Abstract
The ability to recognize a variety of different human faces is undoubtedly one of the most important and impressive functions of the human perceptual system. Neuroimaging studies have revealed multiple brain regions (including the FFA, STS, OFA) and electrophysiological studies have identified differing brain event-related potential (ERP) components (e.g., N170, P200) possibly related to distinct types of face information processing. To evaluate the heritability of ERP components associated with face processing, including N170, P200, and LPP, we examined ERP responses to fearful and neutral face stimuli in monozygotic (MZ) and dizygotic (DZ) twins. Concordance levels for early brain response indices of face processing (N170, P200) were found to be stronger for MZ than DZ twins, providing evidence of a heritable basis to each. These findings support the idea that certain key neural mechanisms for face processing are genetically coded. Implications for understanding individual differences in recognition of facial identity and the emotional content of faces are discussed.
Keywords: N170, P200, Twins, Faces
Though it is undisputed that humans are experts at perceiving and recognizing other human faces, research over the last several decades has generated considerable debate over the neural bases of this ability (Yovel & Kanwisher, 2004; Kanwisher, Stanley, & Harris, 1999; Kanwisher & Yovel, 2006; Gauthier & Logothetis, 2000; Tarr & Gauthier, 2000; McKone, Kanwisher, & Duchaine, 2006; Nelson, 2001; Bentin & Carmel, 2002; Carmel & Bentin, 2002). It is clear from neuroimaging studies that there are structures within the brain that are preferentially responsive to face stimuli--including the fusiform face area (FFA), the superior temporal sulcus (STS), and the occipital face area (OFA); however, the field is divided as to whether the “face-specific” capacity of these neural structures has a constitutional basis, or develops through experience (Kanwisher & Yovel, 2006; Gauthier & Logothetis, 2000). At the forefront of this debate is whether face-processing abilities reflect a domain-specific mechanism, involving face-specific cognitive and neural processes encoded at a basic gene level (i.e., domain-specificity; Carmel & Bentin, 2002; Wilmer, et al., 2010; Yovel & Kanwisher, 2004; Zhu, et al., 2010), or if instead they result from an experience-dependent mechanism, involving neural changes arising from repeated exposure to human face stimuli that facilitate processing of such stimuli (Diamond & Carey, 1986; Gauthier & Logothetis, 2000; Tarr & Gauthier, 2000). Alternatively, it is possible that face processing ability, analogous to the capacity for speech, arises from factors of both types--with inborn, genetically based domain-specific mechanisms requiring specific exposure to faces during a critical period in development (experience-expectant) for face-specific modules to be established and maintained into adulthood (Nelson, 2001).
Recent research investigating the heritability of face processing has attempted to shed light on this debate. For example, Zhu et al. (2010) evaluated whether face-processing abilities are heritable by examining three different cognitive/face-processing phenomena (face-specific recognition ability, face inversion effect, composite-face effect) in monozygotic (MZ) and dizygotic (DZ) twins. Importantly, the tasks these authors employed allowed for specific face-processing abilities to be measured separately from lower level visual processes, attention, or decision-making. Face processing specificity was accomplished by including and contrasting matched non-face stimuli (e.g., houses) in each of the three cognitive/face-processing tasks. Face-specific abilities were quantified based on the difference in performance between the face and non-face conditions. In addition, Zhu et al. (2010) collected measures of high-level cognitive functions (e.g., IQ and global visual processing measures) to contrast and similarly factor out the heritability of so-called “generalist gene” effects. Results of this study clearly demonstrated a prominent genetic component to face processing, distinct from either low-level visual processes or more general cognitive functions.
Similarly, Wilmer et al. (2010) tested whether face processing is a heritable ability, separate from broader visual and memory functions. In this large-sample study, performance scores for same-sex twins MZ and DZ were compared across three different tasks: (1) the Cambridge Face Memory Task (CFMT), which tested subjects’ ability to process and remember facial features in the absence of external head/hair shape and color cues, (2) a newly developed Abstract Art Memory test (AAM), which served as a non-face visual memory control, and (3) the Verbal Paired-Associates Memory test (VPAM), which assessed subjects’ ability to remember non-visual cues. The observed concordance for MZ twins on the CFMT was over twice that for DZ twins, indicating a high degree of genetic contribution to face processing. Results from the other two tasks indicated that this contribution was not attributable to heritability of non-face or non-visual memory abilities.
In other work, Polk et al. (2007) investigated the heritability of functionally defined regions of the ventral visual cortex using functional magnetic resonance imaging (fMRI). In this study, MZ and DZ twins performed a “one-back task” while viewing black and white pictures from five visual categories (faces, places [images of houses], pseudowords, objects [chairs], and phase-scrambled control images) known to activate differing regions of the ventral visual cortex. Though functionally defined regions were not localized with high degrees of selectivity, these authors found higher concordance of activations to face and place stimuli (but not pseudoword or object stimuli) for MZ as compared to DZ twins in the ventral visual cortex.
Another study by Anokhin, Golosheykin, and Heath (2010) investigated the heritability of affective face processing by analyzing brain event-related potential (ERP) responses to continuous presentations of neutral, happy, and fearful faces in MZ and DZ twins. Results demonstrated heritability of facial-affect response effects for two distinct ERP components, the N240 and the P300. Critically, however, due to the nature of the research questions investigated in this study and the experimental design used to address them, a key ERP component of interest in the face processing literature, the N170, was not evaluated for heritability. The N170 is a negative-going brain response that occurs at temporal-parietal electrode sites approximately 170 ms after the presentation of a visual stimulus, which appears to be maximally responsive to the presentation of faces (Bentin et al., 1996). Although the neural generator of the N170 has not been definitively located, multiple studies utilizing simultaneous EEG-fMRI recordings have established a clear correlation between activations of “face areas” in the ventral visual cortex, including the FFA and STS, and face-selectivity of the N170 (Sadeh et al., 2010; Yovel et al., 2008).
Debate surrounding the N170 centers around whether this response reflects face-specific processing or expertise-specific processing, essentially paralleling the broader domain-specific versus experience-dependent face processing debate (Bentin & Carmel, 2002; Rossion, Curran & Gauthier, 2002). A focal point of this debate pertains to findings showing that although the N170 is maximally responsive (has greatest negative peak amplitude) to face stimuli as compared to stimuli of other types (houses, cars, etc.), extensive expertise with a particular category of stimuli (e.g, cars, birds, etc.) tends to result in enhanced N170 response to stimuli of that type in comparison to other “non-expert” stimuli. These results point to the idea that maximal N170 responses to face stimuli simply reflect the very high expertise that adult humans have in general, for the processing of faces, due to widespread exposure to faces of differing types from birth (Rossion, Curran, & Gauthier, 2002).
Building on this prior published work, a major aim of the current study was to evaluate the heritability of the N170 in a face processing context by recording ERP responses to face versus non-face stimuli from MZ and DZ twins and comparing concordance of N170 amplitude to stimuli of each type across the two. Based upon findings summarized above, we hypothesized that if amplitude of the N170 response to faces is determined in part by genetic influences, either domain-specific or experience-expectant, then N170 enhancement for face as compared to non-face stimuli should show higher concordance for MZ as compared to DZ twin pairs.
In addition to gaining further insight into the heritability of the “faceness” component of stimulus processing as indexed by N170 response enhancement for faces versus nonfaces, the current study also investigated an additional component of face-processing that is arguably of equal importance--namely, detection of the emotional content of a face. Face processing tasks have been a dominant methodology in affective neuroscience research for many years, and work aimed at understanding the neural correlates and mechanisms of affective face processing and the etiologic origins of this capacity is a clear priority. Regarding ERP correlates of affective face processing, some evidence exists that the N170 is enhanced for affective (e.g., fearful) as compared to neutral faces (Jiang et al., 2009; Blau et al., 2007), however many argue that affective face processing is more strongly represented at midline scalp sites (e.g., P8; Paulmann & Pell, 2009) rather than temporal-parietal sites. There is also evidence of affective differentiation for later ERP components. For example, Paulmann and Pell (2009) identified an ERP component that appears to reflect processing of the emotional content of a face--specifically, a positive-going component occurring 200 ms after stimulus presentation at midline sites (labeled P200), that was reliably enhanced for affective as compared to neutral face stimuli.
A further ERP component known to be enhanced for visual affective stimuli of differing types--including face stimuli (Eimer & Holmes, 2002) as well as affective non-face stimuli (e.g., Cuthbert et al., 2001; Hajcak et al., 2006; Schupp et al., 2000)--is the late positive potential (LPP). The LPP is broadly defined as a later onset (>250 ms) midline component that reflects sustained attentional-elaborative processing of affective stimuli, following initial registration of the basic affective significance of the stimulus (Schupp et al., 2000; Eimer & Holmes, 2002).
To further advance our understanding of brain ERP indices of affective face processing, the design of the current study included both emotional (fearful) and nonemotional (neutral) face stimuli along with control (scrambled face) stimuli. The twin feature of the design enabled us to evaluate, for the first time, the role of genetic influences in the predicted affective (fear vs. neutral face) differentiation for the N170, P200, and the LPP. Our primary hypothesis for these later ERP components was that they would show (perhaps even more so than the N170) enhancement for fearful as compared to neutral face stimuli. We also predicted that these two ERP components would exhibit enhancement for face stimuli of both types in relation to non-face (scrambled) stimuli, but to a lesser extent than the N170.
Methods
Participants
Participants were 62 pairs of MZ (25 pairs female) and 65 pairs of DZ (20 pairs female) twins recruited from the Minnesota Twin and Family Study database as part of a larger test protocol examining individual differences in affective and cognitive processing. Prior to testing, subjects were screened for impairments in visual acuity. The study was approved by the Institutional Review Board at the University of Minnesota and all subjects received informed consent and were compensated for their participation. All subjects were naïve as to the aims of the study.
Equipment and procedures
Face stimuli selected from the NimStim face stimulus set (Tottenham, et al., 2009), were displayed on a 19″ CRT monitor with a resolution of 1024 × 768 pixels and a refresh rate of 85 Hz. Subjects were seated 100 cm from the screen, yielding a viewing angle of 2.91 by 3.88 degrees for stimuli. Stimulus presentation was controlled using the Psychophysics Toolbox (Psychtoolbox; Brainard, 1997; Pelli, 1997). Face stimuli consisted of 8 different fear faces, their neutral counterparts (i.e., same actors posing neutral expressions), and scrambled versions of the same fear and neutral faces. Scrambled face images were constructed by segmenting the face images into grids (18 × 24 pixels) and randomly resorting the grids within the original image dimensions using the MATLAB software package (The MathWorks, Inc.).
As part of a larger task protocol, subjects wore red/cyan anaglyph glasses and viewed two blocks of face stimuli under standard (dichoptic) presentation conditions, separated by two blocks in which faces were presented to one eye and masked by presentation of 20 Hz Mondrian noise to the other eye, in a continuous flash suppression procedure (Jiang et al., 2009). At all times, participants were instructed to focus their attention on a small black dot (10 by 10 pixels) situated in the center of the screen, and press a button each time the dot doubled in size. The dot doubled in size for 100 ms at random intervals during the procedure, on average once per second. Faces were displayed for 500 ms in a pseudo-random order such that each type of face was presented 36 times, yielding 108 presentations per block. Trials were separated by a random intertrial interval between 300–700 ms. Since this experiment was interested in responses to naturally presented faces, results for the standard dichoptic trials only, are reported here. This condition is most comparable to procedures used in prior relevant work (e.g., Anokhin et al., 2010; Polk et al., 2007).
Electroencephalographic (EEG) activity was recorded from 64 scalp electrodes embedded in a NeuroScan Quik-Cap. EEG recording sites consisted of the standard 10–20 system locations along with additional intermediate positions. Four bipolar facial electrodes, two positioned on the outer canthi of each eye and the others on the inferior and superior regions of each orbit, were used to monitor horizontal and vertical EOG (HEOG and VEOG), respectively. Impedances for each electrode were adjusted to less than 5 kΩ. EEG was continuously recorded at a rate of 1000 Hz using CPz as an online reference. The raw EEG signal was ampli ed using Neuroscan Synamps ampli ers and band-pass ltered online at 0.05–200 Hz. The filtered EEG signal was epoched from 1000 ms before to 2000 ms after stimulus onset and then averaged across trials within condition; the average epoched signal was baseline corrected by subtracting the mean amplitude of EEG activity across a 500 ms pre-stimulus from each aggregate time point. Prior to averaging, epochs were screened for eye blinks and other artifacts and blink corrected using an ocular artifact reduction algorithm developed by Semlitsch et al. (1986). Epochs contaminated by eye blinks, eye movements, or muscle potentials exceeding ±75 μV at any electrode were excluded from averaging. In cases where there were less than 3 epochs meeting the above criteria for an individual at a particular electrode, data from that electrode for that subject were removed from analysis (N = 9 MZ and N = 3 DZ for the P200 and LPP at electrode site PZ).
Data Analyses
Data from selected parietal and temporal-parietal recording sites were selected for analysis based on previous face processing studies (Bentin et al., 1996; Anokhin et al., 2010). In order to measure the most robust effects, temporal-parietal sites were referenced to midline site CPz; these recording sites yielded a characteristic ERP response that included an early positive-going component that peaked around 100 ms, followed by a negative-going peak evident between 50 and 100 ms later (the N170), and ending with a final slower return to baseline. Midline and parietal electrode sites were referenced to linked mastoids; the wave-form response at these sites was marked by a negative-going peak component at approximately 100 ms, followed by a positive-going peak component at approximately 180–220 ms (referenced in this paper as the P200), and ending with a late positive potential. Split-half reliability estimates (Pearson’s r) were computed by creating average waveforms within each stimulus condition, but separately for odd and even trials. Reliabilities for the differing ERP components were as follows: N170 overall = .85, fear = .62, neutral = .71, and scrambled = .64; P200 overall = .76, fear = .59, neutral = .64, and scrambled = .49; and LPP overall = .56, fear = .36, neutral = .46, and scrambled = .26.
To identify face- and affect-related effects in the overall sample, ERP components corresponding to the N170 (150–230 ms), P200 (150–300 ms), and LPP (400–980 ms) were first quantified and compared across stimuli of each type (fear faces, neutral faces, scrambled) for participants as a whole. Next, to evaluate heritability of each ERP component as a whole, in relation to stimuli of each type, concordance faces in amplitude of response were compared for MZ versus DZ twin pairs. Finally, to evaluate the heritability of face-specific variance in components showing differential amplitude for intact versus scrambled face stimuli, and affect specific variance in components showing differential amplitude for fear versus neutral faces, concordances for residual scores reflecting variance specific to these distinct processing components were compared for MZ versus DZ twin pairs.
Results
N170
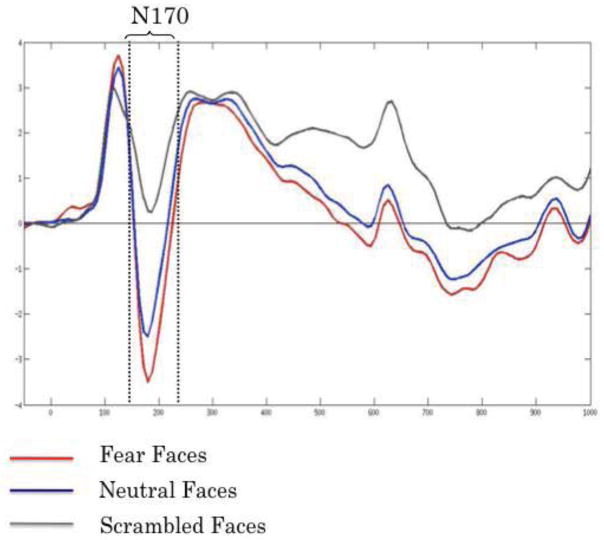
Analyses revealed that at temporal-parietal sites, main effects of condition were significant across participants as a whole (i.e., fear faces more negative than neutral faces, and neutral faces more negative than scrambled faces). However, since (as noted below) N170 amplitude was maximal at electrode P8, and in accordance with previous studies investigating the N170 (Stekelenburg & Gelder, 2004), we report results for this recording site specifically. Two-tailed Student’s t-tests were used to evaluate differences in N170 peak amplitude between different stimulus conditions. These analyses revealed greater negativity of N170 for fear as compared to neutral faces, p<.0005, and for both fear and neutral faces in comparison to scrambled faces, ps<.0005.
As a follow-up to these analyses of overall condition differences, the heritability of responses to stimuli of each type was evaluated through use of the intraclass correlation coefficient. For MZ twin pairs, peak N170 amplitudes showed significant cross-twin concordance in each of the three conditions (fear faces: intraclass r=.56, F(1, 61)=3.52, p<.0005; neutral faces: r=.51, F(1, 61)=3.08, p<.0005; scrambled faces: r=.27, F(1, 61)=1.73, p<.018). In contrast, for DZ twins, peak N170 amplitudes showed significant cross-twin concordance only in the intact neutral face condition (intraclass r=.27, F(1, 61)=1.75, p<.016); concordance was marginal in the fear face condition (r=.17, F(1, 61)=1.40, p<.096) and negligible in the scrambled face condition (r=.07, F(1, 61)=1.15, p<.29; see Table 1). Fisher Z tests for the difference between dependent correlations revealed that concordance for MZ twins significantly exceeded that for DZ twins in the fear face condition only, p<.01.
Table 1.
N170 MZ and DZ Intraclass correlations. Peak amplitude correlations in response to fearful faces, neutral faces, and scrambled stimuli (top row) and corresponding residuals (bottom two rows; see text).
| Fear | Z | Neutral | Z | Scrambled | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||||
| N170 | .56** | .17 ns. | ** | .51** | .27* | ns. | .27* | .07 ns. | ns. |
| Fear-Neutral | Z | Fear-Scrambled | Z | Neutral-Scrambled | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||||
| N170 | −.01 ns. | −.04 ns. | ns. | .46** | .15 ns. | * | .44** | .21* | ns. |
Z > 1.65 (p<05);
Z > 2.33 (p<.01).
Next, given that the pattern of results differed for the intact versus scrambled faces, we compared the spatial distribution of the N170 component for fear, neutral, and scrambled faces. As discussed in Bentin and Carmel (2002), the face-selective N170 response is distinctly maximal at temporal-parietal sites; other ERP components with latencies proximal to the N170 that appear responsive to visual stimuli more generally (e.g., the N1) show maximal amplitude at locations other than temporal-parietal sites. Consistent with this account, inspection of the distribution of peak amplitudes (see Figure 3) indicates that maximal peaks for the intact face condition were centered around temporal-parietal sites (i.e., ordered from most to least negative N170 peak: fear--P8, P7, TP8, TP7, T8; neutral--P8, P7, TP7, TP8, T7), whereas maximal peak amplitudes for scrambled faces were localized more to frontal sites (ordered from most negative to least negative N170 peak: FP2, FPZ, FP1, AF4, AF3). These contrasting topographic patterns suggest that the N170 to scrambled faces reflects a visual processing response, distinct from what is traditionally regarded as the N170 component sensitive to face stimuli specifically (Bentin & Carmel, 2002).
Figure 3.
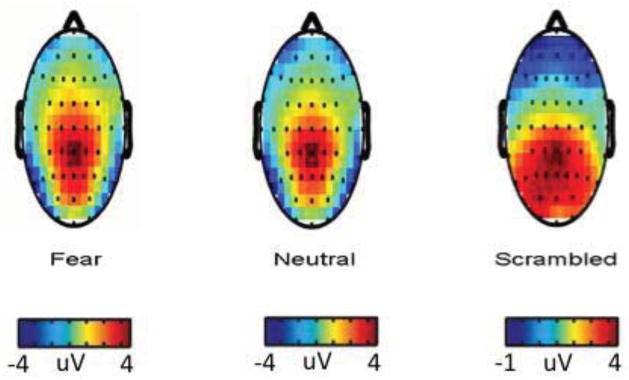
Spatial distribution of N170 peak responses to fearful faces, neutral faces and scrambled stimuli. Red and yellow regions correspond to areas of greater positivity compared to baseline. Regions green and blue in color correspond to areas of greater negativity compared to baseline. Maximal peaks for the intact face condition were centered around occipito-temporal sites (in order from most negative to least negative N170 peak: fear--P8, P7, TP8, TP7, T8; neutral--P8, P7, TP7, TP8, T7), whereas maximal peak amplitudes for scrambled faces were centered more toward frontal sites (in order from most negative to least negative N170 peak: scrambled--FP2, FPZ, FP1, AF4, AF3).
Given that peak N170 responses to nondescript visual stimuli (scrambled faces) differed from those to intact faces both in pattern of results and spatial distribution, and in view of previous research indicating greater concordance in baseline EEG activity for MZ twins as compared to DZ twins (Beijsterveldt and Boomsma, 1994), we undertook follow-up analyses to examine MZ/DZ concordances for amplitude of N170 response to intact faces after removing variability attributable to nonspecific factors (i.e., generic visual processing, baseline EEG, etc.). To do this, we regressed N170 peak amplitudes at electrode site P8 for intact faces as a whole, and for faces of each type (fear, neutral), onto N170 amplitudes for scrambled face stimuli, and saved out residual scores from the analysis--corresponding to variance in the N170 for intact face stimuli not attributable to variability in the scrambled face condition. (Correlations between N170 for fearful and neutral faces with scrambled faces were .38 in each case, indicating ~ 14.4% overlapping variance.) We then computed intraclass correlations to quantify concordance in these standardized residual scores for MZ and DZ twin pairs.
For these residual score variables, concordances for N170 amplitude remained higher for MZ (intraclass rs for Fearful/Neutral faces combined, Fear faces alone, and Neutral faces alone, in each case controlling for variance in common with Scrambled faces, were .51, .46, and .44, respectively, all ps<.0005) than for DZ twins (rs = .20, .15, and .21, respectively, ps < .05, .12, and .05) with the difference in concordance for MZ versus DZ twins achieving significance for all faces and for fear faces (ps<.05), and approaching significance in the neutral face condition (p<.08; see Table 1). These results indicate a contribution of genetic influence to the variance in N170 response that is distinct from variance associated with generic visual processing.
In addition, to similarly evaluate the heritability of variance in N170 amplitude specifically related to affective processing, we regressed N170 peak amplitude for fearful faces onto N170 amplitude for neutral faces, and saved out residual scores corresponding to variance in N170 for fear face stimuli not attributable to variance in the neutral face condition. Concordances for this Fear-Neutral residual variable were small and nonsignificant for both MZ and DZ twin pairs (intraclass rs = −.01 and −.04, respectively, ps>.5), indicating negligible contribution of genetic influence to affective differentiation (fear faces vs. neutral faces) in the N170 response.
P200
Following N170 analysis, we identified a second component of interest using a mastoid reference, characterized by a positive peak occurring at approximately 200 ms after stimulus onset (P200), located at midline sites and peaking at midline site PZ. This component was identified based on previous research by Paulmann and Pell (2009), who reported the presence of a P200 component located at midline sites, as well as Anokhin et al. (2010) who also identified a component at electrode site PZ using the same reference as the currently reported P200 (quantified as a mean-within-window score) which was sensitive to the emotional expression contained within a face. Though the temporal peak of this positive component is similar to that of the N170 and is likely influenced by the vertex positive potential (VPP), or complementary dipole of the N170, we found evidence that the P200 for participants in the current study--defined in the manner described by by Paulmann and Pell (2009) and Anokhin et al. (2010)-- captured an affect-related process not reflected in the N170/VPP.
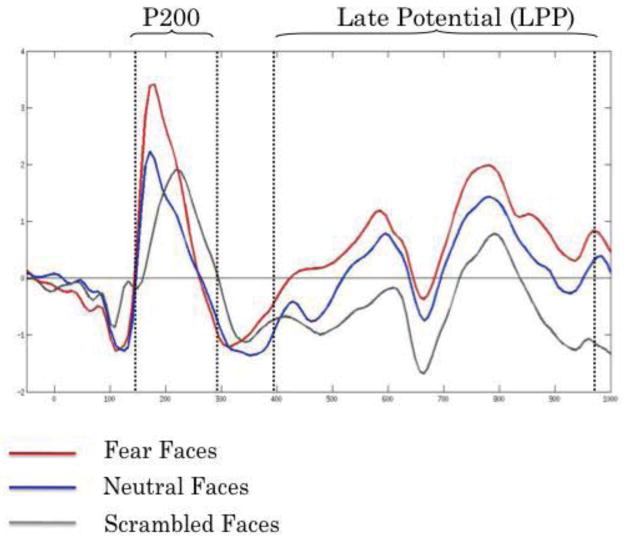
Specifically, a 2-tailed Student’s t-test for dependent samples revealed effects of stimulus condition consistent with previous research indicating that the P200 is reflective of emotional expression information more so than face identity information (i.e., P200 for fear faces exceeded that for neutral faces at p<.0005, and that for scrambled faces at p<.005, with P200 for neutral faces not differing from scrambled, p=.27).
Following main effects analyses, intraclass correlations between MZ and DZ twin pairs were compared. As observed for the N170, mean P200 amplitude showed significant cross-twin concordance for MZ twin pairs in each of the three stimulus conditions (fear faces: r=.58, F(1, 52)=3.78, p<.0005; neutral faces: r=.39, F(1, 52)=2.29, p<.003 scrambled: r=.33, F(1, 52)=1.99, p<.008). In contrast, mean P200 amplitude showed significant cross-twin concordance for DZ twins only in the intact neutral face condition (intraclass r=.29, F(1, 58)=1.82, p<.02); concordance was marginal in the fear face (r=.21, F(1, 58)=1.52, p<.055) and scrambled stimuli conditions (r=.19, F(1, 58)=1.46, p<.077; see Table 2). The difference in MZ/DZ concordance levels was only significant (p<.05) in the fear face condition, indicating heritability for variance in the P200 that is associated specifically with the affective-expressive (fear) component of faces.
Table 2.
P200/LPP MZ and DZ Intraclass correlations. Mean amplitude correlations in response to fearful faces, neutral faces, and scrambled stimuli and corresponding residuals (see text).
| Fear | Z | Neutral | Z | Scrambled | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||||
| P200 | .58** | .21 ns. | * | .39** | .29* | ns. | .33** | .19 ns. | ns. |
| Fear- Neutral | Z | Fear- Scrambled | Z | Neutral- Scrambled | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||||
| P200 | .28* | −.04 ns. | * | .56** | .16 ns. | ** | .33** | .25* | ns. |
| Fear | Z | Neutral | Z | Scrambled | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||||
| LPP | .30* | .23* | ns. | .14 ns. | .02 ns. | ns. | .23* | .06 ns. | ns. |
| Fear- Neutral | Z | Fear- Scrambled | Z | Neutral- Scrambled | Z | ||||
|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | ||||
| LPP | .33** | .23* | ns. | .28* | .24* | ns. | .13 ns. | .02 ns. | ns. |
Z > 1.65 (p<.05);
Z > 2.33 (p<.001).
Analysis of residual score variables revealed that unlike the N170, concordances for P200 face-specific effects were only marginally greater for MZ (intraclass r for fearful and neutral faces combined after controlling for variance in common with Scrambled faces was .52, p<.0005) than DZ twins (r=.27, p<.02), Z = 1.54, p < .062. However, a highly significant difference in MZ versus DZ concordances for P200 was evident for fear faces alone after controlling for covariance with scrambled faces, rs = .56 (p<.001) and .16 (p=.10), respectively, Z = 2.42, but not neutral faces alone, rs = .33 (p<.008) and .25 (p<.03), respectively, Z = .45 (see Table 2).
Furthermore, an analysis of residuals reflecting variance in P200 response to fear faces after controlling for variance P200 to neutral faces revealed significantly higher concordance for MZ (Fear-Neutral: r=.28, F(1, 52)=1.77, p<.023) than DZ twins (Fear-Neutral: r= −.04, F(1, 58)=.93, p<.61) in mean P200 responses rMZ>rDZ(p<.05), rs = .28 (p<.001) and −.04 (p=.60), respectively, Z = 1.68,. These results demonstrate even more directly a contribution of genetic influence to the variance in P200 response that reflects emotional expression information specifically.
LPP
Finally, we analyzed the “late-positive potential” (LPP) quantified as the mean area under the curve of the ERP waveform within a window of 400 to 980 ms following the P200, at midline electrode PZ with a linked-mastoid reference. Main effects analysis (2-tailed paired Student’s t-test) revealed condition effects similar to the N170 (i.e., fear > neutral, and neutral > scrambled, ps<.0005).
In addition, for both twin types (MZ and DZ twins), concordances were stronger in fear face conditions as compared to neutral face conditions. However, the mean of the LPP was not significantly greater between MZ than between DZ twins in any of the face (MZ twins: Fearful: r=.30, F[1, 52]=1.86, p<.014; Neutral: r=.14, F[1, 52]=1.32, p<.16 Scrambled: r=.23, F[1, 52]=1.61, p<.043; DZ twins: Fearful: r=.23, F[1, 58]=1.52, p<.039; Neutral: r=.019, F[1, 58]=1.04, p<.44 Scrambled: r=.064, F[1, 58]=1.14, p<.31 [see Table 2]) or residual conditions (MZ twins: Fearful-Scrambled: r=..28, F[1, 52]=1.77, p<.021; Neutral-Scrambled: r=.13, F[1, 52]=1.30, p<.18; Fearful-Neutral: r=.33, F[1, 52]=1.99, p<.008; DZ Twins: Fearful-Scrambled: r=.23, F[1, 58]=1.61, p<.036; Neutral-Scrambled: r=.007, F[1, 58]=1.01, p<.48; Fearful-Neutral: r=.23, F[1, 58]=1.59, p<.039, see Table 2) when compared using a Fisher’s Z-test.
Discussion
Results of the current study demonstrate for the first time that the amplitude of the N170 response to faces is in part heritable. The peak of the N170 response to intact faces as a whole (fearful and neutral) was more concordant for MZ as compared to DZ twin pairs. Although the “N170” response to scrambled faces was also concordant for MZ than DZ twin pairs, this difference was not significant, and the correlations were much lower for Mz pairs compared with the intact face conditions and negligible for Dz pairs.
Moreover, the spatial distribution of this component for scrambled faces differed in comparison to the distribution of the N170 for intact faces, suggesting that the “N170” to scrambled faces is perhaps generated from a different underlying source and thus may not be indicative of an actual N170 response. Indeed, after controlling for variance in responsivity to scrambled faces, concordance for the residual variance in N170 to faces of each type remained significantly higher for MZ than for DZ twins. Finally, though we found that the N170 response was more pronounced on average to fearful than neutral faces, our concordance analyses demonstrated that this difference was not heritable.
This study was also able to identify a positive-polarity component peaking around 200 ms after stimulus presentation that, consistent with previous research (Paulmann & Pell, 2009), appears to encode the emotional content contained within a face. The amplitude of this component was found to be more highly concordant for MZ than for DZ twins for fear faces only (i.e., concordances did not differ for neutral or scrambled faces). Even more interesting, after controlling for variance in common with the scrambled and neutral conditions, residual variance in response to fear faces showed higher concordance for MZ than DZ twin pairs, indicating that part of the heritable variance in this response component is specific to the emotional content contained within a face.
To summarize, the current study quantified differing ERP components related to face processing, including the N170, an early temporal-parietal component that appears to index processes involved in encoding the presence of faces in general, followed by the P200, a midline component that appears sensitive to the emotional content of a face, and ending with a late potential (LPP) that also appears sensitive to emotional information in the stimuli. With respect to these three variables, our results demonstrate that the amplitude of earlier components of response to faces (N170 and P200) is partially heritable, indicating that certain aspects of face processing capacity have an inborn, constitutional basis. However, lower reliability for the LPP as compared to the N170 and P200 in the present study may have contributed to reduced twin correlations for this component.
In view of previous studies demonstrating heritability for face coding processes associated with the ventral visual cortex (Polk et al., 2007), and data from combined neuroimaging/EEG studies indicating covariance between activity in FFA and STS brain regions and face-selective N170 response (Sadeh et al., 2010; Yovel et al., 2008), it will be interesting in future research to compare twins for concordance in responses across neuroimaging and ERP response domains (e.g., degree of resemblance, on average, of N170 peak scores for one twin with FFA or STS response in his/her co-twin). Evidence for associations of this kind would not only reinforce the notion of heritability for face processing, but would help to further elucidate the specific role that genes play in identifying and interpreting faces.
Figure 1.
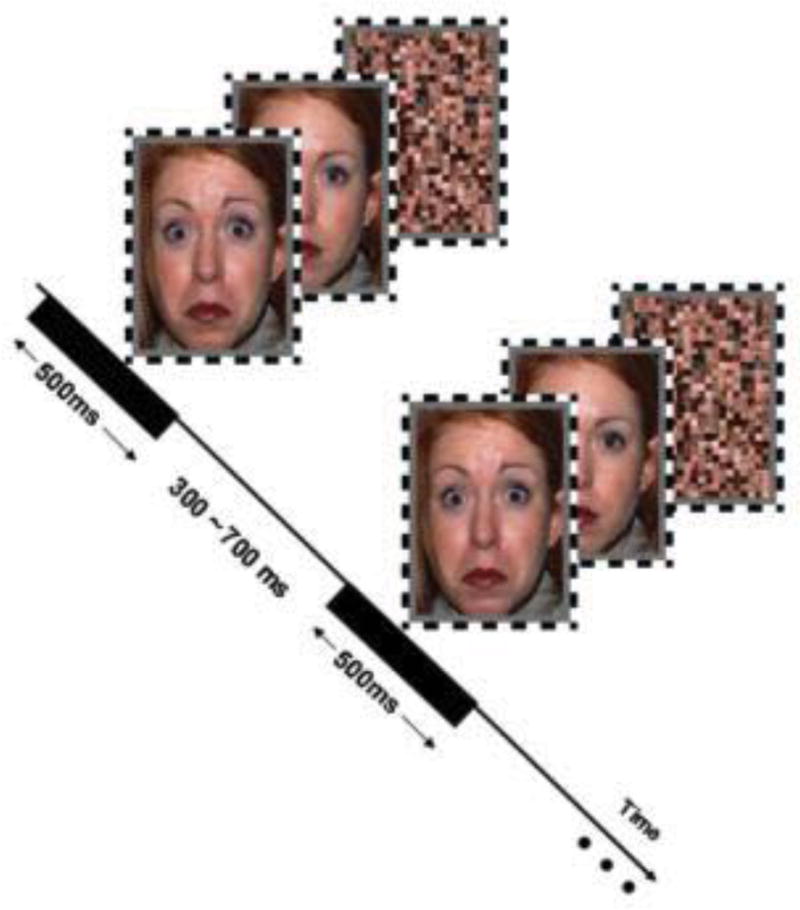
Experimental design and stimuli. Face stimuli consisted of 8 different fear faces, their neutral counterparts (i.e., same actors posing neutral expressions), and scrambled versions of the fear and neutral faces. Faces were displayed for 500ms in a pseudo-random order such that each type of face was presented 36 times, yielding 108 presentations per block. Trials were separated by a random intertrial interval between 300–700ms.
Figure 2.
The N170. Peak responses (150–230 ms) at right occipito-temporal electrode P8, to fearful faces (red), neutral faces (blue) and scrambled stimuli (gray) referenced to midline electrode (CPz).
Figure 4.
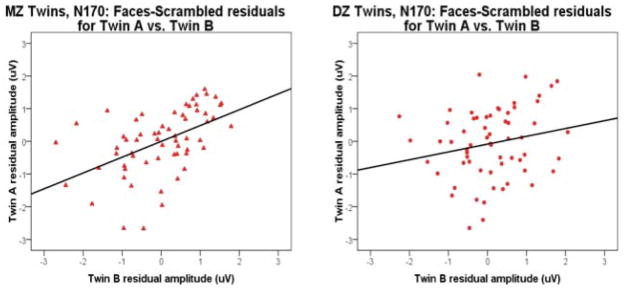
Variance in N170 for intact face stimuli not attributable to variability in the scrambled face condition. Representative residual N170 amplitudes for MZ twins (left) and DZ twins (right). Concordances for N170 amplitude remained higher for MZ (intraclass r for All Faces minus Scrambled =.51, p<.0005 than for DZ twins r= .20 [p<.05]). Difference in concordance for Mz versus Dz twins achieved significance (p<.05).
Figure 5.
The P200 and Late positive potential (LPP). Mean responses at midline electrode PZ, to fearful faces (red), neutral faces (blue) and scrambled stimuli (gray) referenced to averaged linked mastoids.
Figure 6.
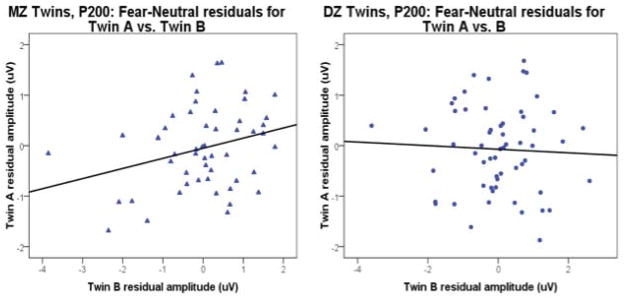
Variance in P200 for fear face stimuli not attributable to variability in the neutral face condition. Representative residual P200 amplitudes for MZ twins (left) and DZ twins (right). Concordances for P200 amplitudes remained higher for MZ (intraclass r for Fear faces minus Neutral =.28, p<.023) than for DZ twins (Fear-Neutral: r= −.04, p<.61). Difference in concordance for Mz versus Dz twins achieved significance (p<.05).
Highlights.
The N170 has a heritable basis
Early processing of affective face information (i.e. the P200) has a heritable basis
Heritable face processing components are more evident earlier versus later in time
Acknowledgments
This work was supported by NIMH grants MH072850 and MH089727. We are grateful to Megan Lucy for coordinating the project; Uma Vaidyanathan, Lindsay Nelson, Marianna Gasperi, Melissa Johnson, Elisabeth Kallenberger, Saaraa Ameri, Beth Dicks, and Michael Storlie for assisting with data collection; Melanie Fuhrman, Siri Scott, and Genevieve Ryczek for assisting with recruitment; Jennifer Cermak for coordinating diagnostic and questionnaire data coding and entry; Justin Jobelius for assisting with physiological data processing; Paul Arbisi for participating in diagnostic consensus meetings; and Mark Kramer, Robert Krueger, William Iacono, and Matt McGue for their assistance in accessing and characterizing the participant sample.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anokhin AP, Golosheykin S, Heath AC. Heritability of individual differences in cortical processing of facial affect. Behav Genet. 2010;40:178–185. doi: 10.1007/s10519-010-9337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijsterveldt CEM, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94:319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8(6):551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Carmel D. Accounts for the N170 face-effect: a reply to Rossion, Curran, & Gauthier. Cognition. 2002;85:197–202. doi: 10.1016/s0010-0277(02)00102-6. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spatial Vision. 1997;10:433–436. [PubMed] [Google Scholar]
- Carmel D, Bentin S. Domain specificity versus expertise: factors influencing distinct processing of faces. Cognition. 2002;83:1–29. doi: 10.1016/s0010-0277(01)00162-7. [DOI] [PubMed] [Google Scholar]
- Diamond R, Carey S. Why faces, are and are not special: An effect of expertise. Journal of Experimental Psychology: General. 1986;115(2):107–117. doi: 10.1037//0096-3445.115.2.107. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Logothetis NK. Is face recognition not so unique after all? Cognitive Neuropsychology. 2000;17(1/2/3):125–142. doi: 10.1080/026432900380535. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Simons RF. Attending to affect: Appraisal strategies modulate the electrocortical response to arousing pictures. Emotion. 2006;6:517–522. doi: 10.1037/1528-3542.6.3.517. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Shannon RW, Vizueta N, Bernat E, Patrick CP, He S. Dynamics of processing invisible faces in the brain: Automatic neural encoding of facial expression information. Neuroimage. 2009;44(3):1171–1177. doi: 10.1016/j.neuroimage.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Stanley D, Harris A. The fusiform face area is selective for faces not animals. NeuroReport. 1999;10:183–187. doi: 10.1097/00001756-199901180-00035. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Phil Trans R Soc B. 2006;361:2109–2128. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone E, Kanwisher N, Duchaine BC. Can generic expertise explain special processing for faces? Trends in Cognitive Sciences. 2006;11(1):8–15. doi: 10.1016/j.tics.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The development and neural bases of face recognition. Inf Child Dev. 2001;10:1–2. 3–18. [Google Scholar]
- Paulmann S, Pell MD. Facial expression decoding as a function of emotional meaning status: ERP evidence. NeuroReport. 2009;20(18):1603–1608. doi: 10.1097/WNR.0b013e3283320e3f. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial Vision. 1997;10:437–442. [PubMed] [Google Scholar]
- Polk TA, Park J, Smith MR, Park DC. Nature versus nurture in ventral visual cortex: A functional magnetic resonance imaging study of twins. The Journal of Neuroscience. 2007;27(51):13921–13925. doi: 10.1523/JNEUROSCI.4001-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Curran T, Gauthier I. A defense of the subordinate-level expertise account for the N170 component. Cognition. 2002;85:189–196. doi: 10.1016/s0010-0277(02)00101-4. [DOI] [PubMed] [Google Scholar]
- Sadeh B, Podlipsky I, Zhdanov A, Yovel G. Event-related potential and functional MRI measures of face-selectivity are highly correlated: A simultaneous ERP-fMRI investigation. Human Brain Mapping. 2010;31:1490–1501. doi: 10.1002/hbm.20952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23(6):695–699. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Stekelenburg JJ, Gelder B. The neural correlates of perceiving human bodies: an ERP study on the body-inversion effect. NeuroReport. 2004;15(5):777–780. doi: 10.1097/00001756-200404090-00007. [DOI] [PubMed] [Google Scholar]
- Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nature Neuroscience. 2000;3(8):764–769. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka J, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson CA. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmer J, Germine L, Chabris C, Chatterjee G, Williams M, Loken E, Nakayama K, Duchaine B. Human face recognition ability is specific and highly heritable. PNAS. 2010;10711:5238–5241. doi: 10.1073/pnas.0913053107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yovel G, Kanwisher N. Face perception: Domain specific, not process specific. Neuron. 2004;44(5):889–898. doi: 10.1016/j.neuron.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Song Y, Hu S, Li X, Tian M, Zhen Z, Dong Q, Kanwisher N, Liu J. Heritability of the specific cognitive ability of face perception. Current Biology. 2010;20:1–6. doi: 10.1016/j.cub.2009.11.067. [DOI] [PubMed] [Google Scholar]