Type 1 diabetes (T1D) is considered an autoimmune disease, predominantly mediated by autoreactive T cell responses, which over time leads to the destruction of pancreatic beta cells and insulin deficiency. Symptoms often appear in childhood or adolescence, but the disease can develop in adult patients as well 1. Both therapeutic management and clinical outcomes have dramatically improved during the last few decades, yet T1D remains a major cause of suffering, a burden to society, and its incidence appears to be increasing, especially in younger children 2. Although we can identify individuals at increased risk among patients' relatives, the disease cannot be prevented and there is no definitive cure 3. Pancreas and islet transplantation can restore insulin independence, at least for a period of time, but are not universally available and require chronic immunosuppression 4.
The partial success of human clinical trials aimed at ameliorating islet autoimmunity likely reflects our incomplete knowledge of etiological factors and the limited understanding of the key pathogenic mechanisms that cause the disease 1,3. Our ability to study the disease has been hampered by scarce access to the pancreas and other disease-related tissues. The limited data available from studies of human pancreata from patients have been to a large extent, from pancreata obtained several decades ago that were not studied with modern technologies. Furthermore, they may no longer reflect current disease, particularly as they relate to etiological factors if these vary over time. Our views of the disease pathogenesis have been largely shaped by studies in experimental rodent models of the disease, especially the non-obese diabetic (NOD) mouse 5. Yet critical questions regarding disease pathogenesis are specific to humans and cannot be easily investigated in experimental animals. Among these are: (i) the nature (phenotypically and functionally) of autoreactive T and B cells, which may be therapeutically targeted; (ii) the role of viral infections, which could perhaps be averted by vaccination if responsible viruses were definitively identified; (iii) potential pathways of beta cell regeneration; and (iv) additional hitherto undefined pathogenic mechanisms. Thus, there is a clear need to study human pancreata and related tissues from T1D patients. Such efforts could identify novel therapeutic targets and define possible strategies for combinatorial therapies that target multiple disease pathways, both immune and non-immune related.
In 2007, the JDRF recognized such a need and supported the creation of the JDRF Network for the Pancreatic Organ Donors with Diabetes (JDRF nPOD; www.JDRFnPOD.org). This article describes nPOD's operational model and some recent preliminary and novel findings that have emerged from research studies utilizing nPOD specimens.
The mission of the JDRF nPOD and its operational model
The JDRF nPOD has three main strategic goals:
Obtain specimens from organ donors with T1D (diagnosed or subclinical), and establish a research resource of pancreas and disease relevant tissues (pancreatic lymph nodes, spleen, thymus, blood, and other) from organ donors with T1D, obtained at any point after clinical diagnosis, or during the prediabetes phase, when islet autoimmunity silently leads to beta cell destruction (donors identified by screening for islet autoantibodies).
Distribute specimens to nPOD approved investigators, anywhere in the world, for comprehensive and diversified investigations of human T1D.
Promote collaboration, by using tissue- and real-time data-sharing, and by developing and managing synergistic project interactions as well as focused working groups, all to facilitate a comprehensive understanding of human T1D.
Table1 lists the donors sought for collection by nPOD and types of tissues recovered. Donors with T1D, of any age and disease duration, are first priority, to investigate the human pathology of T1D. nPOD has established autoantibody screening centers to enable Organ Procurement Organizations (OPO) to rapidly screen organ donors who do not have diabetes for the presence of islet autoantibodies to identify those who might have been developing T1D. Currently, laboratories can simultaneously test for three islet autoantibodies to the autoantigens GAD65, IA-2, and ZnT8, using a modified and customized assay kit based on commercial, standardized, enzyme-linked immunosorbent assays (ELISAs) (Kronus, Boise, ID, USA), and determine autoantibody status in approximately 3 h. Donors with type 2 diabetes (T2D) are studied as controls for hyperglycemia, and also for changes that affect beta cells and the pancreas (which may be relevant to T1D). nPOD also accepts donors with gestational diabetes, cystic fibrosis, and non-diabetic donors as controls.
Table 1.
nPOD donor inclusion criteria and specimens recoverable
| Donor groups* |
| T1D (any age and disease duration) |
| AAb positive, non-diabetic (≥1 AAb, <30 years old) |
| Pancreas transplant recipients (with T1D) |
| T2D with/without incretin therapy |
| Normal pregnancy |
| Gestational diabetes |
| Cystic fibrosis-related diabetes, Turner syndrome, Prader–Willi |
| Bariatric surgery |
| Controls |
| Specimens recoverable |
| Pancreas |
| Spleen† |
| Pancreatic lymph nodes† |
| Non-pancreatic lymph nodes† |
| Whole blood† and serum |
| Bone marrow† |
| Thymus† |
| Duodenum |
| Ampulla Vater |
| Skin |
AAb positive, autoantibody-positive; nPOD, Network for Pancreatic Organ Donors with Diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
Criteria last updated on 30 April 2013. Revision history available at http://jdrfnpod.org/sops/donor-criteria.pdf.
Cryopreserved cells available.
The nPOD operational model is illustrated in Fig. 1. In the USA, nPOD works with all OPO (currently 58), tissue banks, and medical examiners to obtain referrals of organ donors who meet its inclusion criteria. The OPOs also play a vital role in the recovery process through providing a description of the program's purpose to family members of potential donors during the emotionally difficult process of obtaining consent for research. A similar infrastructure is being developed in some European countries as part of the nPOD-Europe initiative (Italy, Sardinia, Finland, and Sweden). Recognizing that T1D may not be diagnosed until late [e.g., diabetic ketoacidosis (DKA) presenting to Emergency Departments], nPOD also initiated a strategic partnership with the Pediatric and Critical Care sections of the American College of Emergency Physicians (ACEP); this, to increase awareness of all deaths involving a child with T1D. Pediatric physicians can play a key role in the identification and subsequent referral of these unfortunate cases to the local OPO or directly to nPOD.
Figure 1.
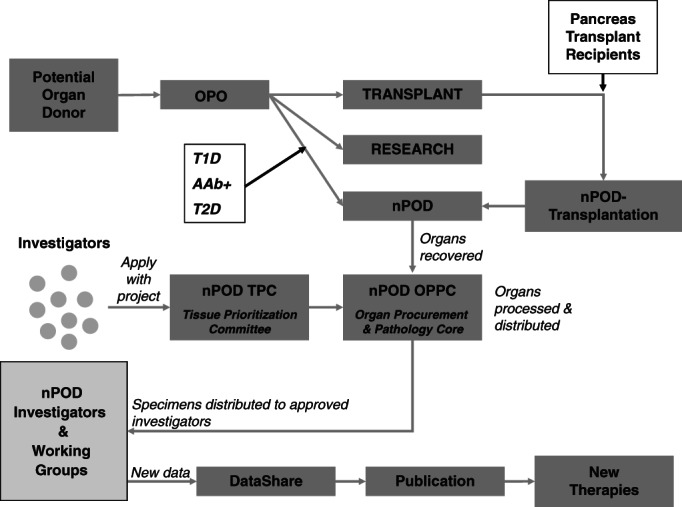
The Network for Pancreatic Organ Donors with Diabetes (nPOD) operational model. The diagram illustrates the current strategy used to obtain organ donors for diabetes research described in the main text.
There are critical ethical considerations associated with organ donation. The ability for nPOD to obtain such tissues, in addition to their provision to investigators, was made possible by the program submitting to a thorough ethical evaluation of its standard operating procedures, affirmation of its ability to protect subject confidentiality and to meet regulatory standards with respect to the ‘chain of custody’ (i.e., in settings of a regulatory audit, to be in a position to provide exact details regarding the disposition of every tissue). Indeed, nPOD repeatedly undergoes such evaluations at an administrative and core laboratory level through evaluation by the University of Florida Institutional Research Board (IRB); moreover, institutional IRBs throughout the world examine the research performed by their investigators on specimens provided by nPOD. Each and every process of review, be it by an OPO or IRB, represents an event of high importance given the need to meet ethical standards. As often noted, the process of organ donation represents a ‘gift’ and as such, both a strong rationale and demonstration of high-performance standards by the organization are key to address a variety of ethical issues (e.g., the use of tissues for research vs. clinical transplantation, obtaining prenatal or tissues from very young individuals, etc.).
nPOD usually accepts organs when processing is possible within 24 h of cold ischemia time (since the organ is cooled with a cold perfusion solution), and in the absence of donor positive tests for serious infectious diseases (HIV, HTLV, VDRL, HbSAg, and anti-HCV). OPOs routinely test donors for multiple infectious diseases as part of their evaluation for potential transplant, and information about other infections is disclosed prior to acceptance of an offer.
Upon acceptance of a donor offer, recovered organs are sent from an OPO partner to the nPOD Organ Procurement & Pathology Core (OPPC) in Gainesville, Florida. Here, tissues are processed according to nPOD's standard operating procedures (SOP) 6–8 (Note: all nPOD SOPs are available at www.JDRFnPOD.org). Routine stains are performed to assess pancreas pathology (insulin, glucagon, CD3, hematoxylin, eosin, etc.). All stained sections can be viewed by investigators using the internet-based Aperio viewer, which effectively works like a microscope. Tissues are recorded and archived before distribution to investigators worldwide.
Like all tissue banks supporting a multitude of studies, nPOD faces the challenge that a variety of donor-specific factors need to be documented and recognized by investigators as potential source of bias in study design and interpretation. Such donor factors include cause of death, concomitant acute or chronic illnesses, including infections, and medications. These apply to the nPOD collection as a whole, but possibly in different ways or variable extent, to the cases that are utilized in any specific investigator-initiated study. As an organ donor tissue bank, nPOD continuously addresses these issues at both levels: (i) by conducting periodic analyses to identify donor factors that may emerge, in general, as potential confounding factors; (ii) by collecting clinical donor information and making it available to investigators in DataShare, de-identified and in full respect of privacy laws, so that investigators can select cases and controls taking into account multiple donor factors. Table2 shows nPOD recoveries, by donor category, as of 31 July 2013. The nPOD resource includes tissues from 70 donors with T1D and from 20 non-diabetic donors with islet autoantibodies. There are now 15 nPOD T1D cases with demonstrated insulitis (Table3), the inflammatory lymphocytic infiltrate of the pancreatic islets that is considered T1D's pathologic hallmark, including donors of pediatric age. This resource has enabled nPOD to develop a statement on insulitis in humans (see Emerging data from nPOD supported studies section, below). nPOD has also recovered organs from rare donors, such as donors who pass away near the time of T1D diagnosis, an occurrence that is thankfully quite rare with improved treatment of DKA and its complications 9,10, and a T1D donor who carried a strongly diabetes-protective human leukocyte antigen (HLA) haplotype typically found in less than 1% of T1D patients.
Table 2.
nPOD recoveries (as of 31 July 2013)
| Donor group | Number | Mean age (years) |
|---|---|---|
| No diabetes | 89 | 21.9 |
| Autoab Pos | 20 | 34.2 |
| T1D | 70 | 28.2 |
| T1D medalist | 12 | 76.3 |
| T2D | 17 | 44.5 |
| T2D with incretin therapy | 9 | 58.4 |
| Gestational diabetes | 3 | 32.6 |
| Pregnancy | 1 | 38.0 |
| Cystic fibrosis | 2 | 31.1 |
| Other | 7 | 28.8 |
| Pending classification | 12 | 31.1 |
| Pancreas transplant with recurrent T1D | 3 | 43.0 |
| Total | 245 |
nPOD, Network for Pancreatic Organ Donors with Diabetes; T1D, type 1 diabetes; T2D, type 2 diabetes.
Table 3.
nPOD donors with insulitis
| Case ID | Donor group | Autoantibodies detected | Age (years) | T1D duration | Gender | Race |
|---|---|---|---|---|---|---|
| 6197 | AAb+ | GAD, IA-2 | 22 | n/a | M | AA |
| 6209 | T1D | IA-2, ZnT8, IAA | 5 | 0.25 | F | C |
| 6052 | T1D | IA-2, IAA† | 12 | 1 | M | AA |
| 6113 | T1D | IAA† | 13.1 | 1 | F | C |
| 6063 | T1D | IAA† | 4.4 | 3 | M | C |
| 6198 | T1D | GAD, IA-2, ZnT8, IAA | 22 | 3 | F | H |
| 6088 | T1D | GAD, IA-2, ZnT8, IAA | 31.2 | 5 | M | C |
| 6195 | T1D | GAD, IA-2, ZnT8, IAA | 19.2 | 5 | M | C |
| 6212 | T1D | IAA | 20 | 5 | M | C |
| 6062 | T1D | No serum available | 10.7 | 6 | M | AA |
| 6070 | T1D | IA-2, IAA | 22.6 | 7 | F | C |
| 6046 | T1D | IA-2, ZnT8 | 18.8 | 8 | F | C |
| 6039 | T1D | GAD, IA-2, ZnT8, IAA | 28.7 | 12 | F | C |
| 6076 | T1D | GAD, IAA | 25.8 | 14 | M | C |
| 6228 | T1D | GAD, IA-2, ZnT8 | 13 | 0 (onset) | M | C |
AA, African American; AAb+, autoantibody-positive; C, Caucasian; H, Hispanic; nPOD, Network for Pancreatic Organ Donors with Diabetes; T1D, type 1 diabetes.
Insulitis was defined as ≥6 CD3-positive T cells adjacent or within islets (n ≥ 3 islets/section) with insulin deficient islets within the pancreas.
IAA, or insulin autoantibodies, if measured after the institution of insulin therapy are likely induced by insulin injections.
Starting in 2012, nPOD initiated a programmatic expansion, called nPOD-transplantation (nPOD-T), to allow patients with T1D who are recipients of pancreas transplants to consent for directed, postmortem donation to the nPOD program (Fig. 1). The rationale for this expansion stems from studies demonstrating that islet autoimmunity can be reactivated in pancreas transplants, despite HLA mismatch and chronic immunosuppression that prevent rejection 11. Moreover, chronic immunosuppression could allow for some beta cell replication to occur in the native pancreas, but this possibility cannot be easily proved or disproved by clinical studies 12; access to native pancreas from immunosuppressed transplant recipients could help address this question. Since its inception, the nPOD-T program has also implemented strategies for recovering both native and transplanted pancreas from transplant recipients, not only after postmortem donation, but also through biopsy of living patients. In doing so, nPOD-T has obtained specimens from pancreas transplant recipients with well-documented diabetes recurrence, and showed that insulitis was also present.
Investigators interested in accessing nPOD tissues submit a request online, with a description of the scientific project focused on the study of donor specimens. The project is reviewed by one of two nPOD Tissue Prioritization Committees (TPC); the academic TPC or the industry TPC. The interaction with either TPC is the first and a most critical interaction for prospective nPOD investigators. They receive constructive feedback, in terms of scientific merit and feasibility. Once approved, investigators join nPOD and receive tissues. nPOD has provided and/or currently provides specimens in support of more than 100 research studies worldwide. Projects have a broad scope including, but not limited to the immunopathology of T1D, beta cell physiology and dysfunction, pancreas development, beta cell regeneration, trans-differentiation and dedifferentiation, environmental factors, and imaging. Investigators study nPOD samples with a variety of state of the art methods, such as high-throughput methods, sequencing, genotyping, gene expression, multiparameter flow cytometry, fluorescence and electron microscopy, proteomics, and more.
The nPOD research model for open collaboration and data sharing
A critical aspect of the nPOD program is the emphasis on identifying synergies among projects and on actively promoting collaboration. Upon approval of their project, investigators effectively join nPOD and are made aware of possible collaborative opportunities within the network; nPOD staff also facilitates interactions among investigators by helping, establishing, and maintaining contact. There are periodic web-based meetings (webinars), and an nPOD annual meeting, where data are presented.
Recognizing that several investigators are operating with synergistic goals in many key research areas, and that there are critical questions that are not likely to be addressed by a single investigator, nPOD has developed working groups. Interested investigators coalesce around a major topic, and one or more fundamental questions, with multiple studies and approaches. For example, the role of viruses, i.e., enteroviruses in T1D etiology remains controversial 13–22. Through nPOD promoted web-based discussions, nPOD investigators assembled a group with the unified goal of defining a strategy to address the critical role of viruses via multidisciplinary approaches and the joint study of nPOD specimens. The group, known as the nPOD-virus group, also aims at cross-validating reagents and standardizing readouts, which are critically needed. Data exchange is fully supported by nPOD's sample/data sharing framework. The nPOD-virus group is pioneering group interactions and dynamics, and developing an effective model for collaboration. Additional groups are being organized in the key areas of T cell receptor sequences and antigens, beta cell regeneration, trans-differentiation and dedifferentiation, and the study of the extracellular matrix; moreover, coordinated studies of pancreas transplant biopsies identified through the nPOD-T program are ongoing.
nPOD is implementing ‘real-time’ data sharing; that is, as investigators produce results from their analyses of nPOD cases, those results are shared, prior to publication, among members of the nPOD network. Information is available in a more expedited fashion and strategic changes, or reconciliation of discordant data, may be implemented during the course of the studies. This philosophy stems also from recognition of the unique circumstances of nPOD, where many investigators are studying samples from the same patients. Real-time data sharing is proving of critical importance, especially for the coordinated working groups. To facilitate data sharing, nPOD has deployed and continues to develop DataShare, a collaborative web-based tool for data communication organized around the nPOD specimen repository. Investigators use DataShare to deposit data and share their findings with other investigators looking at related or unrelated parameters, even matching by donors. DataShare also provides access to detailed donor information, including demographics, lifestyle and medical history, diabetes related information, labs, medications, serologies, transfusions and infections, HLA, and histopathological data. It is hoped that this approach will accelerate the rate of discovery and promote more robust advances.
Emerging data from nPOD supported studies
Several manuscripts reporting novel observations made on nPOD specimens have now been published 23–44. Due to space limitations, we highlight a few below:
Insulitis 24 has not been found to date in nPOD non-diabetic donors who express a single autoantibody. This is consistent with investigations of pancreas organ donors from Europe (based on examination of small tissue fragments) 45 and with the low risk of disease progression observed in prospective studies of relatives with a single islet autoantibody 46.
Insulitis is usually not as severe and frequent in human as it is in NOD mice, the closest mouse model of autoimmune diabetes 5, even in patients with recent onset diabetes. However, it is evident that insulitis may be present in some cases years after diagnosis 29 (Fig. 2).
Insulin-positive beta cells and the expression of glucose transporters 27 may also persist for many years after diagnosis (Figs 2 and 3) 29. The expression of the survivin molecule was linked to the persistence of beta cells in the pancreas of patients with long disease duration 25. This is direct pathological evidence of the chronicity of the disease process, which challenges the classical concept that beta cells destruction is almost complete by the time of diagnosis and is aligned with recent clinical studies demonstrating long-term C-peptide persistence in some patients with T1D 26,47. These findings suggest that patients might benefit from effective therapies for a longer period of time than previously thought.
The demonstration, in the insulitis, of islet antigen-specific CD8 T cells in the pancreas of nPOD donors directly links those T cells with the disease and validates those T cells and antigen specificities as bona fide therapeutic targets 29. Early after diagnosis, no islets were observed that contained more than a single specificity of islet-autoreactive CD8 T cells. In long-standing disease, inflamed islets usually contain multiple islet reactive specificities. Epitope spreading may explain the evolution from T cell monospecificity to multispecificity within islets, as disease progresses. This might involve the progressive release of novel antigens as a consequence of ongoing apoptosis caused by a primary population of diabetogenic T cells. The ensuing secondary generation and influx of a new wave of islet-specific T cells could contribute to disease progression.
A role for complement is also suggested from the observation that C4d deposition was elevated in pancreata of patients with T1D 43. C4d deposition was observed in the blood vessel endothelium and extracellular matrix surrounding blood vessels and exocrine ducts.
Several investigators are addressing mechanisms by which immunological self-tolerance to islet cell autoantigens may be lost in T1D patients. Earlier studies had reported that insulin and other self-molecules are expressed in the thymus, by thymic epithelial cells and bone marrow-derived antigen presenting cells 48–50. The expression of multiple self-molecules, including those with tissue-restricted expression, was largely ascribed to the function of the Aire transcription factor, or autoimmune regulator 51. Cells capable of expressing self-molecules and promoting immune self-tolerance were also reported in peripheral lymphoid tissues 49. Studies by Yip et al. using nPOD samples showed that the expression of self-molecule genes in the pancreatic lymph node is depressed in T1D patients 23; this reduced expression results from the alternative splicing of the Deaf1 transcription factor, which could be driven by inflammation, via impaired expression of the eukaryotic translation initiation factor 4 gamma 3 (Eif4g3) 41; these findings provide clues about mechanisms that suppress the tolerogenic expression of self-molecules (e.g., insulin itself, which is an autoantigen in T1D) and impair peripheral immune tolerance in the pancreatic lymph node, a site that plays a critical role in the regulation and activation of islet autoimmune responses. Moreover, nPOD tissues have been used to better characterize the phenotype of cells that express self-molecules in peripheral lymphoid tissues, specifically the extrathymic Aire-expressing cells 52, which have now been shown to be a distinct phenotype of bone marrow origin 44. Accompanying studies in mice showed that these cells inactivate CD4 T cells independently from regulatory T cells.
The pathology of the diabetic pancreas is heterogeneous, exhibiting distinct patterns of beta cell loss, implicating heterogeneity in the disease pathogenesis and multiple factors 25. A series of studies have identified novel molecules associated with disease pathogenesis, including selected chemokines 36, mediators of endoplasmic reticulum stress 34, certain components of the extracellular matrix 30, and cathepsins as mediators of peri-islet basement membrane degradation and loss of integrity, which in turn facilitates T cell infiltration of the islets 40. New evidence links the expression of IL-15 and IL-15Rα in the pancreatic islets and serum of patients with T1D, and manipulation of this pathway with tofacitinib reversed diabetes in mice, pointing at a new therapeutic option to explore in clinical trials 42.
Studies are obtaining additional evidence for an association of enteroviruses with T1D pathogenesis using nPOD samples 37, including in cases with longer disease duration, which might suggests viral persistence, or multiple infections; as mentioned, these studies are now developing as part of the nPOD-virus group activities.
Investigations are exploring mechanisms of beta cell replication, showing that beta cell replication is more evident in early life and yet replication is possible but rare even in adult patients with T1D; in patients with T2D, incretin therapy was linked to the appearance of double hormone positive cells (i.e., insulin-glucagon), which may represent a transitional state perhaps related to regeneration or remodeling 39.
The analysis of pancreas weight suggests that not only the pancreas of T1D patients is smaller than those on non-diabetic donors, but that a weight reduction was also noted in donors with islet-associated autoantibodies 32. This suggests that exocrine abnormalities might exist in T1D and could precede its development, as opposed to being a simple consequence of pancreatic atrophy due to insulin deficiency.
Figure 2.
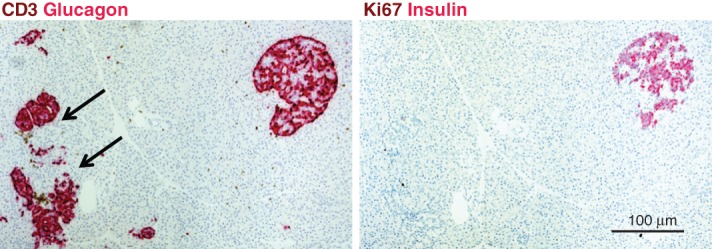
Pancreas pathology from Network for Pancreatic Organ Donors with Diabetes (nPOD) T1D donor 6195. Pancreas serial sections were stained with double immunohistochemistry (IHC) stains (CD3 and glucagon, Ki67 and insulin, brown and red, respectively). Pseudoatrophic islets (glucagon-positive cells only) are located adjacent to an insulin-positive islet in this section. Insulitis is detected by numerous CD3+ T cells adjacent to and infiltrating two islets (arrows). The donor was a 19-year-old Caucasian male with 5 years T1D duration.
Figure 3.
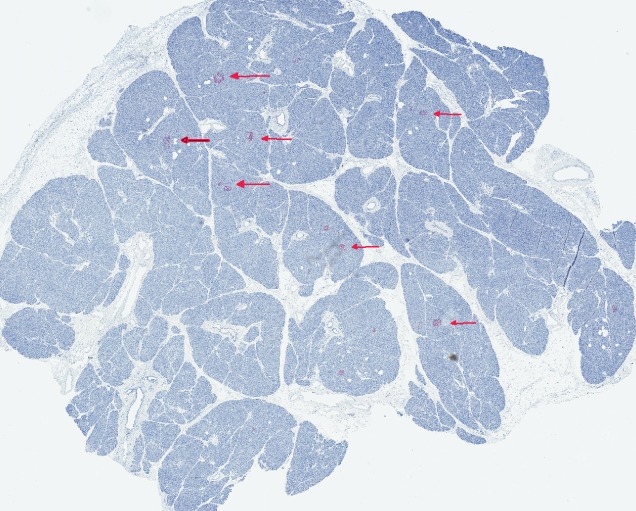
Pancreas pathology from T1D donor nPOD 6046. Pancreas section stained for insulin (dark) by immunohistochemistry and counterstained with hematoxylin. The donor was an 18-year-old Caucasian female, who had developed T1D 8 years prior. A significant numbers of islets stain well for insulin, indicating the presence of beta cells, at least in some lobules, despite 8 years of disease duration.
Summary
nPOD actively promotes a multidisciplinary and unbiased approach toward a better understanding of T1D and identify novel therapeutic targets, through its focus on the study of human samples. Unique to this effort is the coordination of collaborative efforts and real-time data sharing. Studies supported by nPOD are providing direct evidence that human T1D is a complex and heterogeneous disease, in which a multitude of pathogenic factors may be operational and may contribute to the onset of the disease. Importantly, the concept that beta cell destruction is almost completed and that the autoimmune process is almost extinguished soon after diagnosis is being challenged. nPOD investigators are exploring the hypothesis that beta cell dysfunction may also be a significant cause of hyperglycemia, at least around the time of diagnosis, and are uncovering novel molecules and pathways that are linked to the pathogenesis and etiology of human T1D. The validation of therapeutic targets is also a key component of this effort, with recent and future findings providing new strategic direction for clinical trials.
Acknowledgments
The authors are deeply grateful to the organ donors and their families whose generous gift supports diabetes research, and for the trust they placed in this effort. nPOD is grateful to the partnering OPOs. The authors acknowledge the contribution of the late George S. Eisenbarth, who inspired and supported the creation of nPOD. The program is supported by JDRF research grants 25-2013-268, 17-2012-3, and 25-2012-516.
Conflict of interest
No conflicts of interest are noted.
References
- Pugliese A. The multiple origins of type 1 diabetes. Diabet Med. 2013;30:135–146. doi: 10.1111/dme.12081. [DOI] [PubMed] [Google Scholar]
- Cizza G, Brown RJ, Rother KI. Rising incidence and challenges of childhood diabetes. A mini review. J Endocrinol Invest. 2012;35:541–546. doi: 10.3275/8411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold KC, Vignali DA, Cooke A, Bluestone JA. Type 1 diabetes: translating mechanistic observations into effective clinical outcomes. Nat Rev Immunol. 2013;13:243–256. doi: 10.1038/nri3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickels MR. Recovery of endocrine function after islet and pancreas transplantation. Curr Diab Rep. 2012;12:587–596. doi: 10.1007/s11892-012-0294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- Campbell-Thompson M, Wasserfall C, Kaddis J. Network for Pancreatic Organ Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab Res Rev. 2012;28:608–617. doi: 10.1002/dmrr.2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Thompson ML, Montgomery EL, Foss RM. Collection protocol for human pancreas. J Vis Exp. 2012;63:e4039. doi: 10.3791/4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Thompson ML, Heiple T, Montgomery E, Zhang L, Schneider L. Staining protocols for human pancreatic islets. J Vis Exp. 2012;63:e4068. doi: 10.3791/4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewers A. Current concepts and controversies in prevention and treatment of diabetic ketoacidosis in children. Curr Diab Rep. 2012;12:524–532. doi: 10.1007/s11892-012-0307-2. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AL. The management of diabetic ketoacidosis in children. Diabetes Ther. 2010;1:103–120. doi: 10.1007/s13300-010-0008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke GW, III, Vendrame F, Pileggi A, Ciancio G, Reijonen H. Pugliese A. Vol. 1. Curr Diab Rep: Recurrence of autoimmunity following pancreas transplantation; 2011. pp. 229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu EH, Digon BJ, III, Hirshberg B. Pancreatic beta cell function persists in many patients with chronic type 1 diabetes, but is not dramatically improved by prolonged immunosuppression and euglycaemia from a beta cell allograft. Diabetologia. 2009;52:1369–1380. doi: 10.1007/s00125-009-1342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene LC, Oikarinen S, Hyoty H. Enterovirus infection and progression from islet autoimmunity to type 1 diabetes: the Diabetes and Autoimmunity Study in the Young (DAISY) Diabetes. 2010;59:3174–3180. doi: 10.2337/db10-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempainen J, Tauriainen S, Vaarala O. Interaction of enterovirus infection and Cow's milk-based formula nutrition in type 1 diabetes-associated autoimmunity. Diabetes Metab Res Rev. 2011;15:10. doi: 10.1002/dmrr.1294. [DOI] [PubMed] [Google Scholar]
- Oikarinen S, Martiskainen M, Tauriainen S. Enterovirus RNA in blood is linked to the development of type 1 diabetes. Diabetes. 2011;60:276–279. doi: 10.2337/db10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauriainen S, Oikarinen S, Oikarinen M, Hyoty H. Enteroviruses in the pathogenesis of type 1 diabetes. Semin Immunopathol. 2011;33:45–55. doi: 10.1007/s00281-010-0207-y. [DOI] [PubMed] [Google Scholar]
- Oikarinen M, Tauriainen S, Honkanen T. Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol. 2008;151:71–75. doi: 10.1111/j.1365-2249.2007.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen KK, Vuorinen T, Oikarinen S. Isolation of enterovirus strains from children with preclinical type 1 diabetes. Diabet Med. 2004;21:156–164. doi: 10.1111/j.1464-5491.2004.01097.x. [DOI] [PubMed] [Google Scholar]
- Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Immunohistochemical analysis of the relationship between islet cell proliferation and the production of the enteroviral capsid protein, VP1, in the islets of patients with recent-onset type 1 diabetes. Diabetologia. 2011;54:2417–2420. doi: 10.1007/s00125-011-2192-7. [DOI] [PubMed] [Google Scholar]
- Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. The prevalence of enteroviral capsid protein vp1 immunostaining in pancreatic islets in human type 1 diabetes. Diabetologia. 2009;52:1143–1151. doi: 10.1007/s00125-009-1276-0. [DOI] [PubMed] [Google Scholar]
- Dotta F, Censini S, van Halteren AG. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc Natl Acad Sci USA. 2007;104:5115–5120. doi: 10.1073/pnas.0700442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S, Drescher KM, Chapman NM. Enteroviruses and type 1 diabetes. Diabetes Metab Res Rev. 2011;27:820–823. doi: 10.1002/dmrr.1255. [DOI] [PubMed] [Google Scholar]
- Yip L, Su L, Sheng D. Deaf1 isoforms control the expression of genes encoding peripheral tissue antigens in the pancreatic lymph nodes during type 1 diabetes. Nat Immunol. 2009;10:1026–1033. doi: 10.1038/ni.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, Peakman M. Post-mortem analysis of islet pathology in type 1 diabetes illuminates the life and death of the beta cell. Clin Exp Immunol. 2009;155:125–127. doi: 10.1111/j.1365-2249.2008.03864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianani R, Campbell-Thompson M, Sarkar SA. Dimorphic histopathology of long-standing childhood-onset diabetes. Diabetologia. 2010;53:690–698. doi: 10.1007/s00125-009-1642-y. [DOI] [PubMed] [Google Scholar]
- Keenan HA, Sun JK, Levine J. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59:2846–2853. doi: 10.2337/db10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters KT, Wiberg A, Amirian N, Kay TW, von Herrath MG. Persistent glucose transporter expression on pancreatic beta cells from longstanding type 1 diabetic individuals. Diabetes Metab Res Rev. 2011;27:746–754. doi: 10.1002/dmrr.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green-Mitchell SM, Cazares LH, Semmes OJ, Nadler JL, Nyalwidhe JO. On-tissue identification of insulin: in situ reduction coupled with mass spectrometry imaging. Proteomics Clin Appl. 2011;5(7–8):448–453. doi: 10.1002/prca.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppieters KT, Dotta F, Amirian N. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J Exp Med. 2012;209:51–60. doi: 10.1084/jem.20111187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeby M, Ichise M, Harris PE. Vesicular monoamine transporter, type 2 (vmat2) expression as it compares to insulin and pancreatic polypeptide in the head, body and tail of the human pancreas. Islets. 2012;4:393–397. doi: 10.4161/isl.22995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Thompson M, Wasserfall C, Montgomery EL, Atkinson MA, Kaddis JS. Pancreas organ weight in individuals with disease-associated autoantibodies at risk for type 1 diabetes. JAMA. 2012;308:2337–2339. doi: 10.1001/jama.2012.15008. [DOI] [PubMed] [Google Scholar]
- Gregg BE, Moore PC, Demozay D. Formation of a human beta-cell population within pancreatic islets is set early in life. J Clin Endocrinol Metab. 2012;97:3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marhfour I, Lopez XM, Lefkaditis D. Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia. 2012;55:2417–2420. doi: 10.1007/s00125-012-2604-3. [DOI] [PubMed] [Google Scholar]
- Dai C, Brissova M, Hang Y. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2012;55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SA, Lee CE, Victorino F. Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes. 2012;61:436–446. doi: 10.2337/db11-0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson SJ, Leete P, Bone AJ, Foulis AK, Morgan NG. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia. 2013;56:185–193. doi: 10.1007/s00125-012-2745-4. [DOI] [PubMed] [Google Scholar]
- Taylor-Fishwick DA, Weaver JR, Grzesik W. Production and function of IL-12 in islets and beta cells. Diabetologia. 2013;56:126–135. doi: 10.1007/s00125-012-2732-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. doi: 10.2337/db12-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpos E, Kadri N, Kappelhoff R. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes. 2013;62:531–542. doi: 10.2337/db12-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip L, Creusot RJ, Pager CT, Sarnow P, Fathman CG. Reduced DEAF1 function during type 1 diabetes inhibits translation in lymph node stromal cells by suppressing Eif4g3. J Mol Cell Biol. 2013;5:99–110. doi: 10.1093/jmcb/mjs052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Feigenbaum L, Awasthi P. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Ralpha. Proc Natl Acad Sci USA. 2013;110:13534–13539. doi: 10.1073/pnas.1312911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe P, Wasserfall C, Croker B. Increased complement activation in human type 1 diabetes pancreata. Diabetes Care. 2013;36:3815–3817. doi: 10.2337/dc13-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JM, Metzger TC, McMahon EJ. Extrathymic aire-expressing cells are a distinct bone marrow-derived population that induce functional inactivation of CD4 T cells. Immunity. 2013;13:10. doi: 10.1016/j.immuni.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- In't Veld P, Lievens D, De Grijse J. Screening for insulitis in adult autoantibody-positive organ donors. Diabetes. 2007;56:2400–2404. doi: 10.2337/db07-0416. [DOI] [PubMed] [Google Scholar]
- Orban T, Sosenko JM, Cuthbertson D. Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care. 2009;32:2269–2274. doi: 10.2337/dc09-0934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenbaum CJ, Beam CA, Boulware D. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes. 2012;61:2066–2073. doi: 10.2337/db11-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugliese A, Zeller M, Fernandez AJ. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet. 1997;15:293–297. doi: 10.1038/ng0397-293. [DOI] [PubMed] [Google Scholar]
- Pugliese A, Brown D, Garza D. Self-antigen-presenting cells expressing diabetes-associated autoantigens exist in both thymus and peripheral lymphoid organs. J Clin Invest. 2001;107:555–564. doi: 10.1172/JCI10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter J, Brors B, Hergenhahn M, Kyewski B. Medullary epithelial cells of the human thymus express a highly diverse selection of tissue-specific genes colocalized in chromosomal clusters. J Exp Med. 2004;199:155–166. doi: 10.1084/jem.20031677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MS, Venanzi ES, Klein L. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- Gardner JM, DeVoss JJ, Friedman RS. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]


