Abstract
Alzheimer’s disease (AD) is an increasingly prevalent, fatal neurodegenerative disease that has proven resistant, thus far, to all attempts to prevent it, forestall it, or slow its progression. The ε4 allele of the Apolipoprotein E gene (APOE4) is a potent genetic risk factor for sporadic and late-onset familial AD. While the link between APOE4 and AD is strong, many expected effects, like increasing the risk of conversion from MCI to AD, have not been widely replicable. One critical, and commonly overlooked, feature of the APOE4 link to AD is that several lines of evidence suggest it is far more pronounced in women than in men. Here we review previous literature on the APOE4 by gender interaction with a particular focus on imaging-related studies.
1. Introduction
Alzheimer’s disease (AD) is an increasingly prevalent, ultimately fatal neurodegenerative disorder for which there are no disease-modifying treatments. In the wake of several large, costly, and negative phase III studies of antibodies against beta-amyloid, there is a critical need for novel approaches to understanding AD and developing alternative targets for treatment. The ε4 allele of the Apolipoprotein E gene (APOE4) is a potent genetic risk factor for sporadic and late-onset familial AD(Corder et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993). While estimates vary across studies and ethnic backgrounds, the APOE4 allele is typically present in more than 50% of AD patients but is found only in about 15% of healthy older controls(Ward et al., 2012). Basic science research has suggested several roles that the ε4 isoform of apolipoprotein E (ApoE4) may play in augmenting the development of AD. Cell culture and animal models have identified potential pathogenic mechanisms related to beta-amyloid clearance, tau hyperphosphorylation, and synaptic function, among others(Brecht et al., 2004; Castellano et al., 2011; Dumanis et al., 2009). In the clinical realm, several drug trials have suggested that efficacy and side effect profiles may differ between APOE4 carriers and non-carriers though the findings have not always been replicated(Farlow, 2010; Petersen et al., 2005; Risner et al., 2006; Salloway et al., 2009). Some, but not all, imaging and cerebrospinal fluid (CSF) biomarker studies have shown early AD-like findings in healthy older APOE4 carriers(Machulda et al., 2011; Peskind et al., 2006; Reiman et al., 2001; Shaw et al., 2009; Sheline et al., 2010; Sunderland et al., 2004; Westlye, Lundervold, Rootwelt, Lundervold, & Westlye, 2011). Some, but not all, longitudinal studies suggest that among patients with mild cognitive impairment (MCI) APOE4 carriers are more likely to convert to AD (Devanand et al., 2005; Landau et al., 2010; Petersen et al., 1995; Tierney et al., 1996).
Thus, while the link between APOE4 and AD is strong, many expected effects, like increasing the risk of conversion from MCI to AD, have not been widely replicable. One critical, and commonly overlooked, feature of the APOE4 link to AD is that several lines of evidence suggest it is far more pronounced in women than in men. Shortly after the initial linkage studies, a prominent interaction between APOE and gender was reported(Payami et al., 1994). The first large meta-analysis of APOE4 studies found that women in their sixties with one APOE4 allele had a 4-fold increased risk whereas male APOE4 heterozygotes did not bump their risk much, if at all(Farrer et al., 1997). Among APOE4 homozygotes, men and women both showed a pronounced increase in risk compared to homozygous carriers of the risk-neutral ε3 allele (APOE3). Even among the APOE4 homozygotes, however, there appeared to be a gender interaction in that female APOE4 homozygotes’ risk peaked around 12-fold compared to 10-fold in the male APOE4 homozygotes. This finding has been replicated several times and yet is almost never considered or investigated in clinical AD research where male and female APOE4 carriers are generally viewed as having equal risk(Bretsky et al., 1999; Payami et al., 1996). In the few recent studies that have examined this interaction between APOE4 and gender, female, but not male, APOE4 carriers have shown more pronounced AD-like changes in neuroimaging, neuropathological, and neuropsychological measures when compared to their APOE3 homozygous peers(Corder et al., 2004; Damoiseaux, Seeley, et al., 2012; Fleisher et al., 2005; Lehmann et al., 2006). In mouse models designed for AD research, the same interaction between APOE and gender was identified years ago, so that today many studies only carry out experiments in female mice(Andrews-Zwilling et al., 2010; Raber, Bongers, LeFevour, Buttini, & Mucke, 2002; Raber et al., 1998).
Here we review previous literature on the APOE4 by gender interaction with a particular focus on imaging-related studies.
2.1 Genetics of Alzheimer’s Disease
There are genes besides ApoE that have been linked to increased AD frequency, but compared to ApoE, they occur either rarely or their effects are less potent. On one hand autosomal dominant AD—linked to mutations in presenilin-1 (PS1), presenilin-2 (PS2), or the amyloid precursor protein (APP)— is caused by genes that are fully penetrant, but their occurrence is rare; they are thought to account for less than 5% of AD cases(Bertram & Tanzi, 2012). In contrast, a slew of recent genome-wide association studies (GWAS) have identified polymorphisms that are more common, but the risk conferred by any one of these genes has been marginal (Guerreiro & Hardy, 2011). The more potent genes reported in GWAS publications typically result in a roughly 0.1 to 0.15-fold change in risk compared to the 4-fold increased risk associated with APOE4(Bertram, Lill, & Tanzi, 2010).
In contrast, APOE4 is found in more than half of AD patients across most studies and in closer to 65% of patients of Northern European descent(Ward et al., 2012). The reports linking late-onset familial and sporadic AD to APOE4 provided a compelling new inroad into AD pathogenesis(Corder et al., 1993; Saunders et al., 1993; Strittmatter et al., 1993). The main findings—APOE4 increases AD risk 2–3 fold in heterozygotes and 10-fold in homozygotes while moving the age of onset earlier—have been replicated numerous times across a variety of ethnic groups(Farrer et al., 1997; Ward et al., 2012). It has also been shown that the less common ε2 allele (APOE2) is protective against AD(Corder et al., 1994; Talbot et al., 1994). Briefly, the product of the APOE gene is the 299 amino acid long apoE protein. The three major isoforms (ε2, ε3, and ε4) differ only in two single nucleotide polymorphisms leading to changes in the encoded amino acids at positions 112 and 158, respectively. The consequence is that at the protein level, the ε4 isoform is more prone than the ε2 or ε3 isoforms to an interaction between the N-terminal and C-terminal domains, resulting in a pronounced conformational change that is presumed to underlie the functional differences(Zhong & Weisgraber, 2009). In mouse models combining APP mutations with either APOE3 or APOE4 mutations, the APOE4 mice showed increased deposition of beta-amyloid plaques(Bales et al., 2009). Subsequent work suggests that the increased beta-amyloid deposition is due to reduced clearance in the APP/APOE4 mice(Castellano et al., 2011). Several monogenic APOE mouse models have been created and show a variety of beta-amyloid independent, but AD-relevant changes including impaired learning and memory, impaired synaptogenesis, and tau hyperphosphorylation(Huang, 2010). Though the predictive power of the APOE4 allele is less than that of a PS1 mutation, it is sufficiently high to merit the consideration of presymptomatic trials in older APOE4 homozygotes(Reiman et al., 2011).
2.2. APOE Effects on the Clinical Course of Healthy Aging, MCI and AD
In addition to increasing the risk of developing AD, APOE4 tends to move the age of onset 5–15 years earlier(Corder et al., 1993; Gomez-Tortosa et al., 2007). In studies with long-lived subjects, the APOE4 effect is detectable in age of onset but is diminished in terms of overall risk, such that beyond age 80 there is little additional risk attributable to APOE4(Blacker et al., 1997; Dickson et al., 2008; Farrer et al., 1997; Khachaturian, Corcoran, Mayer, Zandi, & Breitner, 2004). Whether or not APOE4 speeds the clinical course of AD is less clear. Several studies have suggested that patients with APOE4 have a shorter survival after diagnosis than their APOE3 counterparts(Dal Forno et al., 2002; Tilvis, Strandberg, & Juva, 1998). A number of other studies, however, have failed to replicate this effect(Corder et al., 1995; Slooter et al., 1999; van Duijn et al., 1995). Studies are similarly split as to whether cognitive and behavioral scores decline more quickly in APOE4 carriers. Some studies suggest APOE4 speeds cognitive decline, some studies suggest no effect, and some studies suggest APOE4 slows cognitive decline(Craft et al., 1998; Hirono, Hashimoto, Yasuda, Kazui, & Mori, 2003; Kleiman et al., 2006; Stern et al., 1997). The data are no clearer on the question of whether APOE4 increases the risk or rate of conversion from MCI to AD. The first study to examine this question found that APOE4-carrying MCI patients had a 4-fold increased risk of converting to AD over 5 years compared to non-carriers(Petersen et al., 1995). The following year it was reported that APOE4, in isolation, was not predictive of conversion from MCI to AD but was predictive when used in combination with measures of memory performance(Tierney et al., 1996). Subsequent studies have suggested that, in isolation, APOE4 status is not a reliable predictor of conversion from MCI to AD(Devanand et al., 2005; Landau et al., 2010). Most recently, APOE4 has been shown to be a strong predictor of conversion from MCI to AD (and even from healthy aging to AD) in a large sample of Chinese patients(P. N. Wang, Hong, Lin, Liu, & Chen, 2011). Regarding cognitive decline in healthy aging, there are, again, studies supporting both possibilities: APOE4 carriers decline more rapidly and APOE4 carriers do not decline more rapidly(Anstey & Christensen, 2000; Beaudreau, Kaci Fairchild, Spira, Lazzeroni, & O’Hara, 2012; Caselli et al., 2009; Jorm et al., 2007; Mayeux, Small, Tang, Tycko, & Stern, 2001; Van Gerven, Van Boxtel, Ausems, Bekers, & Jolles, 2012). Overall, therefore, the literature is decidedly mixed on the issue of APOE4 hastening the progression of disease, whether measured as decline in healthy aging, conversion from MCI to AD, progression of cognitive decline in AD, or survival in AD.
2.3. APOE Effects on AD Biomarkers
Clinical data are noisy, a fact which could account for the lack of consensus on whether APOE4 speeds cognitive and functional decline along the AD spectrum. Endophenotypes or biomarkers are meant to reduce noise in clinical research by providing a biological measure that tracks closely with disease status but is more reliably measured(Meyer-Lindenberg & Weinberger, 2006). In AD research, the field has adopted several imaging and CSF protein biomarkers that can be used alone, or in conjunction with clinical information, to guide research and improve diagnostic accuracy(Albert et al., 2011; McKhann et al., 2011; Sperling et al., 2011). These biomarkers are also now being routinely used in clinical trials in the hopes that they may provide an early signal predictive of later clinical response(Aisen, 2011; Cummings, 2010).
In the imaging domain most research has focused on three measures: hippocampal volume measured on T1-weighted structural MRI, glucose metabolism measured with fluorodeoxyglucose positron emission tomography (FDG PET), and amyloid plaque burden measured with Pittsburgh compound B (PIB) PET or one of the F-18 amyloid agents such as florbetapir (AV45 PET)(Jack, 2012). Resting-state functional MRI (fMRI) is a fourth candidate biomarker, which is also gaining some traction in the field and has recently been added to the Alzheimer’s Disease Neuroimaging Initiative (ADNI, discussed below)(Greicius, Srivastava, Reiss, & Menon, 2004; Jack et al., 2010). CSF biomarkers include levels of beta-amyloid, tau, and phosphorylated tau(p-tau)(Trojanowski et al., 2010). As a general summary across measures, AD patients show reduced hippocampal volumes; reduced glucose metabolism in the posterior cingulate cortex (PCC) and temporoparietal cortex; increased PIB signal (plaque deposition) in the PCC, medial prefrontal cortex, and temporoparietal cortex; reduced functional connectivity between the PCC, temporoparietal cortex, and hippocampus; reduced CSF levels of beta-amyloid; and increased CSF levels of tau and p-tau(Jack, 2012; Trojanowski et al., 2010). Remarkably, most of these biomarkers have shown similar AD-like patterns in MCI patients and even in presymptomatic carriers of genetic mutations causing autosomal dominant AD(Bateman et al., 2012; Damoiseaux, Seeley, et al., 2012; Drago et al., 2011; Sorg et al., 2007).
Following this pattern, one would then expect to see similar AD-like biomarker patterns when studying healthy older APOE4 carriers. As described above with the clinical progression literature, however, the biomarker studies in healthy older APOE4 carriers are also somewhat mixed. Structural MRI studies of healthy older controls are fairly evenly split between those that show an effect of APOE4 on hippocampal volume and those that do not(den Heijer et al., 2002; Lu et al., 2011; Moffat, Szekely, Zonderman, Kabani, & Resnick, 2000; Reiman et al., 1998; Tohgi et al., 1997; Tupler et al., 2007). Some studies of healthy older controls have shown an effect of APOE4 on hippocampal volume loss over time but no effect on baseline hippocampal volume(Jak, Houston, Nagel, Corey-Bloom, & Bondi, 2007; Moffat et al., 2000). Other studies of older controls have found no effect of APOE4 on hippocampal volume loss over time(Du et al., 2006). In two of the larger studies, Mazoyer and colleagues have found that APOE4 homozygotes have reduced baseline hippocampal volumes and show more volume loss over time whereas APOE4 heterozygotes do not differ from non-carriers in baseline hippocampal volume or volume loss over time(Crivello et al., 2010; Lemaitre et al., 2005).
While we do not intend to do an exhaustive review of the task-based fMRI findings in healthy APOE4 carriers, suffice to say that a similar pattern of mixed results can be found in this domain as well. The first study of task-based activation during memory encoding found that older E4 carriers (aged 47 to 82) showed increased activation in the hippocampus (Bookheimer et al., 2000). A subsequent study of healthy E4 carriers with a mean age of 60 also showed increased hippocampal activation in the E4 carriers (Kukolja, Thiel, Eggermann, Zerres, & Fink, 2010). Two more recent studies, however, reported reduced hippocampal activation in older APOE4 carriers (mean age around 64 in both studies) (Adamson, Hutchinson, Shelton, Wagner, & Taylor, 2011; Filippini et al., 2011). In younger APOE4 carriers, there are fewer studies but the results are more consistent, suggesting that E4 carriers have increased hippocampal activation during memory encoding (Dennis et al., 2010; Filippini et al., 2009).
The resting state fMRI studies related to APOE4 are as mixed as the task-based memory studies. Previous resting state fMRI studies have shown that functional connectivity changes in the default mode network (DMN) in MCI (Sorg et al., 2007) and AD (Greicius et al., 2004; K. Wang et al., 2007; Zhang et al., 2009) and that this default mode functional connectivity deteriorates as the disease progresses (Bai et al., 2011; Damoiseaux, Prater, Miller, & Greicius, 2012). Recent resting state fMRI studies have investigated whether we can detect similar changes in brain functional connectivity in healthy older APOE4 carriers (Machulda et al., 2011; Sheline et al., 2010; Trachtenberg et al., 2012; Westlye et al., 2011). Trachtenberg et al. (2012) found no APOE-related differences in DMN connectivity. The other three studies show significant DMN connectivity differences between APOE4 carriers and APOE3 homozygotes, although there is substantial variability in their results. Sheline et al. (2010) found increases and decreases in DMN connectivity in e4 carriers, Machulda et al. (2011) only found decreases, Westlye et al. (2011) only found increases, and Trachtenberg et al. (2012) found no differences in the DMN but reported differences in two hippocampal networks. None of these fMRI studies of APOE, task-based or resting state, examined whether the observed default mode functional connectivity differences varied by gender.
The imaging literature has numerous examples of healthy older APOE4 carriers with AD-like changes in glucose metabolism(Reiman et al., 1996; Small et al., 2000; Small et al., 1995). An early study by Reiman and colleagues reported that, in the 50–65 age range, APOE4 homozygotes showed reduced glucose metabolism in several regions typically targeted in AD, including the PCC and lateral parietal cortex(Reiman et al., 1996). Intriguingly, Figure 2 in that study also demonstrates hypometabolism in the APOE4 carriers in many additional regions not typically affected by AD. A subsequent study by the same group reported similar findings for a much younger group of healthy APOE4 heterozygotes(Reiman et al., 2004). Here again, AD-targeted regions showed hypometabolism but many additional regions (gray-green clusters in Figure 1 of that study) also showed reduced metabolism in the APOE4 carriers. These studies suggest a non-specific effect of APOE4, involving regions typically affected by AD as well as regions commonly spared in AD. More recent work, using large sample sizes and subject-specific control databases, has found that glucose metabolism in AD-relevant regions did not differ between healthy older APOE4 carriers and non-carriers(Samuraki et al., 2012). The literature is, thus far, more consistent when it comes to amyloid imaging in older APOE4 carriers. Several studies have reported that healthy older APOE4 carriers are more likely to harbor amyloid plaques on imaging either with PIB or AV45(Aizenstein et al., 2008; Drzezga et al., 2009; Morris et al., 2010; Reiman et al., 2009; Rodrigue et al., 2012).
Figure 2.
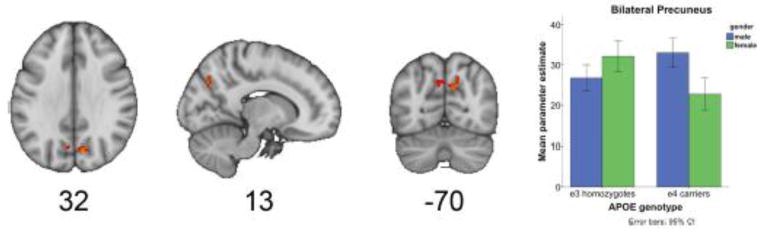
Gender modulates the APOE effect on default mode network connectivity. An ApoE by gender interaction was found in the precuneus region of the default mode network. The bar graph shows the mean parameter estimates for this cluster, for male and female E3 homozygotes and male and female E4 carriers. The greatest reduction was observed in the female 4 carriers. (This is adapted from Figure 3 of Damoiseaux et al.)
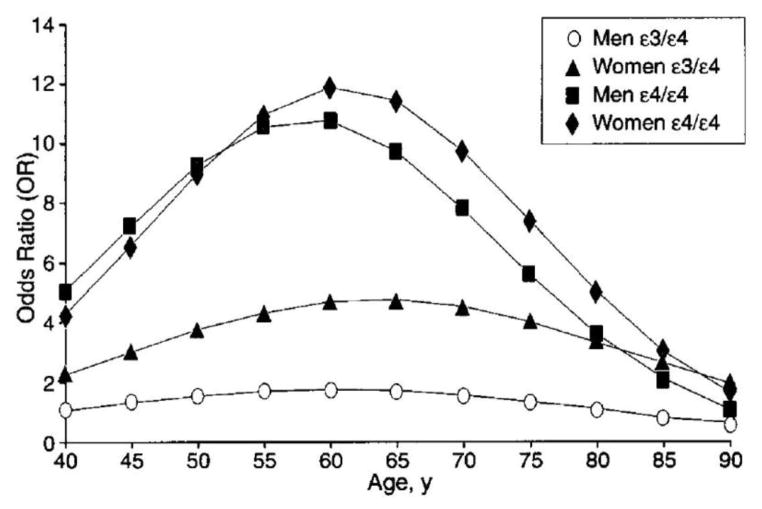
Figure 1. APOE4 risk is greater in women.
Compared to background risk in APOE3 homozygotes, female APOE3/4 heterozygotes’ odds ratio peaks near 4 whereas the odds ratio in male APOE3/4 heterozygotes barely exceeds 1. The odds ratio for both men and women APOE4 homozygotes peaks near 10 though remains somewhat higher in women. (This is adapted from Figure 2 in the meta-analysis by Farrer and colleagues).
The CSF protein studies are also fairly consistent when it comes to beta-amyloid levels. Two large studies have both reported reduced beta-amyloid levels in healthy older APOE4 carriers compared to non-carriers(Morris et al., 2010; Shaw et al., 2009). Importantly, neither study found a significant effect of APOE4 on total tau levels in the CSF. In ADNI MCI patients it has been reported that APOE4 carriers have lower beta-amyloid and higher tau levels than non-carriers, though it is not clear that the model used controlled for potential cognitive differences in APOE4 carriers(Shaw et al., 2009). One study, reporting on CSF data in ADNI, found that APOE status had no effect on annual change in beta-amyloid or total tau levels in healthy controls, MCI patients, or AD patients(Vemuri et al., 2010).
In sum, APOE4 has a pronounced and widely-replicated effect on AD risk. Convergent evidence from amyloid imaging and CSF studies suggests that APOE4 drives beta-amyloid plaque deposition. Despite the strength of these findings, the numerous additional predicted effects of APOE4—hastening decline in healthy aging, speeding conversion from MCI to AD, increasing CSF tau levels in healthy controls, reducing glucose metabolism in healthy controls and several others described above—have not been consistently demonstrated. We hypothesize that this marked lack of replicability in many predicted effects of APOE4 is due to the fact that most researchers overlook, or are unaware of, the potent interaction between APOE and gender. Given that the amyloid findings are among the most consistent, it is possible that the APOE4 effect on amyloid processing is equivalent in the two genders but that downstream effects are still more prominent in women.
3.0 APOE and Gender: Historically
The discovery of the interaction between APOE and gender dates to just after the discovery of the effect of APOE on AD. Just one year following the initial studies linking APOE4 to AD risk, it was shown that, for APOE4 heterozygotes, the risk was mainly seen in women(Payami et al., 1994). Initially, this finding was viewed as controversial, with some studies attributing the increased prevalence of APOE4 alleles in AD women to the longer survival of AD women carriers.(Corder et al., 1995) However, the significance of the interaction, even accounting for survival, was replicated in a larger sample size in 1996, and in 1997 Farrer et al. reported their results from a large meta-analysis of over 5000 patients and 8000 controls(Farrer et al., 1997; Payami et al., 1996). Figure 1, adapted from Farrer et al., makes a compelling case for the interaction between APOE and gender. Two additional studies of AD risk conducted later confirmed the increased AD risk in female compared to male APOE4 carriers. One study used a logistic regression model to show that the significance of a term for an interaction between sex and APOE4 carrier status (sex-by-E4 interaction) was preserved both including and excluding female E4/E4 homozygotes,(Bretsky et al., 1999). The second study found, even more provocatively, that the main effect of sex on AD vanished when accounting for the sex-by-E4 interaction, with the association between female sex and AD applying exclusively to female E4 carriers(Breitner et al., 1999). Moreover, these data suggest that males with one copy of the APOE4 allele have little to no increased risk compared to male APOE3 homozygotes. Even among APOE4 homozygotes, it appears that women are at increased risk compared to men.
Despite being potent and widely-replicated, however, the interaction between APOE and gender is almost never considered either in the clinical or research setting. Below we present the available research on this interaction, organized by modality.
3.1 APOE and Gender: Animal Models and Pathophysiology
The neurobiological relevance of this interaction is supported by the fact that it is also manifested in mouse models of APOE(Raber et al., 1998). The original study of transgenic APOE4 mice demonstrated this interaction, finding that female but not male transgenic APOE4 mice show impairments in memory and learning in special tasks (Raber et al., 1998). In a subsequent study the same group found that the effects of androgen treatment depended on the mice’s APOE4 carrier status and gender (Raber et al., 2002). Further studies found that E4 leads to an age related decline in spatial memory tasks in female but not male mice (Bour et al., 2008; Raber et al., 2000; Reverte, Klein, Ratner, Domingo, & Colomina, 2012). Even more recently, a study suggested a neuropathological correlate of these behavioral findings, demonstrating that female but not male E4 mice have decreased presynaptic density in the their hippocampi (Rijpma et al., 2013). As the interaction became widely replicated and recognized within the APOE mouse model research field, some subsequent studies restricted experiments to female mice, conceding that male mice are typically less affected by the APOE genotype (Andrews-Zwilling et al., 2010; van Meer, Acevedo, & Raber, 2007).
3.2 APOE and Gender: Clinical Outcomes in Healthy Aging and MCI
Though more commonly addressed in the APOE mouse model literature, some human APOE studies have pursued the APOE by gender interaction. These studies can be separated, broadly speaking, into two categories by focusing on their patient population of interest, with the first group of studies examining cognitive performance or clinical conversion in healthy aging and the second examining cognitive performance or clinical conversion in MCI. We will consider a selection of these studies below.
Even in healthy aging, it appears that female E4 carriers have worse cognitive performance, especially in memory tasks. One study of 189 Danish subjects ages 50–80, who did not have dementia, showed that APOE4 was associated with a rapid cognitive decline (performance IQ and verbal IQ) in women after the age of 70, but not in men (Mortensen & Hogh, 2001). Another larger study of episodic memory, including 2181 Norwegian healthy elderly patients aged 70–74, reproduced this finding, evincing that episodic memory was more impaired in heterozygote E4 carrying women than in heterozygote E4 carrying men. Among homozygotes, however, the effect was stronger in men, thus representing one of the few studies showing a worse phenotype in E4 carrying men (Lehmann et al., 2006). Additionally, Reinvang et al. found that the interaction effects not only episodic memory, but working memory as well (Reinvang, Winjevoll, Rootwelt, & Espeseth, 2010). Finally, Swan et al. showed, more generally, that APOE4 is associated with cognitive decline differently in men than in women across a number of tasks. This study measured cognitive performance in men and women with average ages of 79 and 75 respectively at study onset, and again in those same men and women after four years. (Note the older age of these subjects than those in many other studies, and that the men in this study were significantly older than women.) Initially, the expected interaction was demonstrated, with female E4 carriers performing more poorly on short delay cued recall than women without E4. Longitudinally, however, the results were more complex and task dependent, with E4 men demonstrating a greater decline in executive function and verbal memory tasks compared to men without an E4 allele, whereas women with an E4 allele exhibited a greater decline in the Trail Making test compared to women without an E4 allele (Swan, Lessov-Schlaggar, Carmelli, Schellenberg, & La Rue, 2005).
Two recent, prospective studies have examined the interactive effect of APOE and gender on clinical conversion from healthy aging to MCI or AD. Beydoun et al. found a main effect of APOE on conversion from healthy aging to AD (Beydoun et al., 2012). Though the effect was numerically greater in women than men, the formal interaction was not significant. The authors pointed out that they may have been underpowered to detect the interaction. Note that this paper is also informative in that it includes a brief review of the extensive list of studies on APOE and cognitive decline (both with and without an APOE-by-gender interaction term). In a larger prospective study, Altmann et al. found a significant interaction, with female APOE4 carriers more likely to convert from healthy aging to MCI or AD over a mean interval of about 4 years (Altmann, Tian, Henderson, & Greicius, 2013).
In MCI, we were only able to find one study that examined the effect of the APOE by gender interaction on cognition. In a study of 193 subjects with MCI, Fleisher et al. demonstrated that APOE4 genotype status has a greater deleterious effect on memory performance in women than men (Fleisher et al., 2005). In terms of prospective studies of conversion from MCI to AD, we are only aware of one study that explicitly modeled the interaction. In the prospective study by Altmann et al., there was a main effect of APOE4 in increasing the risk of conversion from MCI to AD. The interaction term showed a substantial trend, with a p-value < 0.06 suggesting a greater risk of converting from MCI to AD in female APOE4 carriers (Altmann et al., 2013).
3.3 APOE and Gender: AD pathological and CSF biomarkers
Compared to the ample research on the interaction in animal models and clinical outcomes, the literature on the interaction on AD biomarkers is relatively sparse. With a few prominent exceptions in the AD biomarker domain, the interaction has been overlooked or neglected entirely. Those exceptions are notable, however. In terms of pathological biomarkers, a large autopsy series found that female APOE4 carriers had greater amyloid plaque and neurofibrillary tangle pathology (Corder et al., 2004). A smaller autopsy series evinced a similar but subtler result, showing an interaction between APOE4 and gender on senile plaques in women between the ages 60–79 but not in women older than 80, but an association between APOE4 and increased senile plaques across men of all ages.(Ghebremedhin et al., 2001) Intriguingly, this demonstration that age may modify the interaction of APOE and gender on senile plaque formation echoes a similar modification by age of the APOE by gender interaction on cognitive tasks in Swan et al. 2005, mentioned in section 3.2, suggesting that age may be a modifying factor vis-a-vis the APOE-by-gender interaction generally. In terms of CSF biomarkers, Damoiseaux et al. recently demonstrated that there are higher spinal fluid levels of Tau in female APOE4 carriers, and the difference between female APOEE4 carriers and female APOE3 heterozygotes was significant, whereas the same comparison in men showed no significant differences between male APOE4 carriers and male homozygotes.(Damoiseaux, Seeley, et al., 2012)
3.4 APOE and Gender: Neuroimaging biomarkers
Neuroimaging biomarker research on the interaction is also quite sparse but telling nonetheless. In terms of structural neuroimaging, in MCI subjects, it has been shown that female, but not male, APOE4 heterozygotes have smaller hippocampal volumes when compared to non-carriers(Fleisher et al., 2005). In terms of functional neuroimaging, Damoiseaux et al also recently showed that healthy older female, but not male, APOE4 carriers have reduced functional connectivity in the PCC, a key hub in the brain’s default-mode network which is targeted early in the course of AD(Damoiseaux, Seeley, et al., 2012; Greicius et al., 2004) (see figure 2). For the most part, however, this potent interaction between APOE and gender has been overlooked in neuroimaging research, particularly taking into consideration the vast array of studies that have examined the effect of APOE4 on AD biomarkers more generally (as in section 2.3). Clearly, neuroimaging biomarkers have a lot to tell us about the effects of APOE and we hope that this review will encourage imaging researchers to include an APOE by gender interaction term in subsequent analyses.
4.0 Implications and Future Research
In the fourth and final part of this review, we consider the explanations for and implications of this interaction, with a view towards pathophysiology, diagnosis, and treatment.
4.1 Mysterious Mechanism: Sex-hormone mediated?
Despite the importance of the interaction, and its having been replicated time-and-time-again in multiple and sundry contexts, the underlying mechanisms remain a mystery. The most obvious pathophysiology would be, in some way, sex-hormone mediated. There is a wide literature exploring the relationship between estrogen and cognitive decline (Henderson, 2009). The results, however, are mixed. The Women’s Health Initiative Memory famously determined that hormone replacement therapy (HRT), at least when initiated post-menopause, is associated with increased, not decreased, rates of dementia (Coker et al., 2010). However, the study did not explicitly examine the interaction’s relationship with hormone replacement therapy, and the studies that have examined that interaction complicate the picture. Yaffe et al. for instance found that estrogen use is associated with less cognitive decline in APOE4 negative healthy older women than in women who are APOE4 carriers (Yaffe, Haan, Byers, Tangen, & Kuller, 2000). Subsequently, Kang et al, in a large and longitudinal study of 16,514 nurses, showed similarly that the fastest rates of cognitive decline were registered by APOE4 carriers who were HRT users (Kang & Grodstein, 2012). These studies together suggest that the jury is still out on the relationship between HRT and cognitive decline, that this relationship may be more subtle than initially suggested, and that the interaction of APOE4 and AD may be crucial to understanding it.
4.2 Implications for diagnosis of APOE4 carriers
The advent of direct-to-consumer (DTC) genetic testing means that more healthy individuals will be “diagnosed” as APOE4 carriers(Messner, 2011). Even outside of research settings, clinical testing for APOE is common enough that guidelines have been developed for genetic counseling(Goldman et al., 2011). Indeed, so serious is the “diagnosis” of APOE4 carrier, or at least its perception, that when James Watson released his sequenced genome, the only part of it he had redacted was his APOE status (Nyholt, Yu, & Visscher, 2008). But what exactly it means, clinically, to be an APOE4 carrier depends, as we have discussed, on a patient’s gender. Elucidating the APOE by gender interaction, then, will help mitigate some of the uncertainty related to both clinical and DTC genetic testing.
4.3 Implications for treatment
Regarding treatment, a better understanding of the APOE by gender interaction could have two important kinds of benefits. First, male and female APOE4 carriers could differ in their response to current and future therapeutics, and understanding how they differ could lead to personalized therapeutic regimens. Indeed, at least two studies have already uncovered such a difference: a placebo-controlled, double-blind study of tacrine (an early acetocholinesterase-inhibitor) evinced a different response in APOE4 women as opposed to men, such that tacrine was less effective in APOE4 carrying women than in non APOE4 carrying women, but equally effective in men across genotypes (Farlow et al., 1998). In contrast, a smaller study of tacrine and galantamine (also an acetylcholinesterase-inhibitor) found the opposite, that APOE4 women carriers were more likely to benefit from acetylcholinesterase-inhibitor therapy (MacGowan, Wilcock, & Scott, 1998). Despite these intriguing early finding, most subsequent studies of AD or MCI treatments did not explore the possible effect of the APOE by gender interaction on treatment response(Petersen et al., 2005; Raskind, Peskind, Wessel, & Yuan, 2000) (Rogers, Farlow, Doody, Mohs, & Friedhoff, 1998; Rosler et al., 1999; Wilcock, Lilienfeld, & Gaens, 2000). One subsequent study of donepezil’s efficacy in AD did formally explore the interaction and though it did not reach significance, it reported a p-value of 0.09 (with an N of only 117), although tantalizingly that study did not report in which direction their results were trending (Rigaud et al., 2002). In any case, an understanding of the importance of the interaction vis-à-vis existing therapies could lead to more effective, personalized therapeutic regimens, even involving current medications.
Second, from a basic science perspective, dissecting the mechanisms behind this interaction should provide novel insights into AD pathogenesis and suggest novel approaches to treatment. AD is a progressive, neurodegenerative disorder that gradually, sequentially robs a patient of their memory, language, personality, and mobility, ultimately resulting in death. The course is typically prolonged, lasting on the order of ten years, so that in addition to the patient’s suffering, there is a considerable emotional, physical, and financial toll paid by family members. From a societal perspective, AD represents a massive threat to the stability of the health care system. With the aging population, it is estimated that between 11 and 16 million Americans will have AD by mid-century. Direct health-care costs for AD patients in the U.S. were estimated at $210 billion in 2012. This figure does not account for the lost wages of the estimated 15 million family members and friends who provided unpaid care to patients in 2011(“2012 Alzheimer’s disease facts and figures,” 2012). The prevalence estimates assume the continued absence of a preventative or curative intervention for AD. Even without being curative, an intervention that delayed disease onset would result in a substantial reduction of the prevalence of AD and, in turn, a substantial cost-savings for the healthcare system(Sloane et al., 2002). Unfortunately, despite decades of research and development, we have no disease-modifying medications available. Novel insights into AD pathogenesis, of just the kind that a fuller understanding of the interaction between APOE and gender will yield, are desperately needed.
References
- 2012 Alzheimer’s disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Adamson MM, Hutchinson JB, Shelton AL, Wagner AD, Taylor JL. Reduced hippocampal activity during encoding in cognitively normal adults carrying the APOE varepsilon4 allele. Neuropsychologia. 2011;49(9):2448–2455. doi: 10.1016/j.neuropsychologia.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisen PS. Clinical trial methodologies for disease-modifying therapeutic approaches. Neurobiol Aging. 2011;32(Suppl 1):S64–66. doi: 10.1016/j.neurobiolaging.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65(11):1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann AA, Tian L, Henderson VW, Greicius MD. Gender Modulates the Effect of APOE e4 on the Risk of Developing Mild Cognitive Impairment and Alzheimer’s Disease. 2013. Submitted. [Google Scholar]
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30(41):13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. 30/41/13707 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstey K, Christensen H. Education, activity, health, blood pressure and apolipoprotein E as predictors of cognitive change in old age: a review. Gerontology. 2000;46(3):163–177. doi: 10.1159/000022153. 22153. [DOI] [PubMed] [Google Scholar]
- Bai F, Watson DR, Shi Y, Wang Y, Yue C, Teng Yuhuan, Zhang Z. Specifically progressive deficits of brain functional marker in amnestic type mild cognitive impairment. PLoS One. 2011;6(9):e24271. doi: 10.1371/journal.pone.0024271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KR, Liu F, Wu S, Lin S, Koger D, DeLong C, Paul SM. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29(21):6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, Morris JC. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med. 2012;367(9):795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudreau SA, Kaci Fairchild J, Spira AP, Lazzeroni LC, O’Hara R. Neuropsychiatric symptoms, apolipoprotein E gene, and risk of progression to cognitive impairment, no dementia and dementia: the Aging, Demographics, and Memory Study (ADAMS) Int J Geriatr Psychiatry. 2012 doi: 10.1002/gps.3868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68(2):270–281. doi: 10.1016/j.neuron.2010.10.013. S0896-6273(10)00837-8 [pii] [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- Beydoun MA, Boueiz A, Abougergi MS, Kitner-Triolo MH, Beydoun HA, Resnick SM, Zonderman AB. Sex differences in the association of the apolipoprotein E epsilon 4 allele with incidence of dementia, cognitive impairment, and decline. Neurobiol Aging. 2012;33(4):720–731. e724. doi: 10.1016/j.neurobiolaging.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacker D, Haines JL, Rodes L, Terwedow H, Go RC, Harrell LE, Tanzi R. ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology. 1997;48(1):139–147. doi: 10.1212/wnl.48.1.139. [DOI] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343(7):450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bour A, Grootendorst J, Vogel E, Kelche C, Dodart JC, Bales K, Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193(2):174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- Brecht WJ, Harris FM, Chang S, Tesseur I, Yu GQ, Xu Q, Huang Y. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci. 2004;24(10):2527–2534. doi: 10.1523/JNEUROSCI.4315-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Wyse BW, Anthony JC, Welsh-Bohmer KA, Steffens DC, Norton MC, Khachaturian A. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53(2):321–331. doi: 10.1212/wnl.53.2.321. [DOI] [PubMed] [Google Scholar]
- Bretsky PM, Buckwalter JG, Seeman TE, Miller CA, Poirier J, Schellenberg GD, Henderson VW. Evidence for an interaction between apolipoprotein E genotype, gender, and Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13(4):216–221. doi: 10.1097/00002093-199910000-00007. [DOI] [PubMed] [Google Scholar]
- Caselli RJ, Dueck AC, Osborne D, Sabbagh MN, Connor DJ, Ahern GL, Reiman EM. Longitudinal modeling of age-related memory decline and the APOE epsilon4 effect. N Engl J Med. 2009;361(3):255–263. doi: 10.1056/NEJMoa0809437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano JM, Kim J, Stewart FR, Jiang H, DeMattos RB, Patterson BW, Holtzman DM. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):89ra57. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker LH, Espeland MA, Rapp SR, Legault C, Resnick SM, Hogan P, Shumaker SA. Postmenopausal hormone therapy and cognitive outcomes: the Women’s Health Initiative Memory Study (WHIMS) J Steroid Biochem Mol Biol. 2010;118(4–5):304–310. doi: 10.1016/j.jsbmb.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann N Y Acad Sci. 2004;1019:24–28. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7(2):180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, et al. Apolipoprotein E, survival in Alzheimer’s disease patients, and the competing risks of death and Alzheimer’s disease. Neurology. 1995;45(7):1323–1328. doi: 10.1212/wnl.45.7.1323. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Craft S, Teri L, Edland SD, Kukull WA, Schellenberg G, McCormick WC, Larson EB. Accelerated decline in apolipoprotein E-epsilon4 homozygotes with Alzheimer’s disease. Neurology. 1998;51(1):149–153. doi: 10.1212/wnl.51.1.149. [DOI] [PubMed] [Google Scholar]
- Crivello F, Lemaitre H, Dufouil C, Grassiot B, Delcroix N, Tzourio-Mazoyer N, Mazoyer B. Effects of ApoE-epsilon4 allele load and age on the rates of grey matter and hippocampal volumes loss in a longitudinal cohort of 1186 healthy elderly persons. Neuroimage. 2010;53(3):1064–1069. doi: 10.1016/j.neuroimage.2009.12.116. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Integrating ADNI results into Alzheimer’s disease drug development programs. Neurobiol Aging. 2010;31(8):1481–1492. doi: 10.1016/j.neurobiolaging.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Forno G, Carson KA, Brookmeyer R, Troncoso J, Kawas CH, Brandt J. APOE genotype and survival in men and women with Alzheimer’s disease. Neurology. 2002;58(7):1045–1050. doi: 10.1212/wnl.58.7.1045. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Prater KE, Miller BL, Greicius MD. Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol Aging. 2012;33(4):828, e819–830. doi: 10.1016/j.neurobiolaging.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A Alzheimer’s Disease Neuroimaging Initiative. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32(24):8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Heijer T, Oudkerk M, Launer LJ, van Duijn CM, Hofman A, Breteler MM. Hippocampal, amygdalar, and global brain atrophy in different apolipoprotein E genotypes. Neurology. 2002;59(5):746–748. doi: 10.1212/wnl.59.5.746. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Browndyke JN, Stokes J, Need A, Burke JR, Welsh-Bohmer KA, Cabeza R. Temporal lobe functional activity and connectivity in young adult APOE varepsilon4 carriers. Alzheimers Dement. 2010;6(4):303–311. doi: 10.1016/j.jalz.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devanand DP, Pelton GH, Zamora D, Liu X, Tabert MH, Goodkind M, Mayeux R. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62(6):975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- Dickson MR, Li J, Wiener HW, Perry RT, Blacker D, Bassett SS, Go RC. A genomic scan for age at onset of Alzheimer’s disease in 437 families from the NIMH Genetic Initiative. Am J Med Genet B Neuropsychiatr Genet. 2008;147B(6):784–792. doi: 10.1002/ajmg.b.30689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drago V, Babiloni C, Bartres-Faz D, Caroli A, Bosch B, Hensch T, Frisoni GB. Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. J Alzheimers Dis. 2011;26(Suppl 3):159–199. doi: 10.3233/JAD-2011-0043. [DOI] [PubMed] [Google Scholar]
- Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, Kurz A. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72(17):1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiol Aging. 2006;27(5):733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanis SB, Tesoriero JA, Babus LW, Nguyen MT, Trotter JH, Ladu MJ, Hoe HS. ApoE4 decreases spine density and dendritic complexity in cortical neurons in vivo. J Neurosci. 2009;29(48):15317–15322. doi: 10.1523/JNEUROSCI.4026-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR. Should the ApoE genotype be a covariate for clinical trials in Alzheimer disease? Alzheimers Res Ther. 2010;2(3):15. doi: 10.1186/alzrt39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR, Lahiri DK, Poirier J, Davignon J, Schneider L, Hui SL. Treatment outcome of tacrine therapy depends on apolipoprotein genotype and gender of the subjects with Alzheimer’s disease. Neurology. 1998;50(3):669–677. doi: 10.1212/wnl.50.3.669. [DOI] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278(16):1349–1356. [PubMed] [Google Scholar]
- Filippini N, Ebmeier KP, MacIntosh BJ, Trachtenberg AJ, Frisoni GB, Wilcock GK, Mackay CE. Differential effects of the APOE genotype on brain function across the lifespan. Neuroimage. 2011;54(1):602–610. doi: 10.1016/j.neuroimage.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci U S A. 2009;106(17):7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher A, Grundman M, Jack CR, Jr, Petersen RC, Taylor C, Kim HT, Thal LJ. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62(6):953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- Ghebremedhin E, Schultz C, Thal DR, Rub U, Ohm TG, Braak E, Braak H. Gender and age modify the association between APOE and AD-related neuropathology. Neurology. 2001;56(12):1696–1701. doi: 10.1212/wnl.56.12.1696. [DOI] [PubMed] [Google Scholar]
- Goldman JS, Hahn SE, Catania JW, LaRusse-Eckert S, Butson MB, Rumbaugh M, Bird T. Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med. 2011;13(6):597–605. doi: 10.1097/GIM.0b013e31821d69b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Tortosa E, Barquero MS, Baron M, Sainz MJ, Manzano S, Payno M, Jimenez-Escrig A. Variability of age at onset in siblings with familial Alzheimer disease. Arch Neurol. 2007;64(12):1743–1748. doi: 10.1001/archneur.64.12.1743. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro RJ, Hardy J. Alzheimer’s disease genetics: lessons to improve disease modelling. Biochem Soc Trans. 2011;39(4):910–916. doi: 10.1042/BST0390910. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Aging, estrogens, and episodic memory in women. Cogn Behav Neurol. 2009;22(4):205–214. doi: 10.1097/WNN.0b013e3181a74ce7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono N, Hashimoto M, Yasuda M, Kazui H, Mori E. Accelerated memory decline in Alzheimer’s disease with apolipoprotein epsilon4 allele. J Neuropsychiatry Clin Neurosci. 2003;15(3):354–358. doi: 10.1176/jnp.15.3.354. [DOI] [PubMed] [Google Scholar]
- Huang Y. Abeta-independent roles of apolipoprotein E4 in the pathogenesis of Alzheimer’s disease. Trends Mol Med. 2010;16(6):287–294. doi: 10.1016/j.molmed.2010.04.004. S1471-4914(10)00055-9 [pii] [DOI] [PubMed] [Google Scholar]
- Jack CR., Jr Alzheimer disease: new concepts on its neurobiology and the clinical role imaging will play. Radiology. 2012;263(2):344–361. doi: 10.1148/radiol.12110433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM Alzheimer’s Disease Neuroimaging Initiative. Update on the magnetic resonance imaging core of the Alzheimer’s disease neuroimaging initiative. Alzheimers Dement. 2010;6(3):212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Houston WS, Nagel BJ, Corey-Bloom J, Bondi MW. Differential cross-sectional and longitudinal impact of APOE genotype on hippocampal volumes in nondemented older adults. Dement Geriatr Cogn Disord. 2007;23(6):382–389. doi: 10.1159/000101340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Mather KA, Butterworth P, Anstey KJ, Christensen H, Easteal S. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21(1):1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- Kang JH, Grodstein F. Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging. 2012;33(7):1129–1137. doi: 10.1016/j.neurobiolaging.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khachaturian AS, Corcoran CD, Mayer LS, Zandi PP, Breitner JC. Apolipoprotein E epsilon4 count affects age at onset of Alzheimer disease, but not lifetime susceptibility: The Cache County Study. Arch Gen Psychiatry. 2004;61(5):518–524. doi: 10.1001/archpsyc.61.5.518. [DOI] [PubMed] [Google Scholar]
- Kleiman T, Zdanys K, Black B, Rightmer T, Grey M, Garman K, van Dyck C. Apolipoprotein E epsilon4 allele is unrelated to cognitive or functional decline in Alzheimer’s disease: retrospective and prospective analysis. Dement Geriatr Cogn Disord. 2006;22(1):73–82. doi: 10.1159/000093316. [DOI] [PubMed] [Google Scholar]
- Kukolja J, Thiel CM, Eggermann T, Zerres K, Fink GR. Medial temporal lobe dysfunction during encoding and retrieval of episodic memory in non-demented APOE epsilon4 carriers. Neuroscience. 2010;168(2):487–497. doi: 10.1016/j.neuroscience.2010.03.044. [DOI] [PubMed] [Google Scholar]
- Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, Jagust WJ. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann DJ, Refsum H, Nurk E, Warden DR, Tell GS, Vollset SE, Smith AD. Apolipoprotein E epsilon4 and impaired episodic memory in community-dwelling elderly people: a marked sex difference. The Hordaland Health Study. J Neurol Neurosurg Psychiatry. 2006;77(8):902–908. doi: 10.1136/jnnp.2005.077818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre H, Crivello F, Dufouil C, Grassiot B, Tzourio C, Alperovitch A, Mazoyer B. No epsilon4 gene dose effect on hippocampal atrophy in a large MRI database of healthy elderly subjects. Neuroimage. 2005;24(4):1205–1213. doi: 10.1016/j.neuroimage.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Lu PH, Thompson PM, Leow A, Lee GJ, Lee A, Yanovsky I, Bartzokis G. Apolipoprotein E genotype is associated with temporal and hippocampal atrophy rates in healthy elderly adults: a tensor-based morphometry study. J Alzheimers Dis. 2011;23(3):433–442. doi: 10.3233/JAD-2010-101398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGowan SH, Wilcock GK, Scott M. Effect of gender and apolipoprotein E genotype on response to anticholinesterase therapy in Alzheimer’s disease. Int J Geriatr Psychiatry. 1998;13(9):625–630. doi: 10.1002/(sici)1099-1166(199809)13:9<625::aid-gps835>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Jones DT, Vemuri P, McDade E, Avula R, Przybelski S, Jack CR., Jr Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch Neurol. 2011;68(9):1131–1136. doi: 10.1001/archneurol.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux R, Small SA, Tang M, Tycko B, Stern Y. Memory performance in healthy elderly without Alzheimer’s disease: effects of time and apolipoprotein-E. Neurobiol Aging. 2001;22(4):683–689. doi: 10.1016/s0197-4580(01)00223-8. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner DA. Informed Choice in Direct-to-Consumer Genetic Testing for Alzheimer and Other Diseases: Lessons from Two Cases. New Genet Soc. 2011;30(1):59–72. doi: 10.1080/14636778.2011.552300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Szekely CA, Zonderman AB, Kabani NJ, Resnick SM. Longitudinal change in hippocampal volume as a function of apolipoprotein E genotype. Neurology. 2000;55(1):134–136. doi: 10.1212/wnl.55.1.134. [DOI] [PubMed] [Google Scholar]
- Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen EL, Hogh P. A gender difference in the association between APOE genotype and age-related cognitive decline. Neurology. 2001;57(1):89–95. doi: 10.1212/wnl.57.1.89. [DOI] [PubMed] [Google Scholar]
- Nyholt Dale R, Yu Chang-En, Visscher Peter M. On Jim Watson’s APOE status: genetic information is hard to hide. Eur J Hum Genet. 2008;17(2):147–149. doi: 10.1038/ejhg.2008.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payami H, Montee KR, Kaye JA, Bird TD, Yu CE, Wijsman EM, Schellenberg GD. Alzheimer’s disease, apolipoprotein E4, and gender. JAMA. 1994;271(17):1316–1317. [PubMed] [Google Scholar]
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, Schellenberg GD. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am J Hum Genet. 1996;58(4):803–811. [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Li G, Shofer J, Quinn JF, Kaye JA, Clark CM, Galasko DR. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol. 2006;63(7):936–939. doi: 10.1001/archneur.63.7.936. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, Schaid DJ, Thibodeau SN, Kurland LT. Apolipoprotein E status as a predictor of the development of Alzheimer’s disease in memory-impaired individuals. JAMA. 1995;273(16):1274–1278. [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S Alzheimer’s Disease Cooperative Study Group. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352(23):2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Raber J, Bongers G, LeFevour A, Buttini M, Mucke L. Androgens protect against apolipoprotein E4-induced cognitive deficits. J Neurosci. 2002;22(12):5204–5209. doi: 10.1523/JNEUROSCI.22-12-05204.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, Mucke L. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc Natl Acad Sci U S A. 1998;95(18):10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Yu GQ, Buttini M, Mahley RW, Pitas RE, Mucke L. Apolipoprotein E and cognitive performance. Nature. 2000;404(6776):352–354. doi: 10.1038/35006165. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Wessel T, Yuan W. Galantamine in AD: A 6-month randomized, placebo-controlled trial with a 6-month extension. The Galantamine USA-1 Study Group. Neurology. 2000;54(12):2261–2268. doi: 10.1212/wnl.54.12.2261. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: A foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci U S A. 2001;98(6):3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2009;106(16):6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Langbaum JB, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Tariot PN. Alzheimer’s Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. J Alzheimers Dis. 2011;26(Suppl 3):321–329. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiman EM, Uecker A, Caselli RJ, Lewis S, Bandy D, de Leon MJ, Thibodeau SN. Hippocampal volumes in cognitively normal persons at genetic risk for Alzheimer’s disease. Ann Neurol. 1998;44(2):288–291. doi: 10.1002/ana.410440226. [DOI] [PubMed] [Google Scholar]
- Reinvang I, Winjevoll IL, Rootwelt H, Espeseth T. Working memory deficits in healthy APOE epsilon 4 carriers. Neuropsychologia. 2010;48(2):566–573. doi: 10.1016/j.neuropsychologia.2009.10.018. [DOI] [PubMed] [Google Scholar]
- Reverte I, Klein AB, Ratner C, Domingo JL, Colomina MT. Behavioral phenotype and BDNF differences related to apoE isoforms and sex in young transgenic mice. Exp Neurol. 2012;237(1):116–125. doi: 10.1016/j.expneurol.2012.06.015. [DOI] [PubMed] [Google Scholar]
- Rigaud Anne-Sophie, Traykov Latchezar, Latour Florence, Couderc Rémy, Moulin Florence, Forette Françoise. Presence or absence of at least one [small element of]4 allele and gender are not predictive for the response to donepezil treatment in Alzheimer’s disease. Pharmacogenetics and Genomics. 2002;12(5):415–420. doi: 10.1097/00008571-200207000-00009. [DOI] [PubMed] [Google Scholar]
- Rijpma A, Jansen D, Arnoldussen IAC, Fang XT, Wiesmann M, Mutsaers MPC, Kiliaan AJ. Sex Differences in Presynaptic Density and Neurogenesis in Middle-Aged ApoE4 and ApoE Knockout Mice. Journal of Neurodegenerative Diseases. 2013;2013:9. doi: 10.1155/2013/531326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risner ME, Saunders AM, Altman JF, Ormandy GC, Craft S, Foley IM, Roses AD. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006;6(4):246–254. doi: 10.1038/sj.tpj.6500369. [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM, Devous MD, Sr, Rieck JR, Hebrank AC, Diaz-Arrastia R, Park DC. beta-Amyloid burden in healthy aging: regional distribution and cognitive consequences. Neurology. 2012;78(6):387–395. doi: 10.1212/WNL.0b013e318245d295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo-controlled trial of donepezil in patients with Alzheimer’s disease. Donepezil Study Group. Neurology. 1998;50(1):136–145. doi: 10.1212/wnl.50.1.136. [DOI] [PubMed] [Google Scholar]
- Rosler M, Anand R, Cicin-Sain A, Gauthier S, Agid Y, Dal-Bianco P, Gharabawi M. Efficacy and safety of rivastigmine in patients with Alzheimer’s disease: international randomised controlled trial. BMJ. 1999;318(7184):633–638. doi: 10.1136/bmj.318.7184.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73(24):2061–2070. doi: 10.1212/WNL.0b013e3181c67808. WNL.0b013e3181c67808 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuraki M, Matsunari I, Chen WP, Shima K, Yanase D, Takeda N, Yamada M. Glucose metabolism and gray-matter concentration in apolipoprotein E epsilon4 positive normal subjects. Neurobiol Aging. 2012;33(10):2321–2323. doi: 10.1016/j.neurobiolaging.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH, et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43(8):1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65(4):403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Morris JC, Snyder AZ, Price JL, Yan Z, D’Angelo G, Mintun MA. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci. 2010;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloane PD, Zimmerman S, Suchindran C, Reed P, Wang L, Boustani M, Sudha S. The public health impact of Alzheimer’s disease, 2000–2050: potential implication of treatment advances. Annu Rev Public Health. 2002;23:213–231. doi: 10.1146/annurev.publhealth.23.100901.140525. [DOI] [PubMed] [Google Scholar]
- Slooter AJ, Houwing-Duistermaat JJ, van Harskamp F, Cruts M, Van Broeckhoven C, Breteler MM, van Duijn CM. Apolipoprotein E genotype and progression of Alzheimer’s disease: the Rotterdam Study. J Neurol. 1999;246(4):304–308. doi: 10.1007/s004150050351. [DOI] [PubMed] [Google Scholar]
- Small GW, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97(11):6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Mazziotta JC, Collins MT, Baxter LR, Phelps ME, Mandelkern MA, et al. Apolipoprotein E type 4 allele and cerebral glucose metabolism in relatives at risk for familial Alzheimer disease. JAMA. 1995;273(12):942–947. [PubMed] [Google Scholar]
- Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, Wohlschlager AM. Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2007;104(47):18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Phelps CH. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Brandt J, Albert M, Jacobs DM, Liu X, Bell K, Mayeux R. The absence of an apolipoprotein epsilon4 allele is associated with a more aggressive form of Alzheimer’s disease. Ann Neurol. 1997;41(5):615–620. doi: 10.1002/ana.410410510. [DOI] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland T, Mirza N, Putnam KT, Linker G, Bhupali D, Durham R, Cohen RM. Cerebrospinal fluid beta-amyloid1–42 and tau in control subjects at risk for Alzheimer’s disease: the effect of APOE epsilon4 allele. Biol Psychiatry. 2004;56(9):670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. J Geriatr Psychiatry Neurol. 2005;18(4):196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer’s disease with apoE epsilon 2. Lancet. 1994;343(8910):1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- Tierney MC, Szalai JP, Snow WG, Fisher RH, Tsuda T, Chi H, St George-Hyslop PH. A prospective study of the clinical utility of ApoE genotype in the prediction of outcome in patients with memory impairment. Neurology. 1996;46(1):149–154. doi: 10.1212/wnl.46.1.149. [DOI] [PubMed] [Google Scholar]
- Tilvis RS, Strandberg TE, Juva K. Apolipoprotein E phenotypes, dementia and mortality in a prospective population sample. J Am Geriatr Soc. 1998;46(6):712–715. doi: 10.1111/j.1532-5415.1998.tb03805.x. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Takahashi S, Kato E, Homma A, Niina R, Sasaki K, Sasaki M. Reduced size of right hippocampus in 39- to 80-year-old normal subjects carrying the apolipoprotein E epsilon4 allele. Neurosci Lett. 1997;236(1):21–24. doi: 10.1016/s0304-3940(97)00743-x. [DOI] [PubMed] [Google Scholar]
- Trachtenberg AJ, Filippini N, Ebmeier KP, Smith SM, Karpe F, Mackay CE. The effects of APOE on the functional architecture of the resting brain. Neuroimage. 2012;59(1):565–572. doi: 10.1016/j.neuroimage.2011.07.059. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Vandeerstichele H, Korecka M, Clark CM, Aisen PS, Petersen RC, Shaw LM. Update on the biomarker core of the Alzheimer’s Disease Neuroimaging Initiative subjects. Alzheimers Dement. 2010;6(3):230–238. doi: 10.1016/j.jalz.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupler LA, Krishnan KR, Greenberg DL, Marcovina SM, Payne ME, MacFall JR, Doraiswamy PM. Predicting memory decline in normal elderly: genetics, MRI, and cognitive reserve. Neurobiol Aging. 2007;28(11):1644–1656. doi: 10.1016/j.neurobiolaging.2006.07.001. [DOI] [PubMed] [Google Scholar]
- van Duijn CM, de Knijff P, Wehnert A, De Voecht J, Bronzova JB, Havekes LM, Van Broeckhoven C. The apolipoprotein E epsilon 2 allele is associated with an increased risk of early-onset Alzheimer’s disease and a reduced survival. Ann Neurol. 1995;37(5):605–610. doi: 10.1002/ana.410370510. [DOI] [PubMed] [Google Scholar]
- Van Gerven PW, Van Boxtel MP, Ausems EE, Bekers O, Jolles J. Do apolipoprotein E genotype and educational attainment predict the rate of cognitive decline in normal aging? A 12-year follow-up of the Maastricht Aging Study. Neuropsychology. 2012;26(4):459–472. doi: 10.1037/a0028685. [DOI] [PubMed] [Google Scholar]
- van Meer Peter, Acevedo Summer, Raber Jacob. Impairments in spatial memory retention of GFAP-apoE4 female mice. Behav Brain Res. 2007;176(2):372–375. doi: 10.1016/j.bbr.2006.10.024. http://dx.doi.org/10.1016/j.bbr.2006.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Trojanowski JQ, Shaw LM, Jack CR., Jr Serial MRI and CSF biomarkers in normal aging, MCI, and AD. Neurology. 2010;75(2):143–151. doi: 10.1212/WNL.0b013e3181e7ca82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Liang M, Wang L, Tian L, Zhang X, Li K, Jiang T. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28(10):967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PN, Hong CJ, Lin KN, Liu HC, Chen WT. APOE epsilon4 increases the risk of progression from amnestic mild cognitive impairment to Alzheimer’s disease among ethnic Chinese in Taiwan. J Neurol Neurosurg Psychiatry. 2011;82(2):165–169. doi: 10.1136/jnnp.2010.209122. [DOI] [PubMed] [Google Scholar]
- Ward A, Crean S, Mercaldi CJ, Collins JM, Boyd D, Cook MN, Arrighi HM. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2012;38(1):1–17. doi: 10.1159/000334607. [DOI] [PubMed] [Google Scholar]
- Westlye ET, Lundervold A, Rootwelt H, Lundervold AJ, Westlye LT. Increased hippocampal default mode synchronization during rest in middle-aged and elderly APOE epsilon4 carriers: relationships with memory performance. J Neurosci. 2011;31(21):7775–7783. doi: 10.1523/JNEUROSCI.1230-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock GK, Lilienfeld S, Gaens E. Efficacy and safety of galantamine in patients with mild to moderate Alzheimer’s disease: multicentre randomised controlled trial. Galantamine International-1 Study Group. BMJ. 2000;321(7274):1445–1449. doi: 10.1136/bmj.321.7274.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Haan M, Byers A, Tangen C, Kuller L. Estrogen use, APOE, and cognitive decline: evidence of gene-environment interaction. Neurology. 2000;54(10):1949–1954. doi: 10.1212/wnl.54.10.1949. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Wang SJ, Xing J, Liu B, Ma ZL, Yang M, Teng GJ. Detection of PCC functional connectivity characteristics in resting-state fMRI in mild Alzheimer’s disease. Behav Brain Res. 2009;197(1):103–108. doi: 10.1016/j.bbr.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Zhong N, Weisgraber KH. Understanding the association of apolipoprotein E4 with Alzheimer disease: clues from its structure. J Biol Chem. 2009;284(10):6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]