Abstract
The PD-1 receptor and ligands PD-L1 and PD-L2, members of the CD28 and B7 families, play critical roles in T cell coinhibition and exhaustion. Overexpression of PD-L1 and PD-1 on tumor cells and tumor-infiltrating lymphocytes, respectively, correlates with poor disease outcome in some human cancers. Monoclonal antibodies (mAbs) blockading the PD-1/PD-L1 pathway have been developed for cancer immunotherapy via enhancing T cell functions. Clinical trials with mAbs to PD-1 and PD-L1 have shown impressive response rates in patients, particularly for melanoma, non-small-cell lung cancer, renal cell carcinoma, and bladder cancer. Further studies are needed to dissect mechanisms of variable response rate, to identify biomarkers for clinical response, to develop small molecule inhibitors, and to combine with other therapies.
Keywords: PD-1, PD-L1, PD-L2, monoclonal antibody, human cancer, immunotherapy
Expression of PD-1 and its ligands
The programmed death 1 (PD-1, CD279; see Glossary) receptor can be detected at the cell surface of T cells during thymic development and in the periphery of several types of hematopoietic cells following T cell receptor (TCR) signaling and cytokine stimulation. PD-1 is expressed on CD4− CD8− thymocytes and inducibly expressed on peripheral CD4+ and CD8+ T cells, B cells, monocytes, natural killer T cells (NK T cells), and some dendritic cells (DCs) [1, 2]. Persistent expression of PD-1 on T cells induces T cell exhaustion [3]. Exhausted CD8 T cells lose their effector function, evidenced by their inability to secrete cytolytic molecules, such as perforin, and their failure to secrete pro-inflammatory cytokines, such as interleukin-2 (IL-2), interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α)[4, 5].
CD4+ Foxp3+ regulatory T cells (Tregs), a highly immunosuppressive subset of CD4+ T cells which are critical in maintaining tolerance and attenuating immune responses, express cell surface PD-1 which contributes to their development, maintenance, and functional response [6]. Ligand binding to the PD-1 receptor on Tregs in the presence of CD3 and TGF-β leads to an increase in the de novo conversion of naïve CD4+ T cells to Tregs. This induction generates heightened suppressive function and maintenance of Foxp3 expression through inhibition of Akt-mammalian target of rapamycin (mTOR) signaling and increasing phosphatase and tensin homolog (PTEN) activity [7, 8]. This indicates that the PD-1 pathway stimulation results not only in a reduction in effector T cell function, but also an increase in immunosuppresive Treg function. This allows for proper control of immune homeostasis and creates a high threshold for T cell activation.
Though PD-1 has best been characterized in T cells, the implications for other cell subsets have been made apparent as well. The regulation of PD-1 expression is tightly controlled during B cell differentiation with levels increasing during the course of differentiation from being undetectable in pro B cells, an early precursor stage of B cell development [9]. Additionally, surface levels of PD-1 can be greatly enhanced in mature B cells following stimulation with Toll like receptor (TLR)-9 agonists. Blockade of PD-1 on B cells has been shown to increase antigen-specific antibody responses, suggesting PD-1 plays a role in inhibiting B cell clonal responses [10].
PD-1 has two binding ligands, PD-L1 (B7-H1, CD274) [11, 12] and PD-L2 (B7-DC, CD273) [13, 14], with PD-L1 being the most prominent in regulation. PD-L1 is inducibly expressed on both hematopoietic cells and non-hematopoietic cells following cell-specific stimulation. Cytokines such as IFN-γ and TNF-α up-regulate the expression of PD-L1 on T cells, B cells, endothelial cells, and epithelial cells, furthering its role in the maintenance of peripheral tolerance [1]. Data also links genetic changes seen in cancer cells to the induction of PD-L1, although this can vary by cancer type. PTEN dysfunction in human glioma cells induces Akt activation and subsequently PD-L1 expression, while human melanoma cells show no association between PTEN or Akt and PD-L1 induction [15, 16]. Recent data shows that PD-L1 binds to B7-1 (CD80) in addition to PD-1 [17]. While PD-L1 expression is induced on a wide array of both hematopoietic and non-hematopoietic cells, PD-L2 expression is restricted to inducible expression on DCs, macrophages, mast cells, and some B cells in response to IL-4 and IFN. The affinity of PD-L2 for PD-1 is three times greater than that of PD-L1, which indicates competition between the two ligands. Recent data confirms a second cognate receptor for PD-L2, repulsive guidance molecule B (RGMb) [18]. Despite recent research efforts surrounding PD-L2, little is known regarding the transcriptional regulation of the ligand.
Structures of PD-1 and its ligands
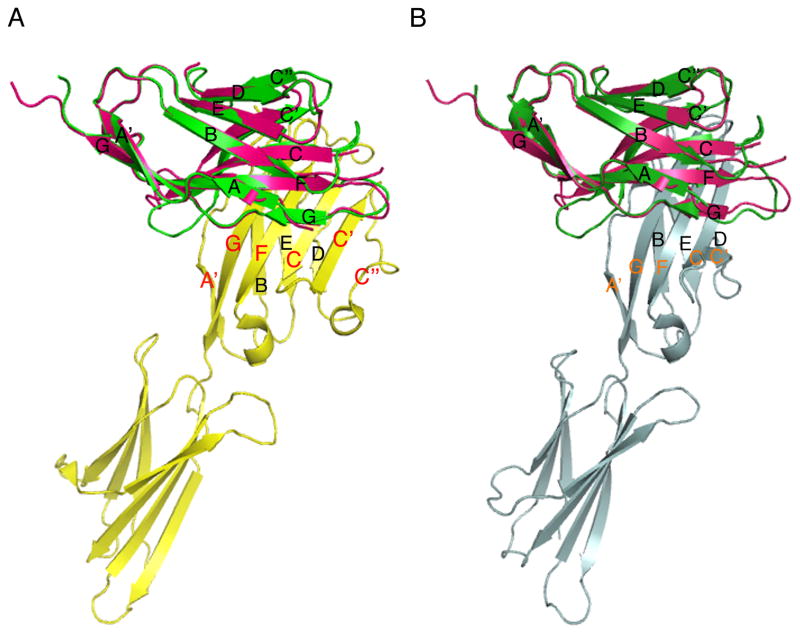
Structurally, PD-1 is a type I transmembrane receptor and belongs to the immunoglobulin superfamily (IgSF). Although it is functionally related to the costimulatory/coinhibitory receptors CD28, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and inducible T-cell co-stimulator (ICOS), PD-1 has important structural and functional differences. Other receptors in the CD28 family are disulfide-linked dimers, however structural and cell surface studies demonstrated that PD-1 is a monomeric glycoprotein [19]. The crystal structure of the extracellular region of mouse PD-1 shows the presence of a typical immunoglobulin variable domain (IgV) composed of front sheets (A′GFCC′C″) and back sheets (ABED; Figure 1), which are stabilized by a disulfide bond linking the F and B strands [19]. This IgV domain is linked to transmembrane and cytoplasmic domains through a 20 amino acid-long stalk region. In contrast to other CD28 family receptors, the absence of an extracellular cysteine residue in the stalk region prevents PD-1 from covalent dimer formation.
Figure 1. Crystal structures of the PD-1/PD-L1 and PD-1/PD-L2 complexes.
(A) Overlay of the crystal structures of human PD-1 (PDB Code 3RRQ) and the mouse PD-1/human PD-L1 complex (3BIK). (B) Overlay of human PD-1 with the mouse PD-1/PD-L2 complex (3BP5). Pink: human PD-1, green: mouse PD-1, yellow: human PD-L1, grey: mouse PD-L2
Human and mouse PD-1 share around 60% overall identity at the protein level, which increases to 75% for the residues forming the IgV domain. It is not surprising, therefore, that crystallographic (3RRQ, PDB) and NMR structures [20] show a high degree of similarity between mouse and human PD-1. Overlay of the crystal structures of mouse and human PD-1 show very similar arrangement (Figure 1). One notable difference between human and mouse PD-1 is the lack of the C″ strand at the edge of the front GFCC′ sheet in human PD-1, as shown by the NMR data [20]. This region presents rather as a highly flexible loop, consistent with the poor electron density observed for that region in the crystallographic dataset, indicating a disordered arrangement (3RRQ, PDB).
Another unique structural feature of PD-1 is that it lacks a consensus complementarity-determining region 3 (CDR3)-like conserved ligand binding motif. The ligand binding site consists of a hydrophobic patch on the front face, contributed by multiple residues from several strands [19]. Crystal structures available for the complexes of mouse PD-1 and human PD-L1 [21] and mouse PD-1 with mouse PD-L2 [22] show similar overall molecular architecture for these inhibitory complexes. Both PD-1 and its ligands interact with their respective surface residues distributed over their front beta sheets (front-to-front binding). In contrast, the FG loop of PD-1, which corresponds to the CDR3 variable region of the immunoglobulin structure, makes little or no contact with PD-L1 or PD-L2 (Figure 1). The crystal structures of the PD-1/PD-L complexes reveal that PD-1 binds its ligands in a 1:1 stoichiometry and forms monomeric complexes. This indicates a distinct ligand binding mode and signaling mechanism which is different from other coinhibitory receptor/ligand interactions such as CTLA-4/B7, where oligomerization plays an important role in signaling. Although crystal structures for the human PD-1/PD-L1 and PD-1/PD-L2 complexes have yet to be solved, the overall similarity of the mouse and human PD-1 structures suggests that mouse and human PD-1 likely form similar complexes. Overlay of human PD-1 with the mouse complexes shows that human PD-1 may bind to its ligands in the same way as mouse PD-1, however a recent study using NMR with binding data and mathematical modeling suggests that PD-1 may be engaged by its two ligands differently [20].
Importantly, the available crystal structures of PD-1 and the PD-1/PD-L1 and PD-1/PD-L2 complexes allow not only for the mapping of the ligand binding sites and mAb blocking epitopes, but also for the design of small molecule inhibitors.
Prognostic relevance of PD-1 and its ligands in human malignancies
Persistent expression of PD-1 by T cells is highly indicative of an exhausted phenotype noted by a decrease in effector function [4, 5]. This phenotype has been observed in various types of tumor-infiltrating lymphocytes (TILs) and linked to poor prognosis and tumor recurrence, highlighting PD-1 as an important molecule in regulating anti-tumor activity (Table 1). Similar to PD-1, PD-L1 and PD-L2 also possess prognostic capacities in some human malignancies (Table 2). Some clinical studies associate high expression of PD-L1 in tumors to tumor size, lymph nodal involvement, grade, and overall survival, while PD-L2 has generally only been tied to a trend in decreased survival, which is not of statistical significance (Table 2). PD-L1 generally has a much broader expression pattern when compared to PD-L2. This indicates that the regulation of PD-L2 depends much more on environmental stimuli than PD-L1. Data from the compilation of all these studies provides a solid rationale for investigating the immunological mechanisms behind the clinical associations. This is important because the poor prognosis indicated by expression of PD-1 on TILs and the expression of PD-L1/2 on tumor cells has been taken into consideration therapeutically.
Table 1.
Prognostic significance of PD-1 expression in human tumor-infiltrating lymphocytes.a
| Tumor | Clinical Correlation | Reference |
|---|---|---|
| Breast | High tumor infiltrating PD-1+ cell counts decreased patient survival. | [23] |
| Breast | PD-1+ TILs associated with tumor size, grade, LN status, and worse overall survival. | [24] |
| Prostate | CD8+ TILs expressed high levels of PD-1 and had restricted TCR Vbeta gene usage. | [25] |
| Thyroid | PD-1+ T cells in LN were indicative of recurrent disease and correlated with Treg frequency. | [26] |
| Melanoma | PD-1+ TILs expressed CTLA-4, displayed an exhausted phenotype, and were functionally impaired compared to PD-1− TILs. | [27] |
| Melanoma | PD-1 expression on CD4+/CD8+ T cells was found in primary tumor with greater expression in distant metastases. | [28] |
| Ovarian | CD8+ PD-1+ T cells were more impaired in IFN-γ/TNF-α secretion compared to CD8+ PD-1− T cells. | [29] |
| RCC | Patients with PD-1+ intratumoral immune cells associated with advanced tumor-node-metastasis stage and at significant risk for cancer-specific death compared to PD-1− patients. | [30] |
| NSCLC | CD8+ TILs increased PD-1 expression resulting in reduced cytokine production and capacity to proliferate. | [31] |
| HCC | CD8+ PD-1+ TILs predicted disease progression tumor recurrence. | [32] |
Abbreviations: CTLA-4, cytotoxic T-lymphocyte-associated protein 4; HCC, Hepatocellular carcinoma; IFN-γ, interferon γ; LN, Lymph node; NSCLC, Non-small-cell lung cancer; PD-1, Programmed death 1; RCC, Renal Cell Carcinoma; TCR, T cell receptor; TILs, Tumor-infiltrating lymphocytes; TNF-α, tumor necrosis factor α; Treg, regulatory T cells.
Table 2.
Prognostic significance and pathological associations of PD-L1 and PD-L2 on human tumor cells.a
| Tumor | Clinical Correlation | Reference |
|---|---|---|
| Colon | PD-L1 expression was associated with TNM staging and predicted prognosis. | [33] |
| Cervical | PD-L1 expressed in only a minority of samples and does influence patient survival. | [34] |
| Pancreatic | PD-L1 positive patients had poorer prognosis than PD-L1 negative patients and inversely correlated with CD8+ TILs; PD-L2 showed no significant correlation with patient survival. | [35] |
| Breast | PD-L1 expression correlated with tumor size, grade, LN status, and significantly worse overall survival. | [36] |
| Ovarian | PD-L1 expression on monocytes in the ascites and blood of patients with malignant ovarian carcinoma was greater than those with borderline/benign disease; PD-L1 expression led to poorer prognosis and inverse correlation with intraepithelial CD8+ T cells while PD-L2 showed poorer prognosis, but not a significant difference. | [37] [38] |
| RCC | Soluble PD-L1 associated with larger tumors, stage, grade, necrosis, and increased risk of death; PD-L1 associates with poor prognosis. | [39] [40] |
| HCC | PD-L1 expression on hematoma cells enriched apoptotic CD8+ T cells; higher expression of PD-L1 had significantly poorer prognosis and was an independent predictor for recurrence while PD-L2 expression correlated with poorer survival but not recurrence. | [32] [41] |
| NSCLC | PD-L1 associated with EGFR mutations and was a negative prognostic factor for disease. | [42] |
| Melanoma | Greater PD-L1 expression correlated with significantly lower overall survival and vertical growth of primary tumors; PD-L1 marks a subset of melanomas with shorter overall survival. | [43] [44] |
| Esophageal | PD-L1 and PD-L2 expression led to significantly poorer prognosis while only PD-L2 expression inversely correlated with CD8+ TILs. | [45] |
Abbreviations: EGFR, Epidermal Growth Factor Receptor; HCC, Hepatocellular Carcinoma; LN, Lymph node; NSCLC, Non-small-cell lung cancer; PD-L1, programmed death ligand 1; RCC, Renal Cell Carcinoma; TILs, Tumor infiltrating lymphocytes; TNM, Tumor-node-metastasis.
Mechanisms of anti-PD-1 and anti-PD-L1 immunotherapy
Appreciating the consequences of the up-regulation of the PD-1/PD-L1/2 axis aids our progress in manipulating an immunosuppressive cancer microenvironment. The cytoplasmic tail of PD-1 contains two signaling motifs. One is an immunoreceptor tyrosine-based inhibitory motif (ITIM), and the other is an immunoreceptor tyrosine-based switch motif (ITSM). Binding of PD-L1 or PD-L2 to PD-1 on activated T cells, along with TCR signaling, leads to phosphorylation of the cytoplasmic domain tyrosines and recruitment of a Src homology 2-containing tyrosine phosphatase (SHP-2) to the ITSM. Consequently, SHP-2 dephosphorylates TCR-associated CD-3ζ and zeta-chain-associated protein kinase 70 (ZAP70), resulting in inhibition of downstream signaling including blocking phosphoinositide 3-kinase (PI3K) and Akt activity, disrupting glucose metabolism and IL-2 secretion [5, 46].
Monoclonal antibodies (mAbs) have been developed for cancer immunotherapy by enhancing T cell function via blockade of the binding between PD-1 and PD-L1 or PD-L2 (Figure 2). Many of these studies have shown that blockade of PD-1 alone or PD-L1 leads to an increase in T cells and IFN-γ at the tumor site [47], along with decreases in the percentages of the highly immunosuppressive myeloid-derived suppressor cell (MDSC) population [48]. Increase in the effector to suppressor cell ratio usually supports an anti-tumor microenvironment. These results demonstrate that the neutralization of PD-1, PD-L1, or PD-L2 can be effective in controlling tumor growth by changing the dynamic of the tumor microenvironment.
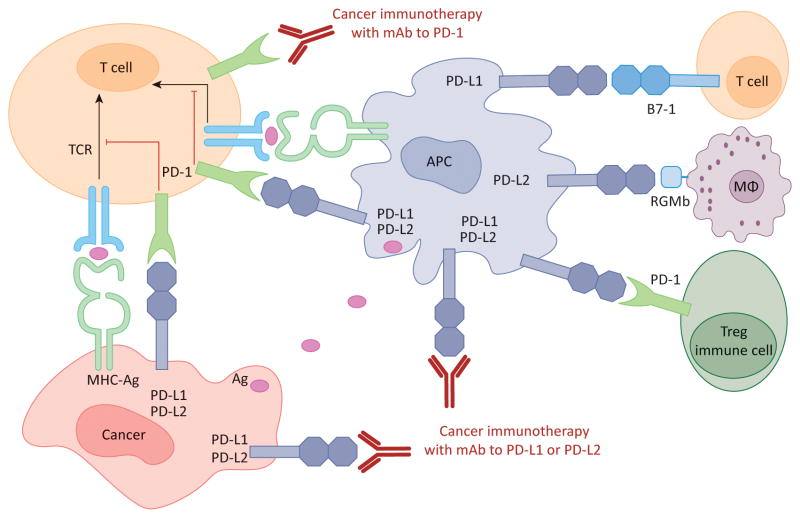
Figure 2. Human cancer immunotherapy with anti-PD-1 and anti-PD-L1/L2 antibodies.
Antigen-presenting cells (APC) take up antigens (Ag) released from cancer cells and present to T cells. Cancer cells can also present Ag to activated T cells in the context of MHC. Upon T cell activation, PD-1 receptors are expressed on T cells and inhibit immune responses by engagement of PD-L1 and PD-L2 ligands on APC and PD-L1 on cancer cells. Therefore, monoclonal antibody (mAb)-mediated specific blockade of the PD-1/PD-L1/PD-L2 pathway can enhance anti-tumor immunity. In addition to binding to PD-1, PD-L1 and PD-L2 also bind B7-1 and repulsive guidance molecule B, respectively. In addition to T cells and APC, PD-1 and PD-L1 can be induced on other immune cells.
Additional approaches generating synergy are the blockade of PD-1 or PD-L1 in combination with other therapeutic agents. Simultaneous blockade of both PD-1 and CTLA-4 leads to expansion of TIL populations while reducing the number of MDSC within the tumor, leading to tumor regression and significant increases in IFN-γ and TNF-α in CD8+ T cells [49]. Furthermore, chemotherapy and radiotherapy are being studied in combination with the blockade of the PD-1/PD-L1 pathway [50, 51]. Together these results set the stage for an optimistic clinical outlook.
Various biological inhibitors of PD-1 and PD-L1 have been developed and are currently being tested in clinical trials with cancer patients (Table 3). These inhibitors include mAbs to PD-1 and PD-L1 as well as PD-L2 fusion protein.
Table 3.
Biologics targeting PD-1 or PD-L1 in cancer clinical trials.
| Biologic | Class | Target | Company | Reference |
|---|---|---|---|---|
| CT-011 (Pidilizumab) | Humanized IgG1 | PD-1 | CureTech | [52] |
| MK-3475 (Lambrolizumab, Pembrolizumab) | Humanized IgG4 | PD-1 | Merck | [53] |
| BMS-936558 (Nivolumab) | Human IgG4 | PD-1 | Bristol-Meyers Squibb | [54] |
| AMP-224 | PD-L2 IgG2a fusion protein | PD-1 | Amplimmune/GlaxoSmithKline | [55] |
| BMS-936559 | Human IgG4 | PD-L1 | Bristol-Meyers Squibb | [56] |
| MEDI4736 | Humanized IgG | PD-L1 | MedImmune | [57] |
| MPDL3280A | Human IgG | PD-L1 | Roche | [58] |
| MSB0010718C | Human IgG1 | PD-L1 | Merck | [59] |
Clinical trials of monoclonal antibody to PD-1
Pidilizumab (CT-011) was the first mAb against PD-1 to reach clinical trials [52] (Table 4). It was initially identified as a mAb binding to the B-lymphoblastoid cell line that stimulated murine lymphocytes, and showed anti-tumor activity in mice [60]. It stimulated human peripheral blood lymphocytes and enhanced cytotoxicity towards human tumor cell lines. The first phase I trial with pidilizumab recruited patients with hematologic malignancies, including acute myeloid leukemia (AML), chronic lymphocytic leukemia (CLL), non-Hodgkin’s lymphoma (NHL), Hodgkin’s lymphoma and multiple myeloma [52]. Dose levels ranged from 0.2 to 6 mg/kg. A maximum tolerated dose (MTD) was not reached and the drug was well tolerated. Of the 17 patients enrolled in the study, one patient experienced a complete response, four had stable disease and one had a mixed response, amounting to a 33% clinical benefit rate. Durable responses of greater than 60 weeks were noted. This was followed by two phase II clinical trials [61, 62]. Patients with diffuse large B-cell lymphoma (DLBCL) or primary mediastinal B-cell lymphoma (PMBCL) who underwent autologous hematopoietic stem cell transplant (ASCT) and who had chemo-sensitive disease were treated with Pidilizumab at 1.5 mg/kg every 42 days for three cycles starting 30 to 90 days post-transplant [62]. The study enrolled 72 patients. Sixteen month progression free survival (PFS) for eligible patients was 72%, meeting the primary endpoint of the study. Intent to treat analysis revealed a 16 month PFS of 68%. Overall response rate for patients with measurable disease after ASCT was 51%. Most common grade 3 or 4 toxicities included neutropenia and thrombocytopenia. Correlative studies of select lymphocyte subsets revealed an increase in the number of activated CD25+PD-L1+ CD4+ T cells, PD-L1+PD-L2+CD14+ monocytes and circulating peripheral and central memory CD8 T cells as well as central memory CD4 T-cells. These results suggest that Pidilizumab may reverse PD-1 mediated inhibition of T-cell survival and proliferation.
Table 4.
Summary of reported clinical trials of monoclonal antibodies that target PD-1 or PD-L1 as monotherapy, or combination therapy with other agents, in human cancer.a
| Monotherapy | |||||
|---|---|---|---|---|---|
| Antibody | Dose | Phase | Cancer | NCT Trial Number | Reference |
| Pidilizumab | 0.2–6 mg/kg | I | AML, CLL, NHL, HL, MM | N/A | [52] |
| Pidilizumab | 1.5 or 6 mg/kg | II | Malignant melanoma | NCT01435369 | [72] |
| Pembrolizumab | 1–10 mg/kg | I | Advanced solid tumors | NCT01295827 | [53] [63] [65] [73] |
| Nivolumab | 0.3–10 mg/kg | I | Advanced solid tumors | NCT00441337 | [67] |
| Nivolumab | 1–10 mg/kg | I | Advanced solid tumors | NCT00730639 | [68] |
| Nivolumab | 1–20 mg/kg | I | Advanced solid tumors | N/A | [74] |
| Nivolumab | 0.3–10 mg/kg | II | RCC | NCT01354431 | [75] |
| Nivolumab | 1 or 3 mg/kg | II | Platinum resistant ovarian cancer | N/A | [76] |
| BMS-936559 | 0.3–10 mg/kg | I | Advanced solid tumors | NCT00729664 | [56] |
| MPDL3280A | 1–20 mg/kg | I | Advanced solid tumors and disease specific cohorts | NCT01375842 | [58] [77] [78] [79] [80] |
| MEDI4736 | 0.1–15 mg/kg | I | Advanced solid tumors | NCT01693562 | [81] |
| MSB0010718C | 1–20 mg/kg | I | Refractory malignancies | NCT01772004 | [59] |
| Combination Therapy | |||||
| Pidilizumab after ASCT | 1.5 mg/kg | II | NHL | NCT00532259 | [62] |
| Pidilizumab + rituximab | 3 mg/kg | II | Follicular lymphoma | NCT00904722 | [61] |
| Nivolumab + Ipilimumab | 1 mg/kg 3 mg/kg |
I | Malignant melanoma | NCT01024231 | [71] |
| Nivolumab + platinum and Nivolumab + ipilimumab | 10mg/kg 1 mg/kg + 3 mg/kg |
I | NSCLC | NCT01454102 | [82] [83] |
| Nivolumab + ipilimumab | 3 mg/kg + 1 mg/kg | I | RCC | NCT01472081 | [84] |
| Nivolumab + multipeptide vaccine | 1–10 mg/kg | I/II | Malignant melanoma | NCT01176461 | [85] |
Abbreviations: AML, acute myeloid leukemia; ASCT, autologous hematopoietic stem cell transplant; CLL, chronic lymphocytic leukemia; HL, Hodgkin’s lymphoma; MM, multiple myeloma; NHL, non-Hodgkin’s lymphoma; NSCLC, Non-small-cell lung cancer; PFS, progression-free survival; RCC, Renal Cell Carcinoma.
The second phase II study with Pidilizumab was a combined treatment with rituximab for follicular lymphoma [61]. Patients with rituximab sensitive disease were treated with four doses of Pidilizumab at 3 mg/kg every four weeks, with the option to continue therapy if they showed a response or stable disease. The study enrolled 32 patients. Patients were fairly well distributed across the three risk groups of the Follicular Lymphoma International Prognostic Index (FLIPI) 1 and 2. Objective response rate was 66%, which met the study endpoint of greater than 60% as compared to a historical response rate of 40% with rituximab alone. Complete response rate was 52%. Responses were durable with a median PFS of 18.8 months. FLIPI 1 or 2 score was not associated with response rates. The regimen was well tolerated with no grade 3 or 4 adverse events. PD-L1 expression was significantly higher on CD4+, CD8+ and CD14+ peripheral blood cells from responding patients, but was not associated with PFS. Gene expression data suggested that intrinsic anti-lymphoma immunity might be predictive of a response to Pidilizumab. Pidilizumab continues to be evaluated in a variety of clinical trials, including solid tumors and hematologic malignancies, both as a single agent and in combination with other regimens including cellular therapies and cancer vaccines.
Pembrolizumab (MK-3475; previously known as Lambrolizumab; Table 4) is a humanized IgG4 PD-1 blocking mAb [53, 63]. It is also the first monoclonal antibody targeting PD-1 that has been granted accelerated FDA approval. A very high affinity mouse anti-human PD-1 antibody was developed, the variable region of which was grafted to a human IgG4 immunoglobulin with a stabilizing S228P Fc mutation. The IgG4 immunoglobulin subtype does not engage Fc receptors or activate complement, and thus avoiding cytotoxic activity against T cells. Pembrolizumab was studied in a phase I trial in patients with advanced solid tumors [53]. The dose range was 1 to 10 mg/kg and a MTD was not identified. In the nine patients enrolled in the study, no grade 3 or 4 toxicities were noted. One patient with melanoma experienced a partial response, with an additional three patients experiencing stable disease. Pembrolizumab activity and safety in melanoma was further explored by recruiting an expansion cohort at the 10 mg/kg dose level [63]. Doses ranged from 2 mg/kg every three weeks to 10 mg/kg every two weeks. Patients with advanced melanoma, including patients with prior ipilimumab treatment, an FDA-approved mAb to CTLA-4, were allowed in this study. Results from 135 treated patients were reported. Response rate across all dose cohorts was 38%, with the highest response rate observed in the 10 mg/kg cohort every two weeks (52%). Responses were durable and overall PFS was longer than 7 months. Median overall survival (OS) was not reached. Treatment related grade 3 or 4 adverse events were reported in 13% of patients. The highest incidence of treatment related adverse events was in the 10 mg/kg every two weeks group. Endocrine toxicities included hypothyroidism in 8.1% of patients, with one case being grade 3 or 4, grade 3 hyperthyroidism, and grade 2 adrenal insufficiency. Correlative studies on available tumor biopsies showed that regressing lesions were densely infiltrated with CTLs, which was consistent with the mechanism of action of the drug. PD-L1 expression by tumor cells was significantly associated with PFS and response rate but not with OS [64]. It is important to note here that despite a very low cutoff for PD-L1 positivity (1% of stained cells), antitumor activity was noted in tumors with low PD-L1 expression. These results cast doubt on the utility of PD-L1 expression as a biomarker and suggest that the mechanism of action of PD-1 targeting antibodies remains to be fully elucidated.
To explore the efficacy of Pembrolizumab in malignant melanoma patients who had progressed after ipilimumab or treatment with a BRAF or mitogen-activated protein kinase kinase (MEK) inhibitor, or both, an open-label, randomized, multicenter expansion cohort of the KEYNOTE-001 trial was performed [65]. The trial randomized 173 patients to receive Pembrolizumab at 2mg/kg or 10 mg/kg every three weeks. Overall response rate was 26% in both groups. Survival at one year was similar in both treatment groups (58% and 63%). Pembrolizumab was well tolerated with drug-related grade 3 or 4 adverse events reported in 12% of patients in both arms. Six patients (3%) discontinued treatment due to adverse events. Three patients experienced immune-related adverse events and were managed with dose interruption and corticosteroid treatment. Results from the KEYNOTE-001 trial served as the basis for the accelerated FDA approval of Pembrolizumab for treatment of patients with advanced or unresectable melanoma that progressed after therapy with ipilimumab or after therapy with a BRAF inhibitor if the tumors carry the BRAF V600 mutation. Of note in this trial is that both dose levels exhibited similar anti-tumor activity and toxicity profiles. Pembrolizumab continues to be evaluated in phase I trials in advanced solid tumors, head and neck cancers, and hematologic malignancies, and in combination with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma, as well as phase II and III trials in microsatellite unstable tumors, in combination with pazopanib in renal cell cancer, and in comparison to docetaxel in non-small cell lung cancer.
Nivolumab (MDX-1106, BMS-936558, ONO-4538; Table 4) is a fully human IgG4 mAb to PD-1. In a humanized in vitro model of melanoma, addition of Nivolumab to human-vaccine induced CD8+ T cells specific to melanoma antigens allowed for the expansion of these lymphocytes [54]. In another study of an ex vivo melanoma model, addition of the same antibody led to the unmasking of CTL inhibition by Tregs and stimulated their proliferation [66]. Nivolumab was first studied clinically in a phase I trial in patients with advanced solid tumors. Thirty-nine patients with metastatic melanoma, colorectal cancer (CRC), castrate-resistant prostate cancer (CRPC), non-small cell lung cancer (NSCLC), or renal cell carcinoma (RCC) were enrolled [67]. Initial treatment entailed a single infusion of Nivolumab in dose-escalating six patient cohorts at 0.3, 1, 3, or 10 mg/kg. This was followed by a 15 patient expansion cohort at the 10mg/kg dose level. Patients who had shown clinical benefit at three months were eligible for repeated therapy. MTD was not reached. One durable complete response of greater than 21 months was noted in a patient with CRC and two partial responses were noted in a patient with melanoma and another with RCC. Two additional responses were noted in one melanoma and one NSCLC patient, but did not meet partial response criteria. Tumor biopsies were available from a small number of patients, and PD-L1 expression appeared to correlate with response to therapy. A cutoff of 5% of tumor cells exhibiting membranous PD-L1 staining was used to define positivity for PD-L1 expression. Nivolumab was well tolerated with one patient experiencing grade 3 inflammatory colitis treated with infliximab and steroids.
Nivolumab was further studied in another large phase I study [68]. Patients were treated with escalating doses ranging from 1 to 10 mg/kg. Analysis of 304 patients, including expansion cohorts, revealed that Nivolumab was well tolerated, with no MTD reached. Objective response rates for melanoma, NSCLC, and RCC were 31%, 16% and 29% respectively. No responses were noted in patients with CRC or CRPC. Responses were durable and lasted over a year. Updated results indicate that responses are durable in patients with NSCLC with OS rates of 12–45% at two years [69]. Similarly, two and three year OS rates in patients with advanced melanoma were 48% and 41% respectively [70]. Examination of PD-L1 expression revealed that responses were noted only in PD-L1 positive tumors (36%). Again, a cutoff of 5% of tumor cells staining positive for membranous PD-L1 expression by immunohistochemistry was used to define positivity. Grade 3 or 4 adverse events were noted in 15% of patients, and 6% discontinued treatment. Adverse events with potential immune related etiology occurred in 45% of patients, 6% of which were grade 3 or 4. They reported three deaths due to pneumonitis. Other studies with Nivolumab reported similar results as detailed in Table 4.
Nivolumab was also studied in combination with ipilimumab in a phase I trial [71]. The trial included a combined regimen arm that enrolled 53 patients, and a sequenced regimen arm that enrolled 33. The MTD was determined to be the 1 mg/kg of nivolumab and 3 mg/kg of ipilimumab dose level (cohort 2) or the 3 mg/kg of Nivolumab and 1 mg/kg of ipilimumab (cohort 2a) of the combined regimen. Response rates were 40% in the combined regimen group, and up to 53% in the MTD cohort. Complete responses were noted as well as 80% tumor reduction in 16 patients. Responses were durable, ranging from 6.1 to 72.1 weeks at the time of analysis. Treatment related grade 3 or 4 adverse events were observed in 53% of patients, and serious adverse events were reported in 49% of patients in the combined regimen group. A total of 21% of patients discontinued therapy due to adverse events. Adverse events were managed with immune suppressants, some requiring infliximab or mycophenolate mofetil. No treatment related deaths were reported. Regarding PD-L1 expression by tumor cells, 21 of 56 patients (38%) were positive for PD-L1 by immunohistochemistry, but this did not correlate with response. This study utilized a different anti-PD-L1 clone and a cutoff of 5% of tumor cells expressing PD-L1 was used to define positivity. Responses were noted in PD-L1 negative tumors.
Ongoing trials with Nivolumab include phase I trials in hematologic malignancies, hepatocellular carcinoma, malignant melanoma, in combination with sunitnib, pazopanib, or ipilimumab in RCC, with ipilimumab in advanced solid tumors, in combination with IL-21 in advanced solid tumors, with a multipeptide vaccine in malignant melanoma, with lirilumab (anti-KIR; BMS-986015) in advanced solid tumors, with dasatinib in relapsed chronic myelogenous leukemia (CML). Ongoing phase II and phase III trials with Nivolumab are conducted with or without ipilimumab versus bevacizumab in glioblastoma and with ipilimumab in malignant melanoma, in comparison to chemotherapy for NSCLC and malignant melanoma, and in comparison to everolimus for RCC.
Clinical trials of monoclonal antibody to PD-L1
BMS-936559 is a fully human monoclonal IgG4 antibody that blocks PD-L1 [56] (Table 4). This blockade was shown to augment T cell proliferation in response to allogeneic dendritic cells in a mixed lymphocyte reaction, as well as antigen-specific T cell responses to cytomegalovirus (CMV) antigen, and antitumor peptide responses in subjects treated with melanoma antigen peptide vaccines. BMS-936559 can reverse in vitro Treg mediated suppression. Use of BMS-936559 in clinical trials was supported by the ability of anti-PD-L1 antibodies to inhibit tumor growth in murine syngeneic tumor models, and long lived antitumor immunity was observed. BMS-936559 did not induce antibody-dependent cytotoxicity or complement-dependent cytotoxicity in PD-L1 positive cells. BMS-936559 was studied in a phase I trial [56]. Patients with a variety of solid tumors were enrolled, with disease specific expansion cohorts enrolled in parallel and treated at the 10 mg/kg dose level. MTD was not reached, and the discontinuation rate due to adverse events was 11%, 6% of which were treatment related. Objective responses were observed in patients treated with a dose level of 1 mg/kg or higher. Responses were noted in patients with melanoma, NSCLC, RCC, and ovarian cancer but none were observed in CRC or pancreatic cancer. Treatment was well tolerated with the expected immune related adverse events that were most grade 1 or 2, and treated with treatment interruption or glucocorticoids. Despite encouraging results, BMS-936559 does not seem to be developed any further by the manufacturer.
MEDI4736 is a humanized PD-L1 blocking monoclonal antibody [57] that showed T cell dependent antitumor activity in an in vivo model where tumor cells were co-implanted with human T cells, or when a surrogate antibody was used in a syngeneic mouse model (Table 4). Combination with oxaliplatin resulted in complete tumor regression in greater than 50% of treated animals. MEDI4736 is being studied in a phase I trial in advanced solid tumors with expansion cohorts in several responding tumor types including NSCLC and melanoma [81, 86]. It was noted to be well tolerated, with tumor shrinkage detectable in many tumor types. It is also being studied in combination with tremelimumab (a mAb to CTLA-4) in patients with advanced solid tumors [87], and also with dabrafenib and trametinib or trametinib alone in patients with malignant melanoma.
Other mAbs to PD-L1 include MPDL3280A which has shown impressive results by shrinking tumors in 43% of patients in a phase I clinical trial in metastatic urothelial bladder cancer patients [58] (http://www.roche.com/media/media_releases/med-cor-2014-05-31.htm) (Table 4). It has been granted FDA breakthrough designation due to its activity in urothelial bladder cancers. It has also shown acceptable tolerability and efficacy in several solids tumors with no MTD reached [77]. Ongoing clinical trials include phase I trials in advanced solid tumors, the phase II BIRCH trial and phase III OAK trial in NSCLC. MSB0010718C is another mAb to PD-L1. It is a fully human IgG1 and is expected to show antineoplastic activity by inhibiting the PD-1/PD-L1 interaction and by antibody-dependent cell-mediated cytotoxicity [59]. MSB0010718C is being studied in a phase I trial in refractory malignancies.
Clinical trials of PD-L2 immunoglobulin fusion protein
As an alternative strategy to monoclonal antibodies, AMP-224 (B7-DC-Ig) was developed as a chimeric fusion protein between the extracellular domain of PD-L2 and an Fc portion of IgG2a (http://www.prnewswire.com/news-releases/glaxosmithkline-and-amplimmune-form-global-strategic-collaboration-99938599.html). In vivo studies suggested that this fusion protein can ameliorate disease by inducing immune responses to pathogens. Furthermore, the murine form of AMP-224 can enhance the therapeutic efficacy of vaccine when combined with cyclophosphamide in a mouse model [55]. AMP-224 exerts its therapeutic effect via a mechanism distinct from the direct blocking of the PD-1/PD-L1 interaction. It is hypothesized that AMP-224 can deplete exhausted effector T cells that express high levels of PD-1, and the T cell pool is replenished with functional T-cells [88]. Ongoing trials with AMP-224 include a phase I trial with cyclophosphamide in advanced solid tumors which has shown no drug-related inflammatory adverse events other than infusion reactions, as well as preliminary tumor responses [88].
Concluding remarks
After success with Ipilimumab in the treatment of malignant melanoma, the field of cancer immunotherapy continues to grow. Blockade of T cell inhibition allows for restored antitumor immunity and has shown impressive results in clinical trials. Beyond the CTLA-4 pathway, T cell inhibition mediated by the PD-1/PD-L1 pathway is now the most studied and clinically developed cancer immunotherapy. Several mAbs to PD-1 or PD-L1 have been studied in phase I trials and continue to be evaluated in phase II and phase III trials. These therapies have been well tolerated, with no MTD reached in most single agent phase I studies. Grade 3 or 4 adverse event rates have been generally acceptable with low treatment-related discontinuation rates. Response rates have been impressive, particularly in melanoma, NSCLC and RCC, with encouraging early reports from urothelial bladder cancer and platinum resistant ovarian cancer. PD-1/PD-L1 blockade has also shown efficacy in hematologic malignancies. What has been striking is the durability of responses. Potential biomarkers for efficacy of PD-1/PD-L1 blockade are being studied, and to date have mainly focused on PD-L1 expression by the tumor. Data from most clinical studies is not yet mature, but preliminary data indicate that PD-L1 expression by tumor cells may correlate with a higher response rate and PFS in some patients. It is encouraging that Pembrolizumab has been granted accelerated FDA approval as second line therapy in advanced melanoma. Integrating immunotherapy into current clinical practice remains to be studied, and many outstanding questions remain (see Box 1). The variability in response both by tumor subtype and by PD-L1 expression indicate that immune checkpoint inhibition remains a field that is open to study, and PD-1/PD-L1 blockade, as CTLA4 blockade was before it, is only the beginning of immunomodulatory therapies.
Text Box 1. Outstanding questions.
Why are the response rates of anti-PD-1 and anti-PD-L1 variable among different cancers?
Can clinical response biomarkers be identified and how can these be integrated into clinical practice?
How can anti-PD-1 and anti-PD-L1 antibodies be integrated into current treatment regimens in upfront and relapsed settings?
Does PD-1 expressed on immune cells other than T cells play a role in anti-PD-1/L1 therapy?
Can we develop small molecule inhibitors of the PD-1/PD-L1 interaction?
Highlights.
The PD-1/PD-L1 pathway inhibits T cell functions
The PD-1/PD-L1 pathway functions as immune evasion in some cancers
Clinical trials targeting PD-1 and PD-L1 show impressive response rates
Acknowledgments
K.C.O is supported by National Institutes of Health (NIH) F31CA183493. X.Z. is supported by NIH R01CA175495, Department of Defense Established Investigator Idea Development Award PC131008 and Dr. Louis Sklarow Memorial Trust.
Glossary
- Cancer immunotherapy
treatments that use the host immune system to inhibit cancer
- Monoclonal antibody
antibodies generated by immune cells which are derived from a same parent cell
- Programmed death 1 (PD-1)
a 288 amino acid cell surface molecule in human encoded by the PDCD1 gene that functions to negatively regulate immune responses
- Programmed death ligand 1 (PD-L1)
a 40 kDa type 1 transmembrane protein encoded in humans by the CD274 gene that suppresses the immune system in cancer, pregnancy, tissue allografts, and autoimmune diseases
- T cell
type of lymphocyte that has an important role in cell mediated immunity that is distinguished by its T cell receptor on the cell surfacel; referred to as T cells because they mature in the thymus
- T cell coinhibition
a signal required for inhibition of activated T cells in the presence of T cell receptor signal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keir ME, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishida Y, et al. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 4.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 5.Hofmeyer KA, et al. The PD-1/PD-L1 (B7-H1) pathway in chronic infection-induced cytotoxic T lymphocyte exhaustion. Journal of biomedicine & biotechnology. 2011;2011:451694. doi: 10.1155/2011/451694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Francisco LM, et al. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haxhinasto S, et al. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thibult ML, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25:129–137. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 10.Nicholas KJ, et al. B cell responses to HIV antigen are a potent correlate of viremia in HIV-1 infection and improve with PD-1 blockade. PLoS One. 2013;8:e84185. doi: 10.1371/journal.pone.0084185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong H, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latchman Y, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 14.Tseng SY, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193:839–846. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atefi M, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res. 2014;20:3446–3457. doi: 10.1158/1078-0432.CCR-13-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parsa AT, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 17.Butte MJ, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao Y, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 2004;20:337–347. doi: 10.1016/s1074-7613(04)00051-2. [DOI] [PubMed] [Google Scholar]
- 20.Cheng X, et al. Structure and interactions of the human programmed cell death 1 receptor. J Biol Chem. 2013;288:11771–11785. doi: 10.1074/jbc.M112.448126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin DY, et al. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc Natl Acad Sci U S A. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lazar-Molnar E, et al. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc Natl Acad Sci U S A. 2008;105:10483–10488. doi: 10.1073/pnas.0804453105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S, et al. PD-1(+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother. 2014;63:395–406. doi: 10.1007/s00262-014-1519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muenst S, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2013;139:667–676. doi: 10.1007/s10549-013-2581-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sfanos KS, et al. Human prostate-infiltrating CD8+ T lymphocytes are oligoclonal and PD-1+ Prostate. 2009;69:1694–1703. doi: 10.1002/pros.21020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.French JD, et al. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E934–943. doi: 10.1210/jc.2011-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmadzadeh M, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114:1537–1544. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chapon M, et al. Progressive upregulation of PD-1 in primary and metastatic melanomas associated with blunted TCR signaling in infiltrating T lymphocytes. J Invest Dermatol. 2011;131:1300–1307. doi: 10.1038/jid.2011.30. [DOI] [PubMed] [Google Scholar]
- 29.Matsuzaki J, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson RH, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13:1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi F, et al. PD-1 and PD-L1 upregulation promotes CD8(+) T-cell apoptosis and postoperative recurrence in hepatocellular carcinoma patients. Int J Cancer. 2011;128:887–896. doi: 10.1002/ijc.25397. [DOI] [PubMed] [Google Scholar]
- 33.Shi SJ, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS One. 2013;8:e76012. doi: 10.1371/journal.pone.0076012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karim R, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15:6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 35.Nomi T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 36.Muenst S, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat. 2014;146:15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maine CJ, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63:215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamanishi J, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frigola X, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.CCR-10-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thompson RH, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 41.Gao Q, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 42.Azuma K, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small cell lung cancer. Ann Oncol. 2014 doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 43.Hino R, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 44.Massi D, et al. PD-L1 marks a subset of melanomas with a shorter overall survival and distinct genetic and morphological characteristics. Ann Oncol. 2014 doi: 10.1093/annonc/mdu452. [DOI] [PubMed] [Google Scholar]
- 45.Ohigashi Y, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11:2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 46.Parry RV, et al. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zang X, Allison JP. The B7 family and cancer therapy: costimulation and coinhibition. Clin Cancer Res. 2007;13:5271–5279. doi: 10.1158/1078-0432.CCR-07-1030. [DOI] [PubMed] [Google Scholar]
- 48.John LB, et al. Anti-PD-1 antibody therapy potently enhances the eradication of established tumors by gene-modified T cells. Clin Cancer Res. 2013;19:5636–5646. doi: 10.1158/1078-0432.CCR-13-0458. [DOI] [PubMed] [Google Scholar]
- 49.Curran MA, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. doi: 10.1073/pnas.0915174107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ding ZC, et al. Immunosuppressive myeloid cells induced by chemotherapy attenuate antitumor CD4+ T-Cell responses through the PD-1-PD-L1 Axis. Cancer Res. 2014;74:3441–3453. doi: 10.1158/0008-5472.CAN-13-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Deng L, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 53.Patnaik A, et al. Phase I study of MK-3475 (anti-PD-1 monoclonal antibody) in patients with advanced solid tumors. ASCO Meeting Abstracts. 2012;30:2512. doi: 10.1158/1078-0432.CCR-14-2607. [DOI] [PubMed] [Google Scholar]
- 54.Wong RM, et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int Immunol. 2007;19:1223–1234. doi: 10.1093/intimm/dxm091. [DOI] [PubMed] [Google Scholar]
- 55.Mkrtichyan M, et al. B7-DC-Ig enhances vaccine effect by a novel mechanism dependent on PD-1 expression level on T cell subsets. J Immunol. 2012;189:2338–2347. doi: 10.4049/jimmunol.1103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brahmer JR, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beck A, et al. 6th Annual European Antibody Congress 2010: November 29–December 1, 2010, Geneva, Switzerland. mAbs. 2011;3:111–132. doi: 10.4161/mabs.3.2.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powles T, et al. Inhibition of PD-L1 by MPDL3280A and clinical activity in pts with metastatic urothelial bladder cancer (UBC) ASCO Meeting Abstracts. 2014;32:5011. [Google Scholar]
- 59.Heery CR, et al. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in advanced solid malignancies. ASCO Meeting Abstracts. 2014;32:3064. [Google Scholar]
- 60.Hardy B, et al. Activation of human lymphocytes by a monoclonal antibody to B lymphoblastoid cells; molecular mass and distribution of binding protein. Cancer Immunol Immunother. 1995;40:376–382. doi: 10.1007/BF01525388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Westin JR, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: a single group, open-label, phase 2 trial. Lancet Oncol. 2014;15:69–77. doi: 10.1016/S1470-2045(13)70551-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Armand P, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013;31:4199–4206. doi: 10.1200/JCO.2012.48.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daud AI, et al. Proceedings of the 105th Annual Meeting of the American Association for Cancer Research. Philadelphia (PA): AACR; 2014. Antitumor activity of the anti-PD-1 monoclonal antibody MK-3475 in melanoma(MEL): Correlation of tumor PD-L1 expression with outcome [abstract] Abstract nr CT104. [Google Scholar]
- 65.Robert C, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014 doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 66.Wang W, et al. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21:1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brahmer JR, et al. Nivolumab (anti-PD-1, BMS-936558, ONO-4538) in patients (pts) with advanced non-small-cell lung cancer (NSCLC): Survival and clinical activity by subgroup analysis. ASCO Meeting Abstracts. 2014;32:8112. [Google Scholar]
- 70.Hodi FS, et al. Long-term survival of ipilimumab-naive patients (pts) with advanced melanoma (MEL) treated with nivolumab (anti-PD-1, BMS-936558, ONO-4538) in a phase I trial. ASCO Meeting Abstracts. 2014;32:9002. [Google Scholar]
- 71.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atkins MB, et al. Phase 2, multicenter, safety and efficacy study of pidilizumab in patients with metastatic melanoma. ASCO Meeting Abstracts. 2014;32:9001. [Google Scholar]
- 73.Garon EB, et al. Safety and clinical activity of MK-3475 in previously treated patients (pts) with non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2014;32:8020. [Google Scholar]
- 74.Felip E, et al. Developmental therapeutics. Annals of Oncology. 2012;23:ix152–ix174. [Google Scholar]
- 75.Motzer RJ, et al. Nivolumab for metastatic renal cell carcinoma (mRCC): Results of a randomized, dose-ranging phase II trial. ASCO Meeting Abstracts. 2014;32:5009. [Google Scholar]
- 76.Hamanishi J, et al. Efficacy and safety of anti-PD-1 antibody (Nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. ASCO Meeting Abstracts. 2014;32:5511. [Google Scholar]
- 77.Herbst RS, et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. ASCO Meeting Abstracts. 2013;31:3000. [Google Scholar]
- 78.Cho DC, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstracts. 2013;31:4505. [Google Scholar]
- 79.Hamid O, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM) ASCO Meeting Abstracts. 2013;31:9010. [Google Scholar]
- 80.Spigel DR, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) ASCO Meeting Abstracts. 2013;31:8008. [Google Scholar]
- 81.Lutzky J, et al. A phase 1 study of MEDI4736, an anti-PD-L1 antibody, in patients with advanced solid tumors. ASCO Meeting Abstracts. 2014;32:3001. [Google Scholar]
- 82.Rizvi NA, et al. A phase I study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus platinum-based doublet chemotherapy (PT-doublet) in chemotherapy-naive non-small cell lung cancer (NSCLC) patients (pts) ASCO Meeting Abstracts. 2013;31:8072. [Google Scholar]
- 83.Antonia SJ, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. ASCO Meeting Abstracts. 2014;32:8023. [Google Scholar]
- 84.Hammers HJ, et al. Phase I study of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma (mRCC) ASCO Meeting Abstracts. 2014;32:4504. [Google Scholar]
- 85.Weber JS, et al. Phase I/II trial of PD-1 antibody nivolumab with peptide vaccine in patients naive to or that failed ipilimumab. ASCO Meeting Abstracts. 2013;31:9011. [Google Scholar]
- 86.Segal NH, et al. Preliminary data from a multi-arm expansion study of MEDI4736, an anti-PD-L1 antibody. ASCO Meeting Abstracts. 2014;32:3002. [Google Scholar]
- 87.Callahan MK, et al. A phase 1 study to evaluate the safety and tolerability of MEDI4736, an anti-PD-L1 antibody, in combination with tremelimumab in patients with advanced solid tumors. ASCO Meeting Abstracts. 2014;32:TPS3120. [Google Scholar]
- 88.Infante JR, et al. Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. ASCO Meeting Abstracts. 2013;31:3044. [Google Scholar]