Abstract
BACKGROUND AND PURPOSE
Quantitative signal targeting with alternating radiofrequency labeling of arterial regions (QUASAR) is a recent spin labeling technique that could improve the reliability of brain perfusion measurements. Although it is considered reliable for measuring gray matter as a whole, it has never been evaluated regionally. Here we assessed this regional reliability.
METHODS
Using a 3-Tesla Philips Achieva whole-body system, we scanned four times 10 healthy volunteers, in two sessions 2 weeks apart, to obtain QUASAR images. We computed perfusion images and ran a voxel-based analysis within all brain structures. We also calculated mean regional cerebral blood flow (rCBF) within regions of interest configured for each arterial territory distribution.
RESULTS
The mean CBF over whole gray matter was 37.74 with intraclass correlation coefficient (ICC) of .70. In white matter, it was 13.94 with an ICC of .30. Voxel-wise ICC and coefficient-of-variation maps showed relatively lower reliability in watershed areas and white matter especially in deeper white matter. The absolute mean rCBF values were consistent with the ones reported from PET, as was the relatively low variability in different feeding arteries.
CONCLUSIONS
Thus, QUASAR reliability for regional perfusion is high within gray matter, but uncertain within white matter.
Keywords: ASL, QUASAR, regional cerebral blood flow, regional reliability
Introduction
Perfusion is an important physiological parameter for probing tissue metabolic activity. The absolute CBF (cerebral blood flow) value has a great clinical importance as a diagnostic and therapeutic indicator. Indeed, while the relative measurement of CBF is of limited use, particularly for conditions in which CBF would alter globally in the brain, the quantitative determination of CBF is of major interest, as it enables, for example, to compare or diagnose subjects among a population.
However, it is necessary to ensure that the measurements methods are reliable; that is, within-subject variation remains within acceptable limits across multiple repeated measures. This is crucial for later clinical use, such as in longitudinal studies with regional CBF alteration, or when planning a comparison of CBF between populations, such as between patients and controls.
Therefore, in the past years, a large effort has been made to achieve high quantitative reliability for modalities of brain perfusion including single photon emission tomography (SPECT), xenon-computed tomography (CT), positron emission tomography (PET), CT-perfusion, but also perfusion-oriented magnetic resonance imaging (MRI).
Arterial spin labeling (ASL) techniques are MRI techniques which have recently shown their potential for quantifying tissue perfusion, and remain under investigation. The mere addition of a labeling pulse to the water proton spin in the blood flow as an endogenous diffusible tracer renders ASL completely noninvasive, unlike other conventional quantitative perfusion techniques which work with exogenous contrast agents or radioactive tracers.1 The fundamental principle of ASL involves labeling the blood by inverting of the spin population in a tissue-feeding artery and acquiring perfusion imaging after the appropriate delay time (inversion time, TI) for the labeled flow to reach the brain parenchyma and change the signal in the region of imaging acquisition. Blood flow of the tissue can be inferred from the magnetization difference (ΔM) subsequently detected between the labeled experiment and a nonlabeled control experiment. At field strength of 1.5 Tesla (T), however, that magnetization change (ΔM) is small, which limits the reliability of quantitative perfusion measurements2 due to poor signal-to-noise ratio (SNR). Furthermore, the T1 relaxation time of the labeled spin is comparatively shorter than the time required for flow throughout the vascular bed of the brain. Such limitations of ASL at 1.5 T may affect its clinical usefulness.
At higher magnetic field strength, recent studies attributed improved ASL estimation of quantitative perfusion to higher SNR and the longer T1 relaxation of arterial blood.3,4 Some investigators have reported good agreement in absolute CBF values in whole gray matter obtained by 3 T ASL techniques and PET.5–8 However, even at 3 T, the regions heterogeneity and longer transit time make the accurate detection of perfusion in white matter and in the watershed zone difficult using conventional, flow-model based ASL methods.9 Furthermore, the quantitative reliability of regional CBF (rCBF) is relatively low even in cortical gray matter using these ASL methods.10 Such suboptimal quantitative reliability may be ascribed to the effects of contaminated residual labeled spin within feeding arteries near the vascular bed, and regional differences in transit time and vascular density.11,12
Quantitative signal targeting with alternating radiofrequency (STAR) labeling of arterial regions (QUASAR) is a quantitative ASL technique designed by Petersen and associates13 that aims at highly accurate quantification and reproducibility of brain tissue perfusion. The method allows voxel-based estimation of regional arterial input functions (AIFs) that are used to obtain perfusion maps by deconvolution. Thus, QUASAR could overcome the broad variation in transit time in structures throughout the brain to improve the reliability of local brain perfusion.
We recently participated in a 22-site study that examined the reproducibility of QUASAR using ASL images obtained in 199 normal volunteers using a unified protocol across centers and 3 T whole-body systems.14 That study reported good reproducibility of CBF in whole gray matter, with values equivalent to those of CT-perfusion or PET perfusion studies.15,16 The study evaluated only the reproducibility of CBF throughout the gray matter as a whole, but did not evaluate the regional variability patterns, nor white matter in QUASAR data. Familiarity with regional variability patterns in QUASAR would be of great importance when ASL perfusion maps are used in diagnosing patients with alterations in local perfusion.
In the current study, we utilized the same protocol design used at our institution for the multicenter study to obtain QUASAR-ASL perfusion data from 10 healthy volunteers to evaluate the regional reproducibility and quantitative reliability of CBF in different anatomical structures in which transit times would be heterogeneous, including the gray and white matter and different arterial territories. We expected QUASAR to correct the heterogeneous variability by region to measure perfusion more accurately.
Methods
Subjects
We analyzed data of 10 healthy Asian volunteers (7 men, 3 women; aged 22-43 years, mean ± standard deviation [SD], 30 ± 8.09 years), who underwent scanning as part of the QUASAR multicenter reproducibility study.14 The local institutional review board approved the study, and all subjects gave written informed consent before participation.
The MRI study including ASL was performed with a 3T whole-body system (Achieva, Philips Healthcare, Best, The Netherlands). All images were obtained using a quadrature body coil as transmission coil and dedicated 8-elements phased-array head coil as receiving coil.
Study Protocol and Data Acquisition
In this study, we analyzed data that we previously acquired as part of the multicenter “QUASAR Reproducibility Study” using a protocol detailed previously.14 The current study consisted of each subject participating in two scanning sessions separated by about 2 weeks (10-18 days, mean ± SD, 13 ± 10 days). All subjects underwent three high-resolution anatomical scans and four ASL scans, two per session. In session 1, the second scan was acquired after repositioning the subject's head in the scanner, whereas for session 2, the second scan was repeated without repositioning. Because anatomical imaging was performed each time a subject was repositioned, two sets of images were acquired during session 1 and one set during session 2. We used the automatic planning tool SmartExam17 equipped with the scanner to minimize human interaction during subject positioning, and improve the consistency of geometrical coverage. To keep physiological perfusion fluctuations as natural as possible, for 8 hours prior to the study, patients abstained from medication and food or beverage, such as coffee, tea, and licorice, that could affect the vascular system. The two sessions were performed in random order across volunteers.14
We obtained high-resolution 3D anatomical images using a magnetization-prepared rapid acquisition gradient-echo (MPRAGE)18 sequence with parameters: TR/TE, 6.7/3.1 milliseconds; TI, .8 seconds; FA, 8°; FOV, 240 × 162 × 190 mm; and voxel size, .9 × .9 × .9 mm. ASL measurements were obtained using the QUASAR sequence, which is detailed elsewhere.13,14,19 Briefly, the sequence was a multislice pulsed ASL method based on the acquisition of a train of multiple images following the labeling scheme. Both tagging and control acquisitions followed a saturation prepulse similar to quantitative imaging of perfusion using a single subtraction (QUIPSS),20 and the readout part consisted of a Lock-Locker sampling strategy where 13 multislices, single-shot gradient-echo (EPI) volumes, with bolus saturation prepulses,21 are acquired in sequence. Additionally, velocity-crushed and noncrushed control-label pairs were acquired in an interleaved manner, in order to estimate the arterial input function (AIF) and arterial blood volume (aBV).19 Perfusion measure was obtained by deconvolution resembling dynamic susceptibility contrast-based techniques. We estimated local AIFs by subtracting the crushed from the noncrushed experiments and estimated local aBVs based on the duration of the bolus of labeled arterial blood. Appropriate scaling of the AIF and subsequent deconvolution of the tissue signal (crushed experiment) by this AIF enabled estimation of CBF from the peak of the resulting function. The scan parameters of the QUASAR sequence were TR/TE/ΔTI/TI1, 4000/23/300/40 milliseconds; 13 inversion times (40-3,640 milliseconds); matrix, 64 × 64; 7 slices; slice thickness, 6 mm; 2-mm gap; FOV, 240 × 240 mm; FA, 35°/11.7°(regular/low); sensitivity encoding (SENSE), 2.5; and 84 averages (48 crushed at 4 cm/second, 24 noncrushed, and 12 low FA used for B1-mapping), all implemented in a single sequence. Postprocessing was done using in-house software provided for the “QUASAR Reproducibility Study” running on IDL 6.1 (ITT Visual Information Solutions, Boulder, CO, USA). These subsequent calculation produced CBF maps.
MR Images Processing
Anatomical imaging data were preprocessed using SPM8 (Wellcome Department of Cognitive Neurology, London, UK).22 Initially, for every subject, all MPRAGE images from the multiple sessions were coregistered together and averaged for higher quality. Those images were then segmented using the “New Segment” procedure of SPM8. This robust algorithm, which is an improvement of “Unified Segmentation”,23 uses a Bayesian, prior-based, model to jointly classify image voxels (gray matter, white matter, or cerebro-spinal fluid), estimates and corrects the nonuniform intensity field, and computes a spatial warping to a common standard space. As a result, for each subject, we could obtain, first, a cortical segmentation map, containing the posterior probability for each voxel to be gray- or white-matter; Second, a high-resolution nonlinear transformation from native space to MNI space.
The ASL images were first used for the computation of rCBF maps for every runs of every subjects. Then, using SPM8, the four CBF maps of every subjects were coregistered to the subject probabilistic segmentation map, as we found that this method yielded the best registration quality. Finally, the nonlinear spatial transformation computed from the MPRAGE processing was applied to those maps, to yield a final set of 40 multiple-sessions, multiple-subjects CBF maps resampled in MNI-space at a resolution of 1.5 × 1.5 × 1.5 mm.
Region of Interest Definitions
In order to focus on specific regions, as well as better compare our results to those from previous reports11,24 we defined some regions of interests: With the CBF images nonlinearly normalized in MNI space, we selected ROIs (region of interest) for gray matter in the flow territories of the anterior (ACA), middle (MCA), and posterior (PCA) cerebral arteries, where the ROIs were defined by closely following Bokkers et al.11 These are depicted in Figure1. Additionally, for the purpose of computing total in-tissue mean CBF, the anatomical probabilistic gray- and white- matter maps defined above were warped to MNI space and further thresholded at 50% probability to obtain hard masks of gray or white matter voxels over which we could average.
Figure 1.
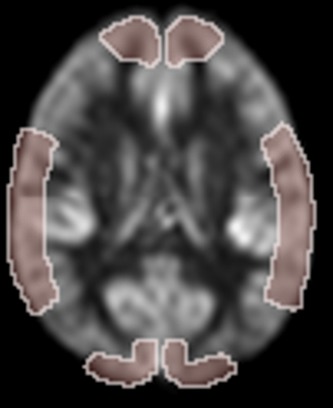
Definition of the regions of interest (ROI) for regional perfusion analysis according to arterial distribution, depicted on an average CBF map. Based on Bokkers 2010, using 12-mm thick, at location z = 21.5 in MNI space. Anterior (top), middle, and posterior ROIs are defined separately for each side.
Measures of Reliability
In order to evaluate the reliability of the QUASAR method, we relied on two statistics. The first was the intraclass correlation coefficient (ICC),25,26 which enables to infer reliability between acquisitions. Essentially, the ICC metric relates the between-session variance to the between-subjects variance of the data. Practically, the ICC statistic is simply assembled from the mean-square (MS) components extracted from the fit of an ANOVA model including subject and session. Hence there exists different variations of the ICC statistic, depending on the type of design and measurement. Following the McGraw and Wong nomenclature, here we computed the ICC(A, 1)-case-2A type, which corresponds to a measure of absolute agreement, where both subjects and sessions are considered random factors with no interactions. The corresponding formula26 reads
with k sessions, n subjects, and where MS_b, MS_w, and MS_e are the mean square of respectively between subjects, within subjects, and residual error of the linear model fit of the two-way ANOVA. McGraw and Wong also provide formula to compute associated significance F-statistics. An ICC of near unity indicates high reliability (1.0 indicates perfect reliability), whereas a value of 0 or less indicates that the multiple intersubject data show no more similarities than data of different subjects. In practice, it is often considered that a value of less than .5 indicates some randomness and limited usefulness. Thus, we also computed the null probability of the ICC under the hypothesis H0: ICC = .5, in addition to the more common but less useful H0: ICC = 0.
The second statistic used was the coefficient of variation (CV), a simple measure of (scaled) variance, which is of common use in reliability studies, defined as the ratio of the standard deviation to the mean. Therefore, for each subject, the within-subject standard deviation divided by the subject mean was computed, the lower the better, and the resulting CV maps were averaged over subjects for display purpose.
Those measures were computed both voxels-wise, in the form of brain maps, and in region of interests, as defined above.
Results
Figure2 depicts a representative example of QUASAR-ASL perfusion images for the same subject obtained in each session, after all session volumes were registered on the structural image.
Figure 2.
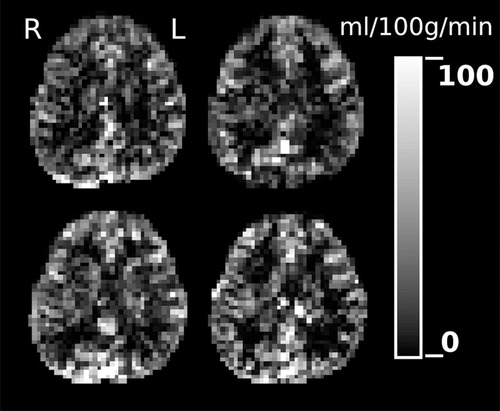
A single slice of the 4 CBF maps of one subject, computed from its 4 QUASAR sessions data, and coregistered together.
From the 40 ASL acquisition of all 10 subjects, the mean CBF of whole gray matter, including cortical and deep gray matter, was 37.74 ± 7.1 mL/100g/minute, with a between-subject standard deviation SDb = 12.86, and a within-subject standard deviation SDw = 4.67. There was a modest negative effect of age (P = .017). The ICC calculated from the mean CBF values of whole gray matter was .703. For whole white matter, the mean CBF was 13.95 ± 2.1, with SDb = 2.93 and SDw = 1.66, and an ICC value of .299.
Figure3 depicts the grand mean rCBF maps of 40 images (3.A) and the two reliability statistics (3.B). The ICC maps (3.B.1) showed high reliability within most region of gray matter. Among the voxels within gray matter area, there were some regions indicating slightly lower reliability corresponding to the watershed area between ACA and MCA, MCA and PCA. The lower ICC values in the voxels of the white matter area revealed predominantly low reliability there. Furthermore, ICC values within deeper layer of white matter voxels were much lower than subcortical white matter. The CV map measuring within-subject variance demonstrated a high reproducibility in almost all regions of the brain including cortical gray matter, watershed areas of gray matter, deep gray matter, and subcortical white matter. But some higher variability, which indicated lower reproducibility, was seen in deep white matter beside ventricles (Fig 3 B.2).
Figure 3.
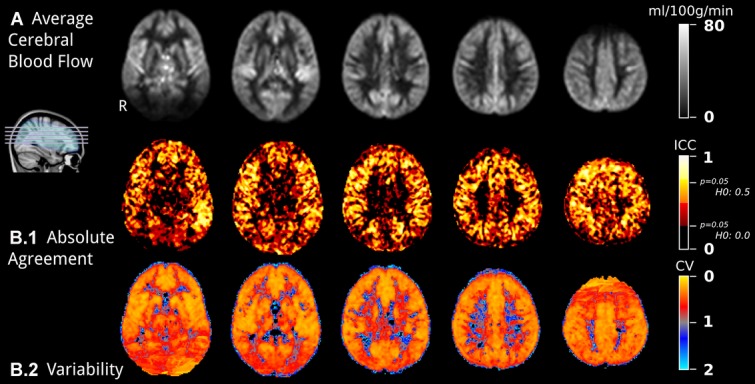
Overview of the cerebral blood flow maps reliability. (A) Grand mean CBF maps, averaging over 10 subjects and 4 sessions, in MNI space, and (B) reliability statistic maps. (B.1) Absolute Agreement map is computed using ICC, where a higher value is better. Significance at 5% threshold is reported for the two null hypotheses of 0 and 0.5 effect (corresponding to ICCs of respectively .23 and .71). (B.2) Variability map, using a coefficient of variation (CV), and averaged over subjects for display purpose.
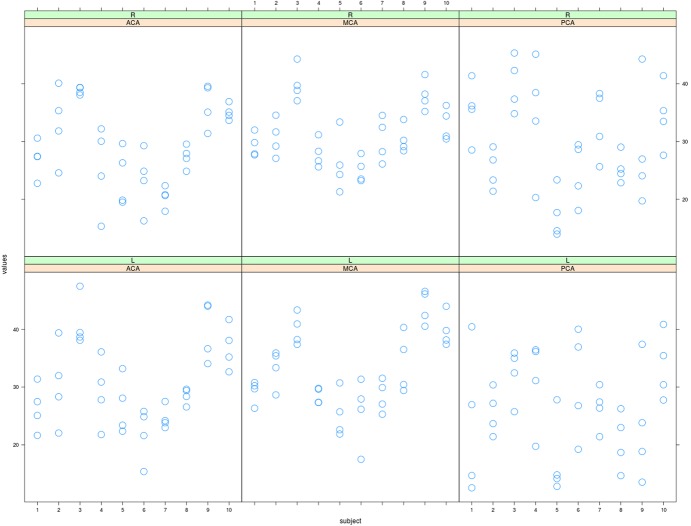
Table1 and Figure4 summarize the distribution of the absolute mean rCBF values in ACA, NCA, and PCA territories obtained by ROI analysis, as well as within-subject standard deviation and reliability values. Left and right ROIs values had high correlation across subjects in ACA (.93), MCA (.91), and a somewhat lower in PCA (.68).
Table 1.
Mean, Within-Subject Standard Deviation (SDw), and Reliability (ICC) of Regional Cerebral Blood Flow (rCBF) [mL/100 g/Minute]
| ROI | Mean | SDw | ICC |
|---|---|---|---|
| ACA | |||
| L | 30.544 | 5.540 | .628 |
| R | 29.071 | 6.124 | .650 |
| meanLR | 29.807 | 5.788 | .669 |
| MCA | |||
| L | 32.855 | 4.065 | .757 |
| R | 31.104 | 4.352 | .680 |
| meanLR | 31.980 | 3.980 | .753 |
| PCA | |||
| L | 26.478 | 6.153 | .188 |
| R | 29.869 | 8.847 | .469 |
| meanLR | 28.173 | 6.391 | .333 |
| Hemisphere | |||
| L | 29.959 | 4.952 | .572 |
| R | 30.015 | 5.414 | .662 |
| meanLR | 29.987 | 4.821 | .646 |
Figure 4.
Summary of ROI analyses. Distribution of the average cerebral blood flow into the region of interests. For three arterial regions (columns) and laterality (rows), the average CBF over the ROI for four sessions are displayed per subject [ml/100g/minute].
Discussion
With voxel-wise comparisons of 1.5 T ASL techniques, Jahng and colleagues reported high reliability of relative rCBF values through most of the brain (ICC close to .80 for both tissue types), but crucially they did not evaluate quantitative determination of rCBF.2 Also, most report evaluating the reproducibility of 3T ASL techniques have analyzed ROIs containing only gray matter and validated the reproducibility of global gray matter only,14,20 and although one recent study performed voxel-based analysis to validate regional reproducibility of several types of 3T ASL methods, QUASAR was not among them.12 In the present study, we conducted voxel-based statistical analysis to examine regional reliability of the 3T QUASAR method, which we believe has never been explicitly done.
Voxel-wise t-test comparisons showed that the mean of the 10 subjects did not vary significantly across session types, and the mean CBF was highly correlated between sessions through subjects, consistent with the results of Petersen's multicenter study,14 which indicates that repositioning, physiological fluctuation, variation in systemic processing, and additionally varied transit time and vascular distribution had little effect on the quantitative value of perfusion measurement with QUASAR. Our primary finding was that on a 3T system, QUASAR could provide satisfactorily reliability for quantitative estimation of CBF: the CV was low throughout the cerebrum regardless of which anatomical structure, including cortical and deep gray matter and subcortical white matter, but not as good in the deep white matter especially in the watershed zone, as demonstrated by the coefficient-of-variation map and absolute agreement (ICC) maps.
For whole gray matter averages of CBF, the high ICC value in our study indicated high reliability between repeated measures, but the value for whole white matter averages remained low, indicating poorer reliability, even though the lower ICC statistic is also due in part to a lower intersubject variance in those area. The statistic ICC and CV maps both reflected this overall good reproducibility in gray matter, and degradation in the deep white matter and the watershed zone. Concerning the regional reliability differences in QUASAR, we presume that the longer transit time required to reach the deep white matter and watershed zone translates to a larger loss of magnetization due to relaxation, and therefore higher noise, even despite the use of an high (3T) field. That problem has been described in several other studies of 3-T ASL perfusion estimation.6,8,9 For example, from a study of flow-sensitive alternating inversion recovery (FAIR) perfusion MRI, Van Gelderen's group concluded that an appropriate AIF model could overcome the transit time effects.9 Thus, quantification of CBF estimation in regions with longer transit time was expected to be more reliable with QUASAR using its Look-Locker-like strategy and crusher gradients for improved AIFs estimation. In the light of our results, although we believe QUASAR had reliably overcome transit time variation in the regions other than white matter, we nonetheless found it unreliable for measuring absolute perfusion in white matter. Two factors may underlie this: image acquisition using a rapid sequence of volumes in QUASAR, as opposed to a single slower volume, might have led to an insufficient SNR to detect significant alteration in the arterial flow of white matter; and, the crusher gradients could have decreased the amount of signal. Nevertheless, Van Osch and associates have suggested that quantitative measurement of white matter perfusion may not be necessary in clinical practice, where the focus is rather the detection of regions with altered hemodynamics versus nonaffected tissues.27 In that sense, the high reproducibility of white matter perfusion on QUASAR would be sufficient for clinical practice, even though it is not sufficiently reliable for the quantitative measurement of rCBF there.
In our study of QUASAR, there was no significant difference in cortical CBF values among the different distributions of the cerebral arteries (F(2,27) = 1.08, P = .352, and see Table1 and Fig 4). Table2 compares the mean cortical CBF values in each arterial territory in our ASL study with those of recent studies using ASL and PET as well as regional CBF ratios of MCA and PCA territories relative to the ACA territory, to appreciate the variability among arteries. Conventional 3T ASL methods have been reported to show heterogeneous regional variability in quantitative estimation of perfusion. Gevers and colleagues performed voxel-based analysis of regional quantitative variability with four different 3T ASL techniques other than QUASAR—continuous ASL (CASL), single TI pulsed ASL (PASL), and pseudo-continuous ASL (p-CASL) with and without background suppression pulse—and found hyperperfusion in vascular regions leading to regional variability in quantification by all four methods.12 In a cross-modality study between a 3T ASL technique (pulsed STAR labeling with Look-Locker-like readout strategy) and H215O-PET, Bokkers’ team compared absolute cortical CBF values in every vascular distribution (ACA, MCA, and PCA territories) and found regional variation in differences between the two modalities, with relative CBF ratios to ACA much higher than those of PET, especially in the PCA distribution (Table2).11 They speculated that these variations could arise from the presence of residual labeled spins inside the arterial vasculature and that CBF values in the PCA territory might be overestimated with that ASL method. They ascribed this overestimation to differences in the courses of main vessels, observing a larger volume of blood vessels of the PCA than the anterior circulation within an axial ASL-MR imaging slice because of the almost parallel coursing of the vessels of the PCA to the imaging plane and the more perpendicular coursing of the anterior circulation vessels.11 In short, regional variation in the volume of feeding vasculature on image acquisition volume and residual spin population within a feeding artery due to heterogeneous transit time could cause variation in estimation of quantitative rCBF on ASL and thereby cause estimation errors of regional CBF even in cortical gray matter. In contrast, in our data, the relative CBF ratios of MCA and PCA to ACA were more consistent with those of PET measurements (Table2).10,11 Thus, QUASAR may well overcome the problem of rCBF overestimation associated with conventional ASL methods and correct the erroneous regional variability of rCBF in cortical gray matter. The two crusher gradients employed in QUASAR experiments may have eliminated signals from rapid vascular flows and residual bolus as a possible source of overestimation to improve quantification.
Table 2.
Comparison of Absolute Regional Cerebral Blood Flow (rCBF) Values from the Literature
|
Average rCBF, (Ratio to ACA Value) |
||||
|---|---|---|---|---|
| Author (Year) | Modality | ACA | MCA | PCA |
| Tatewaki (this study) | QUASAR-ASL | 29.81 (1.00) | 31.98 (1.07) | 28.17 (.95) |
| Bokkers (2010) | H215O-PET | 40.1 (1.00) | 48.0 (1.20) | 48.7 (1.21) |
| ASL* | 50.4 (1.00) | 77.2 (1.53) | 95.6 (1.90) | |
| Bremmer (2011) | H215O-PET | 37.5 (1.00) | 37.4 (.98) | 41.3 (1.09) |
ACA, anterior cerebral artery, ASL: arterial spin labeling, MCA: middle cerebral artery, PCA: posterior cerebral artery.
Pulsed signal targeting with alternating radiofrequency (STAR) labeling technique with Look-Locker-like readout strategy
The absolute rCBF values of each arterial territory in this study were slightly lower than those in PET studies (Table2). However, with our QUASAR study, the mean CBF value of whole gray matter alone, obtained using a T1WI-derivated mask, was 37.74 mL/100 g/minute, which was compatible with the absolute CBF value of whole gray matter generally found for PET. We believe that the lower absolute rCBF values of each arterial territory obtained with our ROI analysis could be explained as partial volume effect between gray matter and subcortical white matter or sulci contained within an identical ROI. In fact, we noticed that the value was not necessarily very robust to changes in ROI definition. Still, overall, QUASAR and PET could be comparable for quantifying perfusion. Additionally, some subjects could locally exhibit high variability between sessions, as illustrated in Figure4. For example, in the anterior cerebral arterial ROI, subject 2 had a CBF estimation ranging from 22 to 40 mL/100 g/minute, which is close to a twofold difference between some runs. More than an intrinsic artifact of averaging over a fixed ROI, we regard that occasional variability as a real possible limitation of the method that all users should have in mind when relying on the estimated rCBF in a clinical context. Even though, due to the numerous advantages of this MRI method, such as its speed and noninvasiveness, we consider that it should not deter users to rely on it.
More accurate quantitative estimation of regional perfusion in QUASAR could allow more precise evaluation of repeated measurements in the same subject, as in longitudinal studies of diseases with regional CBF alteration, such as cerebrovascular disease, encephalitis, epilepsy, and tumor. Further, to compare CBF between populations, as in studies of patients and controls, QUASAR could identify specific pathological conditions with altered whole brain perfusion that could not be detected using only relative evaluation, such as degenerative disorders, certain forms of hydrocephalus, intracranial hypertension, diffuse axonal injury, psychiatric disease, meningitis, and central nervous system lupus.
Limitations
Our study was limited by the image acquisition volume of our QUASAR protocol design that does not cover the whole brain. By partially excluding the parietal and occipital lobes and infratentorial structures, we did not evaluate the reproducibility and reliability of this technique in those regions. We also evaluated only healthy volunteers and not patients with pathological conditions in which transit time might be extremely prolonged, such as carotid arterial stenosis/occlusion or cases following cerebrovascular bypass surgery. Finally, we did not directly compare QUASAR quantification with gold standard methods, such as PET study, in the same subjects. These issues require further study.
Conclusion
Our study suggested that the QUASAR-ASL technique should provide adequately high reliability of local brain perfusion despite variation in transit time, and varied vascular volume across almost the whole brain structure. Further, the absolute regional CBF values with QUASAR for gray matter were consistent with those published for PET regardless of the variation of vascular distributions and transit times, but not yet reliable for white matter. Especially, QUASAR enabled us to overcome the regional variability in estimating quantitative perfusion of gray matter that has been noted using conventional ASL methods, so we believe QUASAR would be a powerful application for absolute quantification of CBF and have broad clinical indications.
Acknowledgments
We wish to thank Yuriko Suzuki, of Philips Electronics Japan, Medical Systems, MR Clinical Science, who provided expert advice.
References
- Detre JA, Leigh JS, Williams DS, et al. Perfusion imaging. Magn Reson Med. 1992;23:37–45. doi: 10.1002/mrm.1910230106. [DOI] [PubMed] [Google Scholar]
- Jahng GH, Song E, Zhu XP, et al. Human brain: reliability and reproducibility of pulsed arterial spin-labeling perfusion MR imaging. Radiology. 2005;234:909–916. doi: 10.1148/radiol.2343031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48:242–254. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhang Y, Wolf RL, et al. Amplitude-modulated continuous arterial spin-labeling 3.0-T perfusion MR imaging with a single coil: feasibility study. Radiology. 2005;235:218–228. doi: 10.1148/radiol.2351031663. [DOI] [PubMed] [Google Scholar]
- Ye FQ, Berman KF, Ellmore T, et al. H215O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med. 2000;44:450–456. doi: 10.1002/1522-2594(200009)44:3<450::aid-mrm16>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Donahue MJ, Lu H, Jones CK, et al. An account of the discrepancy between MRI and PET cerebral blood flow measures. A high-field MRI investigation. NMR Biomed. 2006;19:1043–1054. doi: 10.1002/nbm.1075. [DOI] [PubMed] [Google Scholar]
- Knutsson L, Bloch KM, Holtås S, Wirestam R, Ståhlberg F. Model-free arterial spin labelling for cerebral blood flow quantification: introduction of regional arterial input functions identified by factor analysis. Magn Reson Imaging. 2008;26:554–559. doi: 10.1016/j.mri.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Grossman EJ, Zhang K, An J, et al. Measurement of deep gray matter perfusion using a segmented true-fast imaging with steady-state precession (True-FISP) arterial spin-labeling (ASL) method at 3T. J Magn Reson Imaging. 2009;29:1425–1431. doi: 10.1002/jmri.21794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med. 2008;59:788–795. doi: 10.1002/mrm.21515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremmer JP, van Berckel BM, Persoon S, et al. Day-to-day test-retest variability of CBF, CMRO2, and OEF measurements using dynamic 15O PET studies. Mol Imaging Biol. 2011;13:759–768. doi: 10.1007/s11307-010-0382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokkers RP, Bremmer JP, Berckel BN, et al. Arterial spin labeling perfusion MRI at multiple delay times: a corrective study with H215O positron emission tomography in patients with symptomatic carotid artery occlusion. J Cereb Blood Flow Metab. 2010;30:222–229. doi: 10.1038/jcbfm.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers S, van Osch MJ, Bokkers RP, et al. Intra- and multicenter reproducibility of pulsed, continuous and pseudo-continuous arterial spin labeling methods for measuring cerebral perfusion. J Cereb Blood Flow Metab. 2011;31:1706–1715. doi: 10.1038/jcbfm.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen ET, Lim T, Golay X. Model-free arterial spin labeling quantification approach for perfusion MRI. Magn Reson Med. 2006;55:219–232. doi: 10.1002/mrm.20784. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Mouridsen K, Golay X on behalf of all named co-authors of the QUASAR test-retest study. The QUASAR reproducibility study. Part II: results from a multi-center Arterial Spin Labeling test-retest study. Neuroimage. 2010;49:104–113. doi: 10.1016/j.neuroimage.2009.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K, Terae S, Katoh C, et al. Quantitative cerebral blood flow measurement with dynamic perfusion CT using the vascular-pixel elimination method: comparison with H215O positron emission tomography. AJNR Am J Neuroradiol. 2003;24:419–426. [PMC free article] [PubMed] [Google Scholar]
- Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(1):27–47. doi: 10.1093/brain/113.1.27. [DOI] [PubMed] [Google Scholar]
- Young S, Bystrov D, Netsch T, et al. Proceedings of the SPIE 6144 on Medical Imaging. CA, USA: San Diego; 2006. Automated planning of MRI neuro scans. [Google Scholar]
- Brant-Zawadzki M, Gillan GD, Nitz WR. MP RAGE: a three-dimensional, T1-weighted, gradient-echo sequence–initial experience in the brain. Radiology. 1992;182:769–775. doi: 10.1148/radiology.182.3.1535892. [DOI] [PubMed] [Google Scholar]
- Petersen ET, Zimine I, Ho YC, et al. Proceedings of the 15th. Germany: Berlin; 2007. An improved QUASAR sequence for user-independent quantitative and reproducible perfusion measurements; p. 376. [Google Scholar]
- Wong EC, Buxton RB, Frank LR. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II) Magn Reson Med. 1998;39:702–708. doi: 10.1002/mrm.1910390506. [DOI] [PubMed] [Google Scholar]
- Günther M, Bock M, Schad LR. Arterial spin labeling in combination with a Look-Locker sampling strategy: inflow turbo-sampling EPI-FAIR (ITS-FAIR) Magn Reson Med. 2001;46:974–984. doi: 10.1002/mrm.1284. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, et al. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Tatu L, Moulin T, Bogousslavsky J, et al. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological methods. 1996;1:30–46. [Google Scholar]
- van Osch MJ, Teeuwisse WM, van Walderveen MA, et al. Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med. 2009;62:165–173. doi: 10.1002/mrm.22002. [DOI] [PubMed] [Google Scholar]