Abstract
Complement, part of the innate immune system, is involved with immune protection against invading pathogens as well as cell survival and tissue regeneration. It is known that complement activation is required for timely hepatocyte recovery following an acute toxic injury, but which pathway of complement activation is involved in response to hepatocyte injury has not been identified. In these studies we utilize mice deficient in C1qa, C4 and Factor D, lacking the classical, classical/MBL, and alternative pathways of complement activation, respectively, to identify an essential role for Factor D in the ability of the liver to recover from acute toxic injury. Here we demonstrate that following an acute CCl4-induced injury, the involvement of the alternative complement pathway is essential for efficient liver recovery.
Keywords: complement, alternative pathway, factor D, acute liver injury, necrosis
1. Introduction
Complement is part of the innate immune system serving conventional roles of defending the host against invading pathogens, and unconventional roles of cell survival, growth and differentiation in various tissues (Reca et al., 2003 and Del Rio-Tsonis, 1998), as well as tissue homeostasis, with a critical role in clearing apoptotic cells and scavenging of toxic cellular debris (Munoz et al., 2010).
Complement consists of about 30 soluble and membrane bound proteins and functions in cascades of stepwise protease activation. Upon activation, any one of three distinct pathways (classical, mannose-binding lectin (MBL), and alternative) can lead to the cleavage of the complement protein C3; activation can occur either on pathogen/cellular surfaces or in plasma (Qin and Gao, 2006). C1q and MBL are pattern recognition molecules of the classical and lectin pathway, respectively. C1s, C1r, MBL-associated serine proteases (MASP), C2 and C4 also participate in classical and lectin pathway of activation (Degn and Thiel, 2013). The alternative pathway activates C3 spontaneously in combination with Factor B, Factor D (FD) and properdin (Harboe and Mollnes, 2008) and also serves to amplify complement activation via the classical and lectin pathways. C3 cleavage results in the production of the inflammatory anaphylatoxin C3a and the C3b fragment. Binding of C3b to damaged cells acts as an opsonizing agent and facilitates removal of cellular debris from injured tissue (Munoz et al., 2010). As the activation cascade proceeds, the second anaphylatoxin C5a is generated, along with multiple other protein fragments, leading to the formation of the terminal complex, C5b-9n (Nesargikar et al., 2012).
Acute toxic injury to the liver results in the death of hepatocytes by necrosis and apoptosis. In response to injury, the liver undergoes a series of complex, overlapping phases that are precisely timed in order to re-establish tissue homeostasis. These events are classically divided into three phases: inflammation, proliferation and remodeling. Resident macrophages and recruited immune cells coordinate the inflammatory response, activating multiple cytokine and growth factor networks.
There is a growing appreciation that complement is critical to the liver’s ability to respond to injury at multiple phases of recovery. Following injury, complement anaphylatoxins (C3a and C5a) are also generated, associated with the activation of other innate immune functions. This dynamic orchestration of multiple innate immune pathways serves to regulate hepatocyte proliferation and liver restoration (Taub, 2004). Complement C3a and C5a also contribute to hepatocyte proliferation (Markiewski et al., 2004). Finally, during the tissue regeneration phase, newly formed fibronectin, cell-adhesion proteins and other basement-membrane proteins are also induced, altering liver architecture (Taub, 2004). Normal liver architecture forms only after normal liver mass is restored. Several mechanisms contribute to tissue remodeling and repair after injury, including the serine protease system and matrix metalloproteinases (Nagase, 1999 and Bezerra, 2001). Since complement is involved in the clearance of necrotic debris by cellular scavengers and remodeling of extracellular matrix components in other injury models (Trouw et al., 2008), it is also likely that complement activation contributes to the clearance of debris during hepatic response to injury, so that a suitable scaffold for newly formed cells can develop.
Exposure of animals to carbon tetrachloride (CCl4) is a commonly used model system to study acute toxic injury to hepatocytes. In response to CCl4, C3 is activated biphasically, peaking at 3 and 36 hr; this activation is closely coupled with activation of a network of cytokine signaling (Markiewski, et al., 2004). The early phase of complement activation corresponds with the “priming phase” of hepatocytes after injury (Taub, 2004). The second wave of activation likely facilitates the removal of damaged liver tissue, with C3b acting as an opsonin, flagging damaged tissue for removal by phagocytes (Gullstrand et al., 2009). Reconstitution of C3−/− mice with C3a (Markiewski, et al., 2004) and C5−/− mice with C5a (Mastellos et al., 2001) restores hepatocyte proliferation at 48 hr following CCl4-induced injury.
While it is clear that complement activation is required for timely hepatocyte recovery following an acute toxic injury (Markiewski, et al., 2004), the pathway of complement activation involved in response to hepatocyte injury has not been identified. Here we utilized mice deficient in C1qa, C4 and Factor D, lacking the classical, classical/MBL, and alternative pathways of complement activation, respectively, to identify an essential role for Factor D in the ability of the liver to recover from acute toxic injury. Interestingly, while Factor D-dependent complement activation was dispensable for an adequate proliferative response, FD-deficient mice were unable to adequately clear injured tissue after a toxic injury. Our results provide new insights into the mechanism and importance of timely clearance of damaged cells after acute toxic injury and suggest that interventions leading to local activation of the alternative pathway of complement activation may enhance early clearance of damaged cells, thus avoiding persistent local inflammation and further damage.
2. Materials and Methods
2.1 Materials
Carbon tetrachloride and olive oil were purchased from Sigma-Aldrich (St. Louis, MO). All primers for real-time PCR were synthesized by Integrated DNA Technologies (Coralville, IA). Primary antibodies were purchased from the following sources: proliferating cell nuclear antigen (PCNA, clone PC10), (Chemicon/Millipore,Danvers, MA); C3b/iC3b/C3c (C3b) (Hycult Biotechnology, Uden, The Netherlands); Ki67 (Abcam, Cambridge, MA); phospho(Tyr705)-STAT3 (Cell Signaling Technology, Danvers, MA); HSC70 (Santa Cruz Biotechnology, Inc, Dallas, TX); cytochrome P450 2E1 (CYP2E1 Research Diagnostics, Inc., Flanders, NJ).
2.2 Animals
Ten to 12 week old female C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME). A breeding pair for FD−/− (Xu et al., 2001) was provided by Dr. Gregory Stahl, Harvard University, Boston; and C1qa−/− (Botto et al., 1998) and C4−/− (Koller & Smithies, 1992; Fischer et al., 1996) breeding pairs were provided by Dr. Michael Carroll at Center for Blood Research, Harvard University, Boston. Breeding pairs were used to establish breeding colonies at the Cleveland Clinic.
2.3 Carbon tetrachloride administration and sample collection
CCl4 was prediluted 1:3 in olive oil before administration. Mice received a single dose at 1µl/g body weight of CCl4 administered by intraperitoneal injection using 100 µl Hamilton syringes fitted with 26G 5/8 inch needles. Mice were anesthetized and blood was drawn from the posterior vena cava precisely at 2, 18, 24, 48, and 72 hours (h) after CCl4. Plasma was separated from whole blood by centrifugation. After euthanasia, livers were removed, weighed, and portions were fixed in 10% neutral buffered formalin (Fisher Scientific, Pittsburg, PA), frozen in optimal cutting temperature (OCT) medium (Takura Finetek USA, Torrance, CA), stored in RNAlater (Ambion, Austin, TX) or snap frozen in liquid nitrogen for further analysis.
2.4 Liver histology and plasma alanine aminotransferase (ALT) activities
For histological analysis, formalin-fixed tissues were paraffin-embedded, sectioned (5 µm) and stained with hematoxylin and eosin. Slides were coded prior to examination and reviewed by a liver pathologist blind to the treatment groups. Presence of acidophil bodies (none, few and many), identification and distribution of necrosis (no necrosis, single necrotic cells in zone 3, clusters of necrotic cells in zone 3, diffuse necrosis in zone 3 and zone 3 necrosis extending to zone 2) and neutrophilic inflammation (no neutrophilic inflammation, <2/HPF foci of neutrophilic infiltrate, 2–4 foci/HPF and >4 foci/HPF) were recorded. Images of the histological sections were captured using an Olympus BX41 microscope and digital camera. Plasma samples were assayed for alanine aminotransferase (ALT) activity using Sekisui Chemical Co Ltd enzymatic assay kits (Secaucus, NJ) according to the manufacturer’s instructions.
2.5 Real-time qPCR
Total RNA was isolated from liver and reverse transcribed as previously described (McMullen et al., 2005). Real-time PCR amplification was performed using gene-specific primers: interleukin-6 (IL-6) FWD: TAGTCCTTCCTACCCCAATTTCC; REV: TTGGTCCTTAGCCACTCCTTC; CD68 FWD: GGACCCACAACTGTCACTCAT REV: AAGCCCCACTTTAGCTTTACC; 18S FWD: ACGGAAGGGCACCACCAGGA REV: CACCACCACCCACGGAATCG; as previously described (Barnes M et al., 2013). Relative amount of target mRNA was determined using comparative threshold (Ct) method by normalizing target mRNA Ct values to those of 18S. Statistics were performed on DCt values.
2.6 Immunohistochemistry
C3b/iC3b/C3c and Ki67 immunohistochemical analysis was performed in frozen liver sections as previously described (Roychowdhury et al., 2009). Images were semi-quantified using Image Pro software (Media Cybernetics, Inc, Bethesda, MD).
2.7 Liver homogenate preparation, electrophoresis, and immunoblotting
Liver homogenates were prepared and immunoblotting performed, as previously described (Pritchard et al., 2007).
2.8 Statistical Analysis
Values reported are mean ± standard error of mean. Data were analyzed by general linear models procedure (SAS Institute, Cary, IN). Data were log transformed if needed to obtain a normal distribution. Follow-up comparisons were made by least square means testing. Values in graphs with different alphabetical superscripts are significantly different from one another (p<0.05).
3. Results
3.1 C3b/iC3b/C3c Deposition is Reduced with the Absence of Factor D
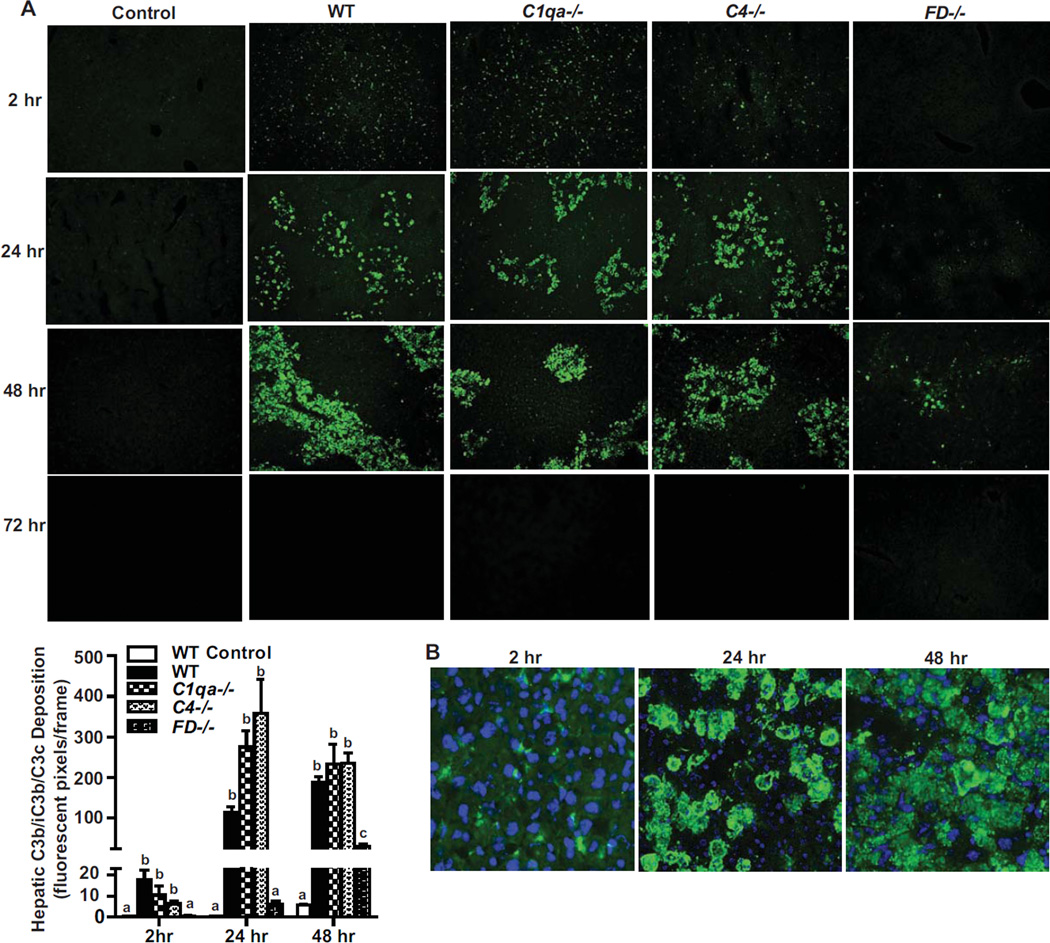
Activation of complement, assessed by C3a in plasma and C3b accumulation, exhibits a biphasic response following an acute CCl4-induced toxic injury (Markiewski et al., 2004). To determine which complement activation pathway responds to acute CCl4 liver injury, C3b/iC3b/C3c deposition in liver parenchyma was assessed in wild-type mice and compared to mice deficient in the classical pathway component C1q, the classical/lectin component C4, or the alternative pathway component Factor D. In the absence of CCl4 exposure, C3b/iC3b/C3c was not detected in WT mice (Figure 1A). In WT mice, C3b/iC3b/C3c accumulated as early as 2 hr after CCl4 in a pattern characteristic of a sinusoidal distribution (Figure 1A). At 24 hr, C3b/iC3b/C3c accumulation increased with a noticeably patchy pattern of staining across the liver parenchyma. These patches of C3b/iC3b/C3c accumulation in the centrilobular area were larger at 48 hr after CCl4 injection, but completely absent by 72 hr. Thus, both the dynamics and pattern of C3b/iC3b/C3c deposition changes over time (Figure 1B). In response to CCl4, mice deficient in C1q and C4 exhibited similar dynamics of C3b/iC3b/C3c accumulation to WT mice. However, mice deficient in Factor D (alternative pathway) did not accumulate C3b/iC3b/C3c at any time point following CCl4 exposure, except for a low level of C3b/iC3b/C3c was detected at 48 h.
Figure 1. Factor D is required for C3b/iC3b/C3c deposition in liver following acute exposure to CCl4.
A. Hepatic C3b/iC3b/C3c (C3b) deposition in mice 2, 24, 48 and 72 hr after a single injection of CCl4 in WT, C1qa−/−, C4−/− and FD−/− mice. WT control mice received injection of olive oil. Immunoreactive C3b/iC3b/C3c was visualized by immunohistochemistry in frozen liver sections. Images are shown at 20× magnification. Total number of fluorescent pixels per 20× field was determined using Image Pro software. Values with different alphabetical superscripts were significantly different from each other, p<.05. B. Representative images of localization of immunoreactive C3b/iC3b/C3c deposition in frozen liver sections of WT mice 2, 24 and 48 hr following treatment with a single injection of CCl4 are shown. Magnification 40×.
3.2 Deficiency of Factor D Leads to Exacerbated Liver Injury and Inflammatory Cells
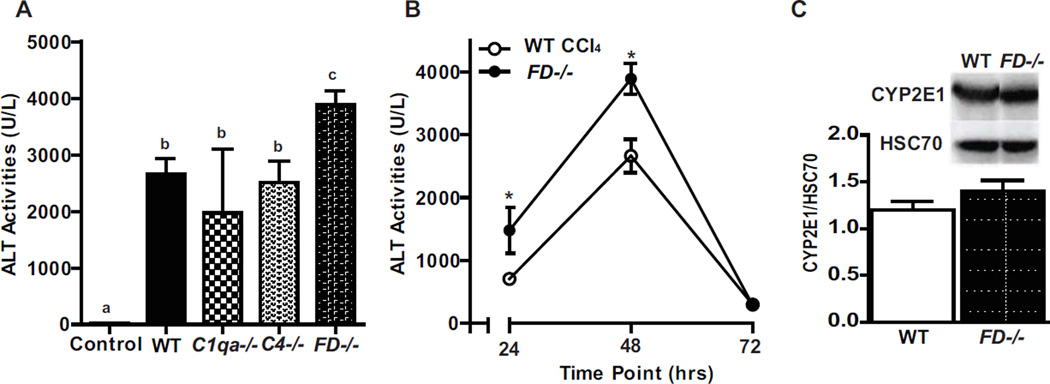
Hepatocyte injury, indicated by release of alanine aminotransferase (ALT) into the circulation, as well as hepatocyte apoptosis and necrosis, following a single injection of CCl4 peaks between 36–48 hr (Markiewski, et al., 2004 and Bezerra et al., 2001). ALT was increased in all genotypes 48 hr after CCl4 exposure (Figure 2A). However, plasma ALT activity was higher in the FD−/− at 48 hr compared to the other genotypes (Figure 2A). In a more detailed time-course, plasma ALT activity was higher in FD−/− mice both at 24 and 48 hr compared to WT. ALT returned to baseline by 72 hr in both genotypes (Figure 2B). Expression of CYP2E1, required to bioactivate CCl4, was not affected by genotype, indicating that the increased injury in FD−/− mice was not due to an altered ability to bioactivate CCl4 (Figure 2C).
Figure 2. Factor D deficient mice have exacerbated CCl4-induced liver ALT activity.
WT, C1qa−/−, C4−/− and FD−/− mice were exposed to a single injection of CCl4 and plasma ALT was measured at A. 48 hr and B. WT and FD−/− mice ALT levels at 24, 48 and 72 hr. N=5–8 mice per group. C. Immunoblots of total hepatic protein from control WT and FD−/− mice were performed to determine baseline expression of cytochrome P450 2E1 (CYP2E1). Quantification of band intensity measured by densitometry after normalization to HSC70 is shown in the bar graphs. n = 4 mice per group. Values represent mean ± standard error of mean. Values with different alphabetical superscripts or points represented by an (*) were significantly different from each other, p<.05.
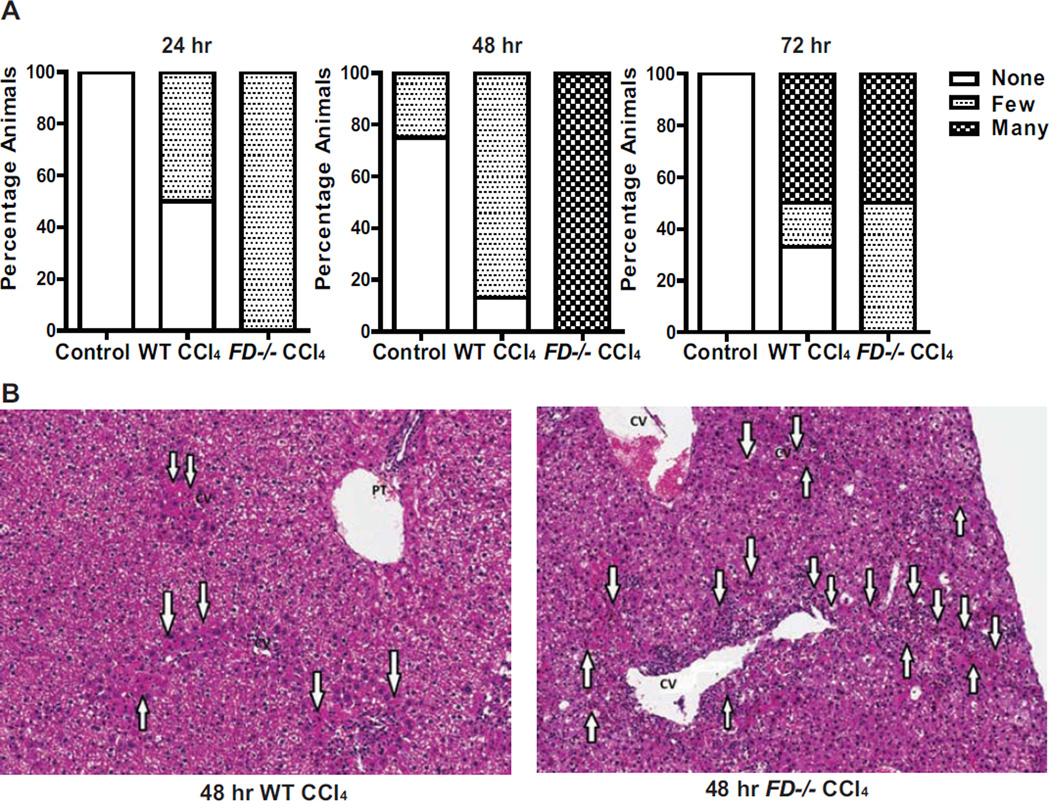
Histological analysis of acute liver injury was performed by a liver pathologist. As expected, WT and FD−/− mice exhibited multiple indications of liver injury following a single CCl4 injection. However, the number of acidophilic bodies, indicative of apoptotic cells, was higher in FD−/− mice at 24, 48 and 72 hr compared to WT mice (Figure 3A). The distribution of acidophilic bodies at 48 hr was more extensive and involved multiple perivenular regions (zone 3) in FD−/− mice compared to the few, very focal acidophilc bodies noted in WT mice (Figure 3B).
Figure 3. Increased and persistent presence of acidophilic bodies in the liver of FD−/− mice following CCl4-induced injury.
A. Evaluation of the quantity of acidophilic bodies evaluated in Hematoxylin and eosin-stained histologic sections of livers from WT and FD−/− mice at 24, 48 and 72 hr following a single injection of CCl4 are shown. WT controls received a single injection of olive oil. Bars represent percentage of mice in each group exhibiting the designated quantity of acidophilic bodies. 24 hr: Control (n=4); WT CCl4 (n=4); FD CCl4 (n=5); 48 hr: Control (n=4); WT CCl4 (n=8); FD CCl4 (n=7); 72 hr: Control (n=4); WT CCl4 (n=6); FD CCl4 (n=6). B. Representative H & E histological sections of livers from WT and FD−/− mice 48 hr after a single injection of CCl4 are shown. A greater number of acidophil bodies was noted in the FD−/− cases. (arrows indicate the acidophilic bodies) (H&E stain, 100×).
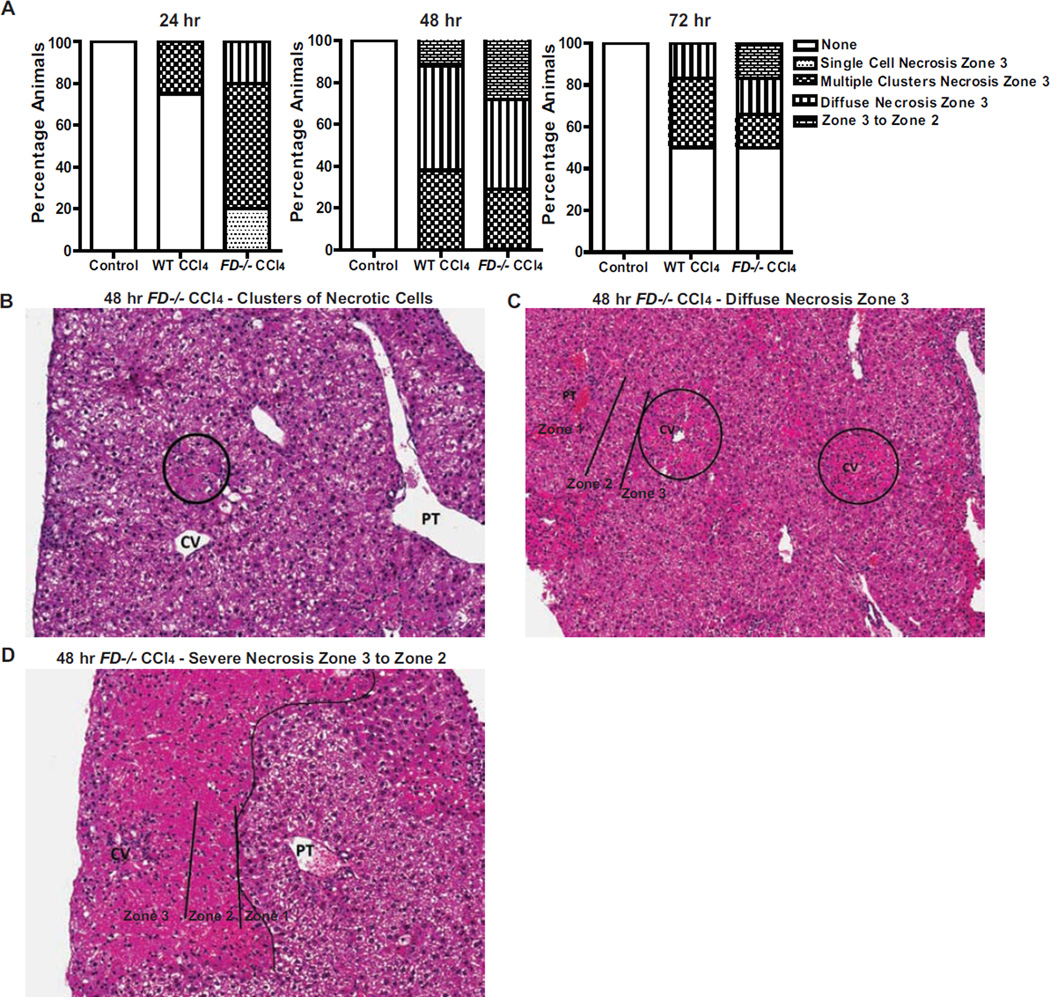
Morphologic features of necrosis were noted in WT and FD−/− mice, but necrosis was more extensive and occurred more frequently in the FD−/− mice (Figure 4A). Livers from WT mice 24 hr after CCl4 injection had either no necrosis (75% of mice) or clusters of necrotic cells in zone 3 of the liver (25% of mice) compared to FD−/− in which more animals exhibited multiple zone 3 clusters of necrotic cells (60% of mice), diffuse necrosis (20% of mice), as well as a few mice with single cell necrosis in zone 3 (20% of mice). 48 hr after CCl4 injection, necrosis was increased in both WT and FD−/− mice, but necrosis was more extensive in FD−/− mice, with more mice demonstrating clusters of necrotic cells (29% of mice) (Figure 4B) or diffuse necrosis (43% of mice) in zone 3 of the liver lobule (Figure 4C), as well as necrosis extending from zone 3 to zone 2 (29% of mice) (Figure 4D). While necrosis resolved in 50% of the mice in WT and FD−/− by 72 hr, the remaining FD−/− mice had more severe necrosis in zone 3 of the liver lobule with continued evidence of clusters of necrotic cells, zone 3 necrosis, as well as necrosis extending from zone 3 to zone 2. Taken together, the histological data indicate elevated cellular debris with presence of damaged tissue persisting in the FD−/− relative to WT mice after acute exposure to CCl4.
Figure 4. FD−/− mouse livers exhibit more severe necrosis following CCl4 exposure.
A. Identification of the presence of cellular necrosis evaluated from histological Hematoxylin and eosin (H&E)-stained sections of livers from WT and FD−/− mice at 24, 48 and 72 hr following a single injection of CCl4 are shown. Bars represent percentage of mice in each group exhibiting the pattern of cellular necrosis described. The number of mice at each time point are the same as those indicated in Figure 3. B–D. Representative histological sections of livers from FD−/− mice exhibiting the predominant patterns of necrosis at 48 hr after a single injection of CCl4 are shown (CV: central vein; PT: portal tract): B. Clusters of necrotic cells in the lobules (H&E stain, 200×) C. Diffuse zone 3 necrosis involving the centrilobular region (also called zone 3) (H&E stain, 100×) D. More extensive necrosis not only involving the zone 3, but also extending to zone 2 (H&E stain, 200×).
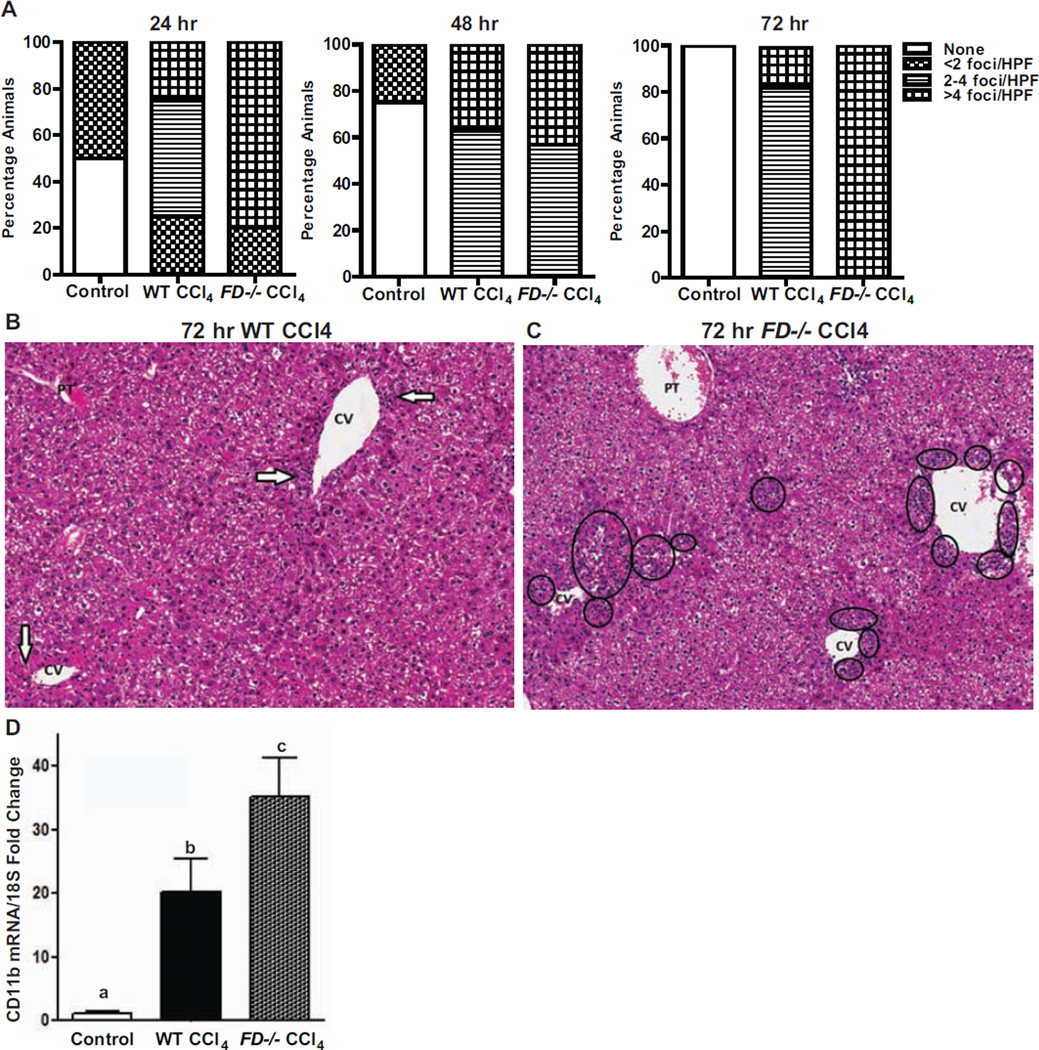
In response to cellular stress and injury, immune cells infiltrate into damaged tissue to assist with its recovery. Liver sections of both genotypes identified the presence of neutrophilic infiltrates predominantly located in zone 3 (Figure 5A). More neutrophilic infiltrates were detected per high power field in FD−/− mice than the WT mice 24 hr following CCl4 injection; however, at 48 hr there was no effect of genotype on neutrophilic bodies. Interestingly, by 72 hr, when other markers of liver injury were resolving in both genotypes, the FD−/− mice continued to exhibit a higher number of foci of neutrophilic infiltrates (>4 foci/HPF) than the WT mice (Figure 5A). The localization of neutrophilic infiltrates also differed between the WT and FD−/− mice at 72 hr; WT mice exhibited scattered foci of neutrophilic inflammation (Figure 5 B) whereas the FD−/− mice exhibited numerous and confluent neutrophilic aggregates around the central veins (Figure 5C). Additionally, mRNA expression of CD11b, a marker of activated neutrophils, was elevated in FD−/− mice at 48 hr (Figure 5D).
Figure 5. FD−/− mice have higher presence of hepatic neutrophilic infiltrates following CCl4- induced injury.
A. Identification of neutrophilic infiltrates evaluated from histological Hematoxylin and Eosin (H&E) sections of livers from WT and FD−/− mice at 24, 48 and 72 hr following a single injection of CCl4 are shown. Bars represent percentage of mice in each group exhibiting the number of foci observed per high power field. The number of mice at each time point are the same as those indicated in Figure 3. B &C. Representative histological H&E sections of livers from WT and FD−/− mice exhibiting the most frequent number of neutrophilic foci at 48 hr after a single injection of CCl4 are shown (H&E stain, 200×) (CV: central vein; PT: portal tract). B. Arrows indicate single foci with scattered neutrophils and rare associated lymphocytes noted in the centrilobular region in WT mice. C. Circles indicate the numerous zone 3 clusters of neutrophils seen in the FD−/− mice D. mRNA expression of CD11b was detected in mouse liver 48 hr after a single injection of CCl4. Values represent means ± SEM. Values with different alphabetical superscripts were significantly different from each other, p<.05.
3.3 Absence of Factor D Does Not Impair Hepatocyte Proliferation
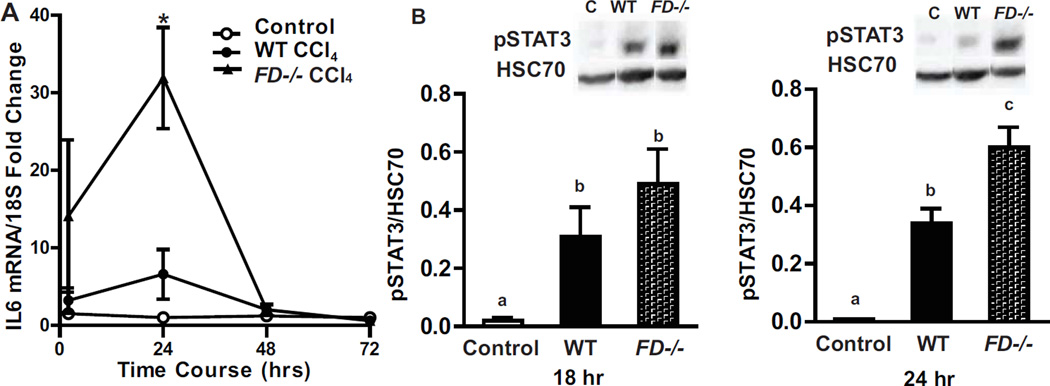
Delayed or reduced hepatocyte proliferation could be one mechanism for impaired recovery of FD−/− mice after CCl4. IL-6/STAT3 signaling is associated with the early priming phase of liver regeneration (Fausto et al., 2006). A single CCl4 injection induced IL-6 mRNA expression in WT and FD−/− mice at 2 and 24 hr; this induction was greater in FD−/− mice compared to WT at 24 hr (Figure 6A). As with the IL-6 mRNA, phosphorylation of STAT3 was elevated in response to a single CCl4 injection in both genotypes; induction was higher in FD−/− mice at 24 hr, corresponding to the time that IL-6 mRNA was also greater in the FD−/− mice (Figure 6B).
Figure 6. IL-6 and pSTAT3 were increased in FD−/− mice 24 hr after CCl4 exposure.
Expression of IL-6 mRNA and phosphorylated STAT3 was detected in mouse livers at after a single injection of CCl4. A. IL-6 mRNA was detected in WT and FD−/− mice 2, 24, 48 and 72 hr following a CCl4 challenge. B. Liver lysates of total hepatic protein were prepared and levels of phosphorylated (Tyr705)-STAT3 was detected 18 and 24 hr following a single injection of CCl4. Quantification of band intensity measured by densitometry after normalization to HSC70 are shown in the bar graphs. n = 4–6 mice per group. Bars are means ± SEM. Values with different alphabetical superscripts or points represented by an (*) are significantly different from each other, p<.05.
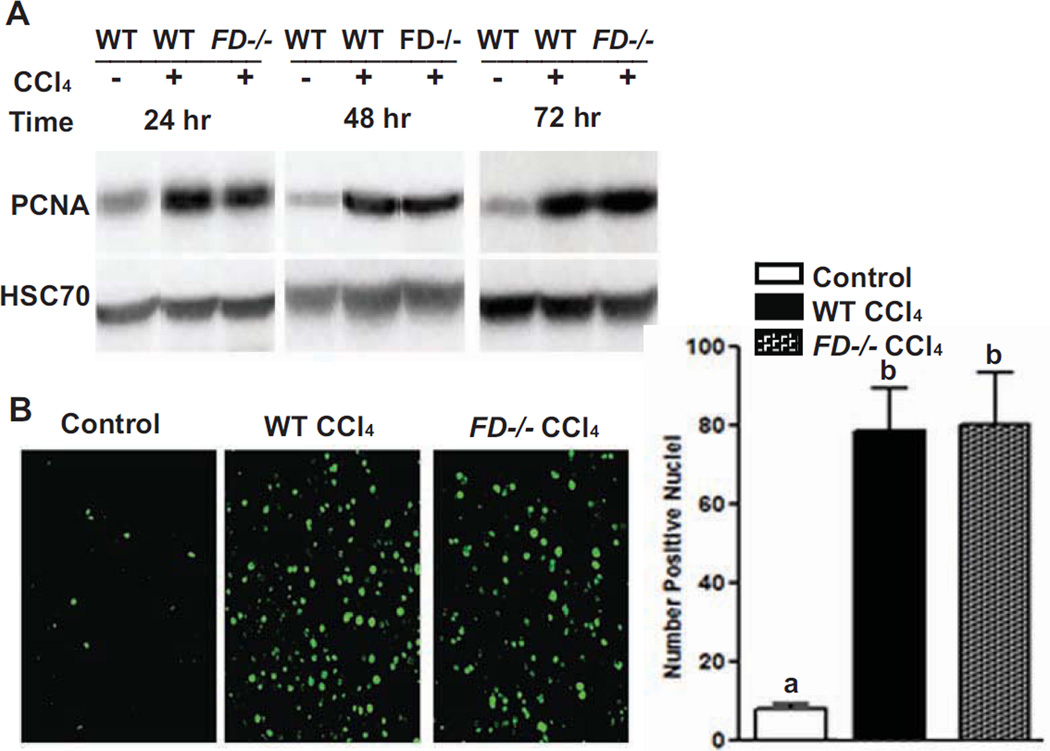
Consistent with markers of IL-6/STAT3 signaling, measures of hepatocyte proliferation were also induced. WT and FD−/− mice mounted a similar proliferative response to CCl4 –induced injury as assessed by proliferating cell nuclear antigen (PCNA) mRNA expression (data not shown) and protein expression via immunoblotting at 24, 48 and 72 hr (Figure 7A). Since liver sections 72 hr following CCl4 induced injury had more neutrophilic infiltrates, we further analyzed if this correlated with Ki67, a cellular marker for proliferation. Frozen liver sections probed for antigen Ki67 revealed Ki67-positive cells to be similarly distributed throughout the liver lobule between genotypes; no difference in the number of Ki67-positive cells was found between WT and FD−/− mice at 72 hr (Figure 7B).
Figure 7. Absence of Factor D does not impair hepatocyte proliferation following CCl4-induced toxic injury.
WT and FD−/− mice were provided with a single injection of CCl4. A. Immunoreactive PCNA (proliferating cell nuclear antigen) protein in liver lysates was assessed by Western blot analysis 24, 48 and 72 hr after injection. Quantification of band intensity measured by densitometry after normalization to HSC70 showed no difference between genotypes (data not shown). B. Ki67 was visualized by immunohistochemistry in frozen liver sections 72 hr following injury. Total number of fluorescent pixels per 10× field was determined using Image Pro software. Images are representative of n = 4–6 mice per group. Bars are means ± SEM. Values with different alphabetical superscripts are significantly different from each other, p<.05.
4. Discussion
The present study identifies the importance of Factor D, a component in the alternative pathway of complement activation, in facilitating complement opsonization and efficient clearance of damaged tissue in the liver in response to acute toxic injury. WT, C1qa−/− and C4−/−, but not FD−/−, mice rapidly activated complement, evidenced by the accumulation of C3b across the liver parenchyma, in response to exposure to CCl4. The failure to activate complement was associated with increased hepatocyte injury in FD−/− mice compared to WT, C1qa−/− and C4−/− mice. Interestingly, while FD−/− were able to mount a normal proliferative response to acute toxic injury, deficiency of FD was associated with increased acidophilic bodies, neutrophilic infiltration and a prolonged accumulation of necrotic tissue. Taken together, these data suggest that the alternative pathway, as indicated by the absence of FD, is critical to the appropriate opsonization of damaged tissue by C3b, facilitating the timely clearance of hepatocellular debris. Impaired clearance of this debris in FD−/− mice exacerbated and prolonged liver injury.
Genetic deficiencies of key molecules of the complement cascade (C3 and C5) are associated with defective liver regeneration and extensive parenchymal necrosis following toxic liver injury induced by CCl4 in mice (Markiewski et al., 2004 and Mastellos et al., 2001). Carbon tetrachloride, a halogenated alkane used to study hepatic injury, (Rahman and Hodgson, 2000, Yamada and Fausto, 1998, Weber et al., 2003) is activated by the cytochrome system (e.g., CYP2E1) to form the trichloromethyl radical, CCl3˙. This radical can react with oxygen to form the trichloromethylperoxy radical CCl3OO˙, a highly reactive species known to induce lipid peroxidation, oxidative stress, hepatic necrosis and apoptosis (Weber et al., 2003 and Taub, 2003). Administration of C3a and C5a in these gene deficient mice restores hepatocyte proliferation, indicating that the anaphylatoxins act as early regulators of normal liver recovery after CCl4 induced injury (Markiewski et al., 2004 and Mastellos et al., 2001).
Tissue repair requires not only generation of new cells, but also efficient removal of damaged cells; complement plays an important role in clearance of cellular debris (Trouw et al., 2008). Following injury and complement activation, C3b is released and deposited on necrotic and apoptotic cells, signaling phagocytes to clear damaged tissue (Takizawa et al., 1996 and Mevorach et al., 1998). While Markiewski, et al. (2004) had identified C3a and C5a as key to hepatocyte proliferation after injury, identification of complement activation products involved in clearing damaged tissue has not yet been studied. We hypothesized that deposition of C3b on damaged tissue is a key mediator of clearance of cellular debris and is necessary for timely liver recovery following CCl4-induced injury.
The identification for a critical role for FD in the alternative pathway for complement activation in the response to acute toxic injury is of particular relevance to clearance of cellular debris. While binding of complement initiation molecules does not typically take place on early apoptotic cells (Trouw et al., 2008); several reports indicate that the alternative pathway plays a role in priming apoptotic cells for safe clearance by phagocytes, being most prominent with late apoptotic/necrotic cells (Mevorach et al., 1998, Tsuji et al., 1994, Gaipel et al., 2001). If apoptotic cells are not rapidly and efficiently removed, they enter the stage of secondary necrosis which can exacerbate inflammatory cellular conditions (Munoz et al., 2010). The present studies indicate that Factor D in the alternative pathway is important for early complement opsonization and plays a significant role in facilitating damaged tissue clearance.
If removal of damaged tissue is inadequate and injury persists, reorganization of the extracellular matrix is impaired. We find the absence of Factor D and lack of C3b/iC3b/C3c deposition following CCl4 -induced injury was associated with more severe and prolonged liver injury. Attracted by multiple signals, neutrophils are amongst the early immune responders to the injury site (Nathan, 2006). Amplifying the local inflammatory environment, neutrophils produce a variety of immunostimulatory effectors involved with tissue repair and regeneration (Chosay et al., 1997). Continuation of tissue injury coincides with inflammation which activates various cell types, including neutrophils. Interestingly, while the WT mice appeared to be recovering from the acute toxic injury at 72 hr, this was associated with resolution of inflammation. In comparison, prolonged tissue injury in FD−/− mice, as indicated by the increased presence of acidophilic bodies and necrosis, was associated with a persistent presence and abundance of neutrophilic infiltrates around the central vein.
The presence of more neutrophils at 24 hr in the FD−/− mice was associated with higher mRNA expression of the proinflammatory cytokine IL-6. There is increased evidence of the importance of cytokines, and in particular IL-6/STAT3 signaling, in having critical roles in liver regeneration (Taub, 2003). Our data reveal that deficiency of Factor D did not impair hepatocyte proliferation, as there was no effect of genotype on IL-6/STAT3, as well as proliferative markers, in response to CCl4-induced injury.
The coagulation cascade is another pathway critical to the clearance of tissue debris in response to toxic liver injury, coordinating timely proteolysis of matrix elements and clearance of tissue debris (Bezerra et al., 2001). In response to CCl4, expression of mRNA for the plasminogen activators, tPA and uPA, was strong in both FD−/− and WT mice, with FD−/− exhibiting an even more robust induction (data not shown). Thus, under the conditions of our study, activation of the coagulation pathway was insufficient to maintain timely clearance of cellular debris, suggesting that clearance is dependent on the activation of both the coagulation cascade and alternative pathway driven complement-dependent opsonization.
5. Conclusion
In summary, complement plays a critical role following an acute toxic liver injury not only in assuring an adequate proliferative response is generated, but also in marking of cellular debris for safe and efficient removal by phagocytes in order for timely tissue repair to proceed. Here we demonstrate the important role of the alternative complement pathway in facilitating opsonization of damaged tissue for a timely reparative process following an acute toxic liver injury induced by CCl4. Early marking of damaged cells for efficient clearance is important to minimize accumulation of necrotic and secondary necrotic cells that could exacerbate and prolong the inflammatory and immune response.
Highlights.
We examine which complement activation pathway is involved for timely liver recovery following an acute toxic injury
We utilized mice deficient in C1qa, C4 and Factor D, lacking the classical, classical/MBL, and alternative pathways of complement activation
Factor D-deficient mice had worse liver injury compared to other genotypes
Factor D-deficient mice were unable to adequately clear injured tissue
We demonstrate the importance of the alternative complement pathway in facilitating opsonization of damaged tissue for a timely reparative process following an acute toxic liver injury
Acknowledgements
We want to thank Dr. Michael Carroll for his generous provision of C1qa−/−, C4−/− mice and Dr. Gregory Stahl for his FD−/− mice.
This work was supported in part by NIH grant F32AA021044 to GC, the Case Western Reserve University/Cleveland Clinic CTSA (UL1RR024989) and NIH grants R01 AA016399 and R37 AA011876 to LEN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gail A. Cresci, Department of Gastroenterology and Pathobiology Cleveland Clinic.
Daniela Allende, Department of Pathology Cleveland Clinic.
Megan R. McMullen, Department of Pathobiology Cleveland Clinic.
Laura E. Nagy, Department of Pathobiology and Gastroenterology Cleveland Clinic.
References
- Barnes MA, McMullen MR, Roychowdhury S, et al. Macrophage migration inhibitory factor contributes to ethanol-induced liver injury by mediating cell injury, steatohepatitis, and steatosis. Hepatology. 2013;57:1980. doi: 10.1002/hep.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezerra JA, Currier AR, Melin-Aldana H, et al. Plasminogen activators direct reorganization of the liver lobule after acute injury. 2001;158:921. doi: 10.1016/S0002-9440(10)64039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto M, Dell’Agnola C, Bygrave AE, et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 1998;19:56. doi: 10.1038/ng0598-56. [DOI] [PubMed] [Google Scholar]
- Chosay JG, Essani NA, Dunn CJ, et al. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am J Physiol. 1997;272:G1195. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- Degn SE, Thiel S. Humoral pattern recognition and the complement system. Scand. J. Immunol. 2013;78:181. doi: 10.1111/sji.12070. [DOI] [PubMed] [Google Scholar]
- Del Rio-Tsonis K, Tsonis PAA, Zarkadis IK, et al. Expression of the third component of complement, C3, in regenerating limb blastema cells of urodeles. J. Immunol. 1998;161:6819. [PubMed] [Google Scholar]
- Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- Fischer MB, Ma M, Goerg S, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. 1996;157:549. [PubMed] [Google Scholar]
- Gaipl US, Kuenkele S, Voll RE, et al. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 2001;8:327. doi: 10.1038/sj.cdd.4400826. [DOI] [PubMed] [Google Scholar]
- Gullstrand B, Martensson Ul, Sturfelt g, et al. Complement classical pathway components are all important in clearance of apoptotic and secondary necrotic cells. Clin Experimental Immunology. 2009;156:303. doi: 10.1111/j.1365-2249.2009.03896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M, Mollnes TE. The alternative complement pathway revisited. J.Cell. Mol. Med. 2008;12:1074. doi: 10.1111/j.1582-4934.2008.00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller BH, Smithies O. Altering genes in animals by gene targeting. Annu Rev Immunol. 1992;10:705. doi: 10.1146/annurev.iy.10.040192.003421. [DOI] [PubMed] [Google Scholar]
- Markiewski MM, Mastellos D, Tudoran R, et al. C3a and C3b/iC3b/C3c activation products of the third component of complement (C3) are critical for normal liver recovery after toxic injury. J Immunology. 2004;173:747. doi: 10.4049/jimmunol.173.2.747. [DOI] [PubMed] [Google Scholar]
- Mastellos D, Papadimitriou JC, Franchini S, et al. A novel role of complement: mice deficient in the fifth component of complement (C5) exhibit impaired liver regeneration. J Immunol. 2001;166:2479. doi: 10.4049/jimmunol.166.4.2479. [DOI] [PubMed] [Google Scholar]
- McMullen MR, Pritchard MT, Wang Q, et al. Early growth response-1 transcription factor is essential for ethanol-induced fatty liver injury in mice. Gastroenterology. 2005;128:2066. doi: 10.1053/j.gastro.2005.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevorach DJ, Mascarenhas O, Gershov K, et al. Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998;188:2313. doi: 10.1084/jem.188.12.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz LE, Lauber K, Schiller M, et al. The role of defective clearance of apoptotic cells in systemic autoimmunity. Nat. Rev. Rheumatol. 2010;6:280. doi: 10.1038/nrrheum.2010.46. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner J. Matrix metalloproteinases. J. Biol Chem. 1999;1999:21491. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nature Reviews Immunology. 2006;6:173. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- Nesargikar PN, Spiller B, Chavez R. The complement system: history, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. (Bp) 2012;2:103. doi: 10.1556/EuJMI.2.2012.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard MT, Roychowdhury S, McMullen MR, et al. Early growth response-1 contributes to galactosamine/lipopolysaccharide-induced acute liver injury in mice. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1124. doi: 10.1152/ajpgi.00325.2007. [DOI] [PubMed] [Google Scholar]
- Qin X, Gao B. The complement system in liver disease. Cellular & Molecular Immunity. 2006;3:333. [PubMed] [Google Scholar]
- Rahman TM, Hodgson HJ. Animal models of acute hepatic failure. Int. J. Exp. Pathol. 2000;81:145. doi: 10.1046/j.1365-2613.2000.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reca R, Mastellos D, Jajka M, et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- Roychowdhury S, McMullen MR, Pritchard MT, et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology. 2009;49:1326. doi: 10.1002/hep.22776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b/iC3b/C3c deposition on apoptotic cells. FEBS Lett. 1996;397:269. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- Taub R. Hepatoprotection via the IL-6/STAT3 pathway. J Clin Investigation. 2003;112:978. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R. Liver regeneration: from myth to mechanism. Nature Reviews. 2004;5:836. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol. Res. 2011;51:45. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouw LA, Bengtsson A, Gelderman K, et al. C4b-binding protein and factor H compensate for the loss of membrane bound complement regulators to protect apoptotic cells against complement attack. J. Biol. Chem. 2007;282:28540. doi: 10.1074/jbc.M704354200. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Kaji K, Nagasawa S. Activation of the alternative pathway of human complement by apoptotic human umbilical vein endothelial cells. J Biochem (Tokyo) 1994;116:794. doi: 10.1093/oxfordjournals.jbchem.a124598. [DOI] [PubMed] [Google Scholar]
- Weber LW, Boll M, Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33:105. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- Xu V, Ma M, Ippolito GC, et al. Complement activation in Factor D-deficient mice. Proc National Acad Sci. 2001;98:14577. doi: 10.1073/pnas.261428398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y, Fausto N. Deficient liver regeneration after carbon tetrachloride injury in mice lacking type 1 but not type 2 tumor necrosis factor receptor. Am. J. Pathol. 1998;152:1577. [PMC free article] [PubMed] [Google Scholar]