Abstract
Purpose
Abuse of opioid analgesics for their psychoactive effects is associated with a large number of fatalities. The effect of making opioid tablets harder to crush/dissolve on opioid-related fatalities has not been assessed. The objective of this study was to assess the impact of introducing extended-release oxycodone (ERO [OxyContin®]) tablets containing physicochemical barriers to crushing/dissolving (reformulated ERO) on deaths reported to the manufacturer.
Methods
All spontaneous adverse event reports of death in the US reported to the manufacturer between 3Q2009 and 3Q2013 involving ERO were used. The mean numbers of deaths/quarter in the 3 years after reformulated ERO introduction were compared with the year before. Changes in the slope of trends in deaths were assessed using spline regression. Comparison groups consisted of non-fatal reports involving ERO and fatality reports involving ER morphine.
Results
Reports of death decreased 82% (95% CI: −89, −73) from the year before to the third year after (131 to 23 deaths per year) reformulation; overdose death reports decreased 87% (95% CI: −93, −78) and overdose deaths with mention of abuse-related behavior decreased 86% (95% CI:−92, −75). In contrast, non-fatal ERO reports did not decrease post-reformulation, and reported ER morphine fatalities remained unchanged. The ratio of ERO fatalities to all oxycodone fatalities decreased from 21% to 8% in the year pre-reformulation to the second year post-reformulation.
Conclusions
These findings, when considered in the context of previously published studies using other surveillance systems, suggest that the abuse-deterrent characteristics of reformulated ERO have decreased the fatalities associated with its misuse/abuse. © 2014 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons, Ltd.
Keywords: OxyContin, extended-release oxycodone, abuse-deterrent, overdose death, pharmacovigilance, pharmacoepidemiology
INTRODUCTION
Opioid analgesics are recommended for the treatment of serious, persistent pain after non-pharmacologic therapies and non-opioid medications have been used.1–5 Extended-release (ER) and immediate-release (IR) opioid analgesics are dispensed to over 4 million and 56 million patients in the USA per year, respectively (IMS health). However, over the last decade, prescription opioid abuse (for psychoactive effects) has increased greatly, resulting in increased deaths and burden to public health.6–9 The addiction potential and sequelae of abuse increase exponentially when tablets are crushed/dissolved for non-oral administration (e.g., snorting, injecting, and smoking) to obtain rapid absorption of the opioid experienced as a “high.”10–12 Initial oral abuse often progresses to non-oral abuse by the time of admission to a substance abuse treatment facility.11,13–15
Scientific innovation of abuse-deterrent formulations to promote safe prescription opioid use is a focus of research and development among pharmaceutical companies.16,17 Pharmacological approaches to incorporate characteristics designed to deter abuse have included: (i) adding an opioid antagonist (e.g., buprenorphine and naloxone [Suboxone®], ER morphine and sequestered naltrexone [Embeda®], as well as pentazocine and naloxone [Talwin® NX]); (ii) adding agents that induce unpleasant symptoms with excessive intake (e.g., IR oxycodone and aversive agent [Oxecta®]); and (iii) incorporating physicochemical barriers intended to confer resistance to tablet tampering (e.g., ER oxycodone [OxyContin®], ER oxymorphone [Opana® ER], and ER tapentadol [Nucynta® ER]).
OxyContin (ERO) is ER-formulated oxycodone approved in the USA in 1995 for the treatment of moderate-to-severe chronic pain,18 which has been widely abused,19–21 especially by snorting/injecting, requiring tablet crushing/dissolving.22–25 In April 2010, the Food and Drug Administration (FDA) approved a reformulated ERO containing physicochemical barriers to breaking, crushing/dissolving to deter abuse, which remains the only available ER form of oxycodone available in the USA. Pre-approval studies demonstrated that reformulated ERO is bioequivalent to, is more difficult to extract oxycodone from, and is less liked by abusers than the original formulation.26–28 All shipments of original ERO to wholesalers stopped on 5 August, and shipments of reformulated ERO started on 9 August 2010. The transition to the reformulation was conducted without notification of the general public. Post-marketing studies have demonstrated a reduction in reported ERO abuse in drug treatment center populations and calls to poison centers.29–32 In April 2013, ERO received FDA-approved labeling, indicating that it is expected to be abuse-deterrent via intranasal and intravenous routes of administration.33 However, the effects on fatality have not been reported.
This report focuses on the impact of reformulated ERO on reports of US fatalities submitted to the manufacturer's pharmacovigilance database. Mortality databases, such as the National Death Index and state medical examiner databases were not used because they do not differentiate between IR and ER oxycodone, and only 5% of patients prescribed oxycodone in the USA received ER oxycodone (IMS Health). However, the ratio of deaths associated with ERO reported to the manufacturer versus all oxycodone deaths reported to the FDA's Adverse Event Reporting System (AERS) was assessed to provide additional context to the findings.
METHODS
Manufacturers receive, archive, and submit spontaneous reports of adverse events on marketed drugs to national drug-regulatory authorities, such as the FDA in the USA.34,35 Searching the manufacturer's adverse event reporting database identified all reports of fatal events originating in the USA involving ERO from 3Q2009–3Q2013. Individual case report narrative descriptions were reviewed and categorized as mentioning an opioid overdose-related event and/or drug abuse-related behavior using criteria developed a priori (Table 1). This review was conducted by the two primary authors with any disagreements resolved by consensus.
Table 1.
Criteria to identify overdose-related event and abuse-related behavior mentions
| Overdose-related event | • Reporter described event using verbatim term “overdose” or a medically related term (e.g., drug poisoning, polydrug toxicity, drug intoxication, and overmedicated); or |
| • Circumstances surrounding death suggest an overdose-related event (e.g., ingested many pills, dosing mistake, tampering/snorting/injection of drug, and drug obtained and ingested at a party); or | |
| • Coroner or physician deemed fatality was associated with opioid overdose or polydrug overdose (with or without toxicology evidence of oxycodone or opioid ingestion). | |
| Abuse-related behavior | • Subject currently or previously manipulated extended-release oxycodone with intention of abuse (e.g., crushed and snorted, dissolved and injected); or |
| • Extended-release oxycodone was not prescribed to the subject and/or subject was obtaining drug via unlawful transfer (e.g., stolen, at a party, from parents supply, and from the street); or | |
| • Subject was obtaining extended-release oxycodone prescriptions from a pill mill, multiple healthcare providers, and/or multiple pharmacies; or | |
| • Reporter states subject has history of addiction disorder and/or drug rehabilitation or indicates that subject is currently addicted; or | |
| • Reporter states subject had been using illicit drugs or alcohol in combination with extended-release oxycodone (e.g., heroin, cocaine, marijuana, and amphetamines), or | |
| • There was evidence of subject exposure to a benzodiazepine, an opioid other than oxycodone, and/or muscle relaxant/hypnotic in absence of mention of prescription. For this purpose, an exposure was defined as: the individual was observed taking drug, or reported to have taken the drug, or drug was revealed in toxicological results. |
The analysis focused on spontaneous fatality reports that included month/year of death, as time trends in mortality cannot be ascertained where this information is unknown and the date when the report was received by the manufacturer does not necessarily correlate with the date of death. Reports associated with post-marketing studies (including the manufacturer's individual patient assistance program), litigation (because these were not spontaneous reports), and those lacking a core reporting element (patient, reporter, suspect product, or adverse event) were excluded. All reports containing month/year of death from 3Q2009–2Q2013 were analyzed using SAS v9.2 (SAS Institute, Inc, Cary, NC).
Fatalities were divided into four periods corresponding to 1 year pre-reformulation (3Q2009–2Q2010) and the first (3Q2010–2Q2011), second (3Q2011–2Q2012), and third (3Q2012–2Q2013) year post-reformulation. The mean fatalities per quarter and changes in the slope of trends in fatalities were calculated by spline regression using a Poisson model with the inflection point corresponding to the time of ERO reformulation.36,37
Several sensitivity analyses were conducted to assess the robustness of the results. To assess the impact of prescription changes on fatalities, counts were adjusted for 100,000 ERO prescriptions dispensed (IMS National Prescription Audit database system).38 Because reporting accuracy varies by source, cases reported by healthcare professionals were analyzed separately. To assess the impact of intentional harm, fatalities excluding suicide/homicide were analyzed separately. The impact of cases without date of death was assessed by combining all cases and using report receipt date as a proxy for date of death. To assess the impact of cases containing missing or nonspecific formulation information, fatality changes were calculated for cases in which the reporter mentioned brand name “OxyContin.” A sensitivity analysis assessed the impact of delayed reporting by removing cases that were reported more than 3 months (or 6 months) after each quarter in the study period.
The ratio of deaths associated with ERO reported to the manufacturer versus all oxycodone deaths reported to the FDA's AERS system (data available through 4Q2012) was calculated. Date of death is not included in the FDA AERS data because of privacy regulations; therefore, report receipt date by the FDA was used in the analysis.
RESULTS
Population characteristics
A total of 326 unique fatalities involving ERO, originating in the USA, were spontaneously reported to the manufacturer with a month/year of death from 3Q2009–2Q2013 (Table 2). Overdose was mentioned in 240 reports, and abuse-related behavior was mentioned in 206 reports. Reports involving fatal overdoses were most frequently received from a healthcare professional, more frequently involving an adult (age 18–64 years) and often involving polysubstance use.
Table 2.
Characteristics of extended-release oxycodone fatality reports received by manufacturer with date of death during 1-year period before and 3-year period after introduction of reformulated extended-release oxycodone
| All fatal cases (N = 326) | Subset of fatal cases of overdose (N = 240) | |||
|---|---|---|---|---|
| Pre-reformulation (3Q2009–2Q2010) | Post-reformulation (3Q2010–2Q2013) | Pre-reformulation (3Q2009–2Q2010) | Post-reformulation (3Q2010–2Q2013) | |
| Fatality reports | ||||
| Total (N) | 131 | 195 | 104 | 136 |
| Gender | ||||
| Male | 63% | 66% | 65% | 68% |
| Female | 37% | 33% | 35% | 32% |
| Unknown | 0% | 1% | 0% | 1% |
| Age distribution | ||||
| <13 years | 2% | 6% | 3% | 7% |
| 13 to <18 years | 5% | 6% | 6% | 9% |
| 18 to <65 years | 69% | 68% | 77% | 71% |
| 65 years or older | 6% | 3% | 3% | 1% |
| Unknown | 18% | 17% | 12% | 13% |
| Reporter type | ||||
| Health care professional | 60% | 50% | 61% | 54% |
| Other | 40% | 50% | 39% | 46% |
| Reporter region | ||||
| Northeast | 17% | 20% | 15% | 18% |
| Midwest | 16% | 19% | 17% | 20% |
| South | 39% | 30% | 40% | 29% |
| West | 18% | 17% | 18% | 18% |
| Not reported (missing) | 10% | 13% | 9% | 14% |
| Oxycodone mention | ||||
| OxyContin | 52% | 52% | 44% | 41% |
| oxycodone NOS* | 48% | 48% | 54% | 57% |
| Other product mentions | ||||
| Alcohol | 15% | 13% | 19% | 19% |
| Benzodiazepine | 34% | 23% | 42% | 33% |
| Opioid | 30% | 18% | 37% | 24% |
| Muscle relaxant/hypnotic | 7% | 8% | 9% | 12% |
| Illicit† | 18% | 16% | 22% | 21% |
| Any of above | 58% | 44% | 73% | 60% |
| Additional findings | ||||
| Autopsy reports | 37% | 35% | 43% | 46% |
| Toxicology reports | 44% | 40% | 49% | 46% |
Oxycodone not otherwise specified. Reports involving oxycodone tablets that do not specify formulation (e.g., immediate-release or extended-release formulation) are implied to have involved extended-release oxycodone (OxyContin) because the reporter has taken the time to specifically transmit the information to the manufacturer. During the evaluation period of this study, only extended-release oxycodone was sold by the manufacturer, and no generic extended-release oxycodone product was approved or sold.
Illicit drugs include marijuana, cocaine, amphetamines, and heroin.
Overall, there were no large differences in report characteristics between the pre-reformulation and post-reformulation periods in terms of report source, gender, age, report source/type, and source of toxicology information. However, for fatal overdoses, notable decreases in the proportion of reports from southern regions (40% to 29%) and those with mentions of benzodiazepines (42% to 33%) or other opioids (37% to 24%) were observed.
Decrease in reports of fatalities
There was a reduction in reports of fatalities involving ERO in the post-reformulation periods, particularly for the subset of cases of overdose and overdose with mention of abuse (Figure 1). These reductions began the first year post-reformulation and were more pronounced in subsequent years. Specifically, the mean of all reports of fatalities in the year pre-reformulation was 32.8 per quarter, which decreased by 82% (95% CI: −89% to −73%) to 5.8 reports per quarter in the third year post-reformulation; the mean of fatality reports involving overdose in the year pre-reformulation was 26.0 per quarter, which decreased by 87% (95% CI: −93% to−78%) to 3.3 reports per quarter in the third year post-reformulation; and the mean number of fatality reports involving both overdose and abuse-related behavior in the year pre-reformulation was 23.3 per quarter, decreasing by 86% (95% CI: −92% to −75%) to 3.3 reports per quarter in the third year post-reformulation (Table 3).
Figure 1.
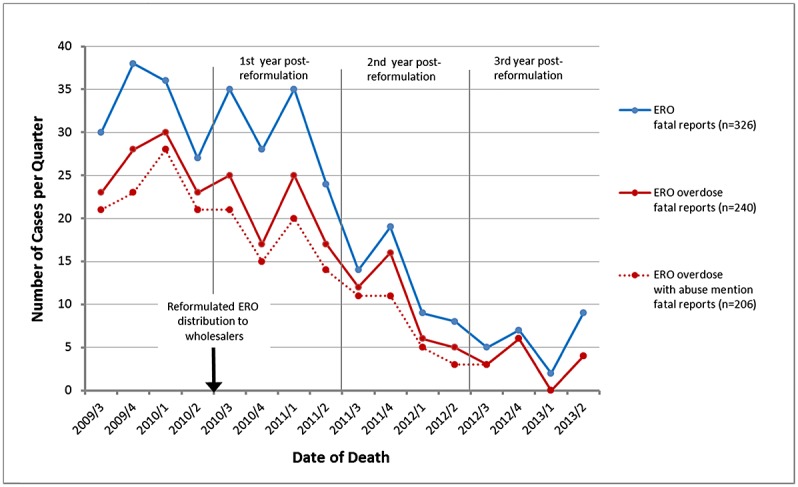
Number of extended-release oxycodone (ERO) fatality reports per quarter. Categories entitled overdose and overdose with mention of abuse-related behavior are defined in methods. Distribution of reformulated ERO to wholesalers was initiated 9 August 2010 (indicated by the arrow).
Table 3.
Changes in the number of extended-release oxycodone fatality reports per quarter received by the manufacturer from 1 year before to 3 years after introduction of reformulated extended-release oxycodone
| 1-year pre-reformulation (3Q2009–2Q2010) | First year post-reformulation (3Q2010–2Q2011) | Second year post-reformulation (3Q2011–2Q2012) | Third year post-reformulation (3Q2012–2Q2013) | ||||
|---|---|---|---|---|---|---|---|
| Mean* | Mean | % change (95%CI) | Mean | % change (95%CI) | Mean | % change (95%CI) | |
| Cases with date of death reported (n = 326) | |||||||
| All fatal reports | |||||||
| All | 32.8 | 30.5 | −7 (−27, 19) | 12.5 | −62 (−72, −47) | 5.8 | −82 (−89, −73) |
| Overdose | 26.0 | 21.0 | −19 (−39, 8) | 9.8 | −62 (−74, −46) | 3.3 | −87 (−93, −78) |
| Abuse-related behavior | 23.3 | 17.5 | −25(−45, 3) | 7.5 | −68 (−79, −51) | 3.3 | −86 (−92, −75) |
| Non-overdose | 6.8 | 9.5 | 41(−14, 130) | 2.8 | −59 (−80, −18) | 2.5 | −63 (−82, −23) |
| All fatal reports, per 100 000 prescriptions of OxyContin† | |||||||
| All | 1.903 | 1.802 | −5 (−26, 21) | 0.794 | −58 (−70, −42) | 0.380 | −80 (−87, −69) |
| Overdose | 1.516 | 1.241 | −18 (−38, 10) | 0.619 | −59 (−72, −41) | 0.213 | −86 (−92, −75) |
| Abuse-related behavior | 1.359 | 1.033 | −23 (−44, 5) | 0.475 | −65 (−77, −47) | 0.213 | −84 (−91, −72) |
| Non-overdose | 0.387 | 0.561 | 43 (−12, 135) | 0.176 | −55 (−78, −10) | 0.167 | −58(−80, −13) |
| Subset of all fatal reports from healthcare professionals | |||||||
| All | 19.8 | 15.8 | −20 (−43, 11) | 6.0 | −70 (−81, −52) | 2.8 | −86 (−93, −74) |
| Overdose | 15.8 | 11.3 | 29 (−51, 5) | 5.0 | −68 (−81, −42) | 2.0 | −87 (−94, −74) |
| Abuse-related behavior | 14.8 | 11.0 | −25 (−50, 10) | 4.0 | −73 (−84, −53) | 2.0 | −86 (−94, −72) |
| Non-overdose | 4.0 | 4.5 | 12 (−43, 121) | 1.0 | −75 (−92, −25) | 0.8 | −81(−95, −36) |
| Subset of all fatal reports mentioning brand name “OxyContin” | |||||||
| All | 17.0 | 15.8 | −7 (−34, 31) | 6.5 | −62 (−76, −40) | 3.0 | −82 (−90, −67) |
| Overdose | 12.0 | 9.0 | −25 (−51, 16) | 4.3 | −65 (−80, −38) | 1.3 | −90 (−96, −74) |
| Abuse-related behavior | 10.5 | 7.8 | −26 (−54, 17) | 3.3 | −69 (−83, −42) | 1.3 | −88 (−95, −70) |
| Non-overdose | 5 | 6.8 | 35 (−24, 141) | 2.3 | −55 (−80, −1) | 1.8 | −65 (−85, −17) |
| Subset of all fatal reports with data confined to cases received during 3-month period following date of death | |||||||
| All | 11.5 | 18.0 | 57 (8, 127) | 4.0 | −65 (−80, −39) | 4.0 | −65 (−80, −39) |
| Overdose | 9.5 | 10.5 | 11 (−29, 71) | 3.3 | −66 (−82, −36) | 2.0 | −79 (−90, −55) |
| Abuse-related behavior | 9.0 | 8.5 | −6 (−41, 51) | 2.8 | −69 (−84, −40) | 2.0 | −78 (−90, −52) |
| Non-overdose | 2.0 | 7.5 | 275 (72, 718) | 0.8 | −62 (−90, 41) | 2.0 | 0 (−62, 166) |
| Subset of all fatal reports with data confined to cases received during 6-month period following date of death | |||||||
| All | 17.3 | 21.5 | 25 (−9, 71) | 7.3 | −58 (−73, −35) | 5.0 | −71 (−82, −52) |
| Overdose | 13.8 | 13.5 | −2 (−33, 43) | 5.3 | −62 (−77, −37) | 2.5 | −82 (−91, −64) |
| Abuse-related behavior | 12.8 | 11.0 | −14 (−42, 29) | 4.8 | −63 (−78, −37) | 2.5 | −80 (−90, 61) |
| Non-overdose | 3.5 | 8.0 | 129 (22, 328) | 2.0 | −43 (−76, 36) | 2.5 | −29 (−68, −61) |
| Cases with date of death reported (n = 326) and not reported (n = 376) | |||||||
| Fatal reports with date of death + fatal reports without date of death (using manufacturer receipt date as proxy) | |||||||
| All | 55.3 | 59.3 | 7 (−11, 29) | 31.8 | −43 (−54, −29) | 29.3 | −47 (−58, −34) |
| Overdose | 40.3 | 39.8 | −1 (−21, 23) | 22.8 | −43 (−56, −27) | 17.0 | −58 (−68, −44) |
| Abuse-related behavior | 31.8 | 29.0 | −9 (−29, 17) | 16.3 | −49 (−62, −31) | 11.3 | −65 (−75, −50) |
| Non-overdose | 22.3 | 26.5 | 19 (−10, 58) | 11.0 | −51 (−66, −29) | 10.5 | −53 (−67, −32) |
Mean number of fatality cases per quarter with values rounded up to one decimal.
IMS National Prescription Audit database (includes retail, mail order and long-term care pharmacy prescriptions).
Increasing trends in mean quarterly fatality reports were observed in the pre-reformulation year. In contrast, in the post-reformulation years, the slope of the 3-year trend for all ERO fatal reports decreased an average of 15.6% (95% CI: −18.7%, −12.3%) per quarter, representing a change of −20.7% (95% CI: −31.3% to −8.5%) from pre-reformulation to post-reformulation, which was statistically significant (p = 0.0015). Similar statistically significant changes in quarterly slopes were observed for reports of fatalities involving overdose (−22.9%; CI: −34.7 % to −8.9%, p = 0.0022) and of fatalities involving both overdose and abuse (−22.2%; CI: −34.9% to −7%, p = 0.0058). Changes for non-overdose fatalities (e.g., death not otherwise specified, suicide/homicide, and cancer) were not statistically significant (−15.8%; CI: −36.8% to 12.3%, p = 0.2421) but trended down.
Trends in comparators
No substantial change in adverse event case handling or pharmacovigilance procedures were made by the manufacturer during the study period. Non-fatal reports to the manufacturer for ERO were 384 per quarter in the year pre-reformulation compared with 3129, 395, and 294 per quarter in the first, second, and third year post-reformulation, respectively. These comparator results suggest that the reductions in fatalities involving ERO post-reformulation were not due to temporal changes in reporting patterns.
A spike in adverse event reports appeared shortly after reformulation, most of which occurred within 3 months of the marketplace transition. A survey of 1967 subjects who reported adverse events at that time indicated that 93% were from individuals who had used ERO for some time and were reporting changes from what they were accustomed to. The transition to the reformulation was conducted without notification of the general public.
Reports of fatalities to the manufacturer for ER morphine (MSContin®) were too few to provide a statistical comparator trend (2.7, 1.5, 2.5, and 2.0 per quarter in the year pre-reformulation, and first, second, and third year post-reformulation, respectively), though there was no substantial decrease.
Fatality reports for extended-release oxycodone versus all oxycodone
The ratio of the numbers of fatalities involving ERO reported to the manufacturer relative to fatalities with any oxycodone categorized as suspect drug reported to FDA decreased significantly (p < 0.0001) from 21% (131/637) in the year pre-reformulation to 22% (122/551), 8% (50/616), and 10% (12/120) in the first, second, and first 6 months of third year post-reformulation, respectively.
Sensitivity analyses
To assess the robustness of the primary results, sensitivity analyses were conducted adjusting for the number of dispensed ERO prescriptions, missing date of death information, reporter type, reporter source, formulation specificity, and reporting time lag (Table 3).
Relative to the pre-reformulation year, the number of ERO prescriptions dispensed in retail, long-term care, and mail-order pharmacies decreased by 2% from 1.72 million to 1.69 million per quarter in the first, by 9% to 1.57 million per quarter in the second, and by 12% to 1.51 million per quarter in the third post-reformulation year (Figure 2). Decreasing trends in fatality counts were detectably, but not substantially, altered when adjusted for ERO prescription numbers. The prescription-adjusted rate of all fatality reports decreased by 80% (95% CI: −87% to −69%) comparing the year pre-reformulation to the third year post-reformulation. Significant decreases in reported fatal overdose cases and fatal overdose cases that also mentioned abuse-related behavior were also observed.
Figure 2.
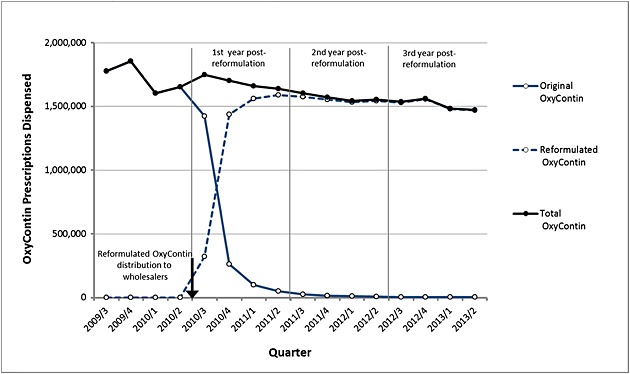
OxyContin prescriptions per quarter. Retail, mail order, and long-term care pharmacy dispensing of OxyContin prescriptions for 3Q2009–2Q2013 extracted from IMS National Prescription Audit database. Distribution of reformulated OxyContin to wholesalers was initiated 9 August 2010 (indicated by the arrow).
Limiting the analysis to reports received from healthcare professionals, confining the analysis to fatality cases where brand name “OxyContin” was mentioned, removing fatality reports with textual mention of suicide or homicide (42 cases), or inclusion of post-marketing studies (82 cases) did not change the results substantially. Imposing a consistent reporting lag period of 3 or 6 months for all quarters across the study period showed similar decreases in fatality reports post-reformulation, suggesting that the observed decreases were not affected by a reporting lag.
During the study period, the manufacturer received 376 fatality cases missing the date of death. In comparison with reports that included this information, these reports, in general, lacked detailed information regarding autopsy findings, toxicology results, patient age, and concomitant drugs. Analysis combining the fatality reports without date of death, using manufacturer report-receipt date as a proxy for date of death, with those reports containing date of death, showed a 47% (95% CI: −58% to −34%) decrease in all fatality reports in the third year post-reformulation. Significant decreases in the number of overdose fatalities, overdose fatalities with mention of abuse-related behavior, and non-overdose fatalities were also observed.
DISCUSSION
The number of spontaneous reports of death involving ERO reported to the manufacturer decreased after introduction of a reformulated ERO that was designed to be abuse-deterrent. As voluntary spontaneous adverse reports do not capture all events, temporal trends in these reports may not be a reliable source for causal interpretation.39–41 However, the large magnitude of the decrease in reported fatalities, while non-fatality adverse events remained unchanged or increased, suggests the decrease was not due to changing processes for reporting/collecting of ERO adverse events. The relative stability of death reports for another opioid product during the same period, though much fewer (because of fewer prescriptions), suggests that there was no systematic process change for reporting or collecting reports of deaths by the manufacturer during this period. Furthermore, analyses conducted to assess whether methodological artifacts might be responsible for the decline showed little impact of potential artifacts, such as changes in prescription numbers, reporter type or source, formulation specificity, missing date of death, and reporting time lag. Therefore, these results suggest a decrease in the number of fatalities associated with ERO abuse/misuse as a result of its reformulation with physicochemical properties that deter crushing/dissolving.
Other studies have reported similar decreases in abuse/misuse of ERO post-reformulation. Cicero.29 reported that in patients with opioid dependence who were entering treatment, the choice of ERO as the primary drug of abuse and use of ERO to get high at least once in the last 30 days, decreased significantly post-reformulation (Survey of Key Informants Patients' Program of the RADARS® System). Butler.30 reported that for individuals assessed for substance abuse treatment, oral and non-oral abuse of reformulated ERO were 17% and 66% lower, respectively, than historic abuse of original ERO (NAVIPPRO Surveillance System), whereas abuse of ER oxymorphone and ER morphine increased or remained relatively unchanged, respectively. Severtson.31 reported that ERO abuse exposure calls to poison centers and reports of diversion of ERO, on a per-catchment area population basis, decreased 38% and 53%, respectively, and that the street price for reformulated ERO was significantly lower than original ERO (Poison Center Study and Drug Diversion Program of RADARS System). Coplan.32 reported that poison center ERO abuse, suicide, therapeutic errors among patients, accidental exposures among children, and adverse reaction exposures decreased significantly post-reformulation but increased or remained steady for other single-entity oxycodone products (National Poison Data System). Havens.42 reported that experienced opioid abusers in a rural Kentucky county self-reported a low frequency of abuse of reformulated ERO while maintaining a consistently high frequency of abuse of IR oxycodone. The Florida Medical Examiners Commission Report showed a decrease in deaths due to all forms of oxycodone, both ER and IR formulations, from a peak of 1516 in 2010 to 735 in 2012.43 However, during this period, a state law was enacted to impose stricter requirements for dispensing of controlled substances targeted specifically at pill mills that were primarily prescribing or dispensing IR oxycodone.50 Nationally, the ratio of fatalities involving ERO versus any oxycodone more than halved (from 21% to 8%) after reformulation, further endorsing the specificity of the effect of the reformulation.
Other researchers have used spontaneous adverse event reports to assess the effects of opioid formulations intended to deter abuse. Post-marketing adverse event reports received for an ER morphine capsule combined with naltrexone (an opioid antagonist) indicated a low number of product tampering reports and no cases of confirmed tampering resulting in fatality.44
There are limitations to causal inference from voluntary spontaneous fatality reports. However, when the results of this study are considered in the context of the five additional published articles and the one state surveillance report that demonstrate an abuse-deterrent impact of reformulated ERO, the Bayesian prior that the observed change in reported fatalities is caused by the reformulation is increased.45
Alternate explanations for the decline in fatality reporting were considered. A reduction in ERO dispensing occurred during the study period. However, combined prescription reductions of original and reformulated ERO (2%, 9%, and 12% in the first, second, and third year post-reformulation, respectively) were insufficient to account for the much larger reduction in fatalities. Dispensing of original ERO gradually decreased in the 18 months after wholesale shipments stopped. Of note, reports received by the manufacturer post-reformulation included exposures to both original and reformulated ERO and therefore a portion of the fatal reports in the post-period likely involved original ERO, which may account for the gradual decrease in fatalities post-reformulation.
Several initiatives to deter opioid abuse/overdose commenced or were ongoing during the study period. The FDA's Risk Evaluation and Mitigation Strategy for ER and long-acting opioids was approved in July 2012.46 However, its primary component is continuing education of prescribers, which began in March 2013, subsequent to the reduction in ERO deaths. State prescription drug monitoring programs were operating or initiated during the study period.47 Preliminary evaluation of these programs indicate a positive impact on opioid abuse/misuse,48 but their effect on fatalities is not clear.49,50 Community-based opioid overdose prevention programs have reduced deaths in the few local regions where implemented but cannot account for large national changes.51,52 Drug disposal programs have been established but have not focused specifically on ERO and have not been shown to impact deaths associated with opioids.53,54 These initiatives may have contributed to decreases in opioid-related fatalities; however, they are unlikely to account for the level of decrease in ERO fatalities. The ratio of fatalities involving ERO versus any oxycodone more than halved after reformulation.
Some authors attributed an increase in heroin abuse to the reformulation of ERO when it was first introduced,29 and this has led to widespread attribution of the cause of the rapidly escalating heroin abuse to ERO in media reports.55 However, currently, 1.7% of individuals dispensed opioid analgesics in the USA receive ERO, and 2.5% of opioid prescriptions dispensed are for ERO. These proportions have remained roughly constant over the past 5 years (IMS Health, unpublished data). Furthermore, approximately 4.3% of diagnosed overdose events in insurance claims databases in 2011 were among people prescribed ERO (MarketScan, unpublished data) and the prevalence of reformulated ERO abuse among prescription opioid abusers in drug treatment centers is 12.1%.22 Therefore, it is unlikely that reduced abuse of ERO, which occurred in a single, abrupt intervention beginning in August 2010, could account for spikes in heroin abuse 3½ years later.
In conclusion, the number of spontaneous reports of fatalities involving ERO has significantly decreased after its reformulation, whereas non-fatal reports involving ERO remained unchanged or increased. These findings, when considered in the context of previously published studies using other surveillance systems, suggest that the abuse-deterrent characteristics of reformulated ERO have decreased fatalities associated with its misuse/abuse. Abuse-deterrent formulations may be a valuable risk management tool, such that innovation, policing, regulation, careful prescribing, and education can be combined to mitigate serious risk and improve the benefit-risk balance of opioid analgesics.56
CONFLICT OF INTEREST
Nelson Sessler, Jerod Downing, Hrishikesh Kale, Howard Chilcoat, Todd Baumgartner, and Paul Coplan are full-time employees of Purdue Pharma L.P.
KEY POINTS
Abuse of prescription opioid analgesics for the psychoactive effects is associated with a large number of fatalities. However, the effect of making opioid tablets harder to crush or dissolve in order to deter abuse on opioid-related fatalities has not been assessed.
The manufacturer's pharmacovigilance database was used to assess changes in fatalities associated with extended-release oxycodone (ERO, OxyContin) after the product was reformulated to be harder to crush or dissolve.
A large decrease in the number of fatality reports associated with ERO occurred following introduction of reformulated OxyContin, especially reports of fatalities involving overdose-related events and involving abuse.
These findings, when considered in the context of previously published studies using other surveillance systems, suggest that the abuse-deterrent characteristics of reformulated ERO have decreased the fatalities associated with its misuse and abuse.
ETHICS STATEMENT
The authors state that no ethical approval was needed.
Acknowledgments
Case review assistance was provided by Barbara Harding. Manuscript preparation assistance was provided by Melinda Philbrook. Writing assistance was provided by Louis Alexander. This study was sponsored by Purdue Pharma L.P.
REFERENCES
- American Academy of Pain Medicine. Use of Opioids for the Treatment of Chronic Pain. Chicago, IL: American Academy of Pain Medicine; 2013. [Google Scholar]
- American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–1346. doi: 10.1111/j.1532-5415.2009.02376.x. . doi. 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- Chou R, Fanciullo GJ, Fine PG. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. . doi:10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management of Opioid Therapy for Chronic Pain Working Group. Washington, DC: Department of Veterans Affairs, Department of Defense; 2010. VA/DoD clinical practice guideline for management of opioid therapy for chronic pain. [Google Scholar]
- National Opioid Use Guideline Group. Hamilton, Canada: National Opioid Use Guideline Group (NOUGG); 2010. Canadian guideline for safe and effective use of opioids for chronic non-cancer pain. Part B: recommendations for practice. [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309:657–659. doi: 10.1001/jama.2013.272. . doi:10.1001/jama.2013.272. [DOI] [PubMed] [Google Scholar]
- Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1983. doi: 10.1056/NEJMp1011512. . doi:10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- White AG, Birnbaum HG, Mareva MN. Direct costs of opioid abuse in an insured population in the United States. J Manag Care Pharm. 2005;11:469–479. doi: 10.18553/jmcp.2005.11.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, White AG, Reynolds JL. Estimated costs of prescription opioid analgesic abuse in the United States in 2001: a societal perspective. Clin J Pain. 2006;22:667–676. doi: 10.1097/01.ajp.0000210915.80417.cf. [DOI] [PubMed] [Google Scholar]
- Raffa RB, Pergolizzi JV. Opioid formulations designed to resist/deter abuse. Drugs. 2010;70:1657–1675. doi: 10.2165/11537940-000000000-00000. . doi:10.2165/11537940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Katz N, Dart RC, Bailey E. Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37:205–217. doi: 10.3109/00952990.2011.569623. . doi:10.3109/00952990.2011.569623. [DOI] [PubMed] [Google Scholar]
- National Drug Intelligence Center. OxyContin diversion and abuse. January 2001. http://www.justice.gov/archive/ndic/pubs/651/abuse.htm [23 February 2012]
- Hays L, Kirsh KL, Passik SD. Seeking drug treatment for OxyContin abuse: a chart review of consecutive admissions to a substance abuse treatment facility in Kentucky. J Natl Compr Canc Netw. 2003;1:423–428. doi: 10.6004/jnccn.2003.0035. [DOI] [PubMed] [Google Scholar]
- Hays LR. A profile of OxyContin addiction. J Addict Dis. 2004;23:1–9. doi: 10.1300/J069v23n04_01. [DOI] [PubMed] [Google Scholar]
- Butler SF, Black RA, Grimes Serrano JM. Characteristics of prescription opioid abusers in treatment: prescription opioid use history, age, use patterns and functional severity. J Opioid Manag. 2010;6:239–252. doi: 10.5055/jom.2010.0022. [DOI] [PubMed] [Google Scholar]
- Romach MB, Schoedel KA, Seller EM. Update on tamper-resistant drug formulations. Drug Alcohol Depend. 2013;130:13–23. doi: 10.1016/j.drugalcdep.2012.12.028. . doi:10.1016/j.drugalcdep.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Moorman-Li R, Motycka CA, Inge LD. A review of abuse-deterrent opioids for chronic nonmalignant pain. PT. 2012;37:412–418. [PMC free article] [PubMed] [Google Scholar]
- OxyContin [full prescribing information]. Stamford, CT: Purdue Pharma L.P. 7/2013.
- Centers for Disease Control and Prevention. CDC grand rounds: prescription drug overdoses—a U.S. Epidemic. Morb Mortal Wkly Rep. 2012;61:10–13. [PubMed] [Google Scholar]
- Cone EJ, Fant RV, Rohay JM. Oxycodone involvement in drug abuse deaths: a DAWN-based classification scheme applied to an oxycodone postmortem database containing over 1000 cases. J Anal Toxicol. 2003;27:57–67. doi: 10.1093/jat/27.2.57. ; discussion 67. [DOI] [PubMed] [Google Scholar]
- Dhalla IA, Mamdani MM, Sivilotti ML. Prescribing of opioid analgesics and related mortality before and after the introduction of long-acting oxycodone. CMAJ. 2009;181:891–896. doi: 10.1503/cmaj.090784. . doi:10.1503/cmaj.090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Black RA, Cassidy TA. Abuse risks and routes of administration of different prescription opioid compounds and formulations. Harm Reduct J. 2011;8:29–45. doi: 10.1186/1477-7517-8-29. . doi:10.1186/1477-7517-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SF, Budman SH, Licari A. National addictions vigilance intervention and prevention program (NAVIPPRO): a real-time, product-specific public health surveillance system for monitoring prescription drug abuse. Pharmacoepidemiol Drug Saf. 2008;17:1142–1154. doi: 10.1002/pds.1659. . doi:10.1002/pds.1659. [DOI] [PubMed] [Google Scholar]
- Katz N, Fernandez K, Chang A. Internet-based survey of nonmedical prescription opioid use in the United States. Clin J Pain. 2008;24:528–535. doi: 10.1097/AJP.0b013e318167a087. . doi:10.1097/AJP.0b013e318167a087. [DOI] [PubMed] [Google Scholar]
- Passik SD, Hays L, Eisner N. Psychiatric and pain characteristics of prescription drug abusers entering drug rehabilitation. J Pain Palliat Care Pharmacother. 2006;20:5–13. [PubMed] [Google Scholar]
- Cone EJ, Giordano J, Weingarten B. An iterative model for in vitro laboratory assessment of tamper deterrent formulations. Drug Alcohol Depend. 2013;131:100–105. doi: 10.1016/j.drugalcdep.2012.12.006. . doi:10.1016/j.drugalcdep.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Perrino PJ, Colucci SV, Harris SC. Attractiveness of reformulated OxyContin(R) tablets: assessing comparative preferences and tampering potential. J Psychopharmacol. 2013;27(9):808–816. doi: 10.1177/0269881113493364. . doi: 10.1177/0269881113493364. [DOI] [PubMed] [Google Scholar]
- Perrino PJ, Colucci SV, Apseloff G. Pharmacokinetics, tolerability, and safety of intranasal administration of reformulated OxyContin® tablets compared with original OxyContin® tablets in healthy adults. Clin Drug Investig. 2013;33:441–449. doi: 10.1007/s40261-013-0085-x. . doi:10.1007/s40261-013-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N Engl J Med. 2012;367:187–189. doi: 10.1056/NEJMc1204141. . doi:10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- Butler SF, Cassidy TA, Chilcoat H. Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain. 2013;14:351–358. doi: 10.1016/j.jpain.2012.08.008. . doi:10.1016/j.jpain.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Severtson SG, Bucher Bartelson B, Davis JM. Reduced abuse, therapeutic errors, and diversion following reformulation of OxyContin® in 2010. J Pain. 2013;14:1122–1130. doi: 10.1016/j.jpain.2013.04.011. . doi:10.1016/j.jpain.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Coplan PM, Kale H, Sandstrom L. Changes in oxycodone and heroin exposures in the national poison data system after introduction of extended-release oxycodone with abuse deterrent characteristics. Pharmacoepidemiol Drug Saf. 2013;22:1274–1282. doi: 10.1002/pds.3522. . doi:10.1002/pds.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug and Administration . 2013b. FDA news release: FDA approves abuse-deterrent labeling for reformulated OxyContin, Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ UCM348252.htm [5 May 2013]
- Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. JAMA. 1993;269:2765–2768. doi: 10.1001/jama.269.21.2765. [DOI] [PubMed] [Google Scholar]
- U.S. Food and Drug and Administration (FDA) Adverse event reporting system (AERS).Rockville, MD: FDA. Available from: http://www.fda.gov/cder/aers/default.htm [28 January 2013]
- Greenland S. Dose response and trend analysis in epidemiology: alternatives to categorical analysis. Epidemiology. 1995;6:356–365. doi: 10.1097/00001648-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd edn. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. p. 410. [Google Scholar]
- IMS health national prescription audit data purchased and received December 2013.
- Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18:57–60. doi: 10.1046/j.1525-1497.2003.20130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons RD, Amatya AK, Brown CH. Post-approval drug safety surveillance. Annu Rev Public Health. 2010;31:419–437. doi: 10.1146/annurev.publhealth.012809.103649. . doi:10.1146/annurev.publhealth.012809.103649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez EM, Staffa A, Graham D. The role of databases in drug post-marketing surveillance. Pharmacoepidemiol Drug Saf. 2001;10:407–410. doi: 10.1002/pds.615. . doi:10.1002/pds.615. [DOI] [PubMed] [Google Scholar]
- Havens JR, Leukefeld CG, DeVeaugh-Geiss AM, Coplan PM, Chilcoat HD. The impact of a reformulation of extended-release oxycodone designed to deter abuse in a sample of prescription opioid abusers. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2014.02.018. [available ahead of print 27 February 2014] doi: 10.1016/j.drugalcdep.2014.02.018. [DOI] [PubMed] [Google Scholar]
- Florida medical examiners commission report for 2012. Available at: http://www.fdle.state.fl.us/Content/getdoc/79241c67-253b-45eb-a238-1a07cf4a2a0c/2012-Drug-Report_Final.aspx [1 October 2013]
- Badalamenti VC, Buckley JW, Smith ET. Safety of EMBEDA (morphine sulfate and naltrexone hydrochloride) extended-release capsules: review of post-marketing adverse events during the first year. J Opioid Manag. 2012;8:115–125. doi: 10.5055/jom.2012.0104. [DOI] [PubMed] [Google Scholar]
- Pearl J. Causality: Models, Reasoning and Inference. Cambridge, United Kingdom: Cambridge University Press; 2009. p. 5. [Google Scholar]
- FDA News Release. FDA introduces new safety measures for extended-release and long-acting opioid medications. July 9, 2012. Available at: http://www.fda.gov/ NewsEvents/ Newsroom/PressAnnouncements/ucm310870.htm [30 March 2013]
- U.S. Department of Justice Drug Enforcement Administration. Prescription drug monitoring programs. Available at: http://www.deadiversion.usdoj.gov/faq/rx_monitor.htm [January 2011]
- Reifler LM, Droz D, Bailey JE. Do prescription monitoring programs impact state trends in opioid abuse/misuse? Pain Med. 2012;13:434–442. doi: 10.1111/j.1526-4637.2012.01327.x. . doi:10.1111/j.1526-4637.2012.01327.x. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12:747–754. doi: 10.1111/j.1526-4637.2011.01062.x. . doi:10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- Sauber-Schatz EK, Mack KA, Diekman ST. Associations between pain clinic density and distributions of opioid pain relievers, drug-related deaths, hospitalizations, emergency department visits, and neonatal abstinence syndrome in Florida. Drug Alcohol Depend. 2013;133:161–166. doi: 10.1016/j.drugalcdep.2013.05.017. . doi:10.1016/j.drugalcdep.2013.05.017. [DOI] [PubMed] [Google Scholar]
- Albert S, Brason FW, Sanford CK. Project Lazarus community-based overdose prevention in rural North Carolina. Pain Med. 2011;12(Suppl 2):S77–S85. doi: 10.1111/j.1526-4637.2011.01128.x. . doi:10.1111/j.1526-4637.2011.01128.x. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Community-based opioid overdose prevention programs providing naloxone—United States, 2010. MMWR Morb Mortal Wkl Rep. 2012;61:101–105. [PMC free article] [PubMed] [Google Scholar]
- National take-back initiative. US Department of Justice, Drug Enforcement Administration, Office of Diversion Control Web site. http://www.deadiversion.usdoj.gov/drug_disposal/takeback/ [11 February 2014]
- Gray JA. Prescription drug abuse and DEA-sanctioned drug take-back events: characteristics and outcomes in rural Appalachia. Arch Intern Med. 2012;172:1186–1187. doi: 10.1001/archinternmed.2012.2374. . doi:10.1001/archinternmed.2012.2374. [DOI] [PubMed] [Google Scholar]
- Silverman G. Heroin abuse: shooting up. Financial Times, February 9, 2014. Available at http://www.ft.com/intl/cms/s/0/d378673a-8fe3-11e3-aee9-00144feab7de.html?siteedition=intl.
- Office of National Control Policy. 2011. Epidemic: responding to America's prescription drug abuse crisis http://www.whitehouse.gov/ondcp/prescription-drug-abuse [30 March 2013]


