Abstract
Drugs-of-abuse may increase the salience of drug cues by sensitizing the dopaminergic (DA) system (Robinson & Berridge, 1993), leading to differential attention to smoking stimuli. Event-related potentials (ERPs) have been used to assess attention to smoking cues but not using an ERP component associated with DA-mediated salience evaluation. In this study the DA-related P2a and the P3, were compared in smokers (N=21) and non-smokers (N=21) during an attention selection cue exposure task including both cigarette and neutral images. We predicted that both the P2a and P3 would be larger to targets than non-targets, but larger to non-target cigarette images than non-target neutral images only in the smokers, reflecting smokers’ evaluation of smoking stimuli as relevant even when they were not targets. Results indicated that smokers showed behavioral cue reactivity, with more false alarms to cigarette images (responding to cigarette images when they were not targets) than non-smokers; however, both smokers and non-smokers had a larger P2a and P3 to cigarette images. Thus, while smokers showed behavioral evidence of differential salience evaluation of the cigarette images, this group difference was not reflected in differential brain activity. These findings may reflect characteristics of the ERPs (both ERP components were smaller in the smokers), the smoking sample (they were not more impulsive, i.e. reward sensitive, than the non-smokers, in contrast to prior studies) and the design (all participants were aware that the aim of the study was related to smoking).
Keywords: event-related potentials, cue reactivity, nicotine, tobacco, smoking
1. Introduction
Tobacco use remains the leading preventable cause of premature death and disability in the United States (CDC, 2008; Mokdad, Marks, Stroup, & Gerberding, 2004). As of 2010, 19% of US adults were cigarette smokers (CDC, 2011b). Most smokers express a desire to quit and engage in quit attempts (CDC, 2011a). However, nicotine dependence is notoriously difficult to treat, and 70-95% of quit attempts are unsuccessful (Fiore et al., 2008; Hughes, Keely, & Naud, 2004). A better understanding of the cognitive, personality, and neural factors that maintain nicotine dependence could contribute to improved screening, intervention, and outcome assessment, including improved targeting of treatments that match individual smoker characteristics. The current study compared smokers and non-smokers on event-related potentials (ERPs) elicited during an attention selection task that included both smoking cues (i.e., images of cigarettes) and neutral cues (i.e., images of flowers, vehicles, and animals), and examined the relationship between ERP amplitude markers of reward sensitivity and trait impulsivity.
1.1 Reactivity and Attention to Smoking Cues
Exposing smokers to smoking-related cues (e.g., images of cigarettes) reliably induces self-reported craving or urge, as well as physiological responses, a phenomenon consistent with classical conditioning theory, and termed “cue reactivity” (Carter & Tiffany, 1999). The degree to which smokers react to smoking cues predicts their ability to remain abstinent in some studies (e.g., Niaura, Abrams, DeMuth, Pinto, & Monti, 1989; Payne, Smith, Adams, & Diefenbach, 2006), indicating a possible link between cue reactivity and addictive processes. In addition to cue-elicited craving and physiological responses, smokers show preferential attention to stimuli related to tobacco use. For example, smokers respond faster to identifying targets that occur in a location preceded by a smoking-related image and slower when preceded by a smoking-related picture presented in a different location from the target (Bradley, Mogg, Wright, & Field, 2003; Ehrman et al., 2002; Waters, Shiffman, Bradley, & Mogg, 2003a). Smokers also exhibit increased reaction time in response to naming the color of smoking-related words relative to neutral words, thereby indicating greater attentional capture by the smoking-related words (Drobes, Elibero, & Evans, 2006; Gross, Jarvik, & Rosenblatt, 1993; Waters, et al., 2003a). This interference effect has also predicted relapse (Waters et al., 2003b), suggesting this effect is related to addiction. However, the neural basis of attentional capture by smoking cues is less well understood.
1.2 Neural Model of Cue Reactivity
Robinson & Berridge’s incentive salience system was proposed as part of a model of cue-related drug craving (Berridge & Robinson, 1995; Robinson & Berridge, 1993). This model proposes that the dopaminergic (DA) reward system functions to attach motivational value to perceptual representations. The incentive salience system has been hypothesized as an attention selection neural mechanism (Potts, Martin, Burton, & Montague, 2006; Potts, 2004). It is hypothesized that drugs-of-abuse, through their impact on the DA system, sensitize incentive salience evaluation to drug cues, thereby resulting in drug-related items being tagged as having high motivational value compared to neutral representations. This sensitization accounts for the preferential attention allocated to drug cues in addicted individuals. Neural systems of cue reactivity have explicitly linked motivation-based selection to the DA reward system (Chase, Eickhoff, Laird, & Hogarth, 2011).
1.3 Using Event-Related Potentials to Assess Cue Reactivity
Event-related potentials (ERPs) provide a functional index of perceptual and attentional neutral systems, and may provide a sensitive assessment of cue reactivity as measured by altered attention to addiction-relevant cues. The P300 (or P3) is a centro-parietal positivity elicited by relatively rare events in an attended stream of stimuli (Donchin et al., 1984; Johnson, 1984; Sutton & Ruchkin, 1984); thus the P3 provides an index of attention: attended stimuli elicit a larger P3 than ignored stimuli. The most prominent theory of the P3 is the context updating theory, which states that we maintain an internal model of our current environment, the context, and when an unexpected (i.e., rare) event occurs, we must update that internal model to accommodate that unexpected event (Donchin & Coles, 1998). For a stimulus to be rare in contrast to the other stimuli in the stream, it must be distinguishable from the other stimuli. As such, the P3 can serve as an index of stimulus distinctiveness or categorization. The P3 is also larger to emotional, compared to neutral, stimuli (Olofsson, Nordin, Sequeira, & Polich, 2008). Thus the P3 may index stimuli that have relevance due to their ability to elicit an emotional response.
The P3 component has frequently been used to assess cue reactivity among individuals with substance use disorders (SUDs) (for a comprehensive review, see Littel, Euser, Munafo, & Franken, 2012), including nicotine dependence (Jang, Lee, Yang, & Lee, 2007; Littel & Franken, 2007, 2011a, 2011b, 2012; McDonough & Warren, 2001; Versace et al., 2012; Versace et al., 2011; Versace et al., 2010; Warren & McDonough, 1999). A recent meta-analysis indicated that the P3 is of significantly larger amplitude in response to drug-related stimuli vs. neutral stimuli, and that the difference in amplitude between drug-related and neutral stimuli is significantly larger among individuals with SUDs relative to controls without SUDs, indicating preferential attention to drug-related stimuli in SUDs (Littel, et al., 2012). Furthermore, larger effects have been observed in heavier users of the drugs (Herrmann, Weijers, Wiesbeck, Böning, & Fallgatter, 2001) or users in a withdrawal period (McDonough & Warren, 2001), implying a relationship between drug craving and attention to drug-related stimuli. However, some prior studies of cigarette smokers have not included a control group of non-smokers (Versace, et al., 2011; Versace, et al., 2010), a particularly important issue as P3 amplitude increases to smoking cues have also occurred in nonsmokers (McDonough & Warren, 2001) and recent work indicates that attention is engaged by drug-related cues in both users and non-users (Oliver & Drobes, in press).
Given that the P3 is a relatively late ERP component, reflecting attention allocation, which occurs after perceptual formation and motivational evaluation, if cue reactivity is associated with incentive salience then effects of drug-related stimuli should occur earlier in processing and be reflected earlier in the ERP. Therefore, in addition to revisiting P3 amplitude reactivity to smoking cues among smokers, in the current study we also examined an earlier ERP component. This component, variously called the anterior P2 (P2a), frontal polar component (FP), and the frontal selection positivity (FSP), responds exclusively to the task relevance of a stimulus, i.e. its motivational value, with an onset at about 150 – 200 ms after the stimulus appears and peaking at about 250 – 300 ms, and has been reliably associated with incentive salience (Guillem, Bicu, & Debruille, 2001; Kenemans, Kok, & Smulders, 1993; Potts, Liotti, Tucker, & Posner, 1996). The P2a has been elicited to visual and auditory stimuli, indicating that it is independent of perceptual modality, and when responses are either overt (e.g. key press) or covert (e.g. silent counting), indicating that a motor response is not required. The critical element in the elicitation of a P2a is that the presented stimulus has motivational relevance (incentive salience) to the individual (e.g., emotional stimuli, Kanske, Plitschka, & Kotz, 2011).
Recent work using an experimental design that delivers or withholds predicted and unpredicted rewards has explicitly linked the P2a to the DA reward system (Potts, et al., 2006). Neurons in the ventral tegmental area (VTA) show enhanced firing when an unexpected reward is delivered and suppress their firing when an expected reward is not delivered (Schultz, Dayan, & Montague, 1997). Likewise, the P2a is most positive to unpredicted rewards and least positive (actually negative) when a predicted reward is not delivered, mirroring the firing pattern of the VTA neurons, supporting the P2a as an index of the DA reward system (Potts, et al., 2006). The current study is the first study to examine P2a responses to drug-related stimuli.
1.5 The Current Study
The current study employed an attention selection cue exposure task using a stimulus set consisting of non-cigarette (animal, flower, vehicle) and cigarette images, with one image category (e.g. “animal”) made explicitly task-relevant on each trial by designating that category as the target, requiring a response from the participant. This design combines aspects of attention selection designs (instructed targets, which have incentive salience) we have used in prior studies (e.g., Potts, 2004) with a cue reactivity assessment of incentive sensitization (presentation of cigarette-related images to smokers). We predicted that both smokers and non-smokers would have an enhanced P2a (and P3) to instructed targets, but that only the smokers would show an enhanced P2a to the cigarette images when they were not instructed targets, i.e. when they were not made explicitly relevant by the task, reflecting incentive salience of the smoking cues in nicotine addiction.
In addition, we examined how individual differences, specifically self-reported impulsivity, might modify these ERP effects. Impulsivity is described as the propensity to make decisions without thoughtful consideration of long-term consequences, and is characterized by rapid responding and a heightened immediate reward preference (Evenden, 1999; Whiteside & Lynam, 2001). Smoking may be considered an impulsive behavior since smokers choose the short-term rewards of smoking cigarettes despite the potential long-term negative consequences. Prior studies have shown that smokers rate themselves as more impulsive, compared to non-smokers, on self-report personality inventories (Dinn, Aycicegi, & Harris, 2004; Mitchell, 1999, 2004; Terracciano & Costa, 2004) and are behaviorally more likely to choose smaller, more immediate rewards over larger delayed rewards (Baker, Johnson, & Bickel, 2003; Bickel, Odum, & Madden, 1999; Reynolds, Richards, Horn, & Karraker, 2004). Like incentive salience and cue reactivity, impulsivity has been related to the dopamine (DA) reward system (Braver & Cohen, 2000; Carli, Evenden, & Robbins, 1985; Matthysse, 1978; Servan-Schreiber, Bruno, Carter, & Cohen, 1998; Smillie & Jackson, 2006; Williams & Dayan, 2005). Therefore we investigated whether self-reported impulsivity might be related to cue reactivity, i.e. whether impulsive smokers might be more susceptible to incentive sensitization, indexed by a larger P2a to cigarette stimuli, and direct more attention two those cigarette stimuli, indexed by a larger P3.
2. Material and Methods
2.1 Participants
Participants were smokers and non-smokers aged 18-55 recruited from the Tampa, Florida community using Craigslist online advertising. Smokers had smoked at least 10 cigarettes per day for at least 1 year (confirmed by breath carbon monoxide of ≥ 8ppm) and were not currently trying to quit smoking; non-smokers had smoked less than 10 cigarettes during their lifetime (breath carbon monoxide of ≤ 5ppm). Exclusion criteria were use of medications that affect physiological responses (e.g., SSRIs, beta blockers), current or lifetime psychiatric disorder (psychosis, major depressive disorder, manic episode, panic disorder, or frequent panic attacks), current psychoactive substance use, or current or lifetime substance dependence (for the smokers, non-nicotine). As smokers have higher rates of psychiatric and non-nicotine substance use disorders than non-smokers (Grant, Hasin, Chou, Stinson, & Dawson, 2004; Lasser et al., 2000), the exclusion criteria were chosen to ensure that any differences found between the two groups could be confidently attributed to differences in nicotine exposure rather than other substance use or psychiatric conditions. This study was approved by the Institutional Review Board of the University of South Florida.
2.2 Screening and Baseline Measures
Interested individuals completed a brief telephone screening to determine preliminary eligibility. Those who denied current psychiatric diagnosis, problematic drug use (for smokers, other than nicotine), or treatment for any substance use disorder were invited to complete an initial assessment session. After providing informed consent, participants were administered a diagnostic interview (SCID; First, Spitzer, Gibbon, & Williams, 2002) to confirm eligibility, carbon monoxide breath test (to confirm self-reported smoking or non-smoking status), and measures of impulsivity including the Barratt Impulsiveness Scale (BIS-11; Patton, Stanford, & Barratt, 1995) and the I-7 (Impulsiveness, Venturesomeness, and Empathy) (Eysenck, Pearson, Easting, & Allsopp, 1985). Smokers additionally completed a brief smoking history, the Fagerström Test for Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991), and the Questionnaire of Smoking Urges (QSU; Tiffany & Drobes, 1991). Other self-report measures were also included that were not relevant to the current analyses.
We screened 182 individuals via telephone, of whom 65 were ineligible, 8 were eligible but were unable to be scheduled for an assessment session, and 43 were scheduled for an assessment session but did not show up or canceled. The remaining 66 individuals (41 smokers and 25 non-smokers) completed the assessment session. Sixteen of the 41 smokers were disqualified from participation in the attention selection cue exposure task (and three other tasks not relevant to the current analyses) based on the results of the diagnostic interview (13 met lifetime criteria for a substance use disorder; 3 met criteria for another psychiatric disorder and also had significant non-nicotine substance use). No non-smokers were eliminated, leaving 25 smokers and 25 non-smokers who were scheduled for appointments to complete the cue exposure task. Of these 50 participants, 6 (3 smokers, 3 non-smokers) did not show up for the appointment or were unable to complete the task (e.g., equipment did not fit properly) and 2 (1 smoker, 1 non-smoker) completed the task but were eliminated for misunderstanding the instructions and excessive artifact in the EEG record, respectively, leaving 21 smokers and 21 non-smokers in the final sample (see Table 1 for demographic, smoking, and impulsivity characteristics of participants).
Table 1.
Participant Characteristics (Means and Standard Deviations) - Smokers vs. Non-Smokers
| Smokers | Non-Smokers | |
|---|---|---|
| N | 21 | 21 |
| Age | 34.3 (11.7) | 32.5 (10.5) |
| Gender (# male) | 13 | 10 |
| Race (# Caucasian) | 19 | 14 |
| Years of education | 13.2 (1.6) | 14.0 (1.8) |
| CO (ppm)a | 30.3 (22.8) | 2.6 (1.2) |
| Years Smoked Daily | 15.4 (12.4) | |
| Cigarettes/day | 21.1 (7.5) | • |
| FTND | 5.4 (2.0) | • |
| WISDM | 54.4 (16.7) | • |
| QSU | 141.7 (25.9) | • |
| BIS-11 | 56.9 (12.4) | 56.0 (12.0) |
| I7 | 4.1 (3.1) | 5.0 (4.7) |
Note. CO (ppm) = carbon monoxide parts per million, FTND = Fagerström Test for Nicotine Dependence, WISDM = Wisconsin Inventory of Smoking Dependence Motives, QSU = Questionnaire of Smoking Urges, BIS-11 = Barratt Impulsiveness Scale, I7 = Eysenck Impulsiveness Scale.
p < .05
2.3 Attention Selection Cue Exposure Task
Participants completed the attention selection cue exposure task on a separate day from the initial assessment session. Sessions occurred between 9:00 am and 5:00 pm. All smoking participants were instructed not to consume alcohol or use any non-nicotine intoxicating substances for 24 hours prior to their session. They were instructed to smoke a cigarette just prior to beginning the task to ensure to standardize and minimize effects of nicotine withdrawal. Stimulus presentation and behavioral response collection were controlled by E-Prime software (PST inc., Pittsburgh). Visual stimuli were presented on a flat-panel display. Behavioral responses were collected with a 4-key keypad. Subjects were seated in an adjustable chair with their eyes approximately 50 cm from the center of the monitor. Subjects were instructed to remain as still as possible and to refrain from eye movement and blinking as much as possible. Rest breaks were provided every 3 - 4 minutes.
The stimuli consisted of 480 photographs, 120 each from 4 categories: animals, flowers, vehicles, and smoking-related images. The non-smoking stimuli were taken from the Broderbund Printshop Clipart collection (Broderbund, Novato, CA) of photographs. The smoking-related stimuli consisted of photographs obtained from the Internet as well as those used by Carter et al. (2006). Images from each category were digitally manipulated to be of equal size. Each trial consisted of a word designating the target stimulus for that trial (Flower, Animal, Vehicle, Cigarette) presented for 500 ms, followed by a fixation cross that varied randomly from 500 to 2200 ms, followed by a picture from one of the four categories presented for 200 milliseconds. The stimulus image was followed by a fixation cross that remained onscreen for 800 ms, providing a 1000 ms response window from stimulus onset, with reaction time measured from stimulus onset. Each picture was presented twice, selected equiprobably and randomly without replacement, for a total of 960 trials. Participants were instructed to press a key whenever they saw a stimulus from the target category and ignore stimuli from all other categories. One quarter of the trials were target-present trials, providing 60 target and 180 non-target trials for each stimulus category.
2.4 EEG acquisition and analysis
EEG data was acquired continuously sampled at 250 Hz. with .1 - 100 Hz. analog filtering, referenced to the vertex, with a 128 channel Electrical Geodesics system consisting of Geodesic Sensor Net electrodes, Netamps, and Netstation software (Electrical Geodesics, Inc., Eugene, OR). The EEG data were digitally filtered offline at 20 Hz to reduce residual noise outside the frequency range of the components of interest. The EEG data was segmented into 1000 ms epochs spanning 200 ms pre- to 800 ms post-stimulus around the image onset. The EEG segments were digitally screened for non-cephalic artifact and contaminated trials removed. The remaining data were averaged to create the ERPs in four categories, cigarette images when they were not targets, cigarette images when they were targets, non-cigarette images when they were not targets, and non-cigarette images when they were targets. Only trials on which the participant responded correctly were included. There were insufficient error trials to create stable ERPs. The mean error rate across the entire sample was 4.12% of 960 trials (SD = 3.38%, range 1% to 14%). There was no significant difference in error rate between smokers and nonsmokers, p > .05. The ERPs were baseline corrected over a 200 ms prestimulus period then rereferenced to an average reference frame to remove topographic bias (Dien, 1998). The subject-averaged ERPs were averaged together to produce the mean waveform across subjects.
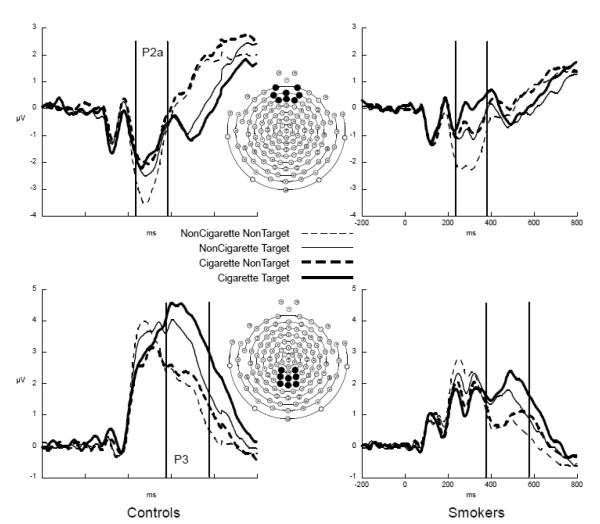
To reduce the dimensionality of an Electrode factor with 128 levels, we extracted the ERPs using a Region Of Interest (ROI) method, averaging voltage across the electrodes in an ROI (Curran, Tucker, Kutas, & Posner, 1993), using an eight electrode medial frontal ROI for the P2a and an eight electrode centroparietal ROI for the P3 (after Potts, Patel, & Azzam, 2004). The P2a was the average ERP amplitude between 240 – 380 ms post-stimulus in the medial frontal ROI; the P3 was the average amplitude between 375 – 575 ms post-stimulus in the centroparietal ROI (see Figure 1 for the ROIs and temporal windows). The P2a and P3 amplitudes were cast into repeated-measures ANOVAs with Group (smoker, non-smoker) as the between-subjects factor and Category (cigarette, non-cigarette (the average of the three non-cigarette stimulus categories)) and Stimulus Type (target, non-target) as within-subjects factors.
Figure 1.
ERPs from the frontal (top row) and centro-parietal (bottom row) regions of interest. (ROIs) from the non-smokers (left column) and smokers (right column) showing the frontal P2a and posterior P3 to the cigarette (thick lines) and non-cigarette (thin lines) stimuli when they were instructed non-targets (dashed lines) and instructed targets (solid lines)
3. Results
3.1 Initial Assessment Session
Group comparisons (smokers vs. non-smokers) on demographics, smoking, and personality measures from the initial assessment session are presented in Table 1. There were no significant differences between smokers and non-smokers in demographics. Contrary to findings from previous research, smokers and non-smokers also did not differ in impulsivity scores on either the BIS-11 or the I71. The BIS-11 and I7 scores were correlated across the full sample, r = .78, p <.001.
3.2 Behavioral Responses to Attention Selection Cue Exposure Task
T-tests conducted on the behavioral data revealed no differences between smokers and non-smokers on response time (RT) to cigarette or neutral images, misses (i.e., failing to respond to a target image), or false alarms to neutral images (i.e., responding to a neutral image that was not a target) (all ps > .05). However, smokers had more false alarms to cigarette images (i.e., responding to a cigarette image that was not a target), t(40) = 2.44, p = .02 (see Table 2).
Table 2.
Behavioral Responses to Attention Selection Cue Exposure Task (Means and Standard Deviations)
| Smokers | Non-Smokers | |
|---|---|---|
| N | 21 | 21 |
| Reaction time - cigarette targets (ms) |
475.2 (63.2) | 489.5 (70.3) |
| Reaction time – non- cigarette targets (ms) |
455.4 (68.7) | 463.2 (71.2) |
| Misses – cigarette targets |
4.4 (5.5) | 5.4 (7.6) |
| Misses – non- cigarette targets |
23.9 (24.4) | 18.6 (19.9) |
| False alarms – cigarette targets* |
3.0 (2.6) | 1.5 (1.2) |
| False alarms – non- cigarette targets |
13.9 (15.4) | 8.6 (4.1) |
Note. p < .05
Signal detection metrics of sensitivity (d’) and response bias (c) (Stanislaw & Todorov, 1999) to cigarette images were also compared between smokers and non-smokers using T-tests. Hit rates of 1.0 were corrected using the formula 1 – 1/(2N) where N was the number of cigarette targets (60). False alarm rates of 0 were corrected using the formula 1/2N (Wixted & Lee). No significant group differences in either metric were found (all ps > .05). Mean sensitivity for smokers was 3.91 (SD = .78) and for non-smokers was 4.11 (SD = .78); mean response bias for smokers was -.27 (SD - .29) and for non-smokers was -.39 (SD = .37).
3.3 ERP
3.3.1 P2a
Visual inspection of the P2a in Figure 1, top indicates a negative deflection in the P2a window, larger in the control participants, with more positivity to instructed targets (solid lines), particularly when those targets were non-cigarette images. Only the smokers appeared to have more positivity to cigarette images when they were targets, compared to when they were non-targets. The ANOVA revealed a trend for Group, F = 3.34, p = .075, with the smokers overall less negative than the non-smokers. There was an effect for Type, F = 9.46, p < .005, with the P2a to the targets more positive than to the non-targets. There was a main effect for Category, F = 53.59, p < .001, with the cigarette stimuli eliciting more positivity than the non-cigarette images. The Type × Category interaction was nearly significant, F = 3.63, p = .064, indicating that the there was the least positivity to the nontargets when they were non-cigarette images. There were no interactions with Group, so the additional positivity to cigarette targets in the smoking participants visible in the waveforms is not a significant effect (see Figure 1).
3.3.2 P3
The waveform plots in Figure 1 bottom show a substantially larger P3 in the controls, F = 8.85, P = .005, and a larger P3 to the instructed targets (solid lines), F = 62.15, p < .001. The P3 was larger to the cigarette images, F = 28.43, p < .001, particularly when they were instructed targets (Category × Type F = 3.52, p = .068). The P3 enhancement to instructed targets was also larger in the control than in the smoking participants, Group × Type F = 5.28, p < .05.
3.3.3 Correlations Between ERPs and Self-Report Measures
Among smokers, there were no significant correlations between smoking characteristics (cigarettes per day, FTND, QSU) and relevant ERP indices (all p’s > .05). Also, there were no significant correlations between impulsivity scores on either the I7 or the BIS-11 and relevant ERP indices for either the smokers or the non-smokers (all p’s >.05).
4. Discussion
Our primary hypothesis was that smokers would exhibit enhanced reactivity to cigarette stimuli, responding to them as targets even when they were nontargets, and that this behavior would be supported in neural systems of stimulus relevance evaluation indexed by the P2a ERP component and in the attention-related P3. We found partial support for these predictions. The smokers did respond behaviorally to cigarette cues, responding to them as targets even when they were instructed non-targets, emitting more false-alarm responses to smoking cues than the non-smoking participants. This indicates that the smokers evaluated the cigarette stimuli as more relevant than the non-smokers did, and were thus more likely to categorize them as targets even when not instructed to. This preferential attention to smoking-related stimuli is consistent with prior findings from the dot-probe task, in which smokers are faster responding to targets at locations where a smoking-related stimulus appeared (Bradley, et al., 2003; Ehrman, et al., 2002; Waters, et al., 2003a) and the Stroop design in which smokers are slower to name the font color of smoking-related words (Drobes, et al., 2006; Gross, et al., 1993; Waters, et al., 2003a).
Results from the ERP analyses revealed that both the P2a and the P3 were more positive to targets, compared to non-targets, reflecting more relevance associated with and more attention paid to targets and replicating standard target detection effects (Potts, 2004). Both the P2a and P3 components were more positive to cigarette than non-cigarette stimuli, indicating preferential processing and more attention paid to cigarette stimuli. Additionally, the P3 showed an interactive enhancement with the target effect larger to cigarette stimuli. Thus the current ERP data showed that attention is differentially allocated to targets, to cigarette stimuli, and to cigarette stimuli when they are targets.
While the P3 is generally enhanced to infrequent targets, a stimulus does not need to be a target to elicit a P3 (Donchin & Coles, 1988). A P3 is elicited by rare stimuli in an attended stream even when those stimuli are not targets in the participant’s task (Donchin, Ritter, & McCallum, 1978; Potts et al., 2004). The P2a shows a similar effect, enhancement to rare stimuli even when they are not instructed targets (Potts et al., 2004). Frequency of occurrence is determined by perceived category, i.e. the observer perceives the stimulus stream as consisting of two distinct categories, one of which is less frequent than the other. For example, in a stimulus stream of red and green items with a 25% of the items red, the red items would elicit a P3 in individuals with normal vision but not in a red/green colorblind individual who is unable to perceive the categories. The current experiment was designed with four equiprobable categories: animals, flowers, vehicles, and smoking-related images, thus no explicit less frequent category. However, as experimenters, we considered three of those stimulus types as control stimuli, thus in the same category, and one of them, the smoking-related stimuli, as a distinct category. The participants may have also perceived the smoking-related images as distinct from the animals, flowers, and vehicles, and categorized those three control stimuli together, creating an implicit, relatively infrequent (25% of the stimuli) stimulus type: smoking related images.
The negative deflection that the P2a is superimposed upon was marginally reduced in amplitude in the smokers and the P3 was significantly reduced. ERP component reduction is generally interpreted as reflecting reduced efficiency in the neurocognitive system indexed by that ERP component (Polich & Herbst, 2000). For the P3, that system is attention orienting and/or context updating (Donchin & Coles, 1998; Donchin, et al., 1984). This would suggest that smokers have reduced efficiency in the neural systems supporting attention orienting, and this is supported by the reduced size of the P3 target effect in the smokers (i.e. the P3 enhancement to instructed targets was smaller in the smokers compared to the controls). This reduction is consistent with prior research showing smaller P3 in smokers (e.g., Anokhin et al., 2000; Evans, Park, Maxfield, & Drobes, 2009). Whether this is a consequence of or contributing factor to nicotine addiction cannot be addressed here.
We predicted a Type × Category × Group interaction in which both the P2a and the P3 would be larger to cigarette stimuli when they were non-targets. The Type × Category interaction was nearly significant for both the P2a and P3 but was not modified by group. The waveforms suggest that both the smokers and non-smokers had larger P2a and P3 amplitude to the smoking stimuli. While we expected the smokers to show enhanced P2a and P3 to cigarette stimuli even when they were not instructed targets, reflecting incentive sensitization of those stimuli and subsequent attention allocation, the presence of these effects in the non-smokers cannot be explained by incentive sensitization since the non-smokers were not exposed to the drug effect simultaneously with the drug-related stimuli, the pairing required for sensitization.
There are a number of reasons why no significant differences in P2a or P3 amplitude were found between smokers and non-smokers in the current study; some related to our procedures and some to characteristics of our sample. With respect to procedures, it is possible that demand effects in the experiment and/or cultural effects contributed to this differential neural response to cigarette stimuli in the non-smokers; these possibilities are a limitation of the current study. The informed consent form read by all participants stated that this was a study of smoking behavior, and this information may have attached additional relevance to the cigarette stimuli, as was also suggested by McDonough and Warren (McDonough & Warren). Cigarettes also have a cultural role with both positive and negative characteristics attributed to them, more so than to the control stimuli used in the study. In addition, recent evidence suggests that mere exposure to smoking in the environment by non-smokers can be associated with increased attention to smoking cues (Oliver & Drobes, in press). Relatedly, some of the cigarette images included people (e.g., person smoking a cigarette) whereas the other three categories of pictures did not include any people. This difference may also have increased the salience of the cigarette images relative to the other categories. In future studies, the use of comparison stimuli with attention-grabbing, appetitive properties among both smokers and non-smokers (e.g., food pictures) may be considered as recent theory suggests that individuals with substance use disorders would have more attentional bias to drug cues than food cues whereas among individuals without substance use disorders the opposite would be expected (e.g., Koob & Volkow, 2010; Robinson & Berridge, 1993).
Nevertheless, we would still expect smokers to attach more relevance to and attend more to the cigarette stimuli than the non-smokers. The behavioral data indicates that the smokers did attach more relevance to the cigarette images, emitting more false alarm responses to those stimuli when they were instructed non-targets. We speculate that the P2a on these false alarm trials would be larger than on the correct rejection trials, unfortunately we had insufficient numbers of these trials to perform an ERP analysis, thus do not have supportive evidence for this speculation. Finally, our smokers were instructed to smoke a cigarette just prior to beginning the task; therefore, it is possible that satiation reduced smokers’ reactivity. Other studies involving measurement of ERPs during cue reactivity tasks have required smokers to abstain for at least 1 hour prior to beginning the task (e.g., Littel & Franken, 2011a).
With respect to the sample, participants in the current study were older relative to previous studies that have included mostly college students. Also, our participants were administered a thorough diagnostic interview whereas most previous studies have not screened out individuals with psychiatric disorders and non-nicotine substance (for a review, see Littel, et al., 2012). The diagnostic interview was intended to ensure isolation of an effect of nicotine, as smokers are more likely than non-smokers to have psychiatric disorders and to be users of other psychoactive substances that can affect ERPs and cue reactivity. Finally, we had initially proposed that smokers would potentially have greater cue reactivity and larger P2a’s to the smoking stimuli, since previous research has found that smokers are more impulsive than non smokers (e.g., Mitchell, 1999) and impulsivity might reflect heightened responsivity of the mesotelencephalic DA reward system (Pine, Shiner, Seymour, & Dolan, 2010), the system theorized to attach motivational salience to stimulus representations. However, contrary to previous research, the smokers who took part in our attention selection cue exposure task were not more impulsive than the non-smokers, and we found no correlation between P2a amplitude and impulsivity in the current sample.
5. Conclusions
The smoking participants in the current study showed enhanced attentional engagement and behavioral responsiveness to cigarette stimuli, responding to these cues as targets even when they were not instructed targets more than the non-smoking control participants. However, the ERPs revealed that both the smokers and the non-smokers evaluated the cigarette stimuli as more salient and allocated more attention to them than the neutral stimuli. These evaluation and attention effects in the non-smokers might be due to demand characteristics of the task or the societal impact of cigarette representations in the culture. The lack of differential ERP effects between the groups might be due, in part, to the smoking sample in the current study, which had reduced amplitude in both ERP components and also lacked the heightened impulsivity found in prior studies of smokers. Subsequent studies could acquire ERPs from high impulsive smokers to test this hypothesis, and use an experimental design that would produce sufficient errors to allow ERP analysis of error trials.
Highlights.
Smokers showed cue reactivity, with more false alarms to cigarette stimuli.
ERPs indicated that all subjects attended more to cigarette than neutral images.
ERPs indicated that all subjects viewed cigarette images as more salient.
ERP indices of salience and attention do not fully account for cue reactivity.
Footnotes
Demographics, smoking, and impulsivity measures were also collected from the smokers who were ineligible for the EEG sessions. These ineligible smokers scored significantly higher on both impulsivity measures compared to non-smokers (both p’s < .05). Comparisons on impulsivity and other measures between eligible and ineligible smokers will be detailed in a separate report (Bloom, Potts, Evans, & Drobes, unpublished manuscript).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anokhin AP, Vedeniapin AB, Sirevaag EJ, Bauer LO, O’Connor SJ, Kuperman S, Rohrbaugh JW. The P300 brain potential is reduced in smokers. Psychopharmacology. 2000;149:409–413. doi: 10.1007/s002130000387. [DOI] [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. Journal of Abnormal Psychology. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. The mind of an addicted brain: Neural sensitization of wanting versus liking. Current Directions in Psychological Science. 1995;4:71–76. [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology. 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bloom EL, Potts GF, Evans DE, Drobes DJ. Impulsivity in Smokers With and Without a Comorbid Non-Nicotine Substance Use Disorder. (unpublished manuscript) [Google Scholar]
- Bradley BP, Mogg K, Wright T, Field M. Attentional bias in drug dependence: vigilance for cigarette-related cues in smokers. Psychology of Addictive Behaviors. 2003;17:66–72. doi: 10.1037/0893-164x.17.1.66. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. On the control of control: The role of dopamine in regulating prefrontal function and working memory. In: Monsell S, Driver J, editors. Attention and Performance XVIII. MIT Press; Cambridge, MA: 2000. pp. 713–738. [Google Scholar]
- Carli M, Evenden J, Robbins T. Depletion of unilateral striatal dopamine impairs initiation of contralateral actions and not sensory attention. Nature. 1985;313:679–682. doi: 10.1038/313679a0. [DOI] [PubMed] [Google Scholar]
- Carter BL, Robinson JD, Lam CY, Wetter DW, Tsan JY, Day SX, Cinciripini PM. A psychometric evaluation of cigarette stimuli used in a cue reactivity study. Nicotine & Tobacco Research. 2006;8:361–369. doi: 10.1080/14622200600670215. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- CDC Smoking-Attributable Mortality, Years of Potential Life Lost, and Productivity Losses - United States, 2000–2004. Morbidity and Mortality Weekly Report. 2008;57:1226–1228. [PubMed] [Google Scholar]
- CDC Quitting smoking among adults --- United States, 2001-2010. Morbidity and Mortality Weekly Report. 2011a;60:1513–1519. [PubMed] [Google Scholar]
- CDC Vital signs: current cigarette smoking among adults aged >/=18 years--United States, 2005-2010. Morbidity and Mortality Weekly Report. 2011b;60:1207–1212. [PubMed] [Google Scholar]
- Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biological Psychiatry. 2011;70:785–793. doi: 10.1016/j.biopsych.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran Tim, Tucker Don M., Kutas Marta, Posner Michael I. Topography of the N400: Brain electrical activity reflecting semantic expectancy. Electroencephalography & Clinical Neurophysiology: Evoked Potentials. 1993;88(3):188–209. doi: 10.1016/0168-5597(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Dien J. Issues in the application of the average reference: Review, critiques, and recommendations. Behavior Research Methods, Instruments, & Computers. 1998;30:34–43. [Google Scholar]
- Dinn WM, Aycicegi A, Harris CL. Cigarette smoking in a student sample: neurocognitive and clinical correlates. Addictive Behaviors. 2004;29:107–126. doi: 10.1016/j.addbeh.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Context updating and the P300. Behavioral and Brain Sciences. 1998;21:152–153. [Google Scholar]
- Donchin E, Heffley E, Hillyard SA, Loveless N, Maltzman I, Ohman A, Siddle D. Cognition and event-related potentials. II. The orienting reflex and P300. Annals of the New York Academy of Sciences. 1984;425:39–57. doi: 10.1111/j.1749-6632.1984.tb23522.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Ritter W, McCallum C. Cognitive psychophysiology: the endogenous components of the ERP. In: Callaway P, Tueting P, Koslow S, editors. Brain-event related potentials in man. Academic Press; New York: 1978. pp. 349–411. [Google Scholar]
- Donchin Emanuel, Coles Michael G. Is the P300 component a manifestation of context updating? Behavioral & Brain Sciences. 1988;11(3):357–427. [Google Scholar]
- Drobes DJ, Elibero A, Evans DE. Attentional bias for smoking and affective stimuli: a Stroop task study. Psychology of Addictive Behaviors. 2006;20:490–495. doi: 10.1037/0893-164X.20.4.490. [DOI] [PubMed] [Google Scholar]
- Ehrman RN, Robbins SJ, Bromwell MA, Lankford ME, Monterosso JR, O’Brien CP. Comparing attentional bias to smoking cues in current smokers, former smokers, and non-smokers using a dot-probe task. Drug and Alcohol Dependence. 2002;67:185–191. doi: 10.1016/s0376-8716(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Evans DE, Park JY, Maxfield N, Drobes DJ. Neurocognitive variation in smoking behavior and withdrawal: genetic and affective moderators. Genes, Brain and Behavior. 2009;8:86–96. doi: 10.1111/j.1601-183X.2008.00445.x. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology. 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Pearson PR, Easting G, Allsopp JF. Age norms for impulsiveness, venturesomeness and empathy in adults. Personality and Individual Differences. 1985;6:613–619. [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF. Clinical Practice Guideline. U.S. Department of Health and Human Services. Public Health Service; Rockville, MD: May, 2008. Treating Tobacco Use and Dependence: 2008 Update; p. 2008. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, non-patient edition (SCIDI/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Gross T, Jarvik M, Rosenblatt M. Nicotine abstinence produces content-specific stroop interference. Psychopharmacology. 1993;110:333–336. doi: 10.1007/BF02251289. [DOI] [PubMed] [Google Scholar]
- Guillem F, Bicu M, Debruille JB. Dissociating memory processes involved in direct and indirect tests with ERPs to unfamiliar faces. Cognitive Brain Research. 2001;11:113–125. doi: 10.1016/s0926-6410(00)00070-7. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Weijers HG, Wiesbeck GA, Böning J, Fallgatter AJ. Alcohol cue-reactivity in heavy and light social drinkers as revealed by event-related potentials. Alcohol and Alcoholism. 2001;36:588–593. doi: 10.1093/alcalc/36.6.588. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99:29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- Jang KW, Lee JS, Yang BH, Lee JH. Changes of brain potentials in response to smoking-induced stimuli in smokers. Cyberpsychol Behav. 2007;10:460–463. doi: 10.1089/cpb.2006.9932. [DOI] [PubMed] [Google Scholar]
- Johnson R. P300: A model of the variables controlling its amplitude. Annals of the New York Academy of Sciences. 1984;425:223–229. doi: 10.1111/j.1749-6632.1984.tb23538.x. [DOI] [PubMed] [Google Scholar]
- Kanske P, Plitschka J, Kotz SA. Attentional orienting towards emotion: P2 and N400 ERP effects. Neuropsychologia. 2011;49:3121–3129. doi: 10.1016/j.neuropsychologia.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Kenemans JL, Kok A, Smulders FT. Event-related potentials to conjunctions of spatial frequency and orientation as a function of stimulus parameters and response requirements. Electroencephalography and Clinical Neurophysiology. 1993;88:51–63. doi: 10.1016/0168-5597(93)90028-n. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Littel M, Euser AS, Munafo MR, Franken IH. Electrophysiological indices of biased cognitive processing of substance-related cues: a meta-analysis. Neuroscience and Biobehavioral Reviews. 2012;36:1803–1816. doi: 10.1016/j.neubiorev.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. The effects of prolonged abstinence on the processing of smoking cues: an ERP study among smokers, ex-smokers and never-smokers. J Psychopharmacol. 2007;21:873–882. doi: 10.1177/0269881107078494. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. Implicit and explicit selective attention to smoking cues in smokers indexed by brain potentials. J Psychopharmacol. 2011a;25:503–513. doi: 10.1177/0269881110379284. [DOI] [PubMed] [Google Scholar]
- Littel M, Franken IH. Intentional modulation of the late positive potential in response to smoking cues by cognitive strategies in smokers. PLoS One. 2011b;6:e27519. doi: 10.1371/journal.pone.0027519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littel M, Franken IH. Electrophysiological correlates of associative learning in smokers: a higher-order conditioning experiment. BMC Neurosci. 2012;13:8. doi: 10.1186/1471-2202-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthysse S. A theory of the relation between dopamine and attention. Journal of Psychiatric Research. 1978;14:241–248. doi: 10.1016/0022-3956(78)90026-2. [DOI] [PubMed] [Google Scholar]
- McDonough BE, Warren CA. Effects of 12-h tobacco deprivation on event-related potentials elicited by visual smoking cues. Psychopharmacology. 2001;154:282–291. doi: 10.1007/s002130000647. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Mitchell SH. Measuring impulsivity and modeling its association with cigarette smoking. Behavioral and Cognitive Neuroscience Reviews. 2004;3:261–275. doi: 10.1177/1534582305276838. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. Journal of the American Medical Association. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Niaura R, Abrams DB, DeMuth B, Pinto R, Monti PM. Responses to smoking-related stimuli and early relapse to smoking. Addictive Behaviors. 1989;14:419. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Oliver JA, Drobes DJ. Visual search and attention bias for smoking cues: The role of familiarity. Experimental and Clinical Psychopharmacology. doi: 10.1037/a0029519. (in press) [DOI] [PubMed] [Google Scholar]
- Olofsson JK, Nordin S, Sequeira H, Polich J. Affective picture processing: An integrative review of ERP findings. Biological Psychology. 2008;77:247–265. doi: 10.1016/j.biopsycho.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Payne TJ, Smith PO, Adams SG, Diefenbach L. Pretreatment cue reactivity predicts end-of-treatment smoking. Addictive Behaviors. 2006;31:702–710. doi: 10.1016/j.addbeh.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. Journal of Neuroscience. 2010;30:8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Herbst KL. P300 as a clinical assay: rationale, evaluation, and findings. International Journal of Psychophysiology. 2000;38:3–19. doi: 10.1016/s0167-8760(00)00127-6. [DOI] [PubMed] [Google Scholar]
- Potts G, Martin L, Burton P, Montague P. When things are better or worse than expected: Medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1–8. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain and Cognition. 2004;56:5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Potts GF, Patel SH, Azzam PN. Impact of instructed relevance on the visual ERP. International Journal of Psychophysiology. 2004;52(2):197–209. doi: 10.1016/j.ijpsycho.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Potts GF, Liotti M, Tucker DM, Posner MI. Frontal and inferior temporal cortical activity in visual target detection: Evidence from high spatially sampled event-related potentials. Brain Topography. 1996;9:3–14. [Google Scholar]
- Reynolds B, Richards JB, Horn K, Karraker K. Delay discounting and probability discounting as related to cigarette smoking status in adults. Behavioural Processes. 2004;65:35–42. doi: 10.1016/s0376-6357(03)00109-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Bruno RM, Carter CS, Cohen JD. Dopamine and the mechanisms of cognition: Part I. A neural network model predicting dopamine effects on selective attention. Biological Psychiatry. 1998;43:713–722. doi: 10.1016/s0006-3223(97)00448-4. [DOI] [PubMed] [Google Scholar]
- Smillie LD, Jackson CJ. Functional Impulsivity and Reinforcement Sensitivity Theory. Journal of Personality. 2006;74:47–83. doi: 10.1111/j.1467-6494.2005.00369.x. [DOI] [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behavior research methods, instruments, & computers : a journal of the Psychonomic Society, Inc. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Sutton S, Ruchkin DS. The late positive complex: Advances and new problems. In: Karrer R, Cohen J, Tueting P, editors. Brain and Information: Event-related potentials. Annals of the New York Academy of Sciences. Vol. 425. 1984. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Costa PT., Jr Smoking and the Five-Factor Model of personality. Addiction. 2004;99:472–481. doi: 10.1111/j.1360-0443.2004.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM. Beyond cue reactivity: blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addict Biol. 2012;17:991–1000. doi: 10.1111/j.1369-1600.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Minnix JA, Robinson JD, Lam CY, Brown VL, Cinciripini PM. Brain reactivity to emotional, neutral and cigarette-related stimuli in smokers. Addict Biol. 2011;16:296–307. doi: 10.1111/j.1369-1600.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Lam CY, Minnix JA, Brown VL, Carter BL, Cinciripini PM. Cigarette cues capture smokers’ attention: evidence from event-related potentials. Psychophysiology. 2010;47:435–441. doi: 10.1111/j.1469-8986.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren CA, McDonough BE. Event-related brain potentials as indicators of smoking cue-reactivity. Clinical Neurophysiology. 1999;110:1570–1584. doi: 10.1016/s1388-2457(99)00089-9. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Bradley BP, Mogg K. Attentional shifts to smoking cues in smokers. Addiction. 2003a;98:1409–1417. doi: 10.1046/j.1360-0443.2003.00465.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003b;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Personality and Individual Differences. 2001;30:669–689. [Google Scholar]
- Williams J, Dayan P. Dopamine, Learning, and Impulsivity: A Biological Account of Attention-Deficit/Hyperactivity Disorder. Journal of Child and Adolescent Psychopharmacology. 2005;15:160–179. doi: 10.1089/cap.2005.15.160. [DOI] [PubMed] [Google Scholar]
- Wixted J, Lee K. [Retrieved August 1, 2013];Signal Detection Theory. from http://kangleelab.com/signal detection theory.html.