Abstract
Background
Fewer than 1 in 5 patients receive hepatocellular carcinoma surveillance; however, most studies were performed in racially and socioeconomically homogenous populations and few used guideline-based definitions for surveillance.
Aims
To characterize guideline-consistent hepatocellular carcinoma surveillance rates and identify determinants of hepatocellular carcinoma surveillance among a racially and socioeconomically diverse cohort of cirrhotic patients.
Methods
We retrospectively characterized hepatocellular carcinoma surveillance among cirrhotic patients followed between July 2008 and July 2011 at an urban safety-net hospital. Inconsistent surveillance was defined as at least one screening ultrasound during the 3-year period, annual surveillance as screening ultrasounds every 12 months, and biannual surveillance as screening ultrasounds every 6 months. Univariate and multivariate analyses were conducted to identify predictors of surveillance.
Results
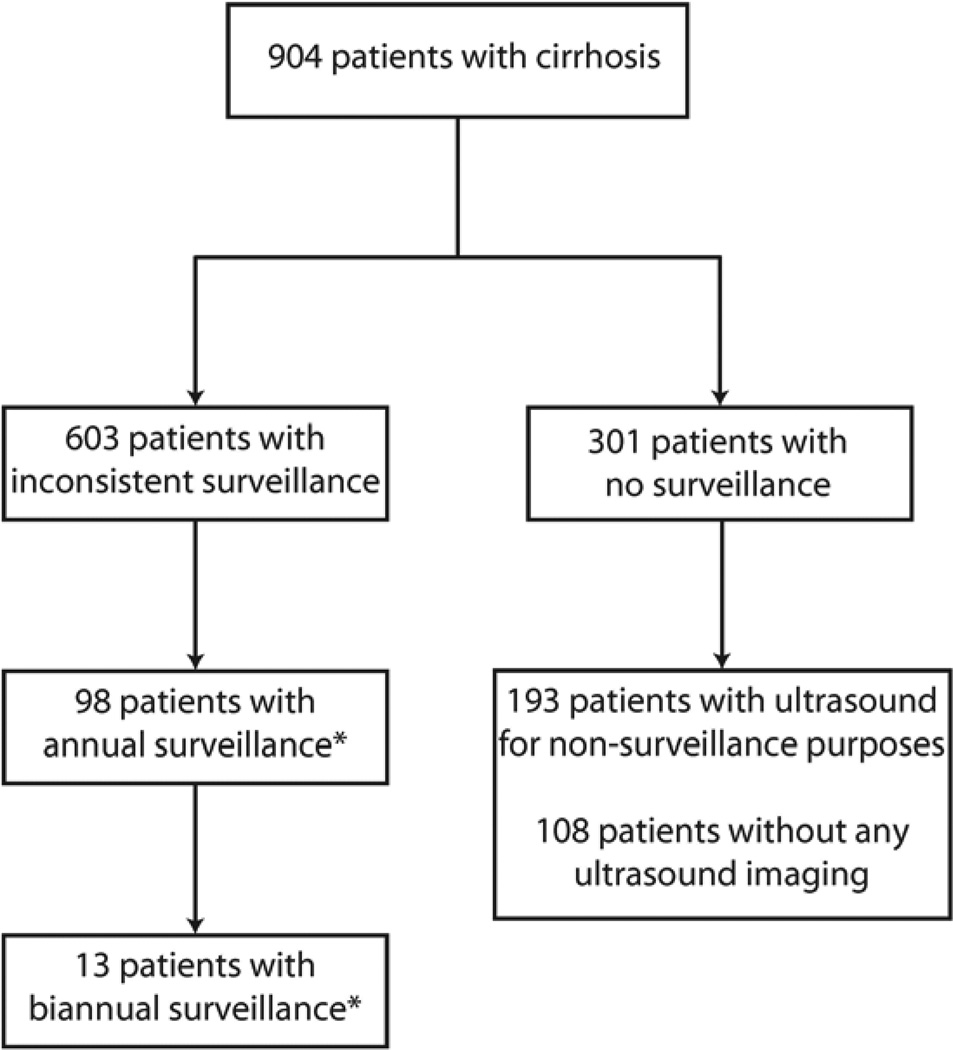
Of 904 cirrhotic patients, 603 (67%) underwent inconsistent surveillance. Failure to recognize cirrhosis was a significant barrier to surveillance utilization (p<0.001). Inconsistent surveillance was associated with insurance status (OR 1.43, 95%CI 1.03–1.98), multiple primary care visits per year (OR 2.63, 95%CI 1.86–3.71), multiple hepatology visits per year (OR 3.75, 95%CI 2.64–5.33), African American race (OR 0.61, 95%CI 0.42–0.99), nonalcoholic steatohepatitis etiology (OR 0.60, 95%CI 0.37–0.98), and extrahepatic cancer (OR 0.43, 95%CI 0.24–0.77). Only 98 (13.4%) of 730 patients underwent annual surveillance, and only 13 (1.7%) of 786 had biannual surveillance.
Conclusions
Only 13% of patients with cirrhosis receive annual surveillance and less than 2% receive biannual surveillance. There are racial and socioeconomic disparities, with lower rates of hepatocellular carcinoma surveillance among African Americans and underinsured patients.
Keywords: Liver cancer, cirrhosis, surveillance, disparities, underuse
INTRODUCTION
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death worldwide and leading cause of death in patients with cirrhosis. Furthermore, its incidence is anticipated to continue increasing over the next two decades1. Prognosis for patients with hepatocellular carcinoma depends on tumor stage, with curative options only available for patients diagnosed at an early stage2. Patients with early hepatocellular carcinoma achieve 5-year survival rates near 70% with resection and liver transplantation3, whereas patients with advanced hepatocellular carcinoma have median survival below one year4.
The American Association for the Study of Liver Diseases (AASLD) and National Comprehensive Cancer Network (NCCN) recommend hepatocellular carcinoma surveillance at six-month intervals in patients with cirrhosis5. Despite being efficacious and standard of care in patients with cirrhosis6, 7, hepatocellular carcinoma surveillance has not been adopted into clinical practice. Whereas colon and breast cancer screening rates are greater than 60%, fewer than 20% of patients with cirrhosis undergo hepatocellular carcinoma surveillance8–10. However, a systematic review found most studies used operational definitions for hepatocellular carcinoma surveillance, e.g. one ultrasound or alpha-fetoprotein (AFP) in a two-year period, and few reported guideline-adherent definitions10.
Hepatocellular carcinoma disproportionately affects socioeconomically disadvantaged populations, with higher age-specific rates and worse survival among racial/ethnic minorities and patients of low socioeconomic status (SES) than their counterparts11–13. Reasons for differences in survival are likely multi-factorial, involving both medical and social factors. While disparities in utilization rates have been well documented for other cancer screening modalities, such as mammography and colonoscopy14–17, less is known about patient-level factors associated with hepatocellular carcinoma surveillance10, 18. Past hepatocellular carcinoma studies have been conducted in highly uniform populations with most patients being male, Caucasian, and insured10. The aims of our study were to 1) characterize guideline-consistent hepatocellular carcinoma surveillance rates among a cohort of patients with cirrhosis, 2) characterize surveillance rates among those with recognized cirrhosis, and 3) identify patient-level determinants of hepatocellular carcinoma surveillance among a racially and socioeconomically diverse cohort of patients with cirrhosis.
METHODS
Study Population
We conducted a retrospective cohort study of cirrhotic patients followed at Parkland Health and Hospital System, the safety-net system for Dallas County. Parkland is an integrated system with eleven primary care provider clinics in low-income neighborhoods, a multidisciplinary hepatology outpatient clinic, and a tertiary hospital-all sharing one electronic medical record system. Parkland provides inpatient and outpatient care for most cirrhotic patients and approximately 50% of hepatocellular carcinoma patients in Dallas.
For inclusion, patients were required to have one outpatient primary care provider clinic visit between July 2008 and July 2011, with continued follow-up through the last year of the study period (August 2010 – July 2011). Patients were identified by a set of ICD-9 codes, which are highly sensitive and specific for cirrhosis (456.0, 456.1, 456.2, 456.21, 567.23, 571.2, 571.5, 572.2, 572.3, and 572.4)19. One author (A.S.) adjudicated cases to confirm they met diagnostic criteria for cirrhosis, defined as Batts Ludwig stage 4 fibrosis on liver biopsy or a cirrhotic-appearing liver on abdominal imaging with signs of portal hypertension (e.g., varices, ascites, splenomegaly). This study was approved by the Institutional Review Board of UT Southwestern Medical Center.
Data Collection
Patient demographics, clinical history, laboratory data and imaging results were obtained through review of computerized medical records. Two authors (M.N. and P.K.) extracted information using standardized forms, with a third investigator (A.S.) available to resolve discrepancies.
Hepatocellular carcinoma Surveillance Outcomes
Dates of all hepatocellular carcinoma surveillance testing with abdominal ultrasound between July 2008 and July 2011 were abstracted. We did not assess receipt of surveillance prior to July 2008 as Parkland’s electronic medical record was not implemented at that time. Given that recognition of cirrhosis is an important mediator of surveillance underutilization, we performed a subgroup analysis among patients who had recognized cirrhosis during the entire study period. Recognition of cirrhosis was defined as mention of pathologic, radiologic, or clinical signs of cirrhosis in providers’ clinical notes.
We characterized patients based on receipt of hepatocellular carcinoma surveillance, which was our primary outcome of interest, using three definitions. Inconsistent surveillance was defined as one abdominal ultrasound, for surveillance purposes, over the study period. Consistent annual surveillance was defined as at least one abdominal ultrasound study, for surveillance purposes, every 12 months. For this analysis, patients were required to have greater than one year of care at Parkland so annual surveillance rates could be assessed. Finally, we assessed consistent biannual surveillance rates, requiring the receipt of consistent surveillance every 6 months. For this analysis, patients were required to have greater than six months of follow-up. Imaging was determined to be for surveillance purposes through review of imaging reports and clinical notes. Imaging exams performed for diagnostic reasons, e.g. abdominal pain or elevated liver enzymes, were not included as surveillance exams.
Covariates
Age, gender, race, ethnicity, preferred language, marital status, and insurance type were recorded. We detailed drug, alcohol and smoking history, with active alcohol abuse defined as drinking more than 40 grams/day. Data regarding underlying etiology and presence of decompensation (ascites or hepatic encephalopathy) were abstracted from laboratory data and clinical notes. We classified patients according to etiology of liver disease, including hepatitis C virus, hepatitis B virus, alcohol-related liver disease, nonalcoholic steatohepatitis, and other. Dates of liver disease diagnosis and cirrhosis diagnosis were abstracted. Date of first medical encounter and number of primary care provider or hepatology clinic visits were documented. Laboratory data of interest included platelet count, creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, albumin, international normalized ratio (INR), and alpha fetoprotein (AFP). Multivariate imputation was used to address missing data (<10% missing data for all laboratory data).
Statistical Analysis
In univariate analysis, Fisher exact and Mann Whitney rank-sum tests were performed to identify patient-factors associated with receipt of hepatocellular carcinoma surveillance. Multivariate logistic regression models included variables of a priori clinical importance (e.g., race, insurance status, Child Pugh class, and receipt of hepatology care) and any factors significant on univariate analysis. Predictor variables with p<0.10 in univariate analysis were included in multivariate models to minimize type II error. Statistical significance was defined as p< 0.05 for multivariate analyses. All data analysis was performed using Stata 11 (StataCorp, College Station, TX) and SAS 9.3 (SAS Institute, NC).
RESULTS
Patient Characteristics
We identified 904 patients with cirrhosis who met inclusion criteria. The median age of patients was 54.8 years (range 21.0–84.2), and 592 (65%) were men. Our population was racially diverse, with 22% African Americans, 36% non-Hispanic Caucasians, and 40% Hispanic Caucasians. Nearly 43% of patients were uninsured/underinsured, 53% had Medicare or Medicaid, and only 4% had private health insurance. The most common etiologies of cirrhosis were hepatitis C virus (53%), alcohol-induced liver disease (28%), and non-alcoholic steatohepatitis (13%). The median Child-Pugh score at diagnosis was 7 (range 5–14), with 43% of patients having Child-Pugh A cirrhosis.
Patients had been followed at Parkland for a mean of 2.3 years and median of 3 years (range 0.5–3 years). Sixty patients had been followed for less than 1 year, 108 patients for 1 year, 281 patients for 2 years, and 455 had been followed for the entire 3-year period. Patients had a median of 2 (range 0–14) primary care provider visits per year and 0.67 (range 0–18) hepatology visits per year.
Receipt of Inconsistent Surveillance
Inconsistent surveillance had been performed in 603 (66.7%) patients. Of the other 301 patients who had not undergone surveillance, 193 had received an ultrasound for non-surveillance purposes. Only 22 patients had an ultrasound ordered by their provider but had not completed the test. Alpha fetoprotein had been performed at least once in 486 (80.6%) of patients with inconsistent surveillance and 174 (56.9%) of those without surveillance. Inconsistent surveillance rates were significantly different according to length of follow-up (p=0.01). Inconsistent surveillance rates were 43.5% among the 108 patients followed for 1–2 years, 62.3% among the 281 patients followed for 2–3 years, and 70.6% among the 455 patients with 3 years of follow-up.
In univariate analysis, inconsistent surveillance was positively associated with insurance status (p=0.05), number of primary care provider visits per year (p<0.001) and number of hepatology visits per year (p<0.001). It was negatively associated with African American race (p=0.07), nonalcoholic steatohepatitis etiology (p=0.003), Child Pugh C cirrhosis (p=0.005), ongoing alcohol use (p<0.001), and presence of an extrahepatic cancer (p=0.004). Inconsistent surveillance was not associated with age (p=0.72), gender (p=0.37), marital status (p=0.62), or Eastern Cooperative Oncology Group (ECOG) performance status (p=0.32). Although surveillance rates were lower in those with extrahepatic malignancy, there was no association with other comorbidities including congestive heart failure (p=1.0), cerebrovascular disease (p=0.86), or chronic obstructive pulmonary disease (p=0.90).
In multivariate analysis, inconsistent surveillance was positively associated with insurance status (OR 1.43, 95%CI 1.03–1.98), having more than one primary care provider visit per year (OR 2.63, 95%CI 1.86–3.71), and having more than one hepatology visit per year (OR 3.75, 95%CI 2.64–5.33). Inconsistent hepatocellular carcinoma surveillance was inversely associated with African American race (OR 0.61, 95%CI 0.42–0.99), nonalcoholic steatohepatitis etiology (OR 0.60, 95%CI 0.37–0.98), and the presence of extrahepatic cancer (OR 0.43, 95%CI 0.24–0.77) (Table 1). These risk factors discriminated between the presence and absence of inconsistent surveillance with fair accuracy, with a c-statistic of 0.72 (95%CI 0.68–0.76).
Table I.
Predictors of inconsistent hepatocellular carcinoma surveillance*
| Variable* | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR | 95% CI | Adjusted OR |
95% CI | |
| Insurance status | 1.32 | 1.00–1.74 | 1.43 | 1.03–1.98 |
| More than one hepatology visit per year | 3.24 | 2.37–4.43 | 3.75 | 2.64–5.33 |
| More than one primary care visit per year | 2.21 | 1.66–2.95 | 2.63 | 1.86–3.71 |
| African American race | 0.73 | 0.53–1.02 | 0.61 | 0.42–0.99 |
| Nonalcoholic steatohepatitis etiology | 0.55 | 0.37–0.82 | 0.60 | 0.37–0.98 |
| Extrahepatic cancer | 0.46 | 0.27–0.78 | 0.43 | 0.24–0.77 |
| Child Pugh C cirrhosis | 0.63 | 0.46–0.86 | 0.75 | 0.52–1.08 |
| Active alcohol use | 0.58 | 0.42–0.80 | 0.80 | 0.56–1.15 |
Inconsistent surveillance was defined as at least one ultrasound for surveillance purposes over the three-year period
Inconsistent Surveillance among Patients with Recognized Cirrhosis
Failure to recognize cirrhosis was a major barrier to surveillance utilization, with significantly higher inconsistent surveillance rates in those who had recognized cirrhosis during the first year of the study period (76.4% vs. 62.7%, p<0.001). Among the 347 patients with recognized cirrhosis during the entire study period, hepatocellular carcinoma surveillance was significantly associated with having more than one primary care provider visit per year (OR 3.80, 95%CI 2.06–7.01) and having more than one hepatology visit per year (OR 2.30, 95%CI 1.20–4.39) in multivariate analysis. Hepatocellular carcinoma surveillance rates were inversely associated with African American race (OR 0.40, 95%CI 0.21–0.77), nonalcoholic steatohepatitis etiology (OR 0.34, 95%CI 0.15–0.77), and the presence of Child Pugh C cirrhosis (OR 0.47, 95%CI 0.24–0.90).
Receipt of Annual Surveillance
Of 730 patients with greater than one year of follow-up, 98 (13.4%) had consistent annual surveillance (Table 2). Annual surveillance was significantly associated with duration of follow-up, with surveillance rates of 21.0% (59/281) among patients followed for 2 years but only 8.7% (39/449) among those followed for 3 years (p<0.001). Patients with known cirrhosis during the entire study period had annual surveillance performed in 20.4% (66/323) of patients, compared to 7.4% (25/339) in patients with unrecognized cirrhosis (p<0.001).
Table II.
Patient characteristics stratified by consistent annual hepatocellular carcinoma surveillance
| Patient Characteristics | Patient
with consistent hepatocellular carcinoma surveillance (n=98) |
Patients
without consistent hepatocellular carcinoma surveillance (n=632) |
p-value |
|---|---|---|---|
| Age | 54.6 ± 11.3 | 55.3 ± 10.6 | 0.56 |
| Gender (% Male) | 72 (73.5%) | 391 (61.9%) | 0.03 |
| Race/Ethnicity | 0.95 | ||
| Caucasian | 32 (32.7%) | 219 (34.7%) | |
| Black | 20 (20.4%) | 139 (22.0%) | |
| Hispanic | 44 (44.9%) | 256 (40.5%) | |
| Asian | 2 (2.0%) | 15 (2.4%) | |
| Etiology | 0.07 | ||
| Hepatitis C | 57 (58.2%) | 327 (51.7%) | |
| Hepatitis B | 4 (4.1%) | 21 (3.3%) | |
| Alcohol | 30 (30.6%) | 166 (26.3%) | |
| Nonalcoholic steatohepatitis | 6 (6.1%) | 99 (15.7%) | |
| Insurance status | 0.49 | ||
| Medicare | 33 (33.7%) | 230 (36.5%) | |
| Medicaid | 18 (18.4%) | 126 (20.0%) | |
| Private Insurance | 7 (7.1%) | 24 (3.8%) | |
| Uninsured | 40 (40.8%) | 250 (39.7%) | |
| Preferred language (% English) | 72 (73.5%) | 473 (75.1%) | 0.71 |
| Functional status (% ECOG 0–2) | 79 (98.8%) | 495 (98.4%) | 1.0 |
| Alcohol (% active) | 18 (19.0%) | 139 (23.2%) | 0.69 |
| HIV status (% positive) | 9 (16.1%) | 50 (12.4%) | 0.40 |
| Presence of ascites | 38 (38.8%) | 233 (36.9%) | 0.72 |
| Presence of hepatic encephalopathy | 25 (25.5%) | 126 (19.9%) | 0.21 |
| Platelet count * 1000/mm3 | 88 (4–234) | 98 (3 – 476) | 0.008 |
| AST (U/L) | 54 (16 – 280) | 55 (9 – 1008) | 0.41 |
| ALT (U/L) | 37 (11 – 333) | 38 (5 – 2253) | 0.68 |
| Bilirubin (mg/dL) | 1.3 (0.2 – 32.8) | 1.0 (0.1 – 30.0) | 0.14 |
| Albumin (g/dL) | 3.4 (1.2 – 4.8) | 3.5 (1.2 – 4.8) | 0.84 |
| Creatinine (mg/dL) | 0.9 (0.4 – 10.3) | 0.9 (0.1 – 12.8) | 0.11 |
| INR | 1.2 (0.9 – 4.0) | 1.2 (0.9 – 6.3) | 0.94 |
| Child Pugh score | 7 (5–13) | 7 (5 – 14) | 0.54 |
| Child Pugh classification | 0.63 | ||
| Child Pugh A | 39 (40.2%) | 266 (44.5%) | |
| Child Pugh B | 42 (43.3%) | 228 (38.1%) | |
| Child Pugh C | 16 (16.5%) | 104 (17.4%) | |
| Number of primary care clinic visits per year | 2.3 (0 – 12.7) | 2.3 (0 – 13.2) | 0.50 |
| Number of hepatology clinic visits per year | 1.0 (0 – 11.0) | 0.7 ( 0 – 10.5) | 0.002 |
All data are expressed as median (range) unless otherwise specified
ALT – alanine aminotransferase; AST – aspartate aminotransferase; ECOG – Eastern Cooperative Oncology Group; INR – international normalized ratio
In univariate analysis, annual surveillance was associated with male gender (p=0.03), nonalcoholic steatohepatitis etiology (p=0.01), and number of hepatology clinic visits per year (p=0.002). Annual surveillance was not associated with Child Pugh C cirrhosis (p=0.35) or ECOG performance status (p=1.0). Although not significant on univariate analysis, we included a priori variables including race, insurance status, and Child Pugh class. In multivariate analysis, annual surveillance was significantly associated with male gender (OR 1.63, 95%CI 1.00–2.67) and number of hepatology clinic visits per year (OR 1.99, 95%CI 1.28–3.10) and inversely associated with nonalcoholic steatohepatitis etiology (OR 0.41, 95%CI 0.17–0.99) (Table 3). Patients with less than two hepatology clinic visits per year had surveillance performed in 10.9% of patients, compared to 19.3% in patients with two or more visits per year. Annual surveillance rates were 5.7% among patients with nonalcoholic steatohepatitis cirrhosis, compared to 14.7% among those with other etiologies of liver disease.
Table III.
Predictors of annual hepatocellular carcinoma surveillance*
| Variable | Multivariate Analysis | Effect size | |
|---|---|---|---|
| OR | 95% CI | ||
| Male gender | 1.63 | 1.00 – 2.67 | 15.6% vs. 9.7% |
| Two or more hepatology visits per year | 1.99 | 1.28 – 3.10 | 19.3% vs. 10.9% |
| Nonalcoholic steatohepatitis etiology | 0.41 | 0.17 – 0.99 | 5.7% vs. 14.7% |
| Caucasian race** | 1.21 | 0.76 – 1.92 | 13.8% vs. 12.8% |
| Insurance status** | 1.18 | 0.76 – 1.84 | 13.8% vs. 13.2% |
| Child Pugh class C cirrhosis** | 0.70 | 0.39 – 1.23 | 11.0% vs. 14.1% |
Annual surveillance was defined as at least one ultrasound for surveillance purposes every 12 months
Race, insurance status, and Child Pugh class were entered into multivariate model given a priori importance
Receipt of Biannual Surveillance
There were 786 patients with greater than six months of follow-up, of whom only 13 (1.7%) had consistent surveillance every 6 months. Biannual surveillance rates were 22% (11/50) among patients followed for one year, 0.4% (1/281) among those followed for two years, and 0.2% (1/455) among patients followed for three years. In univariate analysis, biannual surveillance was associated with Child Pugh score (p=0.02) and number of hepatology clinic visits per year (p=0.004). In multivariate analysis, the only factor associated with receipt of biannual surveillance was the number of hepatology clinic visits per year (OR 8.38, 95% CI 2.28 – 30.7). Biannual surveillance rates were 0.5% (3/556) among those with less than two hepatology clinic visits per year, 2.7% (5/186) among those with 2–5 visits per year, and 11.4% (5/44) among those with 5 or more visits per year.
DISCUSSION
Although a meta-analysis found less than 20% of patients in the United States undergo hepatocellular carcinoma surveillance10, the estimate was limited by heterogeneity of operational definitions for surveillance. Clear consistent definitions and measures are necessary to interpret and quantify hepatocellular carcinoma surveillance rates20. To the best of our knowledge, our study is the first to report guideline-adherent surveillance rates in a large cohort, with nearly 1000 patients followed up to 3 years. In our study, two-thirds of patients had inconsistent surveillance but only 13% had annual surveillance. Even more concerning, biannual surveillance rates were disappointingly low at only 1.7%.
Our results are consistent with the complexity of the surveillance process, with multiple steps that are prone to failure21. Providers must accurately identify high-risk patients and order surveillance testing, the healthcare system must schedule the tests, and patients must adhere with surveillance recommendations22–25. Furthermore, the surveillance process must be repeated every 6 months to be effective. A breakdown at any step results in screening failure, which is associated with higher rates of advanced tumor stages26. The most common reasons for hepatocellular carcinoma surveillance underuse in clinical practice include under-recognition of cirrhosis and lack of provider orders for hepatocellular carcinoma surveillance in those with known cirrhosis, with patient non-compliance being found in a minority of cases24. In our study, patient noncompliance accounted for less than 10% of cases in which surveillance were not completed (data not shown). This process may be particularly challenging for safety-net institutions, which can be overwhelmed with the large number of patients relative to limited clinic availability.
Several studies have reported racial and socioeconomic disparities in hepatocellular carcinoma surveillance10 and treatment utilization27, with lower surveillance rates in non-Caucasians and patients of low socioeconomic status. However, studies to date have been conducted in highly homogenous populations. Our study is the first to quantify surveillance rates among a large racially and socioeconomically diverse cohort. We demonstrated both racial and socioeconomic disparities in receipt of inconsistent surveillance in a safety-net population, with lower rates among underinsured patients and African American patients, after adjusting for several factors including liver function and clinic access. Unfortunately, we were unable to identify determinants of surveillance underutilization in this subgroup of patients, and studies are needed to determine if racial disparities in hepatocellular carcinoma surveillance are driven by provider-level or patient-level attitudes and behaviors.
We also found hepatology subspecialty care was associated with consistent hepatocellular carcinoma surveillance. A similar finding was reported among patients from SEER-Medicare, in which 27.3% of patients receiving subspecialty care underwent surveillance compared to only 10.7% of those seen by primary care proviers9. Given limited availability of subspecialty care in some areas, including safety-net hospitals, referring every cirrhotic patient to subspecialists is not a viable option. In fact, primary care providers follow most cirrhotic patients in the United States, with only 20–40% being followed by gastroenterologists/hepatologists28. However, it is unknown if this disparity relates to differences in provider knowledge or reflects a selection bias. Further studies are needed to characterize primary care providers’ knowledge, attitudes, and perceived barriers regarding hepatocellular carcinoma surveillance.
Most data regarding hepatocellular carcinoma surveillance underutilization has been derived from automated electronic data, such as administrative and registry data8, 9. These databases capture large numbers of patients, allowing for generalizable results with tight confidence intervals; however, automated data fail to capture data that is needed to determine potential exceptions to care. A study assessing performance measures among patients with hepatitis C virus infection highlighted how failure to account for care exceptions can miscode high quality care as poor quality29. We found that hepatocellular carcinoma surveillance was negatively associated with the presence of extrahepatic cancer on multivariate analysis. Interestingly, patients with Child Pugh C cirrhosis or poor functional status underwent surveillance at similar rates, despite lack of proven benefit in these subgroups. Further studies should continue to characterize potential exceptions to care that might partly explain hepatocellular carcinoma surveillance underutilization.
Our study has several limitations. Our conclusions reflect a retrospective analysis of patients with hepatocellular carcinoma seen at a large urban safety-net hospital, and therefore may not be generalized to other practice settings. Given its retrospective nature, our study was also limited by possible unmeasured confounders and missing data. Although some patients may have received hepatocellular carcinoma surveillance at outside institutions, we believe this is unlikely given that Parkland, as the safety-net health system for Dallas County, is the only option for most indigent patients. The retrospective nature of our study could have also led to measurement bias, such as inaccurate estimates of cirrhosis recognition and/or ECOG functional status. Overall, we believe the limitations of our study are outweighed by its strengths including its relatively large size, racially and socio-economically diverse population, and well-characterized outcome measures.
In conclusion, we believe this is the first study to report guideline-consistent surveillance rates among a large cohort of racially and socio-economically diverse patients. We found only 13% of patients had annual surveillance and only 1.7% had consistent biannual surveillance. Furthermore, we found racial and socioeconomic disparities in receipt of inconsistent surveillance, with significantly lower rates among African Americans and underinsured patients. Studies are needed to explore reasons for underutilization of surveillance, as these can help identify appropriate intervention targets to increase hepatocellular carcinoma surveillance rates and help reduce socio-demographic disparities.
Figure 1.
Hepatocellular Carcinoma Surveillance Rates
* Consistent annual surveillance was assessed among the 730 patients with greater than one year of follow-up. Consistent biannual surveillance rates were assessed among the 786 patients with greater than six months of follow-up.
Clinical Significance.
-
♦
Less than 5% of patients with cirrhosis undergo guideline-consistent biannual surveillance for hepatocellular carcinoma
-
♦
There are racial and socioeconomic disparities in hepatocellular carcinoma surveillance utilization, with lower surveillance rates among African Americans and underinsured patients
-
♦
Receipt of hepatology subspecialty care is associated with significantly higher hepatocellular carcinoma surveillance rates
-
♦
Potential exceptions to care, such as significant comorbid illnesses, may in part explain hepatocellular carcinoma surveillance underutilization
Acknowledgments
Financial support: This work was conducted with support from the Center for Translational Medicine, NIH/NCATS Grants UL1-TR000451 and UL1TR001105, the ACG Junior Faculty Development Award, and the American Cancer Society and Simmons Cancer Center grant ACS-IRG-02-196 awarded to Dr. Singal. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Center for Translational Medicine, University of Texas Southwestern Medical Center and its affiliated academic and health care centers, the National Center for Advancing Translational Sciences, or the National Institutes of Health.
Abbreviations
- AASLD
American Association for the Study of Liver Disease
- ECOG
Eastern Cooperative Oncology Group
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential Competing Interests: None
Author contributions:
Amit G. Singal involved in study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of manuscript for important intellectual content, and study supervision. He is the guarantor of the article.
Xilong Li involved in analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Jasmin Tiro involved in study design, interpretation of data, and critical revision of the manuscript for important intellectual content.
Pragathi Kandunoori involved in acquisition of data.
Beverley Adams-Huet involved in analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
Mahendra Nehra involved in acquisition of data.
Adam Yopp involved in analysis and interpretation of data and critical revision of the manuscript for important intellectual content.
REFERENCES
- 1.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 2.Padhya KT, Marrero JA, Singal AG. Recent advances in the treatment of hepatocellular carcinoma. Curr Opin Gastroenterol. 2013;29:285–292. doi: 10.1097/MOG.0b013e32835ff1cf. [DOI] [PubMed] [Google Scholar]
- 3.Pelletier SJ, Fu S, Thyagarajan V, Romero-Marrero C, Batheja MJ, Punch JD, Magee JC, Lok AS, Fontana RJ, Marrero JA. An intention-to-treat analysis of liver transplantation for hepatocellular carcinoma using organ procurement transplant network data. Liver Transpl. 2009;15:859–868. doi: 10.1002/lt.21778. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Bru C, Rodes J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatology. 2010;53:1–35. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanwal F, Kramer J, Asch SM, El-Serag H, Spiegel BM, Edmundowicz S, Sanyal AJ, Dominitz JA, McQuaid KR, Martin P, Keeffe EB, Friedman LS, Ho SB, Durazo F, Bacon BR. An Explicit Quality Indicator Set for Measurement of Quality of Care in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2010;8:709–717. doi: 10.1016/j.cgh.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 7.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davila JA, Henderson L, Kramer JR, Kanwal F, Richardson PA, Duan Z, El-Serag HB. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 9.Davila JA, Morgan RO, Richardson PA, Du XL, McGlynn KA, El-Serag HB. Use of surveillance for hepatocellular carcinoma among patients with cirrhosis in the United States. Hepatology. 2010;52:132–141. doi: 10.1002/hep.23615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singal AG, Yopp A, C SS, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27:861–867. doi: 10.1007/s11606-011-1952-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artinyan A, Mailey B, Sanchez-Luege N, Khalili J, Sun CL, Bhatia S, Wagman LD, Nissen N, Colquhoun SD, Kim J. Race, ethnicity, and socioeconomic status influence the survival of patients with hepatocellular carcinoma in the United States. Cancer. 2010;116:1367–1377. doi: 10.1002/cncr.24817. [DOI] [PubMed] [Google Scholar]
- 12.Decker KM, Harrison M, Chateau D. Influence of direct referrals on time to diagnosis after an abnormal breast screening result. Cancer Detect Prev. 2004;28:361–367. doi: 10.1016/j.cdp.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Davila JA, El-Serag HB. Racial differences in survival of hepatocellular carcinoma in the United States: a population-based study. Clin Gastroenterol Hepatol. 2006;4:104–110. quiz 4–5. [PubMed] [Google Scholar]
- 14.Du XL, Fang S, Vernon SW, El-Serag H, Shih YT, Davila J, Rasmus ML. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 15.Du XL, Meyer TE, Franzini L. Meta-analysis of racial disparities in survival in association with socioeconomic status among men and women with colon cancer. Cancer. 2007;109:2161–2170. doi: 10.1002/cncr.22664. [DOI] [PubMed] [Google Scholar]
- 16.Lloyd SC, Harvey NR, Hebert JR, Daguise V, Williams D, Scott DB. Racial disparities in colon cancer. Primary care endoscopy as a tool to increase screening rates among minority patients. Cancer. 2007;109:378–385. doi: 10.1002/cncr.22362. [DOI] [PubMed] [Google Scholar]
- 17.Schueler KM, Chu PW, Smith-Bindman R. Factors associated with mammography utilization: a systematic quantitative review of the literature. J Womens Health (Larchmt) 2008;17:1477–1498. doi: 10.1089/jwh.2007.0603. [DOI] [PubMed] [Google Scholar]
- 18.Singal A, Volk M, Rakoski M, Fu S, Su G, McCurdy H, Marrero J. Patient Involvement is Correlated with Higher HCC Surveillance in Patients with Cirrhosis. J Clin Gastroenterol. 2011;45:727–732. doi: 10.1097/MCG.0b013e31820989d3. [DOI] [PubMed] [Google Scholar]
- 19.Nehra MS, Ma Y, Clark C, Amarasingham R, Rockey DC, Singal AG. Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol. 2013;47:e50–e54. doi: 10.1097/MCG.0b013e3182688d2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vernon SW, Briss PA, Tiro JA, Warnecke RB. Some methodologic lessons learned from cancer screening research. Cancer. 2004;101:1131–1145. doi: 10.1002/cncr.20513. [DOI] [PubMed] [Google Scholar]
- 21.Taplin SH, Rodgers AB. Toward improving the quality of cancer care: addressing the interfaces of primary and oncology-related subspecialty care. J Natl Cancer Inst Monogr. 2010;2010:3–10. doi: 10.1093/jncimonographs/lgq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singal AG, Mukherjee A, Joseph Elmunzer B, Higgins PD, Lok AS, Zhu J, Marrero JA, Waljee AK. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am J Gastroenterol. 2013;108:1723–1730. doi: 10.1038/ajg.2013.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singal AG, Tiro JA, Gupta S. Improving hepatocellular carcinoma screening: applying lessons from colorectal cancer screening. Clin Gastroenterol Hepatol. 2013;11:472–477. doi: 10.1016/j.cgh.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singal AG, Yopp AC, Gupta S, Skinner CS, Halm EA, Okolo E, Nehra M, Lee WM, Marrero JA, Tiro JA. Failure Rates in the Hepatocellular Carcinoma Surveillance Process. Cancer Prev Res (Phila) 2012;5:1124–1130. doi: 10.1158/1940-6207.CAPR-12-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zapka JG, Taplin SH, Solberg LI, Manos MM. A framework for improving the quality of cancer care: the case of breast and cervical cancer screening. Cancer Epidemiol Biomarkers Prev. 2003;12:4–13. [PubMed] [Google Scholar]
- 26.Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, Lok AS, Lee WM. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425–432. doi: 10.1038/ajg.2012.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan D, Yopp A, Beg MS, Gopal P, Singal AG. Meta-analysis: underutilisation and disparities of treatment among patients with hepatocellular carcinoma in the United States. Aliment Pharmacol Ther. 2013;38:703–712. doi: 10.1111/apt.12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 29.Kanwal F, Hoang T, Kramer J, Chrusciel T, El-Serag H, Dominitz JA, Asch SM. The Performance of Process Measures in Hepatitis C. Am J Gastroenterol. 2012 doi: 10.1038/ajg.2012.201. [DOI] [PubMed] [Google Scholar]