Abstract
BACKGROUND
Neoadjuvant hormonal therapy (NHT) is performed to improve the outcome in organ-confined prostate cancer. However, there is little information regarding the relationship between angiogenesis and NHT. The aim of this study was to identify a suitable method to evaluate the angiogenic status of tissue, and to determine the prognostic value of this method for biochemical recurrence in patients who had undergone radical prostatectomy after NHT.
METHODS
We analyzed 108 formalin-fixed specimens from patients treated by radical prostatectomy. NHT was administered in 48 patients (52.9%) and 60 patients who had a similar Gleason score and pT stage were selected as a non-NHT treated control group. Microvessel density (MVD) was measured using anti-CD31, anti-CD34, and anti-CD105 antibodies. The expressions of vascular endothelial growth factor (VEGF)-A and thrombospondin (TSP)-1 were also evaluated by immunohistochemistry. The prognostic value of CD31-, CD34-, and CD105-MVD for biochemical recurrence was investigated.
RESULTS
The mean/SD of CD105-MVD in the NHT group (13.3/4.7) was significantly (P < 0.001) lower than that in the non-NHT group (125.8/7.3). In the NHT group, CD105-MVD was associated with pT stage and it was positively correlated with VEGF-A expression (r = 0.56, P < 0.001) and negatively correlated with TSP-1 expression (r = 0.42, P = 0.003). CD105-MVD was identified as a significant predictor of biochemical recurrence (BCR) in patients treated with NHT (log rank test, P < 0.001). Although CD31- and CD34-MVD were significantly associated with pT stage or Gleason score in non-NHT group, they were not associated with pathological features and BCR in NHT group.
CONCLUSIONS
Our results indicate that CD105-MVD reflects the angiogenic conditions in prostate cancer tissues treated with NHT. CD105-MVD was also identified as a significant and independent predictor of biochemical recurrence in prostate cancer patients who underwent radical prostatectomy with NHT. Prostate 75:84–91, 2015. © 2014 The Authors. The Prostate published by Wiley Periodicals, Inc.
Keywords: neoadjuvant therapy, angiogenesis, biochemical recurrence, vascular endothelial growth factor-A, thrombospondin-1
INTRODUCTION
Neoadjuvant hormonal therapy (NHT) is often performed to decrease the number of residual cancer cells and to improve the outcome of patients with organ-confined prostate cancer; a third of all patients who receive radical treatment subsequently experience recurrence and metastasis, even in patients with organ-confined disease 1. However, the anti-tumor effects of NHT, including improvement in patient outcome, are far from satisfactory. In recent years, various anti-angiogenic agents have been reported to be effective in many different types of malignancy. As such, detailed information regarding the pathological role of angiogenesis, and the mechanisms that regulate it, are important in choosing the most appropriate strategies of observation and treatment. The impact that NHT exerts on angiogenesis and the expression of angiogenesis-related molecules is not fully understood.
To understand the pathological significance and prognostic value of angiogenesis in malignancies, effective methods for determining cancer-related neovascularity in malignancies are essential. In human cancer tissues, the most common method for semi-quantitative evaluation of angiogenesis is to measure microvessel density (MVD) using endothelial markers. MVD has been widely used for the evaluation of angiogenesis in many previous studies, and it is recognized as one of the most useful prognostic markers for tumor progression and survival in patients with various different types of cancer. Immunohistochemistry and antibodies against the endothelial cell markers CD31, CD34, and CD105 have frequently been used in previous studies of cancer tissues. There are many reports that have used these antibodies to investigate the pathological significance of MVD in prostate cancer tissues 2–4. However, there is relatively little published literature regarding whether this semi-quantitative method has any pathological significance in prostate cancer patients treated with NHT; if such a relationship were established, then it is possible that it can be applied to determining the prognosis of prostate cancer patients treated with NHT.
There is general agreement that various factors regulate angiogenesis in prostate cancer. The roles of the pro-angiogenic vascular endothelial growth factor (VEGF)-A and the anti-angiogenic thrombospondin (TSP)-1 have been well characterized in various different malignancies. Interestingly, androgen is a potent stimulator of VEGF-A expression 5,6 and a suppressor of TSP-1 activity in prostate cancer 7. Actually, androgen depletion has been reported to increase VEGF-A expression and decrease TSP-1 expression in prostate cancer 7–9. Thus, various studies support the view that androgen plays an important role in angiogenic processes in prostate cancer; however, the relationship between NHT and angiogenic activities, including MVD, and the expression of angiogenesis-related factors in human prostate cancer tissues are not fully understood.
In this study, we investigated endothelium markers that can be used to reflect the pathological role and clinical significance of angiogenesis in prostate cancer patients treated with NHT prior to radical operation. In order to evaluate cancer-related neovascularity, we also compared the expression of VEGF-A and TSP-1 in prostate cancer specimens from patients who were or were not treated with NHT. Finally, we investigated and discussed the predictive value of MVD for biochemical recurrence (BCR) in patients treated with NHT.
METHODS
Patients
Included in the study were specimens from 108 prostate cancer patients that had undergone a radical prostatectomy (RP) at our hospital. Among them, 48 patients (44.4%) received NHT (NHT group) and 60 patients (55.6%) were not treated with NHT (non-NHT group). In this study, patients who were treated with NHT for <3 months, had clinical or pathological invasion into the seminal vesicle or surrounding tissues, underwent metastasis, had a Gleason score (GS) of 10, or serum prostate antigen (PSA) levels >90 ng/ml were excluded. In order to match the clinicopathological features of the non-NHT and NHT groups, patient's age, PSA levels, GS, and pT stage were collected. In addition, specimens with <300 cancer cells/specimen or with very few or no viable cancer cells were also excluded.
All patients were evaluated using chest radiography, transrectal ultrasonography, bone scanning, and computed tomography (CT) of the pelvis and abdomen. In addition, CT of the lungs and magnetic resonance imaging of the prostate or bone was performed when necessary. A patient's disease stage was assessed using the 2002 tumor-node-metastasis (TNM) staging system and GS. NHT consisted of anti-androgen agent (n = 1, 2.1%), luteinizing hormone-releasing hormone (LH-RH) agonists (n = 20, 41.7%), or a combined androgen blockage therapy consisting of LH-RH agonists and anti-androgen agent (n = 27, 56.3%). The median duration of NHT was 8 months (interquartile range 5–11 months). BCR was defined as serum PSA levels that were >0.2 ng/ml, as measured on two or more occasions. The study protocol was approved by the Human Ethics Review Committee of Nagasaki University Graduate School of Biomedical Science, and a signed consent form was obtained from each subject.
Immunohistochemistry
Sections were cut 5 µm thick, deparaffinized in xylene, and rehydrated in solutions of graded ethanol. Antigen retrieval was performed at 95°C for 40 min in 0.01 M sodium citrate buffer (pH 6.0). Sections were then immersed in 3% hydrogen peroxide for 30 min to block endogenous peroxidase activity. Primary antibodies were obtained from Novocastra (New Castle, United Kingdom; anti-human CD31 antibody), DakoCytomation (Glostrup, Denmark; anti-human CD34 antibody), Vector Laboratories (Burlingame, CA; anti-human CD105 antibody), Santa Cruz Biotechnology (Santa Cruz, CA; anti-VEGF-A antibody), and (Cambridge, UK; anti-TSP-1 antibody). Sections were incubated with primary antibodies at 4°C overnight. Following primary antibody incubation, sections were treated using the labeled polymer peroxidase method (DAKO EnVision™+ Peroxidase Kit, Dako, Carpinteria, CA) for 60 min. Proteins were visualized using a liquid diaminobenzidine substrate kit (Zymed Laboratories, San Francisco, CA). Sections were counterstained with hematoxylin before mounting. Negative controls consisted of adjacent sections from each sample that were processed without the primary antibody. The positive control for all antibodies was kidney tissue, including renal cell carcinoma. The immunohistochemical staining methods used for all of the antibodies have previously been described 10,11.
The analysis of immunohistochemical staining within the tumor area of sections was performed using a light microscope. The expression levels of VEGF-A and TSP-1 were semi-quantitatively assessed using a method similar to that previously described 12. Briefly, VEGF-A staining was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) according to the staining intensity. In addition, the extent of staining was scored as 0 (nothing), 1 (1–25%), 2 (26–50%), 3 (51–75%), or 4 (76–100%) according to the percentage of the positively stained cancer cells 12. TSP-1 expression was scored as 0 (negative), 1 (weak), 2 (moderate), or 3 (strong) depending on the staining intensity in the cancer region 7. The extent of TSP-1 expression was not scored because it is difficult to evaluate the stained area in stromal tissues. The semi-quantitative analysis and immunostaining interpretation was independently performed by two investigators (S.W. and Y.M.) who were blinded to clinical features and survival data.
To determine the MVD, tumor sections stained with each antibody were examined using a Nikon E-400 bright-field microscope (Nikon, Tokyo, Japan). Images were captured using a digital camera (DU100; Nikon) and a 200× magnification objective lens. For each tumor section, 3–5 hot spot fields of view (that had the greatest density of positively stained vessels) were evaluated. MVD was defined as the number of positively stained vessels per high-power field of view (HPF), estimated using computer-aided image analysis (WinROOF version 6.4; Mitani, Fukui, Japan). For statistical analysis, each MVD was divided into two groups: low (median or less) and high (greater than the median).
Statistical Methods
Normality was evaluated by normal distribution. Histograms were prepared for each variable and the results are expressed as the mean and standard deviation (SD). The Student's t-test was performed for continuous variables. The Mann–Whitney U test was also used for some data. The Scheffé test was used for multiple comparisons of data. Pearson's correlation and the correlation coefficient (r) were used to evaluate the relationship between continuous variables, and the corresponding P values are shown. Spearman's rank correlation coefficient was calculated to confirm the Pearson's correlation results. In survival analyses, the Kaplan–Meier survival curve and log-rank test were used, and Cox proportional hazards model multivariate analysis was also performed. All statistical tests were two-sided, and significance was defined as P < 0.05. All statistical analysis was performed on a personal computer using the StatView for Windows statistical package (version 5.0; Abacus Concepts, CA).
RESULTS
The age of the patients ranged from 51 to 71 years (median 64 years); there was no significant difference in age between the non-NHT and NHT groups (P = 0.082). There were also no significant differences between the non-NHT and NHT groups in regard to serum PSA levels at diagnosis (mean 12.3/SD 9.3 and 15.1/SD 10.8 ng/ml, respectively, P = 0.166) or in regard to the pathological diagnosis. There were 17 (28.3%), 25 (41.7%), and 18 (30.0%) non-NHT group patients and 8 (16.7%), 29 (60.4%), and 11 (22.9%) NHT group patients judged as having low, middle, and high GSs, respectively. In the non-NHT group, pT2 and pT3 disease was observed in 36 (60.0%) and 24 (40.0%) patients, respectively; in the NHT group, pT2 and pT3 disease was observed in 31 (64.6%) and 17 (35.4%) patients, respectively. Thus, no significant differences in pT stage were observed between the groups (P = 0.626).
CD31, CD34, and CD105 staining
Representative examples of CD31, CD34, and CD105 positively stained vessels in prostate cancer tissues with NHT are shown in Figure 1A–C, respectively. In addition, CD31, CD34, and CD105 vessel staining in low GS prostate cancer tissues with non-NHT are shown in Figure 1D–F, respectively. Similar vessels in high GS non-NHT tumors are shown in Figure 1G–I. Many CD34 positively stained vessels were clearly detected in the degenerate area of cancer tissues with NHT (Fig. 1B). However, CD105 positively stained vessels were rare in similar areas (Fig. 1C). In low GS non-NHT specimens, CD105 positively stained vessels were rare. We also noted that the endothelial cells of large and mature blood vessels were strongly stained by the anti-CD31 and anti-CD34 antibodies, but not by the anti-CD105 antibody (arrows in Fig. 1D–F). In contrast to the low GS tissue, CD105 positively stained vessels were clearly detected in high GS prostate cancer tissue (Fig. 1I).
Figure 1.
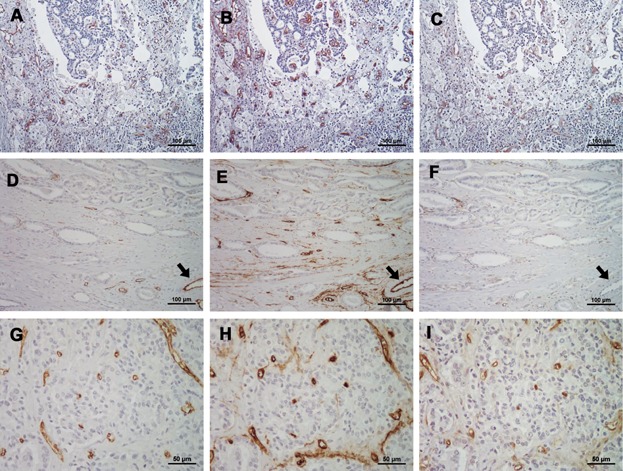
(A–C) Representative examples of CD31, CD34, and CD105 stained vessels in prostate cancer tissues that were not treated with neoadjuvant therapy (NHT), respectively. In degenerated tissues, CD31 and CD34 positive vessels were relatively abundant, but CD105 staining was weak in similar areas. (D–F) CD31, CD34, and CD105 stained vessels in low Gleason's score (GS) non-NHT tissues, respectively. The number of CD105 positive vessels in the tumor area was clearly lower than the number of CD34 positive vessels. The endothelial cells of large and mature blood vessels were strongly stained for CD31 and CD34, but not for CD105 (arrows in D–F). (G–I) CD31, CD34, and CD105 stained vessels in high GS non-NHT tissues, respectively. In contrast to low GS tissue, CD105 positive vessels were clearly detected in high GS prostate cancer (I). In almost of all of the specimens, the intensity and number of CD31 positively stained vessels were with the range of the number of CD105 and CD34 positively stained vessels.
The mean (SD) CD31-, CD34-, and CD105-MVD/HPF in the non-NHT specimens were 38.6 (7.9), 50.2 (9.1), and 25.8 (7.3), respectively. The mean (SD) CD31-, CD34-, and CD105-MVD/HPF in the NHT specimens were 36.5 (9.1), 50.1 (7.4), and 13.3 (4.7), respectively. Although the CD31-, CD34-, and CD105-MVD in the NHT group (Fig. 2A–C) tended to be lower than in the non-NHT group, only CD105-MVD was significantly different (P < 0.001). As shown in Figure 2D and E, a similar difference in VEGF-A and TSP-1 expression between the non-NHT and NHT group was also found.
Figure 2.
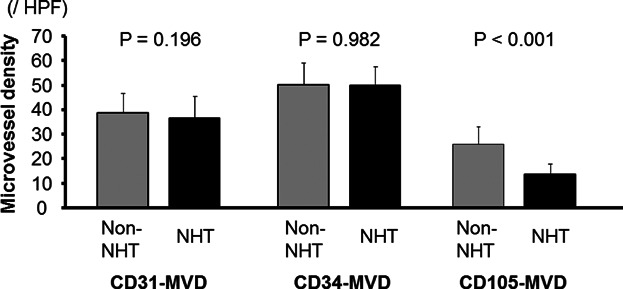
Microvessel densities (MVD) in non-neoadjuvant therapy (non-NHT) and NHT group were showed. Among 3 MVDs, CD105-MVD in NHT was significantly lower than that in non-NHT. Such significant difference was not found in CD31- and CD34-MVD.
Correlation of VEGF-A and TSP-1 Expression with Pathological Features
In the NHT group specimens, CD105-MVD positively correlated with VEGF-A expression (r = 0.56, P < 0.001) while TSP-1 expression negatively correlated with CD105-MVD (r = 0.42, P = 0.003). CD31-MVD was not correlated with VEGF-A (r = 0.21, P = 0.149) or TSP-1 (r = 0.03, P = 0.845) expression in the NHT group samples. Similarly, CD34-MVD was not correlated with VEGF-A (r = 0.11, P = 0.472) or TSP-1 (r = 0.14, P = 0.358) expression in the NHT group.
The relationship between CD31- CD34- and CD105-MVD and the patient's pathological features are showed in Table1. In the non-NHT patients, the CD31-, CD34-, and CD105-MVD were significantly correlated with GS and pT stage. However, in the NHT patients, there was no correlation between CD31- and CD34-MVD and a patient's pathological features. However, CD105-MVD in the NHT patients was significantly correlated with their pT stage, but not with their GS.
I.
Correlation With Pathological Features
| Mean MVD (SD) in non-NHT | Mean MVD (SD) in NHT | |||||||
|---|---|---|---|---|---|---|---|---|
| N | CD31 | CD34 | CD105 | N | CD31 | CD34 | CD105 | |
| GS | ||||||||
| Low | 17 | 34.1 (7.3) | 44.5 (8.0) | 22.0 (6.8) | 8 | 31.5 (7.5) | 48.8 (9.2) | 13.7 (5.3) |
| Middle | 25 | 39.7 (8.0) | 51.7 (8.5) | 26.0 (5.5) | 29 | 37.9 (9.4) | 51.5 (6.7) | 12.9 (4.3) |
| High | 18 | 41.3 (7.0) | 53.3 (8.7) | 28.9 (8.7) | 11 | 36.1 (8.6) | 47.4 (7.4) | 14.2 (5.5) |
| P value† | 0.023 | 0.001 | 0.019 | NS | NS | NS | ||
| P value‡ | 0.035/0.032 | 0.038/0.038 | 0.005/0.007 | 0.894/0.874 | 0.238/0.137 | 0.502/0.428 | ||
| T stage | ||||||||
| pT2 | 36 | 35.9 (5.5) | 45.9 (6.4) | 22.8 (6.0) | 31 | 34.7 (9.0) | 48.8 (7.3) | 12.0 (4.7) |
| pT3 | 24 | 42.6 (9.4) | 56.5 (8.8) | 30.4 (6.8) | 17 | 39.7 (8.7) | 52.5 (7.0) | 15.7 (3.8) |
| P value | 0.001/0.001 | <0.001/<0.001 | <0.001/<0.001 | 0.065/0.091 | 0.092/0.077 | 0.008/0.012 | ||
MVD, microvessel density; NHT, neoadjuvant hormonal therapy; GS, Gleason's score; NS, not significant; SD, standard deviation.
Low versus High, using multiple comparison tests.
‡Low and Middle versus High, respectively, using Student's t-test/Mann–Whitney U test.
Predictive Value for BCR
We analyzed the prognostic value of CD31-, CD34-, and CD105-MVD for BCR after RP in NHT treated patients (Fig. 3A–C). Kaplan–Meier survival curves indicated that CD105-MVD was significantly associated with BCR in patients treated with NHT. However, CD31- and CD34-MVD were not significant predictors in this analysis. Univariate Cox regression analysis indicated that the clinicopathological risk factors GS, pT stage, and serum PSA levels at diagnosis were not associated with BCR in the NHT group patients. As such, only CD105-MVD can be used as a predictive factor for BCR (Table2). This was shown by both univariate and multivariate analyses.
Figure 3.
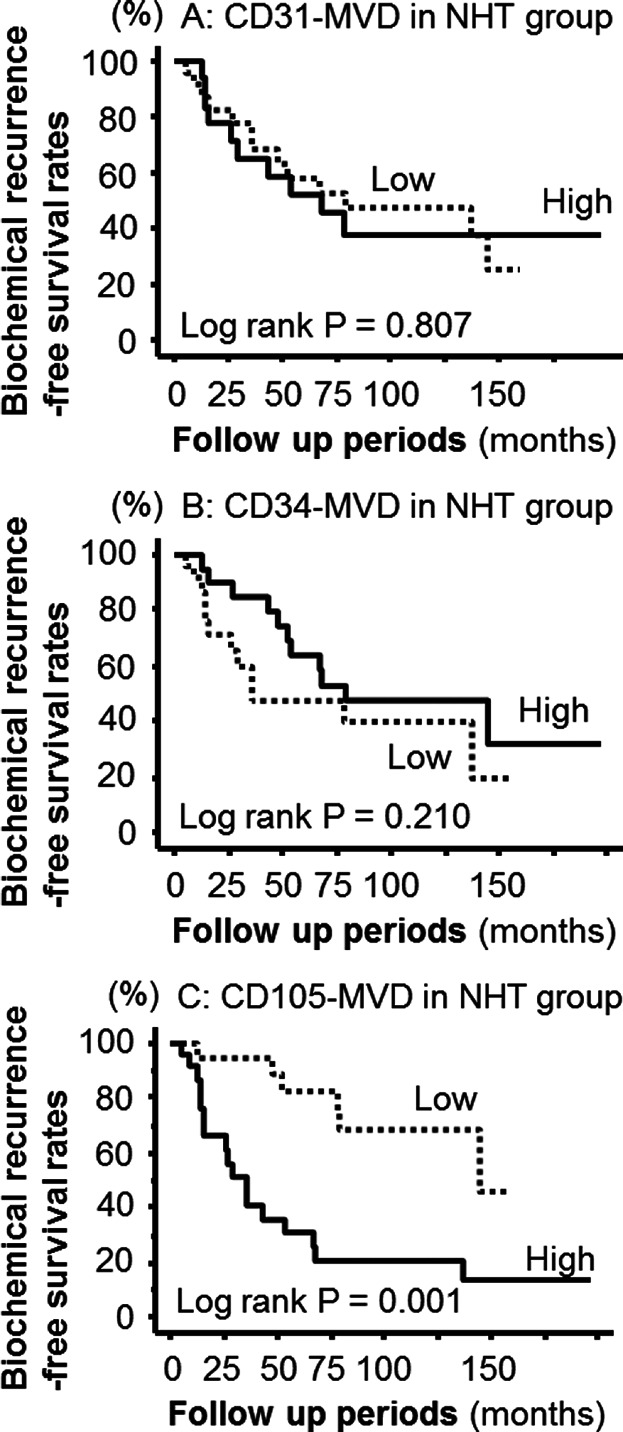
Kaplan–Meier survival curves showed that patients with high CD105-MVD have worse prognosis of biochemical recurrence (BCR) compared to those with low one (log rank P = 0.001). On the other hand, CD31- and CD105-MVD were not associated with BCR.
II.
Predictive Value for Biochemical Recurrence in the Neoadjuvant Group
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Serum PSA level at diagnosis | 1.02 | 0.98–1.05 | 0.396 | 1.01 | 0.96–1.06 | 0.648 |
| High Gleason score (≥8) | 2.28 | 0.56–9.30 | 0.250 | 1.23 | 0.46–3.26 | 0.684 |
| High pT stage (pT3) | 1.86 | 0.80–4.34 | 0.149 | 0.87 | 0.30–2.54 | 0.802 |
| High CD105-MVD (>20/HPF) | 4.68 | 1.83–12.02 | 0.001 | 4.45 | 1.61–12.26 | 0.004 |
HR, hazard ratio; CI, confidential interval; PSA, prostate-specific antigen; MVD, microvessel density.
DISCUSSION
Our results indicate that CD31-, CD34-, and CD105-MVD are positively associated with GS and pT stage in non-NHT group. These results are similar to those of previous studies, which have investigated the relationship between MVD and pathological features in RP specimens from non-NHT treated patients 2,3. However, there is little information regarding the pathological significance and prognostic value of MVD in prostate cancer patients treated with RP following NHT treatment. The present study demonstrates that CD105-MVD is positively correlated with pT stage in NHT group; however, CD31- and CD34-MVD showed no such correlation. Thus, our results suggest that the vascular endothelial cells detected by the anti-CD105 antibody in prostate cancer tissues from NHT treated patients have different pathological characteristics from those detected by the anti-CD31 and anti-CD34 antibodies. Indeed, although CD31 and CD34 are known as pan-endothelial markers, which stain almost all blood vessels, including mature and newly formed blood vessels, CD105 is recognized as a proliferation-associated endothelial marker, which is induced by hypoxia. CD105 is, therefore preferentially expressed in the active dividing endothelial cells of microvessel in cancer tissues 13. Indeed, our results indicated that large and established mature blood vessels did not stain positive for CD105, even though they clearly stained positive for CD31 and CD34. Furthermore, several other studies support the opinion that CD105-MVD may more precisely reflect the tissues angiogenic status of a variety of different cancer tissues than either CD31- or CD34-MVD 10,14,15.
Apart from measuring MVD, many investigators have studied the expression levels of angiogenesis-related molecules, such as VEGF-A and TSP-1, to evaluate the angiogenic activities of human tissues. Androgen deprivation has been reported to inhibit VEGF-A expression in prostate cancer patients 12, and similar results were also found in an in vivo study using a hormone-sensitive prostate cancer cell line 9. However, TSP-1 expression is reported to increase following hormonal therapy in both hormone-sensitive prostate cancer tissues and mouse models 7,16. Our results support the notion that NHT stimulates anti-angiogenic activity in prostate cancer tissues; the CD105-MVD of NHT treated prostate cancer tissues was significantly lower than that of non-NHT treated patients. On the other hand, there was no significant difference in CD31- or CD34-MVD between the NHT and non-NHT prostate cancer tissues. It has previously been reported that in NHT and non-NHT treated human prostate cancer specimens, CD31-MVD does not significantly differ 17. Furthermore, several studies using animal models have also shown that androgen deprivation does not reduced CD31-MVD in cancer tissues 18,19. There is also a report that indicates that the CD34-MVD in prostate cancer tissues at the time of diagnosis does not significantly reduce following androgen deprivation therapy 20. From these facts, we speculate that CD31- and CD34-MVD do not accurately reflect changes in the angiogenic status stimulated by hormonal therapy in prostate cancer patients. We conclude that an anti-CD105 antibody may detect newly formed cancer-related blood vessels more accurately than anti-CD31 or anti-CD34 antibodies in prostate cancer specimens treated with hormonal therapy.
Another interesting result from the present study is that CD105-MVD in RP specimens from patients treated with NHT is a significant predictive factor for BCR. There is some concern that the pathological features following hormonal therapy do not always reflect the malignant potential and aggressiveness of a tumor because androgen deprivation may lead to morphological changes and deformities of the cancer cell. As such, NHT can make it difficult to produce an accurate pathological diagnosis, including the GS and pT stage. Furthermore, these morphological effects exerted by hormonal therapy may affect the outcome of prostate cancer patients treated with NHT. As such, predicting the prognosis of patients who undergo RP following NHT treatment may be more difficult to judge than for non-NHT treated patients. Indeed, our study indicated that GS and pT stage were not useful predictive factors for BCR in the NHT group, and similar results have previously been published 21. Thus, more information regarding appropriate predictive factors for patient prognosis is important in discussing the efficacy and limitations of NHT for organ-confined prostate cancer. The present study has demonstrated that BCR in patients treated with NHT was significantly and independently associated with CD105-MVD, but not with CD31- or CD34-MVD. This might be a consequence of the relationship between the specific marker and the patient's pathological features, VEGF-A expression, or TSP-1 expression.
CONCLUSIONS
Our results show that CD105-MVD may more accurately reflect the angiogenic conditions and malignant potential of prostate cancer tissues than CD31-MVD or CD34-MVD. High CD105-MVD was also correlated with high VEGF-A expression and low TSP-1 expression in NHT treated patients. Furthermore, CD105-MVD was identified as a significant independent predictor of BCR in prostate cancer patients who had undergone RP following NHT treatment.
REFERENCES
- Watson RB, Civantos F, Soloway MS. Positive surgical margins with radical prostatectomy, detailed pathological analysis and prognosis. Urology. 1996;48:80–90. doi: 10.1016/s0090-4295(96)00092-1. [DOI] [PubMed] [Google Scholar]
- Bettencourt MC, Bauer JJ, Sesterhenn IA, Connelly RR, Moul JW. CD34 immunohistochemical assessment of angiogenesis as a prognostic marker for prostate cancer recurrence after radical prostatectomy. J Urol. 1998;160:459–465. [PubMed] [Google Scholar]
- Erbersdobler A, Isbarn H, Dix K. Prognostic value of microvessel density in prostate cancer: A tissue microarray study. World J Urol. 2010;28:687–692. doi: 10.1007/s00345-009-0471-4. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Ohba K, Matsuo T. Tumor-associated stromal cells expressing E-prostanoid 2 or 3 receptors in prostate cancer: Correlation with tumor aggressiveness and outcome by angiogenesis and lymphangiogenesis. Urology. 2013;81:136–142. doi: 10.1016/j.urology.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Joseph IB, Nelson JB, Denmeade SR. Androgens regulate vascular endothelial growth factor content in normal and malignant prostatic tissue. Clin Cancer Res. 1997;3:2507–2511. [PubMed] [Google Scholar]
- Eisermann K, Broderick CJ, Bazarov A, Moazam MM, Fraizer GC. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol Cancer. 2013;12:7. doi: 10.1186/1476-4598-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombel M, Filleur S, Fournier P. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res. 2005;65:300–308. [PubMed] [Google Scholar]
- Joseph IB, Isaacs JT. Potentiation of the antiangiogenenic ability of linomide by androgen ablation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostate cancers. Cancer Res. 1997;57:1054–1057. [PubMed] [Google Scholar]
- Stewart RJ, Panigrahy D, Flynn E. Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: Evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol. 2001;165:688–693. doi: 10.1097/00005392-200102000-00095. [DOI] [PubMed] [Google Scholar]
- Miyata Y, Sagara Y, Watanabe S. CD105 is a more appropriate marker for evaluating angiogenesis in urothelial cancer of the upper urinary tract than CD31 or CD34. Virchows Arch. 2013;463:673–679. doi: 10.1007/s00428-013-1463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Koga S, Takehara K. Expression of thrombospondin-derived 4N1K peptide-containing proteins in renal cell carcinoma tissues is associated with a decrease in tumor growth and angiogenesis. Clin Cancer Res. 2003;9:1734–1740. [PubMed] [Google Scholar]
- Aslan G, Cimen S, Yorukoglu K. Vascular endothelia growth factor expression in untreated and androgen-deprived patients with prostate cancer. Pathol Res Pract. 2005;201:593–598. doi: 10.1016/j.prp.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Kumar P, Wang JM, Bernabeu C. CD105 and angiogenesis. J Pathol. 1996;178:363–366. doi: 10.1002/(SICI)1096-9896(199604)178:4<363::AID-PATH491>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Tanaka F, Otake Y, Yanagihara K. Evaluation of angiogenesis in non-small cell lung cancer: Comparison between anti-CD34 antibody and anti-CD105 antibody. Clin Cancer Res. 2001;7:3410–3415. [PubMed] [Google Scholar]
- Rubatt JM, Darcy KM, Hutson A. Independent prognostic relevance of microvessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: A gynecologic oncology group study. Gynecol Oncol. 2009;112:469–474. doi: 10.1016/j.ygyno.2008.11.030. [DOI] [PubMed] [Google Scholar]
- Fitchev PP, Wcislak SM, Lee C. Thrombospondin-1 regulates the normal prostate in vivo through angiogenesis and TGF-β action. Lab Invest. 2010;90:1078–1090. doi: 10.1038/labinvest.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima H, Goto T, Hosaka Y. Correlation between proliferation, apoptosis, and angiogenesis in prostate carcinoma and their relation to androgen ablation. Cancer. 1999;85:1822–1827. doi: 10.1002/(sici)1097-0142(19990415)85:8<1822::aid-cncr24>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Cheng L, Zhang S, Sweeney CJ. Androgen withdrawal inhibits tumor growth and is associated with decrease in angiogenesis and VEGF expression in androgen-independent CWR22Rv1 human prostate cancer model. Anticancer Res. 2004;24:2135–2140. [PubMed] [Google Scholar]
- Sato F, Furuhara H, Basilion JP. Effects of hormone deprivation and 2-methoxyestradiol combination therapy on hormone-dependent prostate cancer in vivo. Neoplasia. 2005;7:838–846. doi: 10.1593/neo.05145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomić TT, Gustavsson H, Wang W. Castration resistant prostate cancer is associated with increased blood vessel stabilization and elevated levels of VEGF and Ang-2. Prostate. 2012;72:705–712. doi: 10.1002/pros.21472. [DOI] [PubMed] [Google Scholar]
- Ryan CJ, Smith A, LAL P. Persistent prostate-specific antigen expression after neoadjuvant androgen depletion: An early predictor of relapse or incomplete androgen suppression. Urology. 2006;68:834–839. doi: 10.1016/j.urology.2006.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


