Abstract
Background
The aryl hydrocarbon receptor (AHR) mediates the toxic effects of various endocrine disrupting chemicals. In female mice, global deletion of the Ahr (AhrKO) results in slow growth of ovarian antral follicles. No studies, however, have examined whether injection of the Ahr restores the phenotypes of cultured AhrKO ovarian antral follicles to wild-type levels.
Methods
We developed a system to construct a recombinant adenovirus containing the Ahr to re-express the Ahr in AhrKO granulosa cells and whole antral follicles. We then compared follicle growth and levels of factors in the AHR signaling pathway (Ahr, Ahrr, Cyp1a1, and Cyp1b1) in wild-type, AhrKO, and Ahr re-expressed follicles. Further, we compared the response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in wild-type, AhrKO, and Ahr re-expressed follicles.
Results
AdAhr injection into AhrKO follicles partially restored their growth pattern to wild-type levels. Further, Ahr re-expressed follicles had significantly higher levels of Ahr, Ahrr, Cyp1a1, and Cyp1b1 compared to wild-type follicles. Upon TCDD treatment, only Cyp1a1 levels were significantly higher in Ahr re-expressed follicles compared to the levels in wild-type follicles.
Conclusion
Our system of re-expression of the Ahr partially restores follicle growth and transcript levels of factors in the AHR signaling pathway to wild-type levels.
Keywords: aryl hydrocarbon receptor (AHR); ovary; mouse; 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD); adenovirus; AhrKO
Introduction
In recent years, with the use of the aryl hydrocarbon receptor (Ahr) deficient (AhrKO) mouse model, the AHR has been shown to be a key factor involved in organ development and cellular processes (Hernandez-Ochoa et al., 2010, Stockinger et al., 2014). For example, AhrKO mice develop multiple cardiovascular, hepatic, and immune abnormalities (Schmidt and Bradfield 1996). Further, AhrKO female mice exhibit serious reproductive defects, including pregnancy and lactation complications, reduced fertility and litter size, and early reproductive senescence (Abbott et al., 1999, Puga et al,. 2005). Additionally, AhrKO ovaries have significantly lower numbers of preantral and antral follicles compared to wild-type (WT) ovaries. Lastly, the growth of AhrKO antral follicles is significantly compromised compared to WT antral follicles in vitro (Barnett et al., 2007).
Antral follicles mainly consist of multiple layers of granulosa cells, theca cells, and an oocyte. All of these cell types are needed to form a functional ovarian unit that is capable of ovulation and synthesizing sex steroid hormones. Notably, all of these cell types express the Ahr; hence, they are capable of activating the AHR signaling pathway. Briefly, upon ligand binding, the cytoplasmic AHR translocates to the nucleus where it heterodimerizes with its nuclear translocator (ARNT) for further binding to specific DNA sequences and initiation of transcription of downstream target genes. Some of these downstream target genes encode xenobiotic metabolizing enzymes such as cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1a1) and cytochrome P450 1B1 (Cyp1b1). These enzymes are capable of metabolizing estrogen in the mammalian ovary. Termination of the activation of this signaling pathway is by the heterodimerization of ARNT with AHR repressor (AHRR) or by proteosomal degradation of the AHR (Abel and Haarmann-Stemmann 2010, Pollenz 2002, Puga et al., 2009, Schmidt and Bradfield 1996).
The AHR also plays a major role in mediating the toxic effects of various endocrine disrupting chemicals that affect reproductive function. One commonly used endocrine disrupting chemical in the study of AHR signaling pathway is the synthetic AHR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Upon binding of TCDD to the AHR, increased expression of Cyp1a1 and Cyp1b1 occurs, but activation of Cyp1a1 is more apparent due to its low levels at baseline compared to higher levels post-treatment with TCDD. Thus, Cyp1a1 is preferably used as a marker for activation of the AHR signaling pathway (Mandal 2005, Uno et al., 2004, Vanden Heuvel et al., 1993). The increased levels of CYP1A1 aid in detoxifying TCDD by oxygenation (Mimura and Fujii-Kuriyama 2003).
The use of global AhrKO mice has provided data regarding the roles and mechanisms of action of the Ahr in the ovary (Hernandez-Ochoa et al., 2009). Nevertheless, the available research tools (e.g. AhrKO mouse) lack the ability to single out the local and specific effects of the AHR on the ovary. Further, studies have not examined whether the effects of Ahr deletion are permanent or if Ahr re-expression can restore the response to control levels. Therefore, in the current study, we utilized in vitro methods to re-express the Ahr in granulosa cells isolated from AhrKO antral follicles. Following the successful expression in granulosa cells, we implemented similar methods using whole AhrKO antral follicles. We then tested the hypothesis that reexpression of the Ahr in AhrKO antral follicles restores follicle growth and levels of key factors in the AHR signaling pathway to WT levels. Specifically, antral follicle growth and selected expression levels were compared between WT, AhrKO, and Ahr re-expressed follicles. Additionally, as a hallmark of AHR activation, the response to TCDD exposure was compared in WT, AhrKO, and Ahr re-inserted follicles.
Materials and Methods
Chemicals
TCDD dissolved in dimethyl sulfoxide (DMSO) at 50 µg/mL (#ED-901-B, Cambridge Isotope Laboratories, Inc., USA) was further diluted with DMSO (Sigma-Aldrich, USA) to generate a final working dilution of 1.33 µM. This allowed us to add an equal volume of TCDD (0.75 µl/ml) in supplemented media as described below.
Animals
Both WT (Ahr+/+) and AhrKO (official symbol Ahrtm1Bra) mice were generated by intercrossing either heterozygous (Ahr+/−) female and male mice or heterozygous Ahr+/− female mice with AhrKO (Ahr−/−) male mice. The AhrKO mice were originally generated by Schmidt et al. (Schmidt et al., 1996). Mouse genetic screening was performed using ear tissue punches as previously described (Benedict et al., 2000). All mice were housed and bred in the core animal facility located at the College of Veterinary Medicine, University of Illinois. Mice were kept under a 12L:12D photoperiod, at temperature of 22±1°C, with 35% ± 4% relative humidity. Food (Harlan Teklad 8626, USA) and reverse-osmosis purified water were provided ad libitum. Female mice were euthanized at 30–38 days of age by carbon dioxide inhalation followed by cervical dislocation. All procedures and experimental methods involving animals were approved by the University of Illinois Institutional Animal Care and Use Committee.
Cloning of Ahr cDNA
Total RNA was isolated from C57BL/6J mouse ovaries using a RNeasy Mini kit (Qiagen, USA) and subjected to cDNA synthesis using an Omniscript reverse transcription kit (Qiagen, USA). Ahr primers were used according to Ma et al. (Ma et al., 1995). A LongRange polymerase chain reaction (PCR) kit (Qiagen, USA) was used to amplify the Ahr fragment, which is identical to the C57BL/6J mouse Ahr sequence (NM_013464.4; locus: AF405563_1). The 2.5 Kb PCR amplicon was cloned into a commercially available and non-specific PUC19 vector (Invitrogen, USA) that was generously provided by Dr. David Bunick (University of Illinois, USA). The new plasmid was designated pAhr/PUC19 and sequenced for PCR accuracy by the Keck Center at the University of Illinois Urbana-Champaign, USA.
Recombinant adenovirus construction
The Ahr cDNA in pAhr/PUC19 was excised with HindIII and SmaI (New England Biolabs, USA) to generate the orientation for subcloning into a pDC316 shuttle vector (Microbix, Canada) designated as pDC316/Ahr. The new shuttle vector contained a full length 2,418 bp Ahr cDNA with a start codon of ATG and a stop codon of TGA and a highly efficient promoter (MCMV-IE) that it is non-specific for the Ahr. An AdMax adenovirus kit was used to construct the Ahr expression recombinant adenovirus. PDC316/Ahr and pBHGlox (dE1, dE3) Cre (Microbix, Canada) were co-transfected into low passage 293 cells that were purchased at passage 27 and were passaged for 11 additional passages using the calcium phosphate precipitation method according to the adenovirus vector construction manual (Microbix Biosystems Inc., Canada) with minor modifications. Specifically, DNA-calcium precipitates were applied to the low passage 293 cells immediately after precipitation and 5% fetal bovine serum (FBS) was used instead of horse serum in the media. Plaques appeared after 48 hours of transfection and the Ahr expressed adenovirus was designated as AdAhr. AdAhr was harvested when the cytopathic effect was nearly completed. This crude AdAhr suspension was expanded for evaluation by quantitative real-time PCR and western blotting. A high titer of this recombinant adenovirus was prepared and used in the subsequent experiments.
Primary granulosa cell culture
Upon isolation, ovaries were placed in a 35 mm collection dish filled with 2 ml of alpha minimum essential medium (α-MEM; Invitrogen, USA). The collection medium contained 2% antibiotic/antimyotic (final concentrations: 10,000 u/ml penicillin, 10,000 µg/ml streptomycin, 25 µg/ml amphotericin B; Hyclone, USA) and 98% α-MEM. Excess fat and surrounding tissue were mechanically removed under a dissecting microscope and the cleaned ovaries were transferred into a second 35 mm collection dish filled with 2 ml α-MEM medium. After a brief rinse, the ovaries were transferred to a third dish filled with 4 ml α-MEM supplemented medium. Then, antral follicles were punctured with a 23-gauge needle and passed through a 23-gauge needle 3–5 times. The cell suspensions were distributed equally into 4-well plates and cultured in supplemented media (1 ml/well) in a 5% CO2 incubator at 37°C. Supplemented media contained human recombinant follicle stimulating hormone (FSH; Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, USA; 5.85 µl/ml), insulin transferrin selenium (ITS; 10 ng/ml insulin, 5.5 ng/ml transferrin, and 5.5 ng/ml selenium; Sigma-Aldrich, USA), 10% fetal bovine serum (FBS; 100 µl/ml; Atlanta Biological, GA), 1% antibiotic/antimycotic (10 µl/ml), and α-MEM (874 µl/ml). On the next day, the media and floating non-viable cells were rinsed from the plates with 1 ml α-MEM and fresh supplemented media was added to the plates. The cultures were maintained for 2–3 days or until at least 70–90% confluence was observed. Then, the media were removed and replaced with 1 ml of plain supplemented media or supplemented media with AdAhr of 14 MOI (multiplicity of infection) or Ad Green Fluorescent Protein (AdGFP; a gift from Dr. Joan Jorgensen University of Wisconsin, USA) as a positive control for the transfection. Cells were maintained in the incubator for additional 72 hours. Cell viability was determined based on their appearance under the microscope. Then, cells were collected and processed for western blotting.
Follicle culture
Ovaries were removed and antral follicles were mechanically isolated using fine watchmaker forceps and a dissecting microscope. We used at least one mouse per replicate per genotype, and repeated the cultures at least three to four separate times. We obtained 15–40 antral follicles with relative sizes of 230–400 µm from each mouse. Following isolation, follicles were placed in individual wells of 96-well culture plates. Then, follicles were treated with 150 µl of supplemented media with vehicle control (DMSO) or TCDD (1nM). The TCDD concentration was selected based on previous studies indicating that antral follicles were responsive to a 1 nM concentration of TCDD (Karman et al., 2012, Karman et al., 2012). Supplemented media contained (final concentration) 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, and 5.5 ng/ml selenium; Sigma-Aldrich, USA), 5% fetal bovine serum (Atlanta Biological, GA), 1% penicillin/streptomycin (100 U/ml penicillin, 100 µg/ml streptomycin, Sigma-Aldrich, USA), 0.6% human recombinant follicle stimulating hormone (5 IU/ml FSH, Dr. A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, USA), and 92.4% α-MEM. An equal volume of DMSO or TCDD (0.75 µl/ml of media) was added to supplemented α-MEM to keep vehicle concentration at a constant of 0.075% for each treatment. Follicles then were incubated for up to 96 hours, while supplying 5% CO2 at 37°C.
Microinjection of AdAhr into AhrKO follicles
Following isolation, follicles were maintained in α-MEM in a 37°C CO2 incubator. Then, 3–4 follicles were placed in a 20 µl drop of phosphate buffered saline (PBS), while a holding pipet was used on one side of the follicle to prevent it from moving when injection was performed. The AdAhr was injected into the follicles by a microinjection needle, which was connected to a syringe through fine tubing. The volume injected was less than 10% (v/v) of the follicles and with a virus titer of 1.48×1011 pfu/ml. AdGFP was injected into AhrKO follicles to validate the injection success. After injection, the follicles were cultured in supplemented media for 96 hours with DMSO or TCDD (1nM).
Analysis of follicular growth
As a measurement of growth, follicle diameters were measured on perpendicular axes every 24 hours under an inverted microscope equipped with a calibrated ocular micrometer. Follicle diameters were averaged and converted to percent change relative to baseline (0 hour was set as 100%). Data were plotted and statistically analyzed to examine the effects of re-expression of the Ahr into AhrKO follicles compared to WT follicles in terms of growth (n = 4 cultures, with 5–11 follicles per treatment).
Gene expression analyses
At the end of each culture, follicles were snap-frozen and stored at −80°C until further processed for quantitative real-time PCR. Total RNA was extracted from at least 6 pooled follicles per treatment group per biological replicate (n = 3–4) using the RNeasy Micro kit (Qiagen, USA) following the manufacturer’s protocol. The RNA concentration of each sample was determined at 260 nm using a Nanodrop ND1000 UV-Vis spectrophotometer (Nanodrop Technologies, USA). Total RNA (100 ng) was reversed transcribed using an iScript cDNA synthesis kit (Bio-Rad, USA) according to the manufacturer’s instructions. Each cDNA sample was diluted 1:3 with nuclease-free water prior to further analyses. Primer sets were based on previous publications: beta actin (Actb) (Karman et al., 2012), Ahr (Karman et al., 2012), Ahrr (Ziv-Gal et al., 2013), and Cyp1b1 (Karman et al., 2012). Cyp1a1 primers sequences were generously provided by Dr. Tien-Min Lin (University of Wisconsin, USA). Specifically, their sequences were as follows forward: TGTCAGATGATAAGGTCATCACG, reverse: TCTCCAGAATGAAGGCCTCCAG. All gene expression analyses were performed using the CFX96 real-time PCR Detection System C1000 Thermal Cycler (Bio-Rad, USA). All PCR reactions were done in duplicate in a total volume of 10 µl per sample accordingly: 1µl of diluted cDNA, 0.15 µl of gene specific primer mix (50 pM; Integrated DNA Technologies, Inc., USA), 3.85 µl of nuclease free water, and 5 µl of SsoFast EvaGreen Supermix (Bio-Rad, USA). The protocol for the PCR reactions included the following steps: initial denaturation and enzyme activation at 95°C for 1 min, followed by 40 cycles of 10 sec at 94°C, 10 sec at the relevant annealing temperature (Actb 55°C, Ahr 57°C, Ahrr 60°C), a fluorescent absorbance reading, and one final elongation step for 2 min at 72°C. The protocol for Cyp1b1 and Cyp1a1 included an initial denaturation and enzyme activation at 95°C for 5 min, followed by 40 cycles of 10 sec at 94°C, 10 sec annealing at 60°C, elongation for 10 sec at 72°C, a fluorescent absorbance reading, and one final elongation step for 10 min at 72°C. At the end of each run, a heat dissociation curve was performed to confirm specificity of each primer set for the chosen transcript of interest. A standard curve was generated from five serial dilutions of a sample representing the treatment groups to calculate the amplification efficiencies of each primer set. The housekeeping gene Actb was used as a reference gene. Mean relative mRNA expression ratios from 3–4 separate follicle culture experiments normalized to Actb were reported as genomic equivalents (GE).
Western blot analysis
WT, AhrKO, and Ahr re-expressed granulosa cells were scraped from the plates in PBS after removal of the supernatant. The cells were collected and centrifuged at 13,000 rpm, the PBS was discarded, and 50 µl of T-Per plus protease inhibitor was added to the pellet and homogenized. The protein suspensions were centrifuged for 15 minutes at 13,000 rpm, 4°C. The pellets were discarded and the protein supernatant was saved. Protein concentration was measured using a Pierce BCA protein assay kit (Thermo Scientific Inc., USA).
The protein (5 µg) was used in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE Sample Prep kit, USA) and transferred to a polyvinylidene difluoride membrane (Invitrolon PVDF/filter paper sandwich from Invitrogen, USA). After the transfer, the membrane was rinsed with tris-tween buffered saline (TTBS) for 10 min at room temperature, while on a rocker. The membrane was blocked in 5% milk/TTBS for 1 hour at room temperature, while on a rocker. Then, 15 ml of 5% milk/TTBS were added, and AHR antibody rabbit polyclonal (BML-SA210, Enzo Life Sciences, USA) was added at a final concentration 1:5000. The membrane was covered and placed on a rocker at 4°C overnight.
The next day, the membrane was transferred to a new container and washed 3 times for 10 minutes each with TTBS. Later, the membrane was incubated with goat α-rabbit (ab6721; Abcam Inc., USA) at 1:5000 in 1% milk/TTBS for 2 hours at room temperature. Then, the membrane was rinsed 3 times for 10 minutes each with TTBS. Lastly, the membrane was exposed to x-ray to capture the blotting image. Similar blotting procedures were performed with rabbit polyclonal anti-α tubulin antibody (ab4074, Abcam Inc., USA) as a loading control.
Statistical analyses
Data were expressed as the mean ± SEM from at least three separate experiments. Differences between WT, AhrKO, and Ahr re-expressed follicles were statistically analyzed using SPSS software (SPSS Inc., IL). For data on the follicular growth, we have 3–4 biological replicates for each treatment group. These replicates consist of 5–11 follicles per treatment group, per culture. An average of the follicles per treatment group is then calculated per culture to generate data for each biological replicate. In the transcript level analyses, we used 3–4 biological replicates that were generated from at least 6 follicles per biological replicate per treatment with 3 technical replicates (per culture).
All the data were first tested for normal distribution with a Shapiro-Wilk test that requires n≥3. If normal distribution was achieved in all treatment groups, we continued with an ANOVA test and the appropriate post-hoc tests. If at least one of the treatment groups did not meet normality or equal variance criteria (within each examined endpoint), we used the non-parametric Kruskal-Wallis test for overall comparison between the groups followed by Mann-Whitney to detect specific differences between the groups.
Results
Transfection of AhrKO granulosa cells with AdAhr
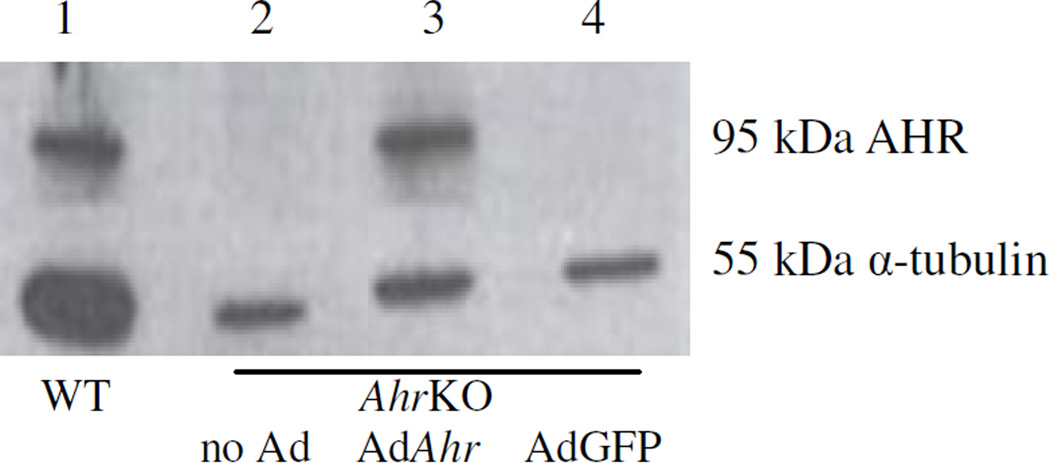
We successfully re-expressed the Ahr in granulosa cells isolated from AhrKO ovaries. We examined the effectiveness of the transfection by western blotting for the AHR (Figure 1). A band in the size of 95 kDa is an indication of successful transfection with the AdAhr that resulted in the translation of the AHR by granulosa cells that originally lacked the Ahr. Specifically, a 95 kDa band of the AHR was detected only in granulosa cells isolated from WT mice (lane 1) and AhrKO mice that were transfected with AdAhr (lane 3). Further, no AHR or any unspecific bands were present in samples of granulosa cells isolated from AhrKO mice (lane 2) or in the AdGFP transfected AhrKO granulosa cells (lane 4). An additional lower band, at the size of 55 kDa is the loading control (α-tubulin) and is present for all samples.
Figure 1.
AHR expression in granulosa cells of wild-type, AhrKO, and AhrKO granulosa cells transfected with AdAhr or AdGFP. Granulosa cells were mechanically isolated from wildtype and AhrKO mouse antral follicles. Some AhrKO granulosa cells were transfected with AdAhr or Ad Green Fluorescent Protein (AdGFP) as transfection control and to account for nonspecific viral induction. Following culture, expression of the AHR (95 kDa) and loading control (α tubulin; 55 kDa) were evaluated by western blotting methods (amount loaded: 5 µg protein per sample).
Re-expression of the Ahr in AhrKO whole antral follicles
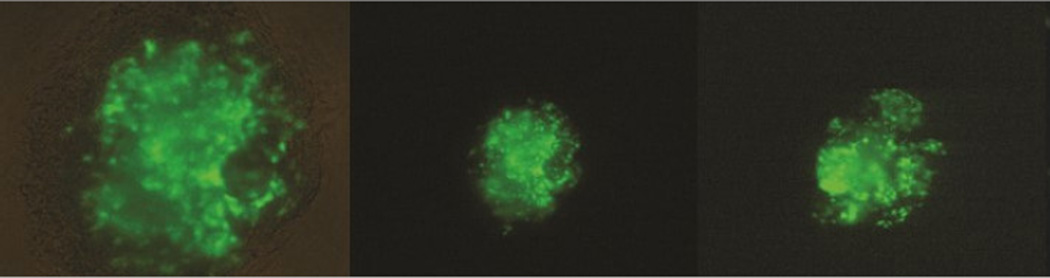
Following the successful expression of the Ahr in granulosa cells, we employed similar techniques on whole antral follicles. Injection of a whole follicle with AdGFP enables us to validate our method as a suitable means for re-expressing the Ahr in an intact ovarian functional unit. In Figure 2, we demonstrate that the AdGFP construct was successfully inserted in AhrKO antral follicles (as evaluated by a detectable fluorescent emission).
Figure 2.
Ad Green Fluorescent Protein (AdGFP) expression in AhrKO antral follicles. AdGFP was injected into AhrKO antral follicles as an insertion control. Green color indicates expression.
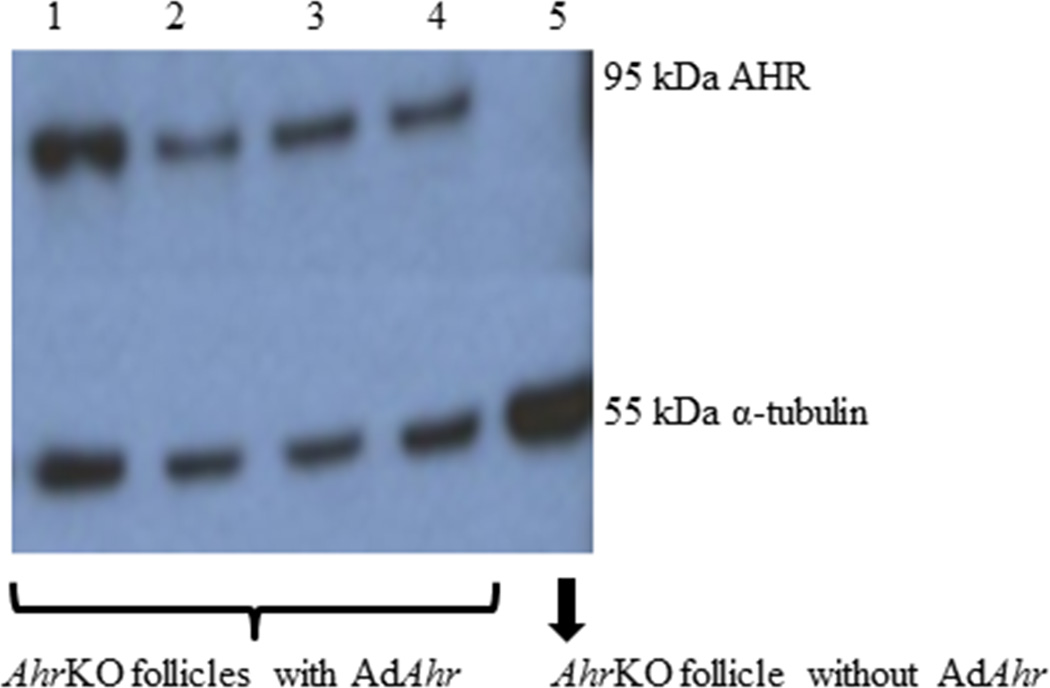
Next, we examined the presence of the AHR in the Ahr re-expressed follicles by western blotting (Figure 3). Similar to the results with the AdAhr transfected granulosa cells, the AhrKO antral follicles were successfully transfected with the AdAhr and this resulted in translation of AHR as indicated by the presence of 95 kDa band in lanes 1–4 and the absence of a similar band in AhrKO follicles that were not transfected with the Ahr virus (lane 5). The loading control (α-tubulin) appears in all lanes, at the size of 55 kDa.
Figure 3.
AHR expression in AhrKO and AdAhr injected mouse antral follicles. AhrKO antral follicles were mechanically isolated from mouse ovaries. The Ahr adenovirus was injected into some of the AhrKO follicles. Expression of the AHR (95 kDa) and loading control (α-tubulin; 55 kDa) were evaluated by western blotting methods.
Growth in WT, AhrKO, and Ahr re-expressed follicles
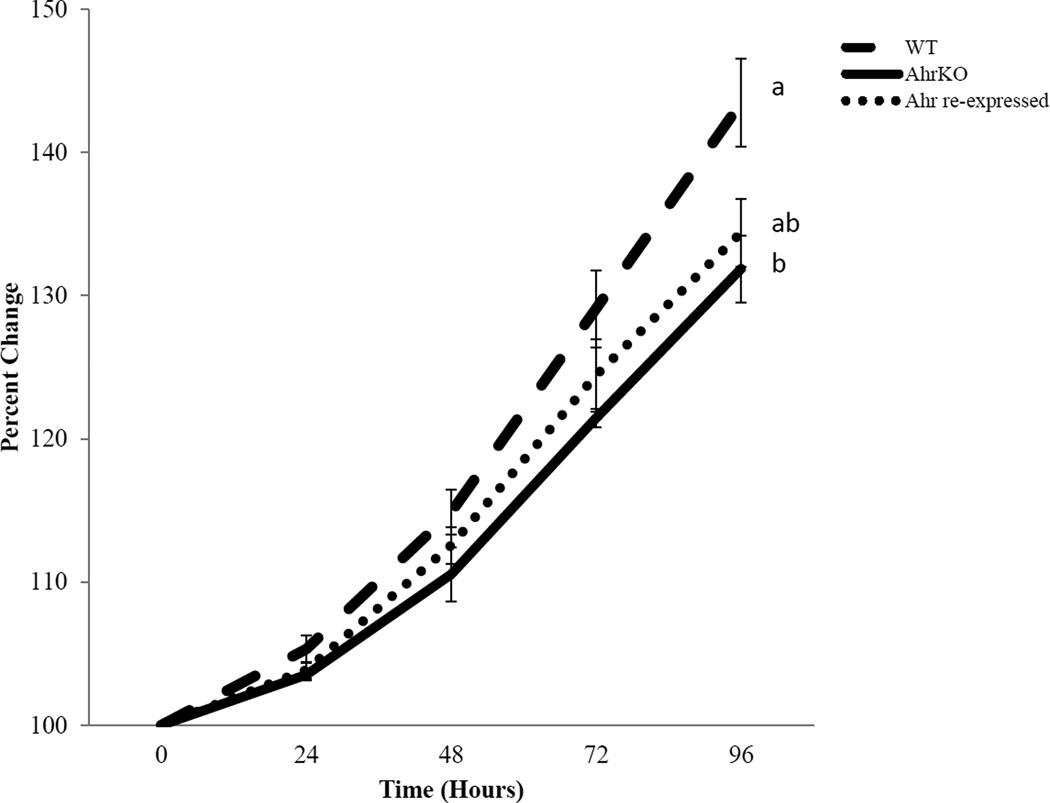
Previous studies indicate that at early reproductive ages (postnatal days 30–38), AhrKO follicles grow slower than WT follicles (Hernandez-Ochoa et al., 2010). Therefore, we examined if re-expressing the Ahr in cultured AhrKO follicles restores the growth of AhrKO follicles to that of WT antral follicles. As shown in Figure 4, WT follicles grew throughout the culture. AhrKO follicles grew similar to the WT follicles at 72 hours, but their growth was significantly inhibited compared to WT follicles at 96 hours. Lastly, growth pattern of Ahr re-expressed follicles was not statistically different from WT or AhrKO follicles at any time-points, indicating that re-expression of the Ahr into AhrKO follicles partially (albeit to a small degree; p=0.03) restored their growth to WT levels, but it was not a full recovery when compared to the growth of the AhrKO follicles (p=0.7) at 96 hours.
Figure 4.
Effect AdAhr injection on the growth of AhrKO antral follicles. Antral follicles were mechanically isolated from wild-type and AhrKO ovaries. The Ahr adenovirus was injected into some of the AhrKO follicles. Wild-type, AhrKO, and Ahr re-expressed follicles were individually cultured for 96 hours. During culture, follicle diameters were measured every 24 hours and were converted to percent change from baseline (time 0). Graph represents means ± SEM from 3–4 separate experiments (n=3–4 separate cultures with 5–11 follicles per treatment group in each experiment). Different letters represent significant differences between the follicle types at 96 hours; p≤0.05.
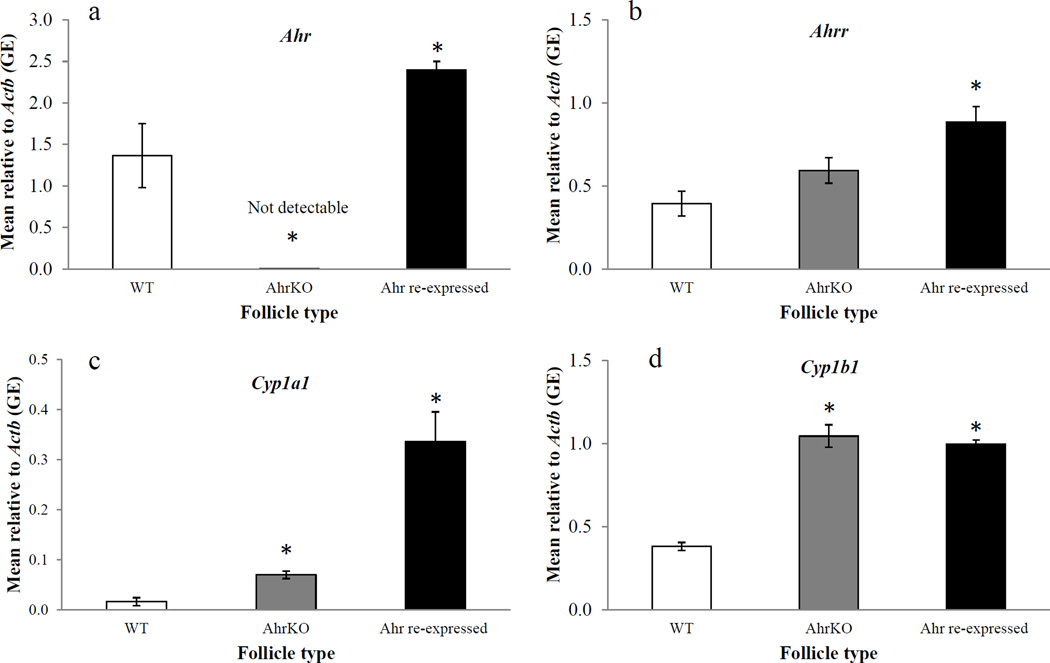
Expression of Ahr, Ahrr, Cyp1a1, and Cyp1b1 in WT, AhrKO, and Ahr re-expressed antral follicles
Expression of several key genes related to the AHR signaling pathway (Ahr, Ahrr, Cyp1a1, and Cyp1b1) was compared in WT, AhrKO, and Ahr re-expressed follicles (Figure 5a–d). Following 96 hours of culture, AhrKO follicles had non-detectable levels of Ahr compared to WT follicles (WT = 1.36 ± 0.39 GE; AhrKO = 0.001 ± 0.00 GE; n = 3–4; p≤0.05; Figure 5a). In contrast, Ahr re-expressed follicles had significantly higher levels of Ahr compared to WT follicles (WT = 1.36 ± 0.39 GE; Ahr re-expressed = 2.40 ± 0.10 GE; n = 4; p≤0.05; Figure 5a).
Figure 5.
Effect of AdAhr injection on expression of Ahr (a), Ahrr (b), Cyp1a1 (c), and Cyp1b1 (d). Wild-type, AhrKO, and Ahr re-expressed follicles were cultured for 96 hours. At the end of the cultures, expression levels of Ahr (a), Ahrr (b), Cyp1a1 (c), and Cyp1b1 (d) were compared using quantitative PCR. Data represent means ± SEM from at least three separate experiments. Asterisks represent significant differences from WT; p≤0.05.
Ahrr levels in AhrKO follicles were similar to the levels of WT follicles (WT = 0.40 ± 0.07 GE; AhrKO = 0.59 ± 0.08 GE; n = 4; p>0.05; Figure 5b). In contrast, Ahr re-expressed follicles had significantly higher Ahrr levels compared to WT follicles (WT = 0.40 ± 0.07 GE; Ahr re-expressed = 0.89 ± 0.09 GE; n = 4; p≤0.05; Figure 5b).
Cyp1a1 levels in AhrKO follicles were significantly higher compared to WT follicles (WT = 0.02 ± 0.01 GE; AhrKO = 0.07 ± 0.01 GE; n = 4; p≤0.05; Figure 5c). Similarly, Cyp1a1 levels in Ahr re-expressed follicles were significantly higher compared to WT follicles (WT = 0.02 ± 0.01 GE; Ahr re-expressed = 0.34 ± 0.06 GE; n = 4; p≤0.05; Figure 5c).
Cyp1b1 levels in AhrKO follicles were significantly higher compared to WT follicles (WT = 0.38 ± 0.02 GE; AhrKO = 1.05 ± 0.07 GE; n = 4; p≤0.05; Figure 5d). Similarly, Cyp1b1 levels in Ahr re-expressed follicles were significantly higher compared to WT follicles (WT = 0.38 ± 0.02 GE; Ahr re-expressed = 1.00 ± 0.03 GE; n =4; p≤0.05; Figure 5d).
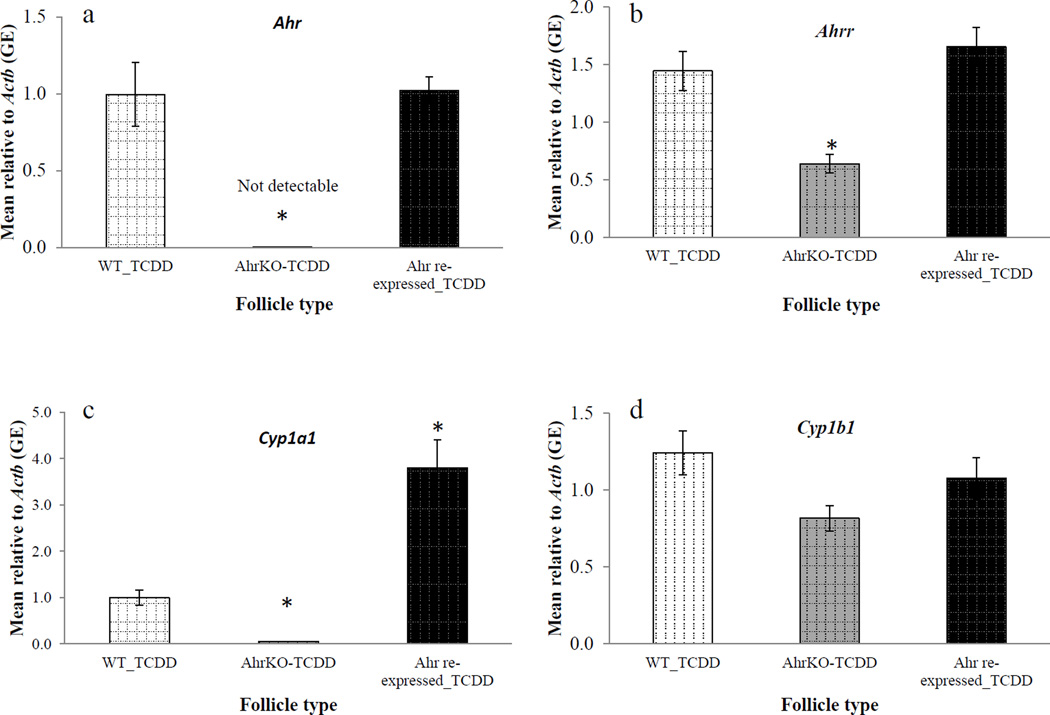
Expression of Ahr, Ahrr, Cyp1a1, and Cyp1b1 in WT, AhrKO, and Ahr re-expressed follicles following TCDD treatment
TCDD is one of the most potent exogenous ligands of the AHR. Therefore, we cultured WT, AhrKO, and Ahr re-expressed follicles with TCDD (1nM) to compare the expression of selected genes following 96 hours of TCDD treatment in WT, AhrKO, and Ahr re-expressed follicles (Figure 6 a–d). Ahr levels were non-detectable in AhrKO follicles compared to WT follicles in response to TCDD (WT = 1.00 ± 0.21 GE; AhrKO = 0.00 ± 0.00 GE; n = 3–4; p≤0.05; Figure 6a). However, Ahr levels in Ahr re-expressed follicles were similar to WT follicles in response to TCDD (WT = 1.00 ± 0.21 GE; Ahr re-expressed = 1.02 ± 0.09 GE; n = 4; p>0.05; Figure 6a).
Figure 6.
Effect of AdAhr injection on expression of Ahr (a), Ahrr (b), Cyp1a1 (c), and Cyp1b1 (d) in response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) treatment. Wild-type, AhrKO, and Ahr re-expressed follicles were cultured with 2,3,7,8-tetrachlorodibenzo-p-dioxin (1nM) for 96 hours. At the end of the cultures, expression levels of Ahr (a), Ahrr (b), Cyp1a1 (c), and Cyp1b1 (d) were measured using quantitative PCR. Data represent means ± SEM from at least three separate experiments. Asterisks represent significant differences from WT_TCDD; p≤0.05.
Ahrr levels were significantly lower in AhrKO follicles compared to WT follicles in response to TCDD (WT = 1.45 ± 0.33 GE; AhrKO = 0.64 ± 0.08 GE; n = 4; p≤0.05; Figure 6b), whereas Ahrr levels in Ahr re-expressed follicles were similar to the levels of WT follicles in response to TCDD (WT = 1.45 ± 0.33 GE; Ahr re-expressed = 1.66 ± 0.17 GE; n = 4; p>0.05; Figure 6b).
Cyp1a1 levels were significantly lower in AhrKO follicles compared to WT follicles in response to TCDD (WT = 1.00 ± 0.17 GE; AhrKO = 0.05 ± 0.001 GE; n = 4; p≤0.05; Figure 6c). In contrast, Cyp1a1 levels in Ahr re-expressed follicles were significantly higher compared to the levels of WT follicles in response to TCDD (WT = 1.00 ± 0.17 GE; Ahr re-expressed = 3.80 ± 0.60 GE; n = 4; p≤0.05; Figure 6c).
Lastly, Cyp1b1 levels were similar in AhrKO follicles compared to WT follicles in response to TCDD (WT = 1.24 ± 0.14 GE; AhrKO = 0.82 ± 0.08 GE; n = 4; p>0.05; Figure 6d). Similarly, Cyp1b1 levels were similar in Ahr re-expressed follicles compared to WT follicles in response to TCDD (WT = 1.24 ± 0.14 GE; Ahr re-expressed = 1.08 ± 0.13 GE; Ahr re-expressed = 1.08 ± 0.13 GE; n = 4; p>0.05; Figure 6d).
Discussion
In this study, we designed and validated in vitro methods to re-express the Ahr in AhrKO granulosa cells and antral follicles. Re-expression of the Ahr in tissues in which the Ahr was deleted at early developmental stages (i.e. AhrKO granulosa cells or antral follicles) provides a novel means to study the role of the AHR in the functional unit of the ovary as well as improving our understanding of whether Ahr deletion effects are permanent or reversible, including the response to TCDD treatment. The current methods complement existing methods such as the use of the global Ahr knockout mouse model. Nevertheless, the current methods, unlike the global knockout mouse model, provide a means for re-expressing the Ahr at selected developmental stages directly in the ovary.
We were able to demonstrate that re-expression of the Ahr by cell transformation or microinjection manipulations successfully restores the synthesis of AHR, as indicated by western blot analysis both in granulosa cells and whole antral follicles. We also showed that injection of AdAhr into AhrKO antral follicles results in a growth pattern similar to that of WT follicles (i.e. no statistical difference between WT and Ahr re-expressing follicles). These findings provide further evidence that the AHR has a key role in maintaining proper ovarian follicle growth (Benedict et al., 2003, Hernandez-Ochoa et al., 2010). However, the recovery of growth was relatively small in magnitude and other factors may be involved in the process of follicle growth. While the mechanism by which Ahr re-expression affects follicle growth is unknown, previous studies indicate that the impaired AhrKO follicle growth is partially due to reduced gonadotropin responsiveness of AhrKO ovaries compared to WT ovaries (Barnett et al., 2007). Further, previous studies show that the AHR can bind to the promoter region of the follicle stimulating hormone receptor (Fshr) as a transcription factor (Hernandez-Ochoa et al., 2010, Hernandez-Ochoa et al., 2013). Therefore, re-expression of the Ahr into AhrKO follicles may have recovered some of the binding ability of FSH and this may have helped restore follicle growth to WT levels. Future studies should examine whether this is the case.
Following the growth evaluation, we compared transcript levels in Ahr re-expressed follicles to WT levels to determine whether there was a full recovery of transcript levels. Interestingly, transcript levels of key factors in the AHR signaling pathway were not fully restored by re-expression of the Ahr in AhrKO follicles. Specifically, at baseline (prior to TCDD induction), Ahr levels in Ahr re-expressed follicles were significantly higher than WT levels. This may be due to the constitutive expression of the Ahr that was introduced into the AhrKO follicles by the adenovirus manipulation. High efficiency in cell transformation and protein synthesis following adenovirus manipulation have been observed in other systems such as HepG2 cell lines (Buning et al., 2008, Ng et al., 1999). It is also possible that deletion of the Ahr at early developmental stages led to permanent altered regulation of transcription of the selected genes that cannot be restored when the re-expression occurs at later times. Perhaps, modifying the timing of re-expression or the length of time of re-expression could result in full recovery of Ahr levels to the levels measured in WT follicles.
Because activation of the AHR signaling pathway can be terminated upon binding of the AHRR to the ARNT, we measured the levels of the Ahrr as another indication of the recovery of the Ahr re-expressed follicles to WT levels. In our current study, Ahrr levels were similar in AhrKO and WT follicles. This finding supports those of Bernshausen et al. (Bernshausen et al., 2006), which indicate that the Ahrr is constitutively expressed in various tissues and at various levels, even in the absence of a functional AHR (i.e. AhrKO mice). Although Bernshausen et al. did not compare the levels of Ahrr in AhrKO and WT ovaries, they found no difference in the Ahrr levels in the livers of WT and AhrKO mice. Therefore, it is possible that in the ovarian antral follicle, the Ahrr is constitutively expressed and is not affected by the absence of the AHR, and that factors other than the AHR induce its constitutive transcription. Interestingly, in the Ahr re-expressed follicles, Ahrr levels were significantly higher when compared to WT follicles. In this case, it may be that unlike in AhrKO follicles, a significant increase in the levels of the Ahr in the Ahr re-expressed follicles does affect the levels of the Ahrr. Additionally, due to the technical limitations, we could not quantify protein levels of any of the transcripts. Hence, we can only speculate that the increased levels of the Ahr in the Ahr re-expressed follicles have led to over-activation of the AHR signaling pathway compared to WT as evidenced by increased levels of Ahrr.
We also observed significantly higher levels of Cyp1a1 and Cyp1b1 in both AhrKO and Ahr re-expressed follicles when compared to WT levels. These findings are in contrast to the study of Shimada et al. (Shimada et al., 2003) in which negligible levels of Cyp1a1 were observed in WT and AhrKO ovaries, and lower levels of Cyp1b1 were observed in AhrKO ovaries compared to WT ovaries. Some of the differences in results may be due to the fact that Shimada et al. examined whole ovaries, whereas we examined cultured isolated antral follicles. Alternatively, it is possible that the isolated mature AhrKO follicles may already have been programmed into a certain functionality that cannot be restored by re-expressing the Ahr. Specifically, it may be that the regulation of the transcription of Cyp1a1 and Cyp1b1 is altered prenatally and thus, increased transcript levels of Cyp1a1 and Cyp1b1 occur in both AhrKO and Ahr re-expressed follicles at later developmental stages. Lastly, the difference between Cyp1a1 levels of Ahr re-expressed and WT follicles is greater (WT 0.02 GE, Ahr re-expressed 0.34 GE) than the difference in Cyp1a1 levels between AhrKO and WT follicles (WT 0.02 GE, AhrKO 0.07 GE). Therefore, it is possible that downstream effects such as an increase in the levels of Cyp1a1 occurs as a result of re-expression of the Ahr into AhrKO follicles.
The response to TCDD is a hallmark of AHR activation that was compared in WT, AhrKO, and Ahr re-expressed follicles. As expected, AhrKO follicles had non-detectable Ahr levels compared to WT levels, whereas Ahr levels were similar between Ahr re-expressed follicles and WT follicles. It is possible that simultaneous re-expression of the Ahr and treatment with TCDD resulted in Ahr levels similar to WT follicles due to a better cellular monitoring of the Ahr transcription when a potent ligand of the AHR is present (i.e. TCDD) in the Ahr re-expressed follicles.
Ahrr levels in AhrKO follicles were significantly lower compared to WT follicles after exposure to TCDD. This is most likely because TCDD exposure results in activation of the AHR signaling pathway only in the WT follicles and not in the AhrKO follicles where the AHR signaling pathway is not induced following treatment with TCDD. Interestingly, Ahrr levels were similar between Ahr re-expressed follicles and WT follicles not exposed to TCDD. This may indicate that our system is able to restore Ahrr transcription in the Ahr re-expressed follicles to WT levels in response to TCDD challenge.
Cyp1a1 levels were lower in AhrKO follicles when compared to WT follicles. This may be due to activation of the AHR signaling pathway in WT follicles and not in AhrKO follicles, similar to what we observed with Ahrr levels following TCDD exposure. In contrast, in Ahr re-expressed follicles, Cyp1a1 levels were significantly higher than in WT follicles following TCDD treatment. This difference may be due to over-activation of the AHR signaling pathway or non-specific activation of the transcription of Cyp1a1 in the Ahr re-expressed follicles. These findings are also suggestive of a functional AHR that is able to activate transcription of Cyp1a1 in the Ahr re-expressed follicles following TCDD treatment.
As for the Cyp1b1, there were no significant differences in expression between any of the follicle types. It is tempting to say that we were able to restore the levels of Cyp1b1 in Ahr re-expressed follicles to WT levels, however, levels in AhrKO follicles were also similar to WT levels. In WT follicles, TCDD increased Cyp1b1 expression compared to vehicle treated follicles, whereas in the AhrKO and the Ahr re-expressed follicles, TCDD did not increase the difference in Cyp1b1 levels compared to vehicle. Hence, it may be that developmental changes (e.g. attenuated response to TCDD) already occurred in the AhrKO and the Ahr re-expressed follicles. This potentially resulted in differences in the effects of TCDD treatment when compared to WT follicles. Additionally, in the AhrKO or the Ahr re-expressed follicles, TCDD may preferably affect Cyp1a1 rather than Cyp1b1 levels in contrast to what we observed in WT follicles. Further studies are needed to elucidate the differences in TCDD action on the different types of follicles.
In conclusion, we generated an in vitro model system for better understanding the role of the Ahr in ovarian follicles. This system can also be used to study the response of the ovarian follicle to Ahr ligands such as TCDD. Additionally, this system can potentially be used in different developmental windows. We partially restored the growth of AhrKO follicles to WT levels upon re-expression of the Ahr during a 96 hours of culture. Based on the transcript levels of key factors relevant to the AHR signaling pathway, it is likely that some of the effects of deletion of the Ahr at early developmental stages result in permanent effects on proper activation of the AHR signaling pathway that cannot be reversed by re-expression of the Ahr at later developmental time-points. Nevertheless, this model allows examination of various endpoints such as the carcinogenic effects of TCDD without the need of maintaining several lines of mice. It also could be used to reduce the use of animals because experiments can be performed using many follicles from the same mouse. Future studies may be able to expand the use of this method to larger tissues.
Highlights.
Ahr re-expressed mouse granulosa cells express AHR.
Growth in Ahr re-expressed mouse antral follicles is partially restored to the levels of wild-type follicles.
Ahr re-expression partially restored expression of factors in the AHR signaling pathway to levels in wild-type follicles.
Ahr re-expressed mouse antral follicles partially respond to TCDD in a manner similar to wild-type follicles.
Acknowledgements
This work was supported by NIH T32 ES 07326 (BNK), NIH R01 HD 047275 (JAF), and a Billie A. Field Fellowship in Reproductive Biology (AZG). The authors thank Dr. Tien-Min Lin (University of Wisconsin, USA) for providing the primer sequences for Cyp1a1, Dr. Joan Jorgensen (University of Wisconsin, USA) for the generous gift of AdGFP, and Dr. David Bunick (University of Illinois, USA) for the generous gift of the plasmid PUC19.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no conflicts of interest or any financial disclosures to declare.
Contributor Information
A Ziv-Gal, Email: zivgal1@illinois.edu.
L. Gao, Email: lgao@illinois.edu.
B.N. Karman, Email: bekarma@gmail.com.
J.A. Flaws, Email: jflaws@illinois.edu.
References
- Abbott BD, et al. Adverse reproductive outcomes in the transgenic Ah receptordeficient mouse. Toxicol Appl Pharmacol. 1999;155(1):62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391(11):1235–1248. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- Barnett KR, et al. The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol Appl Pharmacol. 2007;223(1):66–72. doi: 10.1016/j.taap.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett KR, et al. The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol Reprod. 2007;76(6):1062–1070. doi: 10.1095/biolreprod.106.057687. [DOI] [PubMed] [Google Scholar]
- Benedict JC, et al. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56(2):382–388. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- Benedict JC, et al. Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod. 2003;68(5):1511–1517. doi: 10.1095/biolreprod.102.007492. [DOI] [PubMed] [Google Scholar]
- Bernshausen T, et al. Tissue distribution and function of the Aryl hydrocarbon receptor repressor (AhRR) in C57BL/6 and Aryl hydrocarbon receptor deficient mice. Arch Toxicol. 2006;80(4):206–211. doi: 10.1007/s00204-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Buning H, et al. Recent developments in adeno-associated virus vector technology. J Gene Med. 2008;10(7):717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- Hernandez-Ochoa I, et al. The ability of the aryl hydrocarbon receptor to regulate ovarian follicle growth and estradiol biosynthesis in mice depends on stage of sexual maturity. Biol Reprod. 2010;83(5):698–706. doi: 10.1095/biolreprod.110.087015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ochoa I, et al. Follicle-stimulating hormone responsiveness in antral follicles from aryl hydrocarbon receptor knockout mice. Reprod Biol Endocrinol. 2013;11:26. doi: 10.1186/1477-7827-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77(4):547–559. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman BN, et al. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates the aryl hydrocarbon receptor and alters sex steroid hormone secretion without affecting growth of mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2012;261(1):88–96. doi: 10.1016/j.taap.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karman BN, et al. Dioxin exposure reduces the steroidogenic capacity of mouse antral follicles mainly at the level of HSD17B1 without altering atresia. Toxicol Appl Pharmacol. 2012;264(1):1–12. doi: 10.1016/j.taap.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Dong L, Whitlock JP., Jr Transcriptional activation by the mouse Ah receptor. Interplay between multiple stimulatory and inhibitory functions. J Biol Chem. 1995;270(21):12697–12703. doi: 10.1074/jbc.270.21.12697. [DOI] [PubMed] [Google Scholar]
- Mandal PK. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Comp Physiol B. 2005;175(4):221–230. doi: 10.1007/s00360-005-0483-3. [DOI] [PubMed] [Google Scholar]
- Mimura J, Fujii-Kuriyama Y. Functional role of AhR in the expression of toxic effects by TCDD. Biochim Biophys Acta. 2003;1619(3):263–268. doi: 10.1016/s0304-4165(02)00485-3. [DOI] [PubMed] [Google Scholar]
- Ng P, et al. A high-efficiency Cre/loxP-based system for construction of adenoviral vectors. Hum Gene Ther. 1999;10(16):2667–2672. doi: 10.1089/10430349950016708. [DOI] [PubMed] [Google Scholar]
- Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem Biol Interact. 2002;141(1–2):41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77(4):713–722. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem Pharmacol. 2005;69(2):199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, et al. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci USA. 1996;93(13):6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, et al. Tissue-specific induction of cytochromes P450 1A1 and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in engineered C57BL/6J mice of arylhydrocarbon receptor gene. Toxicol Appl Pharmacol. 2003;187(1):1–10. doi: 10.1016/s0041-008x(02)00035-2. [DOI] [PubMed] [Google Scholar]
- Stockinger B, et al. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- Uno S, et al. Cyp1a1(−/−) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196(3):410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, et al. CYP1A1 mRNA levels as a human exposure biomarker: use of quantitative polymerase chain reaction to measure CYP1A1 expression in human peripheral blood lymphocytes. Carcinogenesis. 1993;14(10):2003–2006. doi: 10.1093/carcin/14.10.2003. [DOI] [PubMed] [Google Scholar]
- Ziv-Gal A, et al. Bisphenol A inhibits cultured mouse ovarian follicle growth partially via the aryl hydrocarbon receptor signaling pathway. Reprod Toxicol. 2013;42:58–67. doi: 10.1016/j.reprotox.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]