bed and peanut SPT sensitization and PA is described in this article's Online Repository at www.jacionline.org.
Table I.
Unadjusted ORs and 95% CIs measuring associations between peanut SPT sensitization and likely PA and log2 EPE units and subject demographic, clinical, and household factors∗
| Peanut SPT sensitization (n = 359 [54.6% positive]) |
Likely PA (n = 209 [42.6% positive]) |
|||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| 4 log2 EPE (μg/g)† | 1.52 | 1.08-2.14 | .01 | 1.46 | 0.92-2.29 | .11 |
| History of infantile AD | 1.83 | 0.82-4.06 | .14 | 1.87 | 0.63-5.51 | .26 |
| Maximum AD severity before entry | ||||||
| No AD (0) | Reference category | Reference category | ||||
| Mild (3-4) | 2.46 | 0.88-6.89 | .09 | 1.31 | 0.31-5.53 | .71 |
| Moderate (5-6) | 1.77 | 0.75-4.19 | .19 | 1.91 | 0.60-6.08 | .28 |
| Severe (7-9) | 1.77 | 0.78-4.01 | .17 | 1.94 | 0.64-5.88 | .24 |
| Nonwhite ethnicity | 1.73 | 0.44-2.21 | .23 | 1.93 | 1.04-3.60 | .04 |
| FLG null mutation (excluding nonwhite subjects) | 0.72 | 0.37-1.39 | .32 | 1.29 | 0.54-3.08 | .56 |
| Parental report of hay fever ever in the child | 3.07 | 1.28-7.32 | .01 | 1.52 | 0.57-4.20 | .39 |
| Male sex | 0.82 | 0.53-1.28 | .38 | 1.49 | 0.81-2.73 | .20 |
| Maternal history of atopy or asthma | 1.29 | 0.83-2.02 | .26 | 1.26 | 0.69-2.28 | .45 |
| Paternal history of atopy or asthma | 1.00 | 0.65-1.54 | 1.0 | 1.00 | 0.56-1.78 | 1.0 |
| Maternal history of AD | 0.94 | 0.56-1.59 | .82 | 1.13 | 0.56-2.28 | .73 |
| Paternal history of AD | 0.74 | 0.42-1.30 | .29 | 0.57 | 0.25-1.28 | .17 |
| Peanut consumption in pregnancy | 1.67 | 0.94-2.26 | .08 | 1.49 | 0.65-3.40 | .34 |
| Peanut consumption while breast-feeding | 0.69 | 0.43-1.10 | .12 | 0.51 | 0.27-0.94 | .03 |
| Peanut butter in house while breast-feeding | 1.04 | 0.64-1.69 | .88 | 0.94 | 0.49-1.78 | .84 |
| Older siblings | 1.34 | 0.87-2.04 | .18 | 1.11 | 0.63-1.95 | .73 |
| Egg SPT wheal diameter (mm) | 1.15 | 1.10-1.21 | <.01 | 1.26 | 1.17-1.35 | <.01 |
| Cow's milk SPT wheal diameter (mm) | 1.07 | 1.03-1.11 | <.01 | 1.21 | 1.09-1.35 | <.01 |
| Duration of breast-feeding (mo) | 1.05 | 1.00-1.10 | .08 | 1.12 | 1.04-1.20 | <.01 |
| Maternal age at baseline (y) | 1.02 | 0.99-1.07 | .12 | 1.02 | 0.97-1.08 | .40 |
| Child's age at baseline assessment (mo) | 1.11 | 1.04-1.19 | <.01 | 1.04 | 0.96-1.14 | .34 |
Statistically significant values (P < .05) are shown in boldface.
Descriptive statistics of subject factors and EPE are shown in Table E1.
ORs for EPE reflect a 4-unit increase in log2 EPE. ORs for other continuous factors reflect a 1-unit increase in the factor unit. These include egg SPT wheal diameter, cow's milk, duration of breast-feeding, maternal age at baseline, and child's age at baseline. ORs for AD severity compare each severity level with the level “no AD.” All other factors are dichotomous. ORs compare yes with no, ever with never, or male with female.
Table II.
Adjusted peanut sensitization (OR [95% CI]) measuring associations between EPE and subject factors (n = 292)∗
| OR | 95% CI | P value | |
|---|---|---|---|
| 4 log2 EPE (μg/g) | 1.71 | 1.13-2.59 | .01 |
| Egg SPT wheal diameter (mm) | 1.17 | 1.11-1.24 | <.001 |
| Maternal peanut consumption in pregnancy | 2.77 | 1.24-6.20 | .01 |
| Maternal peanut consumption while breast-feeding | 0.46 | 0.25-0.85 | .01 |
| Parental report of hay fever ever in the child | 3.88 | 1.35-11.15 | .01 |
Subject factors and EPE values are significant at the 5% level (in boldface). The OR of EPE represents an increase of 4 log2 EPE units (in micrograms per gram).
Sample size was reduced from 359 to 292 because of missing data for some factors in the multivariate analysis.
Table III.
Adjusted likely PA (OR [95% CI]) measuring associations between EPE and subject factors (n = 209)∗
| OR | 95% CI | P value | |
|---|---|---|---|
| 4 log2 EPE (μg/g) | 2.10 | 1.20-3.67 | <.01 |
| Egg SPT wheal diameter (mm) | 1.25 | 1.15-1.36 | <.001 |
| Nonwhite ethnicity | 2.59 | 1.21-5.58 | .02 |
| Cow's milk SPT wheal diameter (mm) | 1.14 | 1.06-1.22 | <.001 |
Subject factors and EPE values are significant at the 5% level (in boldface). The OR of EPE represents an increase of 4 log2 EPE units (in micrograms per gram).
The sample size was reduced from 359 to 209 because of missing data for some factors in the multivariate analysis.
History of AD modifies the effect of EPE on peanut SPT sensitization and PA
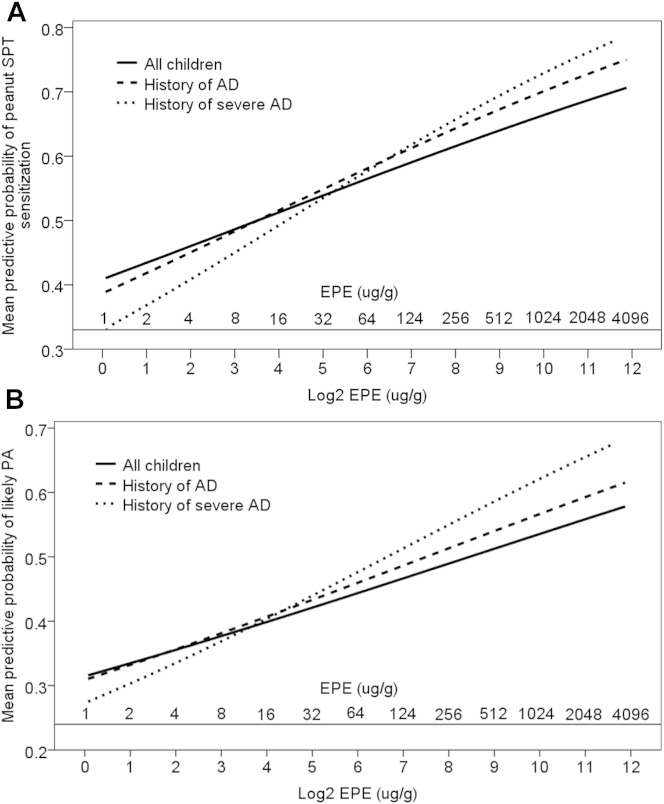
On stratified univariate analysis, the effect of increasing EPE on peanut SPT sensitization and PA was augmented in children with a history of AD and severe AD (Fig 1, A: peanut SPT sensitization; Fig 1, B: PA). On univariate analysis, there was a significant interaction between EPE and AD on the risk of peanut SPT sensitization (OR, 1.41; 95% CI, 1.01-1.97; P < .05) per log2 unit EPE increase; this further increased when comparing the interaction between EPE and a history of severe AD (OR, 1.46; 95% CI, 1.04-2.07; P < .05). The interaction between EPE and a history of AD did not reach statistical significance for PA. There was no association between EPE and peanut SPT sensitization (OR, 0.81; 95% CI, 0.59-1.12) or PA (OR, 0.95; 95% CI, 0.60-1.49) in children without a history of AD.
Fig 1.
A, Univariate stratified predictive probability for the effect of EPE (displayed in log2 [microgram per gram] units and untransformed [microgram per gram] units) for peanut SPT sensitization in all children, children with a history of AD, and children with a history of severe AD. B, Univariate stratified predictive probability for the effect of EPE (displayed in log2 [microgram per gram] units and untransformed [microgram per gram] units) for likely PA in all children, children with a history of AD, and children with a history of severe AD.
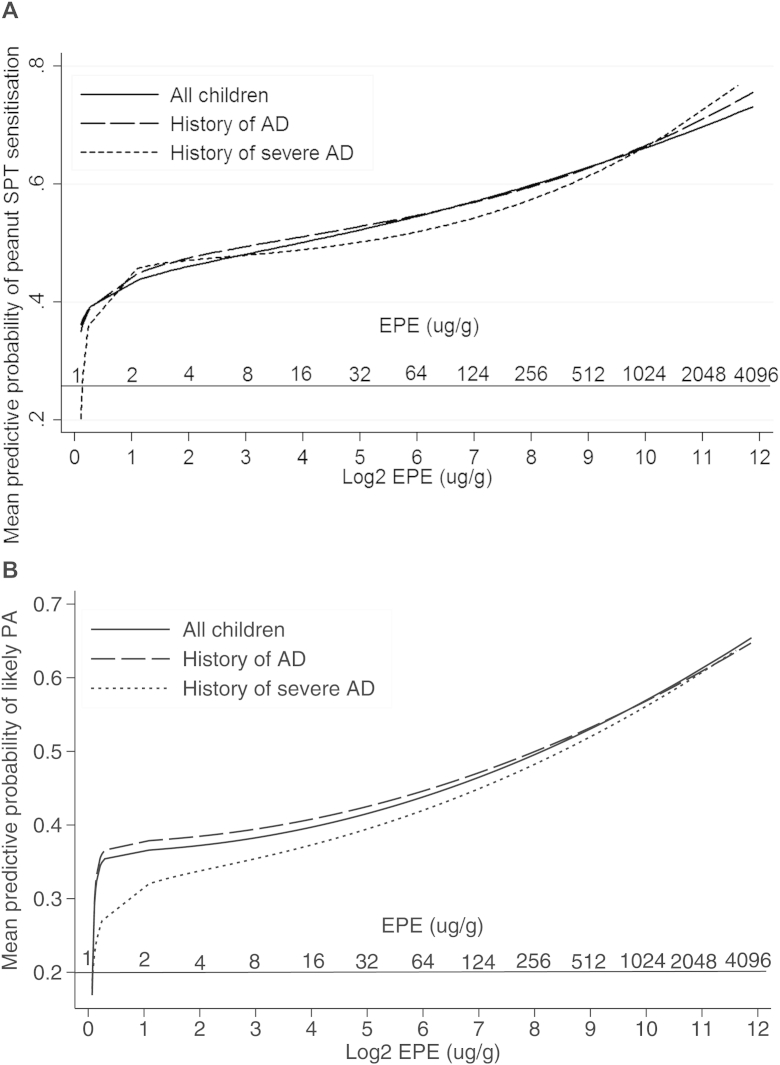
On multivariate LR analysis, the exposure-response relationship of EPE was augmented in children with a history of AD for peanut SPT sensitization (OR, 1.97; 95% CI, 1.26-3.09; P < .01) and PA (OR, 2.34; 95% CI, 1.31-4.18; P < .01; Table IV). For peanut SPT sensitization, the effect of EPE was further augmented in children with a history of severe AD (OR, 2.41; 95% CI, 1.30-4.47; P < .01); however, a similar increase was not observed for PA. In the multivariate predictive probability figures, the association between EPE and peanut SPT sensitization and PA remained; however, there was no longer a clear differentiation of the effect of EPE among all children, children with a history of AD, and children with a history of severe AD (see Fig E1, A, in this article's Online Repository at www.jacionline.org: peanut SPT sensitization; see Fig E1, B: PA).
Table IV.
Stratified LR analysis of the effect of 4 log2 EPE units on peanut SPT sensitization and PA in all children, children with a history of AD, and children with a history of severe AD
| All participants |
Participants with history of AD |
Participants with history of severe AD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No‡ | OR (95% CI) | P value | No. | OR (95% CI) | P value | No. | OR (95% CI) | P value | |
| Peanut SPT sensitization∗ | 292 | 1.71 (1.13-2.59) | .01 | 269 | 1.97 (1.26-3.09) | <.01 | 158 | 2.41 (1.30-4.47) | <.01 |
| Likely PA† | 209 | 2.10 (1.20-3.67) | <.01 | 192 | 2.34 (1.31-4.18) | <.01 | 114 | 2.05 (0.98-4.29) | .06 |
Subject factors and EPE values are significant at the 5% level (in boldface).
Adjusted for parental report of hay ever in the child, egg SPT wheal diameter, maternal peanut consumption during pregnancy, and breast-feeding.
Adjusted for ethnicity, egg, and milk SPT wheal diameter.
The sample size was reduced from 359 to 292 (peanut SPT sensitization) and 209 (likely PA) because of missing data for some factors in the multivariate analysis.
The interaction between EPE and a history of AD for the risk of peanut SPT sensitization remained significant in the multivariate model; the OR was 1.48 (95% CI, 1.01-2.17; P < .05) per log2 unit EPE increase in children with a history of AD versus those with no AD, and the OR was 1.56 (95% CI, 1.04-2.34, P = .03) in children with a history of severe AD versus those with no AD. In the final multivariate model there was a trend toward an interaction between EPE and a history of AD for PA with an OR of 1.68 (95% CI, 0.91-3.12; P = 0.10) and an OR of 1.68 (95% CI, 0.85-3.31; P = .14) in children with a history of severe AD versus those with no AD.
FLG genotype on peanut sensitization and PA
The prevalence of FLG null mutations in white children with AD (with dust available) was 14.9% (41/275); of these children, 37 had FLG heterozygote mutations, 3 had a combined heterozygote mutations, and 1 had a 2282del4 homozygous mutation. There was no significant association between FLG heterozygous or compound heterozygous/homozygous mutations and peanut SPT sensitization/PA; there was also no interaction between FLG genotype and EPE.
Comparisons of the included group (n = 359) with available living room dust and the excluded group (n = 153)
There was no difference in the rate of peanut sensitization or PA between subjects with (n = 359) versus those without (n = 153) available dust; however, there were small but significant differences in the rate of severe AD, ethnicity, number of older siblings, maternal history of AD, maternal peanut consumption during breast-feeding, and peanut present in the home while breast-feeding (see Table E1 in this article's Online Repository at www.jacionline.org).
Discussion
In this high-risk atopic cohort we found that EPE, as assessed by log2 transformed peanut protein (in micrograms) per gram of living room dust was a risk factor for peanut SPT sensitization and PA (peanut sIgE, ≥5 kUA/mL). After adjustment, an increase in 4 log2 EPE units increased the odds of peanut SPT sensitization 1.71-fold (95% CI, 1.13- to 2.59-fold) and the odds of PA 2.10-fold (95% CI, 1.20- to 3.67-fold). The effect of EPE on peanut SPT sensitization and PA increased in an exposure-dependent manner in children with a history of AD, with an increase in odds of 1.97 and 2.34, respectively. The effect of EPE on peanut SPT sensitization was further augmented in children with a history of severe AD; however, this was not the case for PA, which might be due to the smaller sample size of this group. There was a significant interaction between EPE and the history and severity of AD for peanut SPT sensitization, with a trend toward an AD-EPE interaction for PA. Given that peanut sensitization and allergy are more common in children with a history of AD,5,6 these data suggest that environmental exposure to peanut through an impaired skin barrier is a plausible route for peanut sensitization and allergy. The relationship between peanut protein in the infants' bed and peanut SPT sensitization and PA is discussed in this article's Online Repository at www.jacionline.org.
The egg-induced SPT wheal diameter was also associated with peanut SPT sensitization and PA. Egg allergy is known to be a strong predictor of peanut sensitization and allergy.28 The cow's milk–induced SPT wheal diameter was also associated with peanut SPT sensitization and PA; however, it lost significance on multivariate analysis for peanut SPT sensitization, and this might just be another marker of atopy. Environmental exposure to peanut was not a risk factor for egg SPT sensitization or milk SPT sensitization, confirming the specificity of environmental peanut levels on peanut sensitization rather than food sensitization in general. Nonwhite ethnicity (black, Asian, and other nonwhite races combined) was associated with a peanut sIgE level of 5 kUA/L or greater but not peanut SPT sensitization. This supports the findings of the Learning Early About Peanut (LEAP) study, in which black race was associated with a higher peanut sIgE level but a lower peanut SPT response in the baseline screening data from the LEAP study.28
FLG null mutations were not associated with peanut sensitization or PA. This differs from previous published findings; children with 1 of more FLG null mutations were found to have an increased risk of challenge-proven peanut allergy in white individuals from 4 different populations (United Kingdom, Irish, Dutch, and Canadian).29 The lack of association with FLG genotype might be because in CoFAR children already had a 92.5% history of AD and a 54.3% history of severe AD; thus the skin barrier was already impaired, irrespective of whether children had FLG null mutations. In addition, the rate of FLG null mutations was surprisingly low in this cohort (14.9%) given the high rate and severity of AD; previous studies have shown that FLG null mutations are present in up to 56% of children with moderate-to-severe AD.21,30 This might reflect a more varied genetic background in the white American population.31 Another potential explanation for the low FLG mutation rate in this cohort is that 104 children with known PA or peanut sIgE >5 kUA/L were excluded from the CoFAR study before enrollment. If these children had been included, we would have expected a higher rate of FLG null mutations, given the known association between peanut allergy and FLG null mutations.29 A further explanation could be that in children with cow's milk and egg allergy (one of the inclusion criteria for the CoFAR observational cohort), exposure to cow's milk or egg allergens through breast milk or small quantities in food might have led to more severe AD.
Previously, the CoFAR study showed that frequent (≥2 times weekly) maternal peanut consumption during the last trimester of pregnancy was a risk factor for a peanut sIgE level of 5 kUA/L or greater (OR, 2.9; 95% CI, 1.7-4.9).32 In the subgroup of children with available dust samples (n = 359), maternal peanut consumption during pregnancy (any trimester) was associated with peanut SPT sensitization (adjusted OR, 2.66; 95% CI, 1.18-5.99) but not with a peanut sIgE level of 5 kUA/L or greater (P > .3); frequent maternal peanut consumption (≥2 times weekly) in the last trimester showed only a trend toward an association with a peanut sIgE level of 5 kUA/L or greater (P = .15). The lack of significance for this might be due to the smaller sample size of children with available dust; however, maternal peanut consumption during pregnancy might simply be an indirect marker of EPE. Levels of peanut protein in living room dust were significantly greater in households in which mothers consumed peanuts during pregnancy (median, 45.2 μg/g; interquartile range [IQR], 17.5-161.8 μg/g) versus households in which mothers avoided peanuts during pregnancy (median, 16.6 μg/g; IQR, 4.3-72.2 μg/g; P = .001). A prospective study would be required in which maternal peanut consumption during pregnancy was controlled and household peanut consumption was subsequently compared with peanut protein in household dust throughout early childhood to tease out the effect of maternal peanut consumption during pregnancy and EPE during infancy.
The limitations of this study included missing living room dust samples in 153 (30%) of 512 participants, which might have introduced an element of bias. A serologic diagnosis of PA (sIgE, ≥5 kUA/L) rather than one based on oral food challenges was used, which meant that 150 children were excluded because of uncertainty about their peanut allergy outcome; this could also have introduced bias. Children with known peanut allergy were excluded at baseline; these children might have had even higher peanut protein levels in living room dust and thus even steeper predictive probability curves for peanut sensitization and PA. Subjects recruited who did not have moderate-to-severe AD had either cow's milk or egg allergy; this might have led to an unusual association between EPE and peanut SPT sensitization or PA in children with no history of AD. The dust sample obtained was a single baseline collection from one area of the home and thus might be prone to variation; however, previous studies have shown high within-home correlation of peanut protein levels in dust, and peanut protein levels from a single dust collection have been shown to correlate strongly with household peanut consumption over the previous 6-month period.15 Peanut protein levels in dust from the living-room floor were positively correlated with those found in the infants' bed (see Fig E2 in this article's Online Repository at www.jacionline.org). There was no detailed assessment of infant peanut consumption, which could potentially protect a child from high EPE, as per the findings of Fox et al,12 who showed that children who consumed peanut in the first year of life were not affected by high household peanut consumption. Animal data suggest that oral allergen exposure prevents induction of allergy,33 whereas epicutaneous exposure prevents induction of oral tolerance.8 The role of early high-dose peanut consumption on the prevention of peanut allergy is currently being investigated28 but has already been suggested in cross-sectional observational studies.34
In summary, these findings demonstrate a positive association between exposure to peanut protein in dust and peanut SPT sensitization and PA in atopic children. The effect of EPE on peanut sensitization and PA was augmented in children with a history of AD and severe AD for peanut sensitization after adjusting for other covariates. This provides biological plausibility that EPE might be sensitizing children through an impaired skin barrier, thus supporting the hypothesis of epicutaneous sensitization. We demonstrated the specificity of EPE on peanut SPT sensitization and PA by showing that EPE does not increase the risk of egg or cow's milk SPT sensitization; however, it would be interesting to assess the effect of other food allergens in dust and respective sensitization and allergy to these foods. Routes of exposure to food antigens appear to be crucial in determining whether food allergy or tolerance develops as per the dual-allergen exposure hypothesis.35,36 Although early consumption of food will inevitably lead to higher environmental exposure to foods, there are currently studies in place assessing the role of oral tolerance induction in young children (www.leapstudy.co.uk and www.eatstudy.co.uk); should these strategies fail to prevent the development of food sensitization and allergy, the alternative strategy of reducing environmental exposure to food allergens could be considered.
Key messages.
-
•
Increased environmental exposure to peanut protein is associated with an increased risk of sensitization and likely allergy to peanut in atopic children.
-
•
The effect of peanut dust exposure on peanut sensitization is augmented in children with a history of and increasing severity of AD.
-
•
The data are consistent with the hypothesis that allergic sensitization to peanut occurs through an impaired and inflamed skin barrier.
Acknowledgments
We thank the CoFAR Site Investigators F. M. Atkins, D. Y. M. Leung, T. T. Perry, and A. M. Scurlock and the CoFAR coordinators D. Brown, L. Talarico, S. Noone, K. Mudd, S. Knorr, P. Steele, J. Kamilaris, S. Carlisle, P. Mayfield, M. M. Beksinska, A. Hiegel, J. Straw, J. Ellingson, J. Stone, S. Leung, K. Morgan, S. Cushing, K. Brown-Engelhardt, and D. Fleischer. We also thank Dr Marshall Plaut, the medical officer, and J. Poyser for managing the project for CoFAR (NIAID) and Dr R. Lindblad and D. Rosenberg from EMMES. We thank the families who kindly participated. We also thank Professor A. Grieve for his statistical advice.
Footnotes
Funded by Action Medical Research (S/P/4529) and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at Guy's and St Thomas' NHS Foundation Trust and King's College London and National Institutes of Health (NIH)/National Institute of Allergy and Infectious Disease (NIAID) grants U19AI066738 and U01AI066560. The project was also supported by grants UL1 TR-000154 (National Jewish), UL1 TR-000067 (Mount Sinai), UL1 TR-000039 (Arkansas), UL1 TR-000083 (University of North Carolina), and UL1 TR-000424 (Johns Hopkins) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the BRC, NCRR, or NIH. This work was supported by the Wellcome Trust (Intermediate Clinical Fellowship WT086398MA to S.J.B. and Strategic Award 098439/Z/12/Z to W.H.I.M.).
Disclosure of potential conflict of interest: H. A. Brough has received research support from the Department of Health through the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St. Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust, Action Medical Research, UK and Food Allergy Research and Education (FARE), US. S. Sicherer is a member of the American Board of Allergy and Immunology; has consultant arrangements with Food Allergy Research and Education (FARE) and Novartis; has received research support from the National Institute of Allergy and Infectious Disease (NIAID) and Food Allergy Research and Education; has received royalties from UpToDate; and is an Associate Editor for the Journal of Allergy and Clinical Immunology and the Journal of Allergy and Clinical Immunology: In Practice. K. Makinson has received research support from the Immune Tolerance Network, National Institutes of Health (NIH). A. Douiri has received research support from the National Institute for Health Research. S. J. Brown has received research support from the Wellcome Trust Intermediate Clinical Fellowship (WT086398MA) and has received honorarium for speaking at the American Academy of Allergy, Asthma & Immunology annual meetings in 2012 and 2013. W. H. I. McLean has received research support from the Wellcome Trust. R. A. Wood has consultant arrangements with the Asthma and Allergy Foundation of America, is employed by Johns Hopkins University, has received research support from the NIH, and has received royalties from UpToDate. S. M. Jones has received research support from the NIAID, DBV Technologies, and Dyax; has consultant arrangements with the Gerson Lehrman Group; has received payment for lectures from Mercy Children's Hospital, the Greater Kansas City Allergy Society, the European Academy of Allergy and Clinical Immunology, and Riley Children's Hospital. W. Burks is a board member for the American Academy of Allergy, Asthma & Immunology, the Food Allergy Initiative, the Journal of Allergy and Clinical Immunology, the US Food and Drug Administration, and the NIH Study Section; has consultant arrangements with Abbott Laboratories, Dow AgroSciences, McNeill Nutritionals, Merck, Novartis Pharma AG, Schering Plough, GLG Research, ExploraMed Development, Regeneron Pharmaceuticals, and Unilever; is employed by the University of North Carolina; has received research support from Hycor Biomedical; has received payment for lectures from Mylan Specialty; has the following patents: US5558869, US5973121, US6441142, US6486311, US6835824, US7485708, US7879977; receives royalties from UpToDate; has received payment for development of educational presentations from Current Views 2012; and is a minority stockholder in Allertein and Mastcell Pharmaceuticals. P. Dawson has received research support from the NIH. D. Stablein has received research support from the NIH. H. Sampson has received research support from the NIAID and NIH; has received funding supporting clinical trials in milk and wheat allergy from Food Allergy Research and Education; is the chair of the PhARF Award review committee; has consultant arrangements with Allertein Therapeutics, Regeneron, and Danone Research Institute; and has received payment for lectures from Thermo Fisher Scientific, UCB, and Pfizer. G. Lack has received research support from the Department of Health through the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy's & St. Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust and Action Medical Research, UK; is a member of the Scientific Advisory Board for DBV Technologies; has consultant arrangements with the Anaphylaxis Campaign and the National Peanut Board; has received payment for lectures from Sodilac, Novartis, Nestle Nutrition, GlaxoSmithKline, and Serono Symposia International Foundation; and has stock/stock options with DBV Technologies. The rest of the authors declare that they have no relevant conflicts of interest.
Methods
EPE was also quantified from dust collected at baseline from the infant's bed. Families were asked to avoid changing their infant's bed sheet for 3 days before obtaining dust. Peanut protein levels in bed dust versus living room dust (in micrograms per gram) were compared by using the Mann-Whitney U test and the Spearman rank correlation coefficient (rs); additionally, Pearson correlation was used to compare log2 transformed peanut protein levels (in micrograms per gram) in dust from the infant's bed versus living room dust.
Results
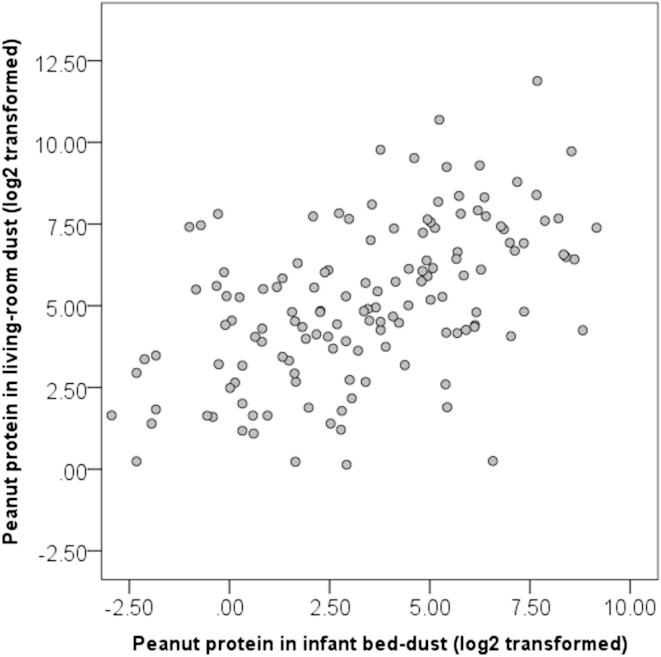
There were only 173 (33.8%) of 512 infant bed dust samples available for peanut protein analysis in the CoFAR observational study. This was because of a large proportion of homes in which no or less than 5 mg of dust was obtained from the infant's bed. Median peanut protein concentrations in the infant's bed (10.7 μg/g; IQR, 2.5-42.38 μg/g) were also significantly lower than peanut protein levels obtained from living room dust, where the concentration was approximately 4 times higher (39.1 μg/g; IQR, 13.4-150.60 μg/g; P < .001). However, peanut protein levels in the infant's bed and living room floor were positively correlated (n = 138; rs = 0.52; 95% CI, 0.39-0.63; P < .001; see Fig E2).
There was a trend toward a significant association between bed dust EPE values and peanut SPT sensitization on univariate analysis (n = 172; OR, 1.08; 95% CI, 0.97-1.21; P = .16); however, this was lost on multivariate analysis (n = 132; OR, 1.05; 95% CI, 0.92-1.20; P = .49) adjusting for ethnicity, egg SPT wheal diameter, and cow's milk SPT wheal diameter. There was no significant association between infant bed dust EPE and PA on univariate LR analysis for EPE (n = 109; OR, 0.93; 95% CI%, 0.80-1.06; P = .27). There was no interaction between AD or AD severity and bed dust EPE on peanut SPT sensitization or likely allergy.
Discussion
The lack of association between peanut protein levels in infant bed dust and peanut sensitization/PA is not surprising because peanut protein levels in bed dust correlate best with individual peanut consumptionE1 and most infants recruited to CoFAR would not have been eating peanut at this stage. This is reflected in the lower median peanut protein concentration found in the infant's bed compared with that in living room dust. The living room is the area that reflects the passage of most members of the family and thus the contribution of household peanut consumption to EPE. Furthermore, with the low number of dust samples and complete data sets we had using infant bed dust samples, we were underpowered to show an effect.
Fig E1.
A, Multivariate stratified predictive probability for the effect of EPE (displayed in log2 [microgram per gram] units and untransformed [microgram per gram] units) for peanut SPT sensitization in all children, children with a history of AD, and children with a history of severe AD. Results are adjusted for egg SPT wheal diameter, hay fever, maternal peanut consumption during pregnancy, and breast-feeding. B, Multivariate stratified predictive probability for the effect of EPE (displayed in log2 [microgram per gram] units and untransformed [microgram per gram] units) for peanut SPT sensitization in all children, children with a history of AD, and children with a history of severe AD. Results are adjusted for egg and milk wheal diameter and ethnicity.
Fig E2.
Scatter plot of peanut protein concentration in bed versus living room dust. The Spearman tank correlation coefficient (rs) was 0.521 (P < .001).
Table E1.
CoFAR demographics from the included group (n = 359) with available living room dust versus the excluded group (n = 153) and whole cohort (n = 512)∗
| Included group | Variable included group | Percentage of variable included group | Excluded group | Variable excluded group | Percentage of variable excluded group | P value | Whole CoFAR cohort | Variable whole cohort | Percentage of variable in cohort | |
|---|---|---|---|---|---|---|---|---|---|---|
| Peanut SPT sensitization ≥3 mm | 359 | 196 | 54.6% | 153 | 80 | 52.3% | .38 | 512 | 276 | 53.9% |
| Peanut sIgE ≥0.35 kU/mL | 359 | 214 | 59.6% | 153 | 91 | 59.5% | .96 | 512 | 305 | 59.6% |
| Likely PA (sIgE ≥5 kU/ml) | 209 | 89 | 42.6% | 99 | 45 | 45.5% | .40 | 308 | 134 | 43.5% |
| History of infantile AD | 359 | 332 | 92.5% | 153 | 139 | 90.8% | .28 | 512 | 471 | 92.0% |
| Maximum AD severity before study entry | ||||||||||
| No AD (0) | 359 | 27 | 7.5% | 153 | 14 | 9.2% | .28 | 512 | 41 | 8.0% |
| Mild (3-4) | 359 | 35 | 9.7% | 153 | 17 | 11.1% | .41 | 512 | 52 | 10.2% |
| Moderate (5-6) | 359 | 102 | 28.4% | 153 | 47 | 30.7% | .34 | 512 | 149 | 29.1% |
| Severe (7-9) | 359 | 195 | 54.3% | 153 | 75 | 49.0% | .04 | 512 | 270 | 52.7% |
| Nonwhite ethnicity | 359 | 81 | 22.6% | 153 | 51 | 33.3% | <.01 | 512 | 380 | 74.2% |
| FLG null mutation (excluding nonwhite subjects) | 275 | 41 | 14.9% | 101 | 15 | 14.9% | .98 | 376 | 56 | 14.9% |
| Parental report of hay fever ever in the child | 353 | 31 | 8.8% | 148 | 11 | 7.4% | .33 | 501 | 42 | 8.4% |
| Male sex | 357 | 240 | 67.2% | 153 | 103 | 67.3% | .97 | 512 | 345 | 67.4% |
| Maternal history of atopy or asthma | 356 | 243 | 68.3% | 153 | 102 | 66.7% | .52 | 509 | 345 | 67.8% |
| Paternal history of atopy or asthma | 350 | 217 | 62.0% | 150 | 89 | 59.3% | .31 | 500 | 306 | 61.2% |
| Maternal history of AD | 245 | 87 | 35.5% | 97 | 28 | 28.9% | .02 | 342 | 115 | 33.6% |
| Paternal history of AD | 215 | 71 | 33.0% | 85 | 27 | 31.8% | .69 | 300 | 98 | 32.7% |
| Peanut consumption during pregnancy | 352 | 295 | 83.8% | 152 | 124 | 81.6% | .28 | 504 | 419 | 83.1% |
| Peanut consumption during breast-feeding | 299 | 179 | 59.9% | 133 | 64 | 48.1% | <.01 | 432 | 243 | 56.3% |
| Peanut present in house while breast-feeding | 358 | 271 | 75.7% | 153 | 107 | 69.9% | .02 | 511 | 378 | 74.0% |
| Older siblings | 359 | 148 | 41.2% | 153 | 54 | 35.3% | .02 | 512 | 202 | 39.5% |
| Egg SPT wheal diameter, median (IQR) | 358 | 7.0 (4.0-10.5) | 153 | 7.5 (4.3-11.0) | .32 | 511 | 7.5 (4.0-10.5) | |||
| Cow's milk SPT wheal diameter, median (IQR) | 356 | 5.0 (0.0-9.0) | 155 | 4.0 (0.0-9.0) | .69 | 511 | 5.0 (0.0-9.0) | |||
| Breast-feeding duration (mo), median (IQR) | 359 | 5.0 (1.0-9.0) | 153 | 5.0 (2.0-9.0) | .15 | 512 | 5.0 (2.0-9.0) | |||
| Maternal age at baseline (y), median (IQR) | 357 | 32.0 (28.0-35.0) | 153 | 32.0 (28.5-35.0) | .78 | 510 | 32.0 (28.0-35.0) | |||
| Child's age at baseline assessment (mo), median (IQR) | 359 | 9.0 (7.0-12.0) | 153 | 9.0 (7.0-12.0) | .64 | 512 | 9 (7.0-12.0) | |||
| EPE (μg/g) in living room dust | 359 | 39.1 (13.4-150.6) | 153 | Not available | 359 | 39.1 (13.4-150.6) | ||||
| EPE (μg/g) in infant bed dust | 172 | 10.7 (2.5-42.8) | 340 | Not available | 172 | 10.7 (2.5-42.8) | ||||
Statistically significant values (P < .05) are shown in boldface.
Numbers and percentages of count data or medians (IQRs) of continuous factors and EPE values are shown.
References
- 1.Leung D.Y. Our evolving understanding of the functional role of filaggrin in atopic dermatitis. J Allergy Clin Immunol. 2009;124:494–495. doi: 10.1016/j.jaci.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 2.Elias P.M., Steinhoff M. “Outside-to-inside” (and now back to “outside”) pathogenic mechanisms in atopic dermatitis. J Invest Dermatol. 2008;128:1067–1070. doi: 10.1038/jid.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spergel J.M., Paller A.S. Atopic dermatitis and the atopic march. J Allergy Clin Immunol. 2003;112(suppl 6):S118–S127. doi: 10.1016/j.jaci.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Zheng T., Yu J., Oh M.H., Zhu Z. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67–73. doi: 10.4168/aair.2011.3.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hill D.J., Sporik R., Thorburn J., Hosking C.S. The association of atopic dermatitis in infancy with immunoglobulin E food sensitization. J Pediatr. 2000;137:475–479. doi: 10.1067/mpd.2000.108207. [DOI] [PubMed] [Google Scholar]
- 6.Lack G., Fox D., Northstone K., Golding J. Avon Longitudinal Study of Parents and Children Study Team. Factors associated with the development of peanut allergy in childhood. N Engl J Med. 2003;348:977–985. doi: 10.1056/NEJMoa013536. [DOI] [PubMed] [Google Scholar]
- 7.Strid J., Hourihane J., Kimber I., Callard R., Strobel S. Disruption of the stratum corneum allows potent epicutaneous immunization with protein antigens resulting in a dominant systemic Th2 response. Eur J Immunol. 2004;34:2100–2109. doi: 10.1002/eji.200425196. [DOI] [PubMed] [Google Scholar]
- 8.Strid J., Hourihane J., Kimber I., Callard R., Strobel S. Epicutaneous exposure to peanut protein prevents oral tolerance and enhances allergic sensitization. Clin Exp Allergy. 2005;35:757–766. doi: 10.1111/j.1365-2222.2005.02260.x. [DOI] [PubMed] [Google Scholar]
- 9.Bartnikas L.M., Gurish M.F., Burton O.T., Leisten S., Janssen E., Oettgen H.C. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J Allergy Clin Immunol. 2013;131:451–460. doi: 10.1016/j.jaci.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fallon P.G., Sasaki T., Sandilands A., Campbell L.E., Saunders S.P., Mangan N.E. A homozygous frameshift mutation in the mouse Flg gene facilitates enhanced percutaneous allergen priming. Nat Genet. 2009;41:602–608. doi: 10.1038/ng.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brough H.A., Simpson A., Makinson K., Sara B., Douiri A., Belgrave D. Peanut allergy: impact of environmental peanut exposure in children with filaggrin loss-of-function mutations. J Allergy Clin Immunol. 2014;134:867–875. doi: 10.1016/j.jaci.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox A.T., Sasieni P., Du Toit G., Syed H., Lack G. Household peanut consumption as a risk factor for the development of peanut allergy. J Allergy Clin Immunol. 2009;123:417–423. doi: 10.1016/j.jaci.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 13.Bertelsen R.J., Faeste C.K., Granum B., Egaas E., London S.J., Carlsen K.H. Food allergens in mattress dust in Norwegian homes—a potentially important source of allergen exposure. Clin Exp Allergy. 2014;44:142–149. doi: 10.1111/cea.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trendelenburg V., Ahrens B., Wehrmann A.K., Kalb B., Niggemann B., Beyer K. Peanut allergen in house dust of eating area and bed—a risk factor for peanut sensitization? Allergy. 2013;68:1460–1462. doi: 10.1111/all.12226. [DOI] [PubMed] [Google Scholar]
- 15.Brough H.A., Santos A., Makinson K., Penagos M., Stephens A.C., Fox A.T. Peanut protein in household dust is related to household peanut consumption and is biologically active. J Allergy Clin Immunol. 2013;132:630–638. doi: 10.1016/j.jaci.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Sicherer S.H., Wood R.A., Stablein D., Burks A.W., Liu A.H., Jones S.M. Immunologic features of infants with milk or egg allergy enrolled in an observational study (Consortium of Food Allergy Research) of food allergy. J Allergy Clin Immunol. 2010;125:1077–1083. doi: 10.1016/j.jaci.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts G., Lack G. Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol. 2005;115:1291–1296. doi: 10.1016/j.jaci.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 18.Sampson H.A. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 19.Maloney J.M., Rudengren M., Ahlstedt S., Bock S.A., Sampson H.A. The use of serum-specific IgE measurements for the diagnosis of peanut, tree nut, and seed allergy. J Allergy Clin Immunol. 2008;122:145–151. doi: 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 20.Sandilands A., Terron-Kwiatkowski A., Hull P.R., O'Regan G.M., Clayton T.H., Watson R.M. Comprehensive analysis of the gene encoding filaggrin uncovers prevalent and rare mutations in ichthyosis vulgaris and atopic eczema. Nat Genet. 2007;39:650–654. doi: 10.1038/ng2020. [DOI] [PubMed] [Google Scholar]
- 21.Palmer C.N.A., Irvine A.D., Terron-Kwiatkowski A., Zhao Y., Liao H., Lee S.P. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2009;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 22.Rajka G., Langeland T. Grading of the severity of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1989;144:13–14. doi: 10.2340/000155551441314. [DOI] [PubMed] [Google Scholar]
- 23.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186:23–31. doi: 10.1159/000247298. [DOI] [PubMed] [Google Scholar]
- 24.Brough H.A., Makinson K., Penagos M., Maleki S.J., Cheng H., Stephens A.C. Distribution of peanut protein in the home environment. J Allergy Clin Immunol. 2013;132:623–629. doi: 10.1016/j.jaci.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Park L.P., Coates S., Brewer V.A., Garber A.E., Abouzied M., Johnson K. Performance tested method multiple laboratory validation study of ELISA-based assays for the detection of peanuts in food. J AOAC Int. 2005;88:156–160. [PubMed] [Google Scholar]
- 26.Poms R.E., Agazzi M.E., Bau A., Brohee M., Capelletti C., Norgaard J.V. Inter-laboratory validation study of five commercial ELISA test kits for the determination of peanut proteins in biscuits and dark chocolate. Food Addit Contam. 2005;22:104–112. doi: 10.1080/02652030400027953. [DOI] [PubMed] [Google Scholar]
- 27.Hornung R.W., Reed L.D. Estimation of average concentration in the presence of nondetectable values. App Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 28.Du Toit G., Roberts G., Sayre P., Plaut M. Identifying infants at high risk of peanut allergy—the LEAP Screening Study. J Allergy Clin Immunol. 2013;131:135–143. doi: 10.1016/j.jaci.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 29.Brown S.J., Asai Y., Cordell H.J., Campbell L.E., Zhao Y., Liao H. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J Allergy Clin Immunol. 2011;127:661–667. doi: 10.1016/j.jaci.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown S.J., Irvine A.D. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128–137. doi: 10.1016/j.sder.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Londin E.R., Keller M.A., Maista C., Smith G., Mamounas L.A., Zhang R. CoAIMs: a cost-effective panel of ancestry informative markers for determining continental origins. PLoS One. 2010;5:e13443. doi: 10.1371/journal.pone.0013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sicherer S.H., Wood R.A., Stablein D., Lindblad R., Burks A.W., Liu A.H. Maternal consumption of peanut during pregnancy is associated with peanut sensitization in atopic infants. J Allergy Clin Immunol. 2010;126:1191–1197. doi: 10.1016/j.jaci.2010.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita H., Takahashi K., Tanaka H., Nagai H., Inagaki N. Overcoming food allergy through acquired tolerance conferred by transfer of Tregs in a murine model. Allergy. 2012;67:201–209. doi: 10.1111/j.1398-9995.2011.02742.x. [DOI] [PubMed] [Google Scholar]
- 34.Du Toit G., Katz Y., Sasieni P., Mesher D., Maleki S.J., Fisher H.R. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J Allergy Clin Immunol. 2008;122:984–991. doi: 10.1016/j.jaci.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 35.Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012;129:1187–1197. doi: 10.1016/j.jaci.2012.02.036. [DOI] [PubMed] [Google Scholar]
- 36.Lack G., Golding J. Peanut and nut allergy. Reduced exposure might increase allergic sensitisation. BMJ. 1996;313:300. doi: 10.1136/bmj.313.7052.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reference
- Brough H.A., Makinson K., Penagos M., Maleki S.J., Cheng H., Stephens A.C. Distribution of peanut protein in the home environment. J Allergy Clin Immunol. 2013;132:623–629. doi: 10.1016/j.jaci.2013.02.035. [DOI] [PubMed] [Google Scholar]