To the Editor:
Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is an inherited syndrome of early-onset systemic autoimmunity and the prototype of immune dysregulatory disorders. It is caused by mutations of forkhead box p3 (FOXP3) gene (Xp11.23), encoding a key transcription factor for natural regulatory T (nTreg) cells.1 Treg cell dysfunction leads to severe multi-organ autoimmune phenomena including enteropathy, dermatitis, endocrinopathy, and other organ-specific diseases.2 Patients often present early in infancy and, without treatment, usually die in the first years of life. The only effective cure is hematopoietic stem cell transplantation (HSCT). However, the outcome is variable. Early HSCT with non-myeloablative conditioning provides the best outcome, before organs are damaged by autoimmunity and/or adverse effects of therapy.3 Complete donor engraftment in all hematopoietic lineages may not be necessary, because the preferential engraftment of donor Treg cells seems to be sufficient to control the disease.4,5
With parental informed consent and Newcastle upon Tyne Hospitals National Health Service Foundation Trust approval, we report the case of an IPEX patient presenting with severe enteropathy, dermatitis, and other signs of autoimmunity since 3 to 4 weeks of age. The identified FOXP3 gene mutation was associated with a reduced protein expression in Treg cells (Figs E1 and E2 available in this article's Online Repository at www.jacionline.org). Therefore, at 6 months of age, he received an unmanipulated, unrelated donor cord blood stem cell transplant (1 DP mismatch) with sub-myeloablative conditioning and graft-versus-host disease (GvHD) prophylaxis (see this article's Methods section and Fig E3 in the Online Repository at www.jacionline.org). He had an uneventful engraftment and a reasonable immune reconstitution (Fig E4 in this article's Online Repository at www.jacionline.org). Chimerism analysis in peripheral blood showed 90% donor T lymphocytes during the first 6 months after the transplant, with a decrease and stabilization at 70% 1 year post-transplant (Fig E5 in this article's Online Repository at www.jacionline.org). Consistent with the sub-myeloablative conditioning regimen the patient has received, mixed myeloid chimerism was also observed; however, as previously reported,4-6 donor myeloid chimerism is not necessary to control the disease. Moreover, FOXP3 protein expression by Treg cells increased over time (Fig E2).
Despite good post-transplant immune reconstitution, the patient continued to suffer from diarrhea and malabsorption and was dependent on parenteral nutrition. He developed episodes of upper intestinal obstruction and, despite anti-inflammatory therapy with an anti-TNF-α agent, required jejunal resections at 3, 4, and 6 months. Histopathology of resected areas revealed severe chronic mucosal injury without histological signs of GvHD, with an improvement of the architecture over time (see Fig E6 in this article's Online Repository at www.jacionline.org).7 The gut dysfunction improved progressively from month 6 to 9, and at 1 year post-transplant, the patient was independent of parenteral nutrition and thriving on enteral feeding.
The intestine has a major interface with the external environment, and its integrity is important in the maintenance of immune homeostasis.8 The intestinal mucosa contains an extensive network of secondary lymphoid tissues and is home to several lymphocyte subsets, including intestine-specific subpopulations. The beta-7 integrins (α4β7 and αEβ7) are selective mediators of lymphocyte homing to the gut-associated lymphoid tissue. In particular, α4β7 is expressed at low levels on naive T and B cells and at high levels on effector and memory T (mainly CD4+) cells.9
Because the persistence of enteropathy in the patient was inconsistent with the transplant outcome, we explored the hypothesis that intestinal immune reconstitution proceeded at a different pace to that in the peripheral blood with a delay in the re-establishment of homeostasis within the gut immune system. We therefore investigated the engraftment of donor lymphocytes in the gut mucosa to evaluate any differences between peripheral blood and gut immune reconstitution that might explain the clinical course.
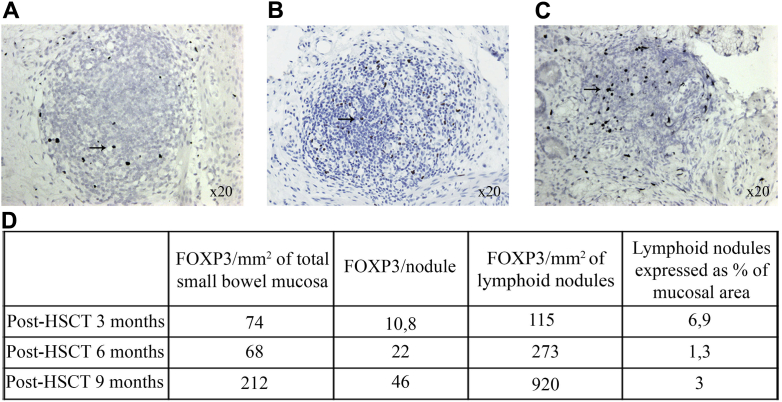
To study gut immune reconstitution, we probed for FOXP3+ and CD4+ T cells on tissue sections of small bowel mucosa at different times after transplant (3, 6, and 9 months). We observed the presence of lymphoid nodules numerically decreasing over time, with an increased proportion of FOXP3+ cells both within nodules and the mucosal area (Fig 1), suggesting a reduction of the small bowel inflammatory state.
Fig 1.
FOXP3 “reactive nuclei” (arrow) in and around lymphoid follicles in small bowel mucosa and submucosa on immunohistochemically stained sections at 3 months (A), 6 months (B), and 9 months (C) post-transplant. (FOXP3 reactive nuclei are stained black, and the sections are counterstained with hematoxylin.) D, Quantitative analysis of FOXP3+nuclei in the examined total small bowel mucosal and submucosal tissue.
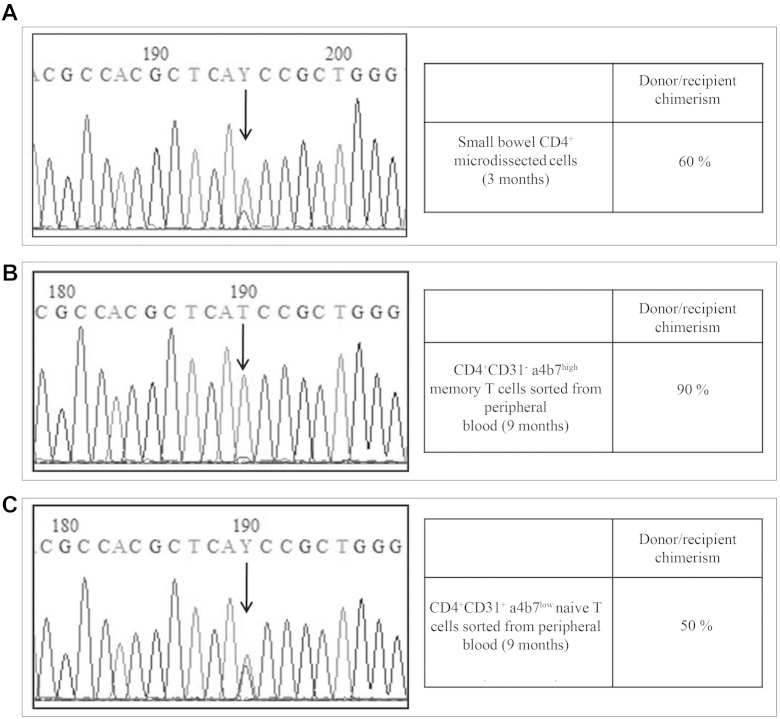
We isolated CD4+ cells from small bowel tissue sections obtained at 3 months post-transplant and investigated their origin by genotyping FOXP3 (donor or recipient) to evaluate the donor chimerism within the relevant cellular compartment. Both wild-type and mutated nucleotides were present at the c.1037 position on the FOXP3 gene, suggesting a mixed population of lymphocytes in the gut. This was confirmed by chimerism analysis of polymorphic markers on the same cell population showing 60% donor, 40% recipient origin (Fig 2, A), while donor chimerism in the blood CD3+T cells was 91% (Fig E5). We set out to study circulating gut-homing lymphocytes in a patient blood sample, which was available at only 9 months after HSCT. We recovered CD4+CD31+α4/β7low naive and CD4+CD31−α4/β7high memory T cells by cell sorting. Sequence analysis showed wild-type FOXP3 sequence and 90% donor chimerism on CD4+CD31−α4β7high gut-homing lymphocytes, whereas wild-type and mutated FOXP3 genes along with a 50% donor chimerism were found on CD4+CD31+α4/β7low naive T cells not specifically committed to the intestine (Fig 2, B and C).
Fig 2.
Exon 9 FOXP3 sequencing and chimerism analysis on laser microdissected CD4+ T cells from small bowel biopsies at 3 months post-transplant (A) and on CD31−CD4+α4β7high memory T cells (B) and CD31+CD4+α4β7low naive T cells (C) sorted from peripheral blood at 9 months post-transplant.
Our results suggest that, in this patient, the gut immune system took longer to recover and function compared with the peripheral immune system (Fig E3). FOXP3 expression assessed in small bowel biopsies taken at different times post-transplant was found to correlate with the patient's clinical condition. Gut function and tolerance of enteral nutrition progressively improved in parallel with the increased FOXP3 expression within the gut mucosa and the appearance of donor CD4+CD31−α4/β7high cells. Furthermore, cells of donor origin were mostly present in the periphery rather than in the gut early after transplant, possibly explaining poor gut function. The increase in donor chimerism within gut-homing lymphocytes was associated with a progressive rise of FOXP3+ cells within the small bowel later on, possibly suggesting that a preferential homing of donor Treg cells to the gut is associated with disease recovery. Further studies in additional patients will be required to determine if this is applicable to other patients with IPEX.
To our knowledge, this is the first study of gut immune reconstitution in a patient affected with an inherited disorder of immune tolerance, and we believe it could be a unique case study that sheds light on the role of the intestine in reconstituting the immune system after HSCT. Further investigation of function of external factors (such as microbiota) in influencing the imprinting of mucosal immunity will help to identify new therapeutic approaches.
Acknowledgments
We thank the patient and his family for their support and cooperation. We greatly appreciated the collaboration with Pamela Pinzani of the Department of Biomedical, Experimental, and Clinical Science, University of Florence, Florence, Italy, for her help in microdissection experiments, and with colleagues at the Institute of Cellular Medicine of Newcastle University and at the Flow Cytometry Core Facility Laboratory, Centre for Life, Newcastle Upon Tyne, United Kingdom, for technical support in cell sorting.
Footnotes
This study was supported by a Telethon grant (GGP07241) and by the Italian Ministry of Health (GR-2007-686104).
Disclosure of potential conflict of interest: P. Lionetti is an advisory board member for AbbVie, has received a fee for delivering a lecture at a European Crohn's and Colitis Organisation meeting/Danone research symposium, and has received payment for development of an educational presentation at a PKG srl Educational packages meeting for pediatricians. B. Haugk has received payment for preparation of a review article for Surgery Oxford in 2013 and a voucher for contribution to a chapter in Underwood's textbook in pathology in 2013. The rest of the authors declare that they have no relevant conflicts of interest.
Methods
Subject
The male child included in this study presented at 3 to 4 weeks of life with severe enteropathy characterized by watery diarrhea that did not respond to different types of enteral feeding. He then developed eczematous-like skin changes. Moreover, he had been noted to be hypotonic at birth, with dysmorphic facial characteristics. Small bowel biopsies evidenced an eosinophilic infiltrate and villous atrophy.
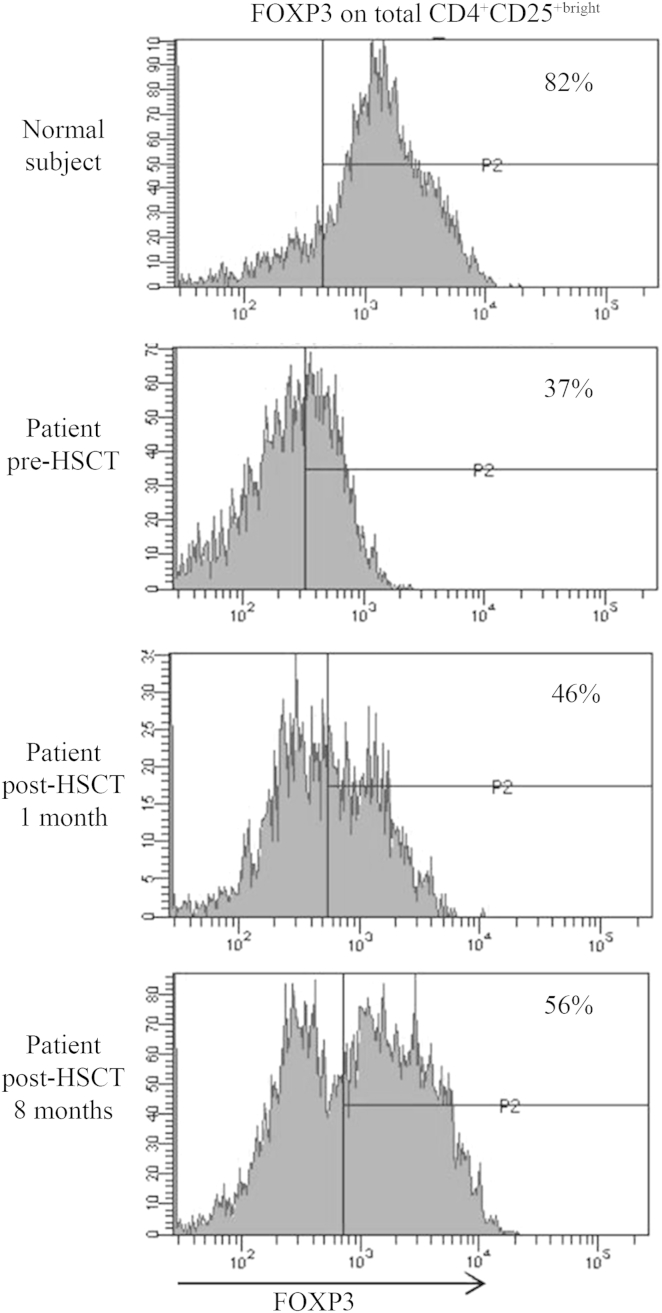
Investigations showed a positive Coombs test, positive islet cell antibodies and high IgE (1278 UI/L). The above-mentioned findings were suggestive for IPEX syndrome. Genetic testing confirmed IPEX with a homozygous missense mutation in exon 9 of FOXP3 (c.1037T>C), causing an amino acid substitution (p.Iso346Thr) in the forkhead domain of the protein.E1 Analysis of FOXP3 protein on peripheral lymphocytes showed that its expression was reduced and was lower in the patient's Treg cells compared with those of the normal control (37% of FOXP3 expressing CD4+CD25bright cells in the patient vs 82% in the control; Fig E2). Accordingly, immunohistochemistry of small bowel biopsies revealed the presence of FOXP3 protein, although presumably it was not functional (Fig E1).
To cure his disease, at 6 months of age, the patient received an unmanipulated, unrelated donor cord blood stem cell transplant (1 DP mismatch) with sub-myeloablative conditioning (Alemtuzumab 0.3 mg/kg, Fludarabine 150 mg/m2, and Treosulfan 36 g/m2) and GvHD prophylaxis (tacrolimus, mycophenolate mofetil).E2
Written informed consent was obtained from the patient's parents, and the study was approved by the local ethics committee.
Immunologic studies
T, B, and natural killer cells were analyzed on peripheral blood at different times after the transplant (Day 0 is considered the day of graft infusion). Monoclonal antibodies specific for CD45RA, CD3, CD4, CD8, CD19, CD15, CD16/56, and CD45 (BD Biosciences, San Jose, Calif) were used to define percentage of cell subset on gated lymphocytes. Absolute numbers of CD8+ cells, total CD4+ and CD4+ naive T cells, B cells, and natural killer cells were calculated.
For identification of Treg cells, PBMCs were stained with the surface molecules CD4 and CD25 and then fixed, permeabilized, and stained with intracellular FOXP3 (eBioscience, San Diego, Calif). Cell fluorescence was evaluated with FACSCanto II flow cytometry and analyzed with FACSDiva software (BD, San Jose, Calif).
Immunohistochemistry and analysis of stained sections
Immunohistochemistry to detect FOXP3 was carried out on tissue sections received from the histopathology archives of Newcastle upon Tyne Teaching Hospitals National Health Service Trust. All tissue was used in accordance with Newcastle and North Tyneside Local Research Ethics Committee approval.
Immunohistochemistry methods were as previously published, with minor modifications.E3 Four μm paraffin sections on positively charged slides were deparaffinized and partially rehydrated to 95% ethanol. Endogenous peroxidase was blocked in methanol/hydrogen peroxide for 10 minutes at room temperature (RT) followed by washing in standard TRIS-buffered saline (TBS) (pH 7.6). Antigen retrieval was achieved at low temperature (65°C) in EDTA buffer (pH 8.0) for approximately 21 hours in a hot air oven. Following a wash in TBS, endogenous biotin was blocked (Vector, Hartfield, United Kingdom), and nonspecific binding of antibodies was minimized by incubation of sections with 20% normal swine serum (NSS) in TBS. Overnight incubation with primary antibody (anti-FOXP3 at 1/10 in NSS) supernatant received from Dr Alison Banham, Oxford, at 4°C was followed by washing in TBS, then incubation at RT for 1 hour with biotinylated goat anti-mouse immunoglobulins (Vector: 1/200 in NSS). Subsequently, the antibody complex was detected with avidin-biotin peroxidase (Vector: 30 minutes at RT) and nickel diaminobenzidine substrate (Sigma-Aldrich, St Louis, Mo) at RT. The counterstain was Mayer's hematoxylin, and slides were dehydrated, cleared, and mounted in distrene plasticizer xylene.
Sections were scanned using an Aperio ScanScope Digital Scanner (Leica Biosystems, Nussloch, Germany). Analysis of digital images was carried out with Imagescope software, and a nuclear algorithm was used to enumerate black FOXP3+ nuclei. Submucosal tissue was excluded from the analysis.
Preparation of membrane-mounted paraffin sections for laser-assisted microdissection: Identification of CD4+ cell populations using immunohistochemistry
Identification of CD4+ cell populations for laser-assisted microdissection was carried out according to Gjerdrum et al.E4 Serial 5 μm paraffin sections, cut with a fresh blade, were placed on APES-coated PEN-membrane slides and dried at 60°C for 2 hours.
Deparaffinization was carried out in fresh xylene for at least 2 × 10 minutes, followed by prolonged washing and partial rehydration in absolute then 95% ethanol. Subsequent steps to detect CD4+ cells were as for FOXP3+ cells above. Primary anti-CD4 antibody was used at 1/50 in NSS (Leica Biosystems, Novocastra).
No counterstain was applied, and slides were dehydrated in fresh ethanol (graded) then stored at RT. Groups of CD4+ T cells were subsequently microdissected and collected by gravity into the lid of a 0.5-mL reaction tube using Leica LMD6500 laser microdissector (Leica Microsystems) or isolated by laser capture and pressure catapulting with the PALM Laser MicroBeam system (Carl Zeiss MicroImaging GmbH, Oberkochen, Germany).
Gut-homing cell sorting
Gut-homing T cells were isolated with cell sorting (FACSAriaII, BD Biosciences). Briefly, 500 uL of whole blood was stained with specific monoclonal antibodies CD3-PerCp-Cy5.5, CD4-PE-Cy7, CD31-PE, CD49d-FITC, beta7-APC (BD Biosciences), and incubated for 15 minutes at room temperature in the dark. Red cells were lysed, and the sample was then washed with PBS. CD4+CD31+α4/β7low naive and CD4+CD31−α4/β7high memory T cells were collected in PBS and stored as a pellet at −80°C.
gDNA isolation from gut microdissected and sorted CD4+ T cells
About 50,000 μm2 of microdissected CD4 T cells and 100,000 sorted CD4+CD31−α4/β7high memory and CD4+CD31+α4/β7low naive T cells were lysed in proteinase K buffer (Qiagen, Germantown, Md). The nucleic acids were purified with Chelex 100 (Bio-Rad, Hercules, Calif) and concentrated with Amicon Ultra 0.5 mL filters (Merck KGaA, Millipore, Darmstadt, Germany).
FOXP3 sequence analysis
Genomic DNA (gDNA) was isolated from peripheral blood using the QIAamp DNA Blood Mini Kit (Qiagen). The FOXP3 coding sequence, including the exon-intron junction and the poly-A region were amplified from gDNA by PCR with specific primer pairs flanking the regions of interest, using a 2700 thermal cycler (Thermo Fisher Scientific, Life Technologies, Waltham, Mass). Primer pair sequences were already reported in the literature.E5
Approximately 50 ng of genomic DNA were amplified with the following conditions: 94°C 4 minutes, 35 cycles at 94°C 30 seconds, 30 seconds at a primer-specific melting temperature, and 68°C for 30 seconds, with a final extension at 68°C for 5 minutes. PCR products were purified using the ExoSAP-IT reagent (Affymetrix, USB, Santa Clara, Calif) and sequenced by using the BigDye Terminator Cycle Sequencing Kit (Thermo Fisher Scientific, Life Technologies) on an automated 3130 × l Genetic Analyzer (Thermo Fisher Scientific, Life Technologies). Sequencing analysis was carried out with BioEdit sequence alignment software (Ibis Therapeutics, Carlsbad, Calif).
Chimerism analysis
Chimerism was evaluated by multiple fluorescent short tandem repeat analysis using the AmpFlSTR Identifiler amplification kit (Thermo Fisher Scientific, Life Technologies) according to the manufacturer's instructions. The tetranucleotide short tandem repeats loci amplified in this reaction included D8S1179, D21S11, D7S820, and CSF1PO (all labeled with 6-FAM blue dye); D3S1358, TH01, D13S317, D16S539, and D2S1338 (all labeled with VIC green dye); D19S3433, vWA, TPOX, and D18S51 (all labeled with NED yellow dye); and D5S818 and FGA (all labeled with PET red dye). In addition, the amelogenin locus was analyzed to differentiate the X and Y chromosomes (labeled with PET red dye). The PCR products were analyzed with an ABI PRISM 310 DNA Genetic Analyzer (Thermo Fisher Scientific, Life Technologies). Fragment size and peak area data were determined by GeneScan software (Thermo Fisher Scientific, Life Technologies). The degree of chimerism was calculated using the method described elsewhere.E6
Fig E1.
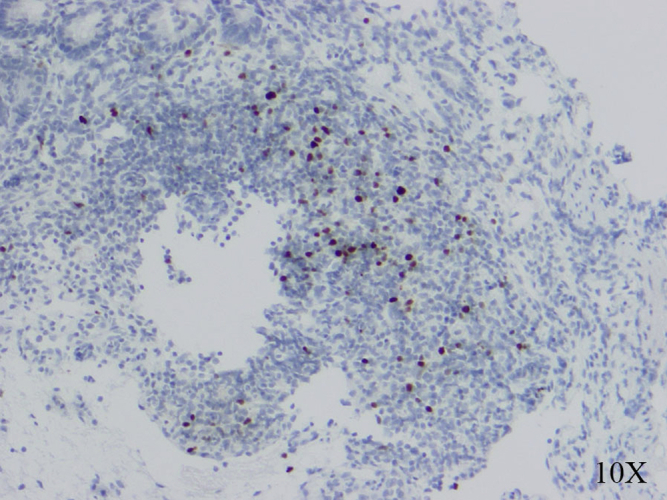
Immunohistochemical staining for FOXP3+ cells in pretransplant small bowel mucosa and submucosa section from small bowel biopsy (FOXP3 reactive nuclei are stained black, and the sections are counterstained with hematoxylin).
Fig E2.
Intracytoplasmic FOXP3 expression in peripheral blood cells before and at indicated time points after transplant. Gates for analysis were set on CD4+CD25+bright T cells. x axis/arrow, Fluorescence intensity; y axis, cell counts.
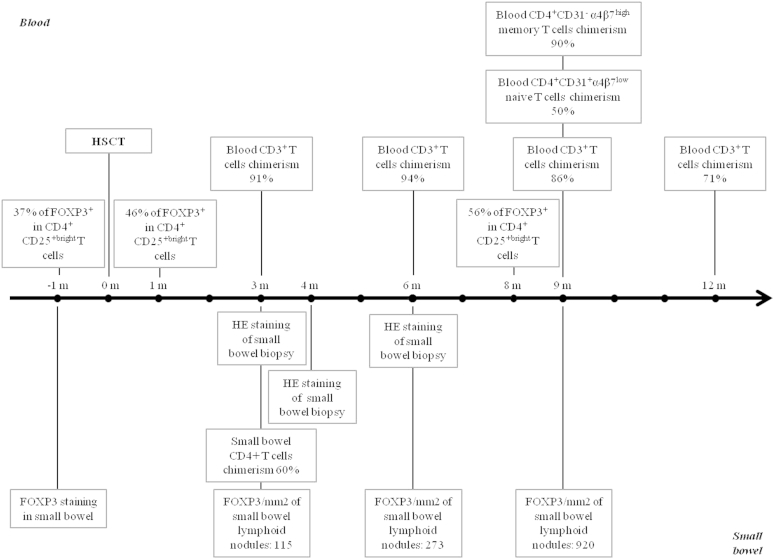
Fig E3.
Timeline representing experiments and analysis performed during this study. HE, Hematoxylin-eosin.
Fig E4.
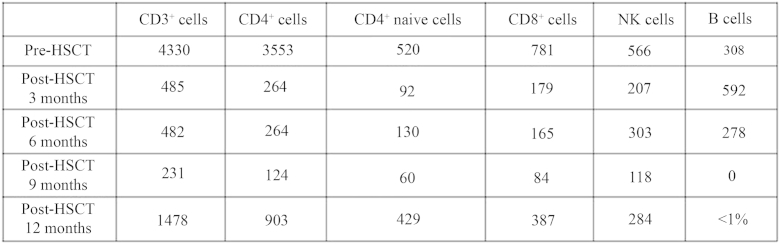
Absolute count of T, B, and natural killer (NK) cells (per uL blood) in peripheral blood pre- and post-HSCT (3, 6, 9, and 12 months).
Fig E5.
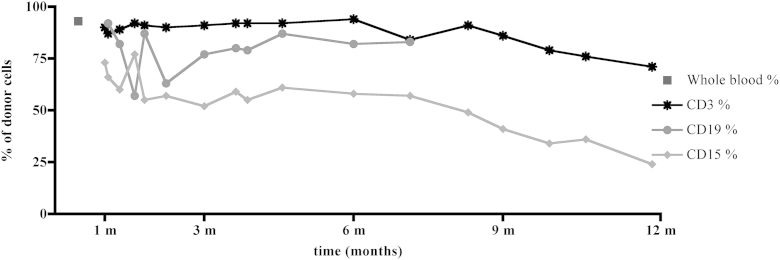
Donor cell chimerism in peripheral blood T cells (CD3+), B cells (CD19+), and monocytes (CD15+) during 1 year post-transplant, shown in months (m).
Fig E6.
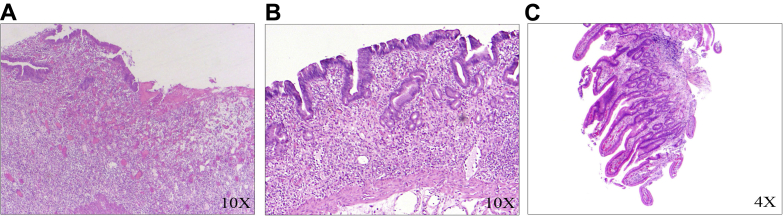
Histopathological findings of the resected small bowel specimens. Hematoxylin-eosin (HE) staining showed loss of mucosal epithelium with total villous atrophy at both 3 months (A) and 4 months (B) post-transplant and long slender villi with normal brush borders at 6 months post-transplant (C). No histologic evidence of GvHD.
References
- 1.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 2.Gambineri E., Torgerson T.R., Ochs H.D. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance (IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T-cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Rao A., Kamani N., Filipovich A., Lee S.M., Davies S.M., Dalal J. Successful bone marrow transplantation for IPEX syndrome after reduced-intensity conditioning. Blood. 2007;109:383–385. doi: 10.1182/blood-2006-05-025072. [DOI] [PubMed] [Google Scholar]
- 4.Seidel M.G., Fritsch G., Lion T., Jurgens B., Heitger A., Bacchetta R. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113:5689–5691. doi: 10.1182/blood-2009-02-206359. [DOI] [PubMed] [Google Scholar]
- 5.Barzaghi F., Passerini L., Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slatter M.A., Cant A.J. Hematopoietic stem cell transplantation for primary immunodeficiency diseases. Ann N Y Acad Sci. 2011;1238:122–131. doi: 10.1111/j.1749-6632.2011.06243.x. [DOI] [PubMed] [Google Scholar]
- 7.Patey-Mariaud de Serre N., Canioni D., Ganousse S., Rieux-Laucat F., Goulet O., Ruemmele F. Digestive histopathological presentation of IPEX syndrome. Mod Pathol. 2009;22:95–102. doi: 10.1038/modpathol.2008.161. [DOI] [PubMed] [Google Scholar]
- 8.Nenci A., Becker C., Wullaert A., Gareus R., van Loo G., Danese S. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 9.Williams M.B., Butcher E.C. Homing of naive and memory T lymphocyte subsets to Peyer's patches, lymph nodes, and spleen. J Immunol. 1997;159:1746–1752. [PubMed] [Google Scholar]
References
- Passerini L., Olek S., Di Nunzio S., Barzaghi F., Hambleton S., Abinun M. Forkhead box protein 3 (FOXP3) mutations lead to increased TH17 cell numbers and regulatory T-cell instability. J. Allergy Clin. Immunol. 2011;128:1376–1379.e1. doi: 10.1016/j.jaci.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Nademi Z., Slatter M., Gambineri E., Mannurita S.C., Barge D., Hodges S. Single centre experience of haematopoietic SCT for patients with immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Bone Marrow Transplant. 2013;49:310–312. doi: 10.1038/bmt.2013.181. [DOI] [PubMed] [Google Scholar]
- Robertson H., Kirby J.A., Yip W.W., Jones D.E., Burt A.D. Biliary epithelial-mesenchymal transition in posttransplantation recurrence of primary biliary cirrhosis. Hepatology. 2007;45:977–981. doi: 10.1002/hep.21624. [DOI] [PubMed] [Google Scholar]
- Gjerdrum L.M., Lielpetere I., Rasmussen L.M., Bendix K., Hamilton-Dutoit S. Laser-assisted microdissection of membrane-mounted paraffin sections for polymerase chain reaction analysis: identification of cell populations using immunohistochemistry and in situ hybridization. J Mol Diagn. 2001;3:105–110. doi: 10.1016/S1525-1578(10)60659-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambineri E., Perroni L., Passerini L., Bianchi L., Doglioni C., Meschi F. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122:1105–1112.e1. doi: 10.1016/j.jaci.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Thiede C., Florek M., Bornhäuser M., Ritter M., Mohr B., Brendel C. Rapid quantification of mixed chimerism using multiplex amplification of short tandem repeat markers and fluorescence detection. Bone Marrow Transplant. 1999;23:1055–1060. doi: 10.1038/sj.bmt.1701779. [DOI] [PubMed] [Google Scholar]