Abstract
As studies uncover the breadth of microbes associated with human life, opportunities will emerge to manipulate and augment their functions in ways that improve health and longevity. From involvement in the complexities of reproduction and fetal/infant development, to delaying the onset of disease, and indeed countering many maladies, microbes offer hope for human well-being. Evidence is emerging to suggest that microbes may play a beneficial role in body sites traditionally viewed as being sterile. Although further evidence is required, we propose that much of medical dogma is about to change significantly through recognition and understanding of these hitherto unrecognized microbe–host interactions. A meeting of the International Scientific Association for Probiotics and Prebiotics held in Aberdeen, Scotland (June 2014), presented new views and challenged established concepts on the role of microbes in reproduction and health of the mother and infant. This article summarizes some of the main aspects of these discussions.
Keywords: Lactobacilli, mastitis, microbiota, reproduction
Introduction
Unless science finds a way to create a human being entirely outside the womb, females will remain central to the survival of humankind. This statement might be delinquent to some, but more human research is performed on males than females,1 and most microbiome work focuses on chronic gut disease rather than the female reproductive tract. In some instances, ethical committees discourage research on pregnant women for fear of damaging the fetus, but more research is needed on issues particularly related to women. Having stated that, relatively few studies have examined the role of the microbiome in male fertility. In this review, we will focus on the female.
The process of reproduction is simple yet strewn with complex processes, any one of which can be problematic and even catastrophic to the fetus or mother. Conception starts when sperm comes into contact with the egg. Until recently, the site of conception and fetal growth (fallopian tubes and uterus) and the whole fetal environment was believed to be sterile and that the baby was first exposed to microorganisms only during birth. Thus, microbes were only pertinent to infections which impacted on fertility or successful gestation. In June, 2014, a meeting of the International Scientific Association for Probiotics and Prebiotics was held in Aberdeen, Scotland, to discuss recent research on the role of microbes in reproduction and health of the mother and infant. This article presents a perspective on these discussions.
Microbes and conception
The failure of the sperm–egg conception process is not infrequent, and the study of infertility has provided useful insights into the details of the fertilization process. Of infertility cases, one-third remain unexplained, while the others are attributed to infection or to anatomical and physiological problems. Some studies investigating unexplained infertility have shown the presence of lactobacilli in human follicular fluid.2 These bacteria, known for their beneficial effects on health and their usage in food, have been recently associated with embryo maturation and transfer.2 This fascinating study also reported a correlation between culturable microbes and the cause of infertility and in vitro fertilization (IVF) outcomes. The authors focused on the problematic pathogens rather than why fertility was associated with lactobacilli. By recommending antimicrobial therapy to increase the success rate of IVF, the authors may have missed an opportunity to discuss the adverse effects of such an approach.
Many of the bacterial types likely present in the cervix and vagina are not easily cultured and thus the study did not provide a clear picture of the population structure in this niche and which bacterial species might need to be eradicated. The options for therapy are either broad-spectrum agents that will also disrupt the indigenous microbes, or narrow spectrum agents that would not affect the entire range of organisms, namely non-pathogenic Gram positive, Gram negative, aerobes and anaerobes. This could represent a failure of antibiotics to penetrate biofilms or an inability to reach suitable minimal inhibitory concentrations. As a consequence, the efficacy of treatments for vaginal infections is often poor.3,4 The normal microbiota does not recover promptly following antimicrobial use,5,6 and thus if lactobacilli are important for IVF, antimicrobial treatment alone would not guarantee success. Moreover, long-term adverse effects of antimicrobial therapy are increasingly recognized, and while the mechanisms are not fully understood, a general reduction in use of these agents is warranted.7
The presence of lactobacilli reported in the ovaries is itself interesting, particularly as an earlier study failed to find any culturable bacteria in ovarian tissue,8 and because the endometrium is protected by physical barriers, antimicrobial peptides, complement, Toll-like receptors (TLRs), and other pattern recognition receptors.9 It seems reasonable to argue that the lactobacilli could have ascended from the vagina and cervix along the fallopian tubes, although systemic access via the gut epithelial membrane and blood supply has been shown for lipopolysaccharides from Gram-negative bacteria.10 The ovary is well protected, with proteins such as long pentraxin 3 (PTX3), produced by innate immunity cells in response to pro-inflammatory signals and Toll-like receptor engagement. The protein recognizes microbes, activates complement, facilitating phagocyte recognition, but it also is essential in female fertility by acting as a nodal point for the assembly of the cumulus oophorus hyaluronan-rich extracellular matrix.11 While neither this or any other ‘protective’ proteins were investigated in the human follicular fluid containing lactobacilli, the fact that the bacteria were reportedly viable and the female remained fertile suggests the organisms are in a quiescent state, are tolerated by the host, or are somewhat metabolically active and perform some important as yet not understood function. Although the fluid contains lysozyme, an enzyme that can be bactericidal to Gram-positive bacteria,12 it did not kill the lactobacilli, possibly because of peptidoglycan modifications13 or organism dormancy. Furthermore, the ovary has epithelial cells, and the follicular fluid itself contains almost 500 proteins,14 various hormones including estrogen, fatty acids, and likely has sufficient nutrient content for bacteria to grow. It seems unlikely that lactobacilli or other organisms are rapidly multiplying or are at high numbers, but are probably present in low numbers similar to the microbiota of the breast.15
While a quiescent state of bacteria adjacent to or within epithelial cells is a known phenomenon,16 it does not mean the lactobacilli are inactive. Indeed, they can maintain esterase activity, intact cytoplasmic membrane, and a pH gradient when dormant,17 as well as potentially other metabolic processes when in the host. Polycystic ovary syndrome (PCOS) is a heterogeneous endocrine disorder which increases the risk of developing type 2 diabetes mellitus and cardiovascular disease. A study of 217 women with PCOS and 48 healthy women as controls demonstrated elevated levels of lactate, long-chain fatty acids, triglyceride and very low-density lipoprotein in plasma, while glucose, phosphatidylcholine, and high-density lipoprotein concentrations were reduced in PCOS patients.18 The levels of alanine, valine, serine, threonine, ornithine, phenylalanine, tyrosine, and tryptophan were also generally increased, while glycine and proline were significantly reduced in PCOS samples. Dietary changes, such as reduction in carbohydrate intake affects insulin and may improve reproductive/endocrine outcomes.19 Processes such as l-carnitine-mediated beta-oxidation of fatty acids have a well-established role in energy supply of oocytes and embryos,20 and also protects bacterial cells from damage due to free radicals.21 The number of bacteria present in the ovary environment is presumably low and it is unknown if they are enough to induce measurable metabolomic changes. However, this is an aspect that is worth pursuing given lactobacilli's metabolic fermentative properties.
While we can only speculate on what functions lactobacilli might perform in the healthy ovary or follicular fluid, other studies suggest a role for lactobacilli in conception. One study found that lactobacilli can protect human spermatozoa from radical oxygen species in the presence of vaginal disorders, thereby improving the fertilization potential of the female host.22 Bacterial vaginosis (BV) is the most prevalent vaginal disorder, and it is associated with not only an increased rate of preterm labor,23 but also reduced ability to conceive.24,25 In a study of 874 women with female factor infertility and 382 asymptomatic fertile women, the prevalence of BV was significantly higher in the former (45.5% versus 15.4%), and the highest prevalence occurred in patients with PCOS (60.1%) and unexplained infertility (37.4%).26 But others disagree that BV affects preterm labor.27 A longitudinal study found no differences in the bacterial taxa, relative abundance and frequency of community state types between women who delivered at term and those who had spontaneous preterm delivery. The inability of metronidazole treatment of BV to alter preterm delivery rates,28 would support the latter view, but the effectiveness of clindamycin counters this.29 The fact that BV therapy improved the pregnancy rate considerably26 suggests that bacteria may play a role in conception and successful pregnancy. Clearly, more studies are needed to confirm this concept and understand the mechanisms, especially the role of lactobacilli in the follicular fluid. Intervention studies that assess whether probiotic lactobacilli could impact fertility and conception would also be highly valid and probably a better approach than antibiotics.
Fetal development and pregnancy duration
A study reporting the presence of bacteria in both preterm and term fetal membranes is interesting for two reasons: (i) it confirmed that there is a greater spread and diversity of bacterial species in tissues of women who had very preterm births; and (ii) it showed that bacteria can be present without any apparent complications.30 A recent report that the fetus in healthy women is indeed exposed to bacteria via the placenta,31 raises many questions. Some have questioned whether appropriate controls included blood and environmental samples, suggested that a placental microbiome has not yet been shown present in other mammalians, and that there is a history of deriving ‘germ-free’ animals, so the finding makes no sense in evolutionary terms. In fairness, few have yet used modern technologies at these sites in animals to test for the presence or remnants of bacteria, either live, dead, or inert. Still, there was more abundance, consistency, and diversity of taxa,31 far exceeding that expected even if maternal blood was contaminated from the oral cavity,32 and numerous other studies have reported culturable bacteria in endometrial curettage samples obtained from fresh hysterectomies,33 in term placentas,34 in fetal membranes,35 and in the basal plate of placentas in women who delivered at term with no evidence of infection.36 In terms of other mammals, a microbiome has been shown in other species.37
Further studies greatly strengthen the position that bacteria reach the placenta and amnion. Oral inoculation of a genetically labelled E. faecium strain to a group of pregnant mice led to its isolation and PCR detection from the amniotic fluid of the inoculated animals.38 The amniotic fluid was obtained by cesarean section 2 days before the expected delivery day to avoid contamination from maternal feces. In contrast, it could not be detected in the samples obtained from a non-inoculated control group. The counts in the samples of amniotic fluid obtained from the treated animals were ∼2.6 log CFU/mL. Bacteria were also isolated from amniotic fluid of the non-treated animals although at a lower level (∼1.7 log CFU/mL). Later, it was found that bacterial translocation from the gut to mesenteric lymph nodes and mammary gland occurred during late pregnancy and lactation in mice.39 During such a period, human breast milk cells and maternal peripheral blood mononuclear cells contain viable bacteria. This mechanism could also explain the presence of bacteria in amniotic fluid obtained from healthy mice. Further studies are required to confirm these findings.
It is easy to state that the purpose of this potential prenatal bacterial transfer is to prepare the fetus for entry to a microbial world, but proving this presents challenges. As an example, the above results may appear contradictory with the fact that hysterectomy has been the method of choice to rederive new strains of germ-free animals.40 The pregnant uterus is clamped off, removed, sterilized, and transferred into a sterile isolator with a recipient germ-free foster mother. The uterus is rinsed with distilled water before removing the pups from the uterus and transferring the surviving pups to the germ-free foster mom. The rinsing step may wash out bacteria as its concentration in amniotic fluid or placenta of healthy hosts seems to be very low. Anyway, to our knowledge, there are no studies on the bacterial load of germ-free derived animals at their fetal stage.
It is obvious that germ-free mice reproduce, so arguably microbes are not critical for conception and pregnancy. However, they may play a key role for a physiological reproduction as it is also true that germ-free animals exhibit pronounced defects in multiple aspects of their organs’ structure and function, often leading to digestive and reproductive disorders. In addition, germ-free animals not only contain abnormal numbers of several immune cell types and immune cell products, but also have relevant deficits in local and systemic lymphoid structures, including hypoplastic Peyer's patches and a decreased number of isolated lymphoid follicles.41 Therefore, any vertical bacterial transmission system involving complex cross talk processes with immune cells will be impaired in these animals.
Although germ-free animals are a wonderful research tool and have greatly contributed to the microbiota field, their use has some major drawbacks. It has been stated that as gut microbiota is crucial for a proper host development, altered responses exhibited by germ-free animals might not reflect what actually occurs in the natural setting.42 Therefore, it may be challenging to transfer results obtained in a germ-free system to the same events occurring in a conventional host.41 In fact, the subsequent addition of bacteria to germ-free animals after birth influences immune development, improves sociability, and reduces anxiety,43–45 emphasizing the importance of the microbes.
Even though confirmative studies are welcomed using sterilized and DNA-free surgical equipment and handling processes, the probability that bacteria are present in the placenta is tantalizing and promotes the concept of a microbiome–human symbiosis throughout conception and gestation. The wide array of non-pathogenic microbiota from the Firmicutes, Tenericutes, Proteobacteria, Bacteroidetes, and Fusobacteria phyla strongly suggests this microbiota is not a minor contaminant, and the sample size of 320 subjects31 suggests this is not a chance finding.
In terms of fetal development, the presence of DNA from Bifidobacterium spp. and Lactobacillus rhamnosus in 34 human placentae (25 vaginal and nine cesarean deliveries) suggested at least the possibility of a degree of mother–fetus microbial signaling.46 Other studies, albeit in mice, have shown that when the gut microbiota of the mother changes, so too can inflammation and energy loss, adiposity and insulin insensitivity, all in turn affecting the fetus.47
As stated earlier, conditions like BV can influence the duration of pregnancy. However, the presence of viruses is potentially even more important. Although few virome studies have been performed on the female reproductive tract, a series of studies has clearly shown that viral infection of the placenta or amnion leads to a predisposition for bacterial induced preterm labor.48–51 The viral infection might alter the normal mechanism mediating the interaction between the placenta/maternal tissues and the normal microbiota present at the implantation site. These changes most likely would induce damage to the protective layers allowing additional bacterial access. However, given the high prevalence of certain viruses in humans, for example a reasonably stable population of around 1200 viral genotypes in the human gut,52 one could speculate that viruses may also give access to lactobacilli or bifidobacteria without subsequent membrane damage and miscarriage. This is worthy of investigation.
It has been proposed that endogenous retroviruses mediate regulatory evolution and that this is an important mechanism underlying the evolution of placental development.53 The suggestion is that retroviruses integrated into the germ cell genome and that viral activity is responsible for establishing the ancestral trophoblast cell type through regulatory recruitment of developmental genes. If true, such host–viral association is intriguing. One implication proposed by the author is that epigenetic aberrations could cause global endogenous retroviral repression and placental defects, such as pre-eclampsia. At the very least, this viral–host association would illustrate the central role of microbes in reproduction.
Other pathogenic viral effects can occur during pregnancy, with disturbances during the second trimester from influenza infection being linked with neurodevelopmental contribution to major affective disorder, especially unipolar depressive disorder and schizophrenia later in life.54 This further illustrates the ability of microbes to influence life, but given the prevalence of influenza infection, it also suggests that protective effects are in place in many instances to prevent this serious condition.
T cells and potentially also B cells have long been studied in reproductive immunology, especially in terms of tolerance mechanisms supporting fetal well-being.55 This topic has been well reviewed elsewhere,56 but can immune cells prepare the newborn to ‘receive’ and tolerate certain microorganisms, and if so could the maternal programming be modulated to optimize the receptivity to certain microbial types? Such questions remain unanswered, but emerging evidence does suggest that microbes can indeed affect fetal development. Could viral vaccines administered to the mother, for example against cytomegalovirus (CMV) and human papilloma virus, affect the response of the newborn to certain organisms? There is at least one study where CMV-infected human fetal brains showed a higher tropism for stem cells/radial glial cells.57 Clearly, infections by a variety of organisms in utero can affect fetal development and the short- and long-term outcomes for the neonate,58 but could some non-pathogenic organisms do the same in a manner that benefits the fetus?
Vitamins are critical for the first 1000 days of life, most notably folic acid for brain and nerve system development.59 A number of these vitamins can be produced by bacteria.60 Processes that involve methionine and one carbon metabolism in response to physiological, nutritional, and hormonal influences are critical for cellular and organ function, and growth of the fetus.61 Indeed, amino acids (lysine and methionine), functional amino acids (histidine and ornithine), and a dipeptide (l-alanyl-l-glutamine) essential for development can all be produced by bacteria.62 Nutrient availability influences total bacterial numbers and species composition in the gut, but to date, no specific nutrients or prebiotics have been examined to boost the microbial production of compounds critical to the fetus. Some developmental stages may be so important that even nutritional interventions post-birth could be too late. Severe malnourishment has been associated with significantly less diverse microbiota that is only partially ameliorated following nutritional interventions in infancy.63 It is already known that diminished microbial diversity in infancy, and colonization with specific pathogenic bacteria, is linked with an elevated risk for allergy,64 and probiotic intervention in the mother and newborn can ameliorate this,65,66 although not all evidence is supportive and many factors are contributory to success or failure.67–69
The supplementation of folic acid during pregnancy came from a recognition of its importance in fetal development. However, other compounds might be worth considering, especially linoleic acid given that the brain is composed of 60% fat. The correlation between lower maternal intake of linoleic acid and autism spectrum disorder,70 and lower levels of linoleic acid and Alzheimer's disease later in life,71 provide examples of how at least these fatty acids are important. The ability of bifidobacteria and lactobacilli to produce linoleic acids72,73 raises the question of whether administration of these strains as probiotics might improve brain development over the first two trimesters and beyond. Environmental chemicals, drugs, and maternal nutritional imbalances can interfere with regulatory pathways involved in heart development early in gestation. No studies have examined probiotic intervention at this stage of life, but the ability of lactobacilli to modulate cardiac modeling74 and counter environmental toxins75 suggest they could be worth investigating to improve heart health.
Learning from mastitis
The importance of maternal milk in development of the newborn is unquestioned. In addition to antibodies, oligosaccharides and nutrients, the neonate also receives microbes in the milk.76,77 It had long been assumed that these comprised skin ‘contaminants’ from the mother, or potentially the baby passed organisms it had acquired at birthing back into the mammary ducts upon feeding. However, recent studies have suggested that bacteria are picked up by dendritic cells in the maternal gut and transferred to the mammary ducts – perhaps as a means to colonize the newborn through breast feeding.78 Although this theory is still somewhat controversial, a Lactobacillus salivarius CECT 5713, isolated from maternal milk and the newborn baby's feces, has been sequenced and shown to have the potential to stimulate the maturation of immature dendritic cells and to inhibit the in vitro infectivity of HIV-1.79 The latter could potentially be important in reducing HIV infection to newborns, a characteristic reported in vaginal lactobacilli.80,81 Another possibility, not yet investigated, is for bacteria to reach the mammary gland via adsorption across the skin surface directly into the fatty tissue or via the bloodstream.82
The latter example cites work on Staphyococcus aureus, better known for its ability to cause bacteremia, wound infections, and mastitis. Over one-third of women can suffer from mastitis during breastfeeding women, resulting in cessation of feeding, and administration of antibiotic and pain therapy.83 It is actually defined as ‘inflammation of the breast’, but invariably is associated with bacterial infection. Interestingly, inflammatory changes of the breast can range from benign self-limited processes such as puerperal mastitis, to highly aggressive malignant inflammatory carcinoma. The diagnosis of mastitis is usually made by patients presenting with focal tenderness in one breast accompanied by fever and malaise. The concept of using probiotic lactobacilli to prevent and help treat mastitis is being considered,77,84 but data from managing the disease in cows suggest that bacteriocins, antibacterial proteins produced by one strain and that are active against usually close relatives, are also worthy of consideration.
Mastitis is a major problem faced by the dairy industry. Over 137 different organisms have been identified as being causative agents of bovine mastitis, including bacteria, viruses, mycoplasma, yeasts, and algae.85 This makes a generalized treatment difficult, but as most cases, at least in the United Kingdom and Ireland, are caused by Escherichia coli, S. aureus, Streptococcus uberis, Strepococcus dysgalactiae, and Streptococcus agalactiae,86 bacteriocin therapy could make a significant impact. In a series of studies, a broad-spectrum bacteriocin produced by the food-grade Lactococcus lactis, Lacticin 3147 which is bactericidal to streptococci and staphylococci in vitro, when applied with a teat seal was found to significantly prevented mastitis.87 Further studies showed reduced incidence of mastitis after experimental challenge with S. dysgalactiae and S. aureus.87,88 Mechanistically, infusion with live Lactococcus lactis led to a rapid and considerable innate immune response which may also form part of the efficacy.89 Given the failures of antibiotic therapy, this intervention provides an illustration of the potential power of the microbes. Such applications to humans are certainly worthy of attempting, especially given the option of local administration as well as via ingestion and immunological modulation. In fact, a preparation of nisin, another lactococcal bacteriocin, has been successfully employed for the treatment of human staphylococcal mastitis and nipple cracking during lactation.90
The ability of naturally occurring microbes to treat infections allows us to revisit the pre-antibiotic era. It is known that some infections naturally resolve, and competition from commensal microbes has been suggested as one of the mechanisms.91 The dense and complex bacterial population that exists in the human colon arguably may offer a wealth of novel antimicrobials,92 including bacteriocins as well as candidate probiotic strains. Recently, Hatziioanou et al.93 provided evidence of a novel bacteriocin-like substance against Bacillus subtilis that is formed by Roseburia faecis, an abundant species in the human colon. The finding of substantial geographical variation in the composition and distribution of bacterial species in human colonic94,95 and vaginal samples96 suggests there is merit in screening human samples collected from various countries and ethnicities for novel bacteriocin-producing bacteria and candidate probiotics. For indigenous people, such as the Hadza in Tanzania, a more close association with the microbial world and their hunter–gatherer lifestyle certainly shapes their microbiome,97 and presumably alters how they prevent, treat and recover from infections, or indeed die from them. In Venezuelan Hadza, infectious disease is a major killer,98 but of those who live a relatively long life, it would be interesting to examine how their microbiome has evolved without vaccination or modern medicine. Analysis of samples collected by Jeff Leach while living with Hadza in Tanzania will soon provide valuable insight into these questions.99
Conclusions
There seems little doubt that microorganisms are central to human life and longevity (Fig.1). While death due to pathogenic microbes has been well documented, recent studies indicate that commensal organisms, and in some cases administered probiotics, are helping to delay morbidity and mortality and are playing a critical role in the continuation of human life. The more we understand about our tiny co-inhabitants, the better able we will be to manipulate and utilize them to our healthy advantage.
Figure 1.
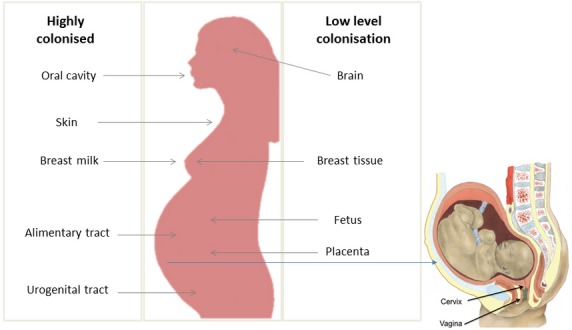
Microbes are being identified at all regions of the human body from external (shown here as abundances: Darryl Leja, NHGRI – http://www.genome.gov/dmd/img.cfm?node=Photos/Graphics&id=85320) sites including sites where bacteria are in high numbers, such as the oral cavity, alimentary tract, skin, urogenital area, and breast milk, to internal sites previously thought to be sterile such as in low numbers in the brain, breast, and placenta/fetus. The origin of microbes reaching interior sites, include food, water, air, skin, the environment, and people. These microbes play a role in reproduction, healthy gestation, and longevity in ways that are now being uncovered.
The study of internal maternal transmission of microbes in mammals is in its infancy due to the enduring influence of the sterile womb paradigm and to the ethical and technical difficulties of collecting samples from healthy pregnancies before birth. Thus, we still know very little about the number and identity of microbes that traverse the placenta, whether they persist in the infant or if their presence has long-term health consequences. The ability of probiotic bacteria, administered to pregnant women, to modulate the fetal intestinal immune gene expression and placental physiology suggests host–microbe interactions take place at this site.100 While maternal transmission of microbes in vertebrate species has only been recognized in the last few years, nearly a century's worth of research is available for vertical transmission of symbionts in invertebrates. Maternal provisioning of microbes to developing offspring is widespread in the metazoan world, with evidence of internal microbial transmission in animal phyla as diverse as Porifera, Cnidaria, Mollusca, Platyhelminthes, Nematoda, Arthropoda and Chordata.101 The presence of maternal transmission at the base of the Animalia Kingdom and the surprising plasticity by which microbes gain access to germ cells or embryos in these systems signifies that maternal symbiont transmission is an ancient and evolutionary advantageous mechanism that is inherent in animals. Therefore, we can no longer ignore the fact that exposure to microbes in the womb is more common than previously thought and may even be a normal part of human pregnancy as the first inoculation of beneficial microbes to an unborn child.
Acknowledgments
Funding provided by ISAPP for authors to meet in Aberdeen, Scotland, and discuss these topics is much appreciated.
References
- 1.Kim AM, Tingen CM, Woodruff TK. Sex bias in trials and treatment must end. Nature. 2010;465:688–689. doi: 10.1038/465688a. [DOI] [PubMed] [Google Scholar]
- 2.Pelzer ES, Allan JA, Waterhouse MA, Ross T, Beagley KW, Knox CL. Microorganisms within human follicular fluid: effects on IVF. PLoS ONE. 2013;8:e59062. doi: 10.1371/journal.pone.0059062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwebke JR, Desmond RA. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin Infect Dis. 2007;44:213–219. doi: 10.1086/509577. [DOI] [PubMed] [Google Scholar]
- 4.Verstraelen H, Swidsinski A. The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Curr Opin Infect Dis. 2013;26:86–89. doi: 10.1097/QCO.0b013e32835c20cd. [DOI] [PubMed] [Google Scholar]
- 5.Reid G, Bruce AW, Cook RL, Llano M. Effect on the urogenital flora of antibiotic therapy for urinary tract infection. Scand J Infect Dis. 1990;22:43–47. doi: 10.3109/00365549009023118. [DOI] [PubMed] [Google Scholar]
- 6.Lazarevic V, Manzano S, Gaïa N, Girard M, Whiteson K, Hibbs J, François P, Gervaix A, Schrenzel J. Effects of amoxicillin treatment on the salivary microbiota in children with acute otitis media. Clin Microbiol Infect. 2013;19:E335–E342. doi: 10.1111/1469-0691.12213. [DOI] [PubMed] [Google Scholar]
- 7.Keeney KM, Yurist-Doutsch S, Arrieta MC, Finlay BB. Effect of antibiotics on human microbiota and subsequent disease. Annu Rev Microbiol. 2014;12:217–235. doi: 10.1146/annurev-micro-091313-103456. [DOI] [PubMed] [Google Scholar]
- 8.Chimura T, Hirayama T, Morisaki N, Nakahara M, Funayama T, Oda T, Kanasugi H, Takahashi H, Saito N. Comparisons of the bacterial flora in genital regions at non-pregnancy. Jpn J Antibiot. 1992;45:1065–1070. [PubMed] [Google Scholar]
- 9.Sheldon IM, Bromfield JJ. Innate immunity in the human endometrium and ovary. Am J Reprod Immunol. 2011;66(Suppl 1):63–71. doi: 10.1111/j.1600-0897.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 10.Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)–a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012;79:104–112. doi: 10.1016/j.mehy.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Bottazzi B, Bastone A, Doni A, Garlanda C, Valentino S, Deban L, Maina V, Cotena A, Moalli F, Vago L, Salustri A, Romani L, Mantovani A. The long pentraxin PTX3 as a link among innate immunity, inflammation, and female fertility. J Leukoc Biol. 2006;79:909–912. doi: 10.1189/jlb.1005557. [DOI] [PubMed] [Google Scholar]
- 12.Stepanović S, Djukić S, Veljković M, Arsić B, Garalejić E, Ranin L. Antimicrobial activity of human follicular fluids. Gynecol Obstet Invest. 2003;56:173–178. doi: 10.1159/000074103. [DOI] [PubMed] [Google Scholar]
- 13.Bernard E, Rolain T, Courtin P, Guillot A, Langella P, Hols P, Chapot-Chartier MP. Characterization of O-acetylation of N-acetylglucosamine: a novel structural variation of bacterial peptidoglycan. J Biol Chem. 2011;286:23950–23958. doi: 10.1074/jbc.M111.241414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambekar AS, Nirujogi RS, Srikanth SM, Chavan S, Kelkar DS, Hinduja I, Zaveri K, Prasad TS, Harsha HC, Pandey A, Mukherjee S. Proteomic analysis of human follicular fluid: a new perspective towards understanding folliculogenesis. J Proteomics. 2013;87:68–77. doi: 10.1016/j.jprot.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Urbaniak C, Cummins J, Brackstone M, Macklaim JM, Gloor GB, Baban CK, Scott L, O'Hanlon DM, Burton JP, Francis KP, Tangney M, Reid G. Microbiota of human breast tissue. Appl Environ Microbiol. 2014;80:3007–3014. doi: 10.1128/AEM.00242-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goneau LW, Yeoh NS, MacDonald KW, Cadieux PA, Burton JP, Razvi H, Reid G. Selective target inactivation rather than global metabolic dormancy causes antibiotic tolerance in uropathogens. Antimicrob Agents Chemother. 2014;58:2089–2097. doi: 10.1128/AAC.02552-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahtinen SJ, Ouwehand AC, Reinikainen JP, Korpela JM, Sandholm J, Salminen SJ. Intrinsic properties of so-called dormant probiotic bacteria, determined by flow cytometric viability assays. Appl Environ Microbiol. 2006;72:5132–5134. doi: 10.1128/AEM.02897-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y, Fu L, Li R, Wang LN, Yang Y, Liu NN, Zhang CM, Wang Y, Liu P, Tu BB, Zhang X, Qiao J. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: plasma metabolomics analysis. BMC Med. 2012;10:153. doi: 10.1186/1741-7015-10-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglas CC, Gower BA, Darnell BE, Ovalle F, Oster RA, Azziz R. Role of diet in the treatment of polycystic ovary syndrome. Fertil Steril. 2006;85:679–688. doi: 10.1016/j.fertnstert.2005.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Várnagy A, Bene J, Sulyok E, Kovács GL, Bódis J, Melegh B. Acylcarnitine esters profiling of serum and follicular fluid in patients undergoing in vitro fertilization. Reprod Biol Endocrinol. 2013;11:67. doi: 10.1186/1477-7827-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atroshi F, Rizzo A, Westermarck T, Ali-vehmas T. Effects of tamoxifen, melatonin, coenzyme Q10, and l-carnitine supplementation on bacterial growth in the presence of mycotoxins. Pharmacol Res. 1998;38:289–295. doi: 10.1006/phrs.1998.0363. [DOI] [PubMed] [Google Scholar]
- 22.Barbonetti A, Cinque B, Vassallo MR, Mineo S, Francavilla S, Cifone MG, Francavilla F. Effect of vaginal probiotic lactobacilli on in vitro-induced sperm lipid peroxidation and its impact on sperm motility and viability. Fertil Steril. 2011;95:2485–2488. doi: 10.1016/j.fertnstert.2011.03.066. [DOI] [PubMed] [Google Scholar]
- 23.Yudin MH. Bacterial vaginosis in pregnancy: diagnosis, screening, and management. Clin Perinatol. 2005;32:617–627. doi: 10.1016/j.clp.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Eckert LO, Moore DE, Patton DL, Agnew KJ, Eschenbach DA. Relationship of vaginal bacteria and inflammation with conception and early pregnancy loss following in-vitro fertilization. Infect Dis Obstet Gynecol. 2003;11:11–17. doi: 10.1155/S1064744903000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Oostrum N, De Sutter P, Meys J, Verstraelen H. Risks associated with bacterial vaginosis in infertility patients: a systematic review and meta-analysis. Hum Reprod. 2013;28:1809–1815. doi: 10.1093/humrep/det096. [DOI] [PubMed] [Google Scholar]
- 26.Salah RM, Allam AM, Magdy AM, Mohamed ASH. Bacterial vaginosis and infertility: cause or association? Eur J Obstet Gynecol Reprod Biol. 2013;167:59–63. doi: 10.1016/j.ejogrb.2012.10.031. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Hassan SS, Gajer P, Tarca AL, Fadrosh DW, Bieda J, Chaemsaithong P, Miranda J, Chaiworapongsa T, Ravel J. The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome. 2014;2:18. doi: 10.1186/2049-2618-2-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brocklehurst P, Gordon A, Heatley E, Milan SJ. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev. 2013;1:CD000262. doi: 10.1002/14651858.CD000262.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliver RS, Lamont RF. Infection and antibiotics in the aetiology, prediction and prevention of preterm birth. J Obstet Gynaecol. 2013;33:768–775. doi: 10.3109/01443615.2013.842963. [DOI] [PubMed] [Google Scholar]
- 30.Jones HE, Harris KA, Azizia M, Bank L, Carpenter B, Hartley JC, Klein N, Peebles D. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS ONE. 2009;4:e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra65. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benítez-Páez A, Álvarez M, Belda-Ferre P, Rubido S, Mira A, Tomás I. Detection of transient bacteraemia following dental extractions by 16S rDNA pyrosequencing: a pilot study. PLoS ONE. 2013;8:e57782. doi: 10.1371/journal.pone.0057782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cowling P, McCoy DR, Marshall RJ, Padfield CJH, Reeves DS. Bacterial colonization of the non-pregnant uterus: a study of pre-menopausal abdominal hysterectomy specimens. Eur J Clin Microbiol Infect Dis. 1991;11:204–205. doi: 10.1007/BF01967084. [DOI] [PubMed] [Google Scholar]
- 34.Hillier SL, Martius J, Krohn M, Kiviat N, Holmes KK, Eschenbach DA. A case–control study of the chorioamnionic infection and histologic chorioamnionitis in prematurity. N Engl J Med. 1988;319:972–978. doi: 10.1056/NEJM198810133191503. [DOI] [PubMed] [Google Scholar]
- 35.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, Reynolds PR, Feldman RG, Sullivan MH. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57:404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 36.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, Roehl KA, Nelson DM, Macones GA, Mysorekar IU. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 208:226. doi: 10.1016/j.ajog.2013.01.018. [Internet]. 2013/01/22 ed. Elsevier Inc.; 2013 Mar [cited 2014 January 2];.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, Blundell P, Alan Harris R, Frias AE, Grove KL, Aagaard KM. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun. 2014;5:3889. doi: 10.1038/ncomms4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C, Narbad A, Olivares M, Xaus J, Rodríguez JM. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51:270–274. doi: 10.1007/s00284-005-0020-3. [DOI] [PubMed] [Google Scholar]
- 39.Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, Segura-Roggero I, Schiffrin EJ, Donnet-Hughes A. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007;119:e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 40.Arvidsson C, Hallen A, Backhed F. Generating and analyzing germ-free mice. Curr Protoc Mouse Biol. 2012;2:307–316. doi: 10.1002/9780470942390.mo120064. [DOI] [PubMed] [Google Scholar]
- 41.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 42.Falk PG, Hooper LV, Midtvedt T, Gordon JI. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol Mol Biol Rev. 1998;62:1157–1170. doi: 10.1128/mmbr.62.4.1157-1170.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsythe P, Kunze WA, Bienenstock J. On communication between gut microbes and the brain. Curr Opin Gastroenterol. 2012;28:557–562. doi: 10.1097/MOG.0b013e3283572ffa. [DOI] [PubMed] [Google Scholar]
- 45.Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Satokari R, Grönroos T, Laitinen K, Salminen S, Isolauri E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett Appl Microbiol. 2009;48:8–12. doi: 10.1111/j.1472-765X.2008.02475.x. [DOI] [PubMed] [Google Scholar]
- 47.Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Bäckhed HK, Gonzalez A, Werner JJ, Angenent LT, Knight R, Bäckhed F, Isolauri E, Salminen S, Ley RE. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell. 2012;150:470–480. doi: 10.1016/j.cell.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, Hassan SS, Yeo L, Yoon BH, Mor G, Barzon L, Franchin E, Militello V, Palù G. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:2002–2013. doi: 10.3109/14767058.2012.683899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes A, Haynes M, Hanson N, Angly FE, Heath AC, Rohwer F, Gordon JI. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–338. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breitbart M, Hewson I, Felts B, Mahaffy JM, Nulton J, Salamon P, Rohwer F. Metagenomic analyses of an uncultured viral community from human feces. J Bacteriol. 2003;185:6220–6223. doi: 10.1128/JB.185.20.6220-6223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chuong EB. Retroviruses facilitate the rapid evolution of the mammalian placenta. BioEssays. 2013;35:853–861. doi: 10.1002/bies.201300059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machón RA, Huttunen MO, Mednick SA, Sinivuo J, Tanskanen A, Bunn Watson J, Henriksson M, Pyhälä R. Adult schizotypal personality characteristics and prenatal influenza in a Finnish birth cohort. Schizophr Res. 2002;54:7–16. doi: 10.1016/s0920-9964(01)00346-2. [DOI] [PubMed] [Google Scholar]
- 55.Fettke F, Schumacher A, Costa SD, Zenclussen AC. B cells: the old new players in reproductive immunology. Front Immunol. 2014;5:285. doi: 10.3389/fimmu.2014.00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang TT, Chaturvedi V, Ertelt JM, Kinder JM, Clark DR, Valent AM, Xin L, Way SS. Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol. 2014;192:4949–4956. doi: 10.4049/jimmunol.1400498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teissier N, Fallet-Bianco C, Delezoide AL, Laquerrière A, Marcorelles P, Khung-Savatovsky S, Nardelli J, Cipriani S, Csaba Z, Picone O, Golden JA, Van Den Abbeele T, Gressens P, Adle-Biassette H. Cytomegalovirus-induced brain malformations in fetuses. J Neuropathol Exp Neurol. 2014;73:143–158. doi: 10.1097/NEN.0000000000000038. [DOI] [PubMed] [Google Scholar]
- 58.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146:R151–R162. doi: 10.1530/REP-13-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elmadfa I, Meyer AL. Vitamins for the first 1000 days: preparing for life. Int J Vitam Nutr Res. 2012;82:342–347. doi: 10.1024/0300-9831/a000129. [DOI] [PubMed] [Google Scholar]
- 60.LeBlanc JG, Laiño JE, del Valle MJ, Vannini V, van Sinderen D, Taranto MP, de Valdez GF, de Giori GS, Sesma F. B-group vitamin production by lactic acid bacteria–current knowledge and potential applications. J Appl Microbiol. 2011;111:1297–1309. doi: 10.1111/j.1365-2672.2011.05157.x. [DOI] [PubMed] [Google Scholar]
- 61.Kalhan SC, Marczewski SE. Methionine, homocysteine, one carbon metabolism and fetal growth. Rev Endocr Metab Disord. 2012;13:109–119. doi: 10.1007/s11154-012-9215-7. [DOI] [PubMed] [Google Scholar]
- 62.Mitsuhashi S. Current topics in the biotechnological production of essential amino acids, functional amino acids, and dipeptides. Curr Opin Biotechnol. 2014;26:38–44. doi: 10.1016/j.copbio.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 63.Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BD, Barratt MJ, VanArendonk LG, Zhang Q, Province MA, Petri WA, Jr, Ahmed T, Gordon JI. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;509:417–421. doi: 10.1038/nature13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bendiks M, Kopp MV. The relationship between advances in understanding the microbiome and the maturing hygiene hypothesis. Curr Allergy Asthma Rep. 2013;13:487–494. doi: 10.1007/s11882-013-0382-8. [DOI] [PubMed] [Google Scholar]
- 65.Bertelsen RJ, Brantsæter AL, Magnus MC, Haugen M, Myhre R, Jacobsson B, Longnecker MP, Meltzer HM, London SJ. Probiotic milk consumption in pregnancy and infancy and subsequent childhood allergic diseases. J Allergy Clin Immunol. 2014;133:165–171. doi: 10.1016/j.jaci.2013.07.032. .e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rautava S, Kainonen E, Salminen S, Isolauri E. Maternal probiotic supplementation during pregnancy and breast-feeding reduces the risk of eczema in the infant. J Allergy Clin Immunol. 2012;130:1355–1360. doi: 10.1016/j.jaci.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 67.Jensen MP, Meldrum S, Taylor AL, Dunstan JA, Prescott SL. Early probiotic supplementation for allergy prevention: long-term outcomes. J Allergy Clin Immunol. 2012;130:1209–1211. doi: 10.1016/j.jaci.2012.07.018. .e5. [DOI] [PubMed] [Google Scholar]
- 68.Loo EX, Llanora GV, Lu Q, Aw MM, Lee BW, Shek LP. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: a 5-year follow-up. Int Arch Allergy Immunol. 2014;163:25–28. doi: 10.1159/000356338. [DOI] [PubMed] [Google Scholar]
- 69.Azad MB, Coneys JG, Kozyrskyj AL, Field CJ, Ramsey CD, Becker AB, Friesen C, Abou-Setta AM, Zarychanski R. Probiotic supplementation during pregnancy or infancy for the prevention of asthma and wheeze: systematic review and meta-analysis. BMJ. 2013;347:f6471. doi: 10.1136/bmj.f6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lyall K, Munger KL, O'Reilly ÉJ, Santangelo SL, Ascherio A. Maternal dietary fat intake in association with autism spectrum disorders. Am J Epidemiol. 2013;178:209–220. doi: 10.1093/aje/kws433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iuliano L, Pacelli A, Ciacciarelli M, Zerbinati C, Fagioli S, Piras F, Orfei MD, Bossù P, Pazzelli F, Serviddio G, Caltagirone C, Spalletta G. Plasma fatty acid lipidomics in amnestic mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2013;36:545–553. doi: 10.3233/JAD-122224. [DOI] [PubMed] [Google Scholar]
- 72.Barrett E, Fitzgerald P, Dinan TG, Cryan JF, Ross RP, Quigley EM, Shanahan F, Kiely B, Fitzgerald GF, O'Toole PW, Stanton C. Bifidobacterium breve with α-linolenic acid and linoleic acid alters fatty acid metabolism in the maternal separation model of irritable bowel syndrome. PLoS ONE. 2012;7:e48159. doi: 10.1371/journal.pone.0048159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Shea EF, Cotter PD, Stanton C, Ross RP, Hill C. Production of bioactive substances by intestinal bacteria as a basis for explaining probiotic mechanisms: bacteriocins and conjugated linoleic acid. Int J Food Microbiol. 2012;152:189–205. doi: 10.1016/j.ijfoodmicro.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 74.Gan XT, Ettinger G, Huang CX, Burton JP, Haist JV, Rajapurohitam V, Sidaway JE, Martin G, Gloor GB, Swann JR, Reid G, Karmazyn M. Probiotic administration attenuates myocardial hypertrophy and heart failure following myocardial infarction in the rat. Circ Heart Fail. 2014;7:491–499. doi: 10.1161/CIRCHEARTFAILURE.113.000978. [DOI] [PubMed] [Google Scholar]
- 75.Monachese M, Burton JP, Reid G. Bioremediation and human tolerance to heavy metals through microbial processes: a potential role for probiotics? Appl Environ Microbiol. 2012;78:6397–6404. doi: 10.1128/AEM.01665-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jeurink PV, van Bergenhenegouwen J, Jiménez E, Knippels LM, Fernández L, Garssen J, Knol J, Rodríguez JM, Martín R. Human milk: a source of more life than we imagine. Benef Microbes. 2013;4:17–30. doi: 10.3920/BM2012.0040. [DOI] [PubMed] [Google Scholar]
- 77.Urbaniak C, Burton JP, Reid G. Breast, milk and microbes: a complex relationship that doesn't end with cessation of lactation. Womens Health (Lond Engl) 2012;8:385–398. doi: 10.2217/whe.12.23. [DOI] [PubMed] [Google Scholar]
- 78.Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013;69:1–10. doi: 10.1016/j.phrs.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 79.Langa S, Maldonado-Barragán A, Delgado S, Martín R, Martín V, Jiménez E, Ruíz-Barba JL, Mayo B, Connor RI, Suárez JE, Rodríguez JM. Characterization of Lactobacillus salivarius CECT 5713, a strain isolated from human milk: from genotype to phenotype. Appl Microbiol Biotechnol. 2012;94:1279–1287. doi: 10.1007/s00253-012-4032-1. [DOI] [PubMed] [Google Scholar]
- 80.Cadieux P, Burton J, Gardiner G, Braunstein I, Bruce AW, Kang CY, Reid G. Lactobacillus strains and vaginal ecology. JAMA. 2002;287:1940–1941. doi: 10.1001/jama.287.15.1940. [DOI] [PubMed] [Google Scholar]
- 81.Petrova MI, van den Broek M, Balzarini J, Vanderleyden J, Lebeer S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol Rev. 2013;37:762–792. doi: 10.1111/1574-6976.12029. [DOI] [PubMed] [Google Scholar]
- 82.Hahn BL, Sohnle PG. Direct translocation of staphylococci from the skin surface to deep organs. Microb Pathog. 2013;63:24–29. doi: 10.1016/j.micpath.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jahanfar S, Ng CJ, Teng CL. Antibiotics for mastitis in breastfeeding women. Cochrane Database Syst Rev. 2013;2:CD005458. doi: 10.1002/14651858.CD005458.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arroyo R, Martín V, Maldonado A, Jiménez E, Fernández L, Rodríguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of lactobacilli isolated from breast milk. Clin Infect Dis. 2010;50:1551–1558. doi: 10.1086/652763. [DOI] [PubMed] [Google Scholar]
- 85.Watts JL. Etiological agents of bovine mastitis. Vet Microbiol. 1988;16:41–46. doi: 10.1016/0378-1135(88)90126-5. [DOI] [PubMed] [Google Scholar]
- 86.Crispie F, Flynn J, Ross RP, Hill C, Meaney WJ. Dry cow therapy with a non-antibiotic intramammary teat seal – a review. Ir Vet J. 2004;57:412–418. doi: 10.1186/2046-0481-57-7-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ryan MP, Flynn J, Hill C, Ross RP, Meaney WJ. The natural food grade inhibitor, lacticin 3147, reduced the incidence of mastitis after experimental challenge with Streptococcus dysgalactiae in nonlactating dairy cows. J Dairy Sci. 1999;82:2108–2114. doi: 10.3168/jds.S0022-0302(99)75453-6. [DOI] [PubMed] [Google Scholar]
- 88.Crispie F, Twomey D, Flynn J, Hill C, Ross P, Meaney W. The lantibiotic lacticin 3147 produced in a milk-based medium improves the efficacy of a bismuth-based teat seal in cattle deliberately infected with Staphylococcus aureus. J Dairy Res. 2005;72:159–167. doi: 10.1017/s0022029905000816. [DOI] [PubMed] [Google Scholar]
- 89.Beecher C, Daly M, Berry DP, Klostermann K, Flynn J, Meaney W, Hill C, McCarthy TV, Ross RP, Giblin L. Administration of a live culture of Lactococcus lactis DPC 3147 into the bovine mammary gland stimulates the local host immune response, particularly IL-1beta and IL-8 gene expression. J Dairy Res. 2009;76:340–348. doi: 10.1017/S0022029909004154. [DOI] [PubMed] [Google Scholar]
- 90.Fernández L, Delgado S, Herrero H, Maldonado A, Rodríguez JM. The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J Hum Lact. 2008;24:311–316. doi: 10.1177/0890334408317435. [DOI] [PubMed] [Google Scholar]
- 91.Reid G, Younes JA, Van der Mei HC, Gloor GB, Knight R, Busscher HJ. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol. 2011;9:27–38. doi: 10.1038/nrmicro2473. [DOI] [PubMed] [Google Scholar]
- 92.Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013;15:337. doi: 10.1007/s11894-013-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hatziioanou D, Mayer MJ, Duncan SH, Flint HJ, Narbad A. A representative of the dominant human colonic Firmicutes, Roseburia faecis M72/1, forms a novel bacteriocin-like substance. Anaerobe. 2013;23:5–8. doi: 10.1016/j.anaerobe.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 94.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, Heath AC, Warner B, Reeder J, Kuczynski J, Caporaso JG, Lozupone CA, Lauber C, Clemente JC, Knights D, Knight R, Gordon JI. Human gut microbiome viewed across age and geography. Nature. 2012;486:222. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB, Reid G. Deep sequencing of the vaginal microbiota of women with HIV. PLoS ONE. 2010;5:e12078. doi: 10.1371/journal.pone.0012078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schnorr SL, Candela M, Rampelli S, Centanni M, Consolandi C, Basaglia G, Turroni S, Biagi E, Peano C, Severgnini M, Fiori J, Gotti R, De Bellis G, Luiselli D, Brigidi P, Mabulla A, Marlowe F, Henry AG, Crittenden AN. Gut microbiome of the Hadza hunter-gatherers. Nat Commun. 2014;5:3654. doi: 10.1038/ncomms4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hill K, Hurtado AM, Walker RS. High adult mortality among Hiwi hunter-gatherers: implications for human evolution. J Hum Evol. 2007;52:443–454. doi: 10.1016/j.jhevol.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 99.de Vrieze J. Gut instinct. Science. 2014;343:241–243. doi: 10.1126/science.343.6168.241. [DOI] [PubMed] [Google Scholar]
- 100.Rautava S, Collado MC, Salminen S, Isolauri E. Probiotics modulate host–microbe interaction in the placenta and fetal gut: a randomized, double-blind, placebo-controlled trial. Neonatology. 2012;102:178–184. doi: 10.1159/000339182. [DOI] [PubMed] [Google Scholar]
- 101.Funkhouser LJ, Bordenstein SR. Mom knows best: the universality of maternal microbial transmission. PLoS Biol. 2013;11:e1001631. doi: 10.1371/journal.pbio.1001631. [DOI] [PMC free article] [PubMed] [Google Scholar]


