Abstract
Background/Aims
Diffuse or segmental irregular narrowing of the main pancreatic duct (MPD), as observed by endoscopic retrograde cholangiopancreatography (ERCP), is a characteristic feature of autoimmune pancreatitis (AIP).
Methods
ERCP findings were retrospectively examined in 40 patients with AIP in whom irregular narrowing of the MPD was detected near the orifice. The MPD opening sign was defined as the MPD within 1.5 cm from the orifice being maintained. The distal common bile duct (CBD) sign was defined as the distal CBD within 1.5 cm from the orifice being maintained. Endoscopic findings of a swollen major papilla and histological findings of specimens obtained from the major papilla were examined in 26 and 21 patients, respectively.
Results
The MPD opening sign was detected in 26 of the 40 patients (65%). The distal CBD sign was detected in 25 of the 32 patients (78%), which showed stenosis of the lower bile duct. The patients who showed the MPD opening sign frequently showed the distal CBD sign (p=0.018). Lymphoplasmacytic infiltration, but not dense fibrosis, was histologically detected in biopsy specimens obtained from the major papilla.
Conclusions
On ERCP, the MPD and CBD adjacent to the major papilla are frequently maintained in patients with AIP involving the pancreatic head. These signs are useful for diagnosing AIP on ERCP.
Keywords: Pancreatitis, chronic, Cholangitis, sclerosing
INTRODUCTION
Autoimmune pancreatitis (AIP) is a recently identified peculiar type of pancreatitis with a presumed autoimmune etiology. AIP is characterized serologically by elevated serum immunoglobulin G4 (IgG4) levels, histologically by abundant infiltration of IgG4-positive plasma cells and lymphocytes with fibrosis, and therapeutically by a dramatic response to steroids. The most common presenting symptom of AIP is obstructive jaundice due to stricture of the bile duct. In many AIP patients, the causative stenosis is located in the lower part of the common bile duct (CBD). As AIP sometimes mimics pancreatic cancer, accurate differentiation of AIP from pancreatic cancer is important to avoid unnecessary surgery.1,2
Irregular narrowing of the main pancreatic duct (MPD) is a characteristic pancreatographic feature of AIP. Diffuse irregular narrowing of the MPD is rather specific to AIP; however, segmental narrowing of the MPD is sometimes difficult to differentiate from stenosis of the MPD caused by pancreatic cancer.3–6 Given their utility in differentiating AIP from pancreatic cancer, pancreatographic findings play an important role in both the international consensus diagnostic criteria (ICDC)7 and the Japanese diagnostic criteria 2011.8 We have prospectively examined pancreatographic findings in AIP patients on endoscopic retrograde pancreatography (ERP). The current study retrospectively examined findings of endoscopic retrograde cholangiopancreatography (ERCP) for AIP patients in whom the pancreatic head was involved, focusing on the opening portion of the MPD and the distal portion of the CBD.
MATERIALS AND METHODS
From 1991 to 2013, a total of 94 patients with type-1 AIP (68 males, 26 females; median age, 64 years) were diagnosed according to the ICDC7 in Tokyo Metropolitan Komagome Hospital. Enlargement of the pancreas on computed tomography (CT) and irregular narrowing of the MPD on ERP were detected in all patients (Fig. 1). ERCP findings were retrospectively examined for 48 patients in whom the pancreatic head was enlarged on CT, and satisfactory imagings from pancreatography were obtained. To achieve the best possible visualization, several pancreatograms of the head of the pancreas were taken in a prone or slightly oblique position. Four patients with pancreas divisum (complete, n=2; incomplete, n=2) and four patients in whom the MPD within 2 cm from the orifice was not involved in irregular narrowing were excluded from the study (Fig. 2). Forty type-1 AIP patients in whom irregular narrowing of the MPD was detected near the orifice were enrolled in this study. Pancreatic enlargement of the pancreas was diffuse (n=22) and segmental in the pancreatic head (n=18). Irregular narrowing of the MPD was diffuse (n=19), skipped in the pancreatic head and body (n=4), and segmental in the pancreatic head (n=17).
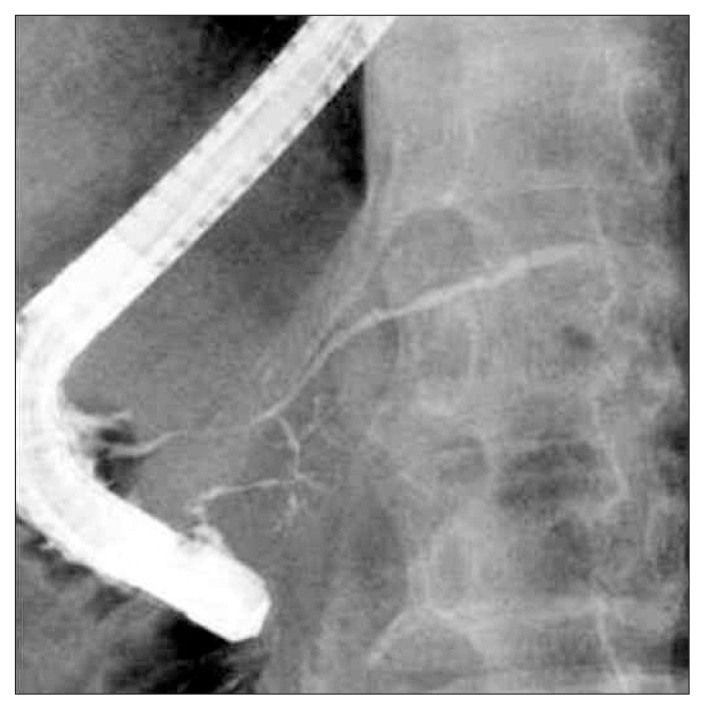
Fig. 1.
Endoscopic retrograde pancreatography finding of diffuse narrowing of the main pancreatic duct involving the orifice in the major papilla.
Fig. 2.
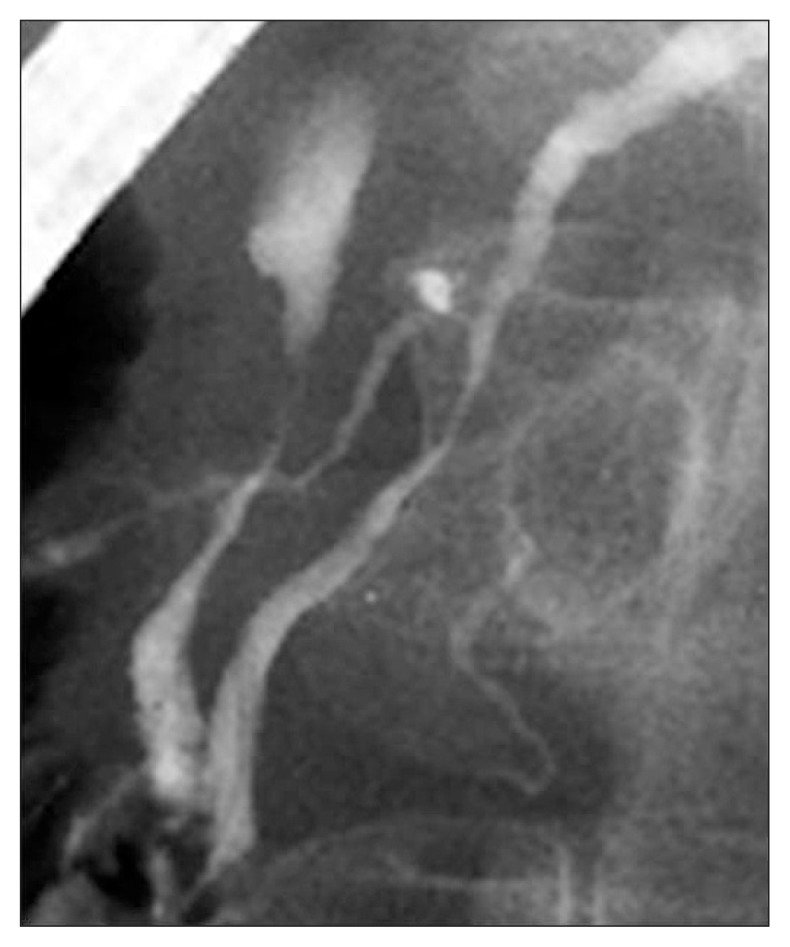
Endoscopic retrograde pancreatography findings of excluded cases showing no irregular narrowing of the main pancreatic duct within 2 cm from the orifice.
ERCP findings were examined in all 40 patients, focusing on the opening portion of the MPD and the distal portion of the CBD. Endoscopic retrograde cholangiography (ERC) was not performed in six patients. Stenosis of the lower bile duct was observed in 32 patients on ERC. MPD opening sign was defined as the MPD within 1.5 cm from the orifice being maintained without irregular narrowing. The preserved MPD showed a spindle (Fig. 3A) or cystic shape (Figs 3B and 4). Distal CBD sign was likewise defined as the distal CBD within 1.5 cm from the orifice being maintained without involvement of stenosis (Figs 4 and 5).
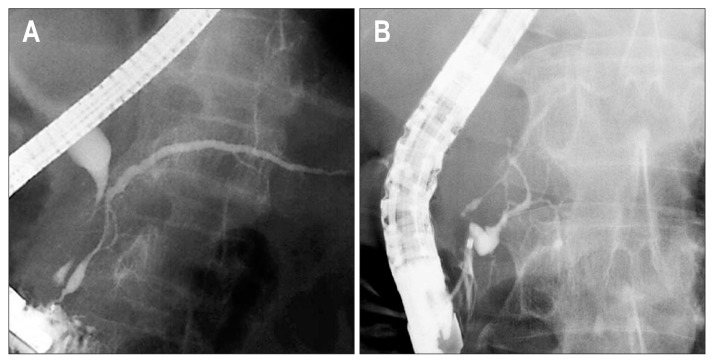
Fig. 3.
Main pancreatic duct opening sign showing that the main pancreatic duct within 1.5 cm from the orifice is maintained without irregular narrowing. The preserved main pancreatic duct is either (A) spindle-shaped or (B) cystic-shaped.
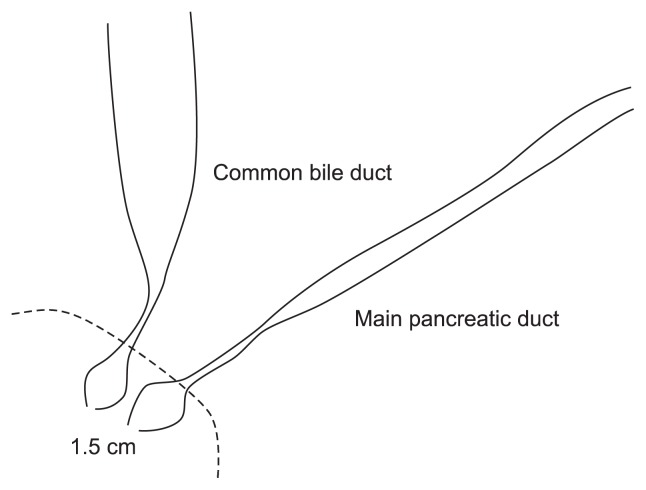
Fig. 4.
Schematic illustration of the main pancreatic duct opening and distal common bile duct signs.
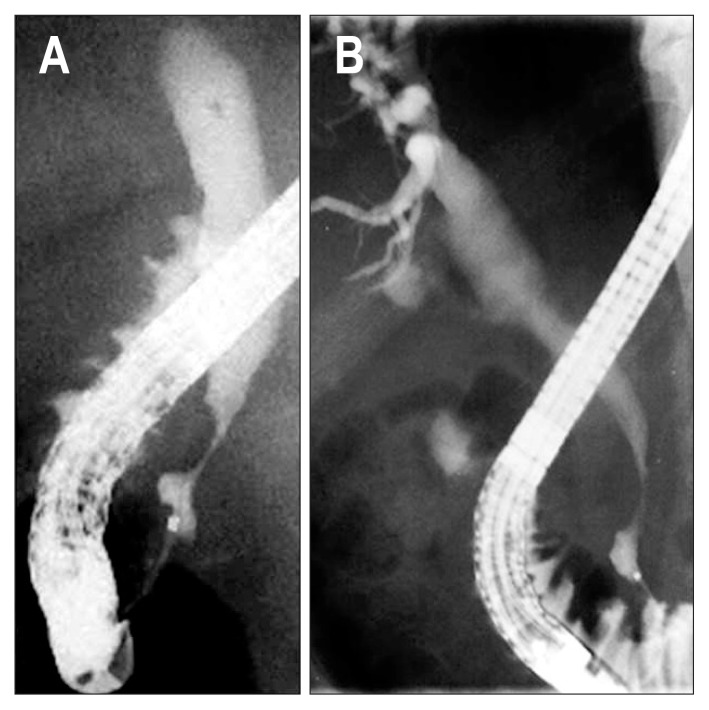
Fig. 5.
Distal common bile duct (CBD) sign showing that the distal CBD within 1.5 cm from the orifice is maintained without involvement of stenosis (A, B).
We examined endoscopic findings of the major papilla in 26 of the 40 AIP patients during ERCP. Swelling of the major papilla was judged during ERCP and/or from photographs of the papilla. A swollen major papilla was diagnosed based on observation of an indistinct border between the major papilla and oral protrusion.9–11 At the end of ERCP, we obtained one or two specimens from the major papilla of 21 patients using biopsy forceps. To avoid biopsy-related acute pancreatitis, biopsy specimens were not obtained near the orifice of the pancreatic duct. Sections cut from paraffin-embedded blocks were stained with hematoxylin and eosin and immunostained using anti-IgG4 antibody (The Binding Site, Birmingham, UK) with avidinbiotin-peroxidase complex. Positive IgG4 immunostaining was defined as >10 IgG4-positive plasma cells in at least one high-power field.11,12
Statistical analysis was performed using chi-square test. A p<0.05 was considered statistically significant.
This study was approved by the Institutional Review Board at Tokyo Metropolitan Komagome Hospital, and informed consent for all invasive procedures was obtained from all patients.
RESULTS
MPD opening sign was detected in 26 of 40 patients (65%). Distal CBD sign was detected in 25 of the 32 patients (78%) who showed stenosis of the lower bile duct on ERC. Patients showing MPD opening sign frequently also showed distal CBD sign (p=0.018) (Table 1).
Table 1.
Main Pancreatic Duct Opening Sign and Distal Common Bile Duct Sign in Autoimmune Pancreatitis
| Distal CBD sign (+) | Distal CBD sign (−) | No stenosis of the lower bile duct | No cholangiogram | Total | |
|---|---|---|---|---|---|
| MPD opening sign (+) | 20* | 2 | 2 | 2 | 26 |
| MPD opening sign (−) | 5 | 5 | 0 | 4 | 14 |
| Total | 25 | 7 | 2 | 6 | 40 |
CBD, common bile duct; MPD, main pancreatic duct.
p=0.018 comparing MPD opening sign and distal CBD sign.
Swelling of the major papilla was detected endoscopically in 9 of 15 patients (60%) with MPD opening sign, but no significant relationship was apparent between MPD opening sign and a swollen major duodenal papilla (p=0.69) (Table 2).
Table 2.
Relationship between the Main Pancreatic Duct Opening Sign and Endoscopic Findings of a Swollen Major Papilla in Autoimmune Pancreatitis
| MPD opening sign | Swelling of the major papilla | |
|---|---|---|
|
| ||
| (+) | (−) | |
| (+) | 9 (60)* | 6 (40) |
| (−) | 5 (45) | 6 (55) |
Data are presented as number (%).
MPD, main pancreatic duct.
p=0.69.
Lymphoplasmacytic infiltration was histologically detected in the biopsy specimen taken from the major papilla of 11 of 21 patients (52%), but dense fibrosis was not detected in any patients. Abundant infiltration of IgG4-positive plasma cells in the major papilla was detected histologically in 8 of the 13 patients (62%) with MPD opening sign, but no significant relationship was found between MPD opening sign and abundant infiltration of IgG4-positive plasma cells (p=0.38) (Table 3).
Table 3.
Relationship between the Main Pancreatic Duct Opening Sign and Histological Findings of Abundant Infiltration of IgG4-Positive Plasma Cells in the Major Papilla in Autoimmune Pancreatitis
| MPD opening sign | Abundant infiltration of IgG4-positive plasma cells in the major papilla | |
|---|---|---|
|
| ||
| (+) | (−) | |
| (+) | 8 (62)* | 5 (38) |
| (−) | 3 (38) | 5 (62) |
Data are presented as number (%).
MPD, main pancreatic duct.
p=0.38.
DISCUSSION
In our previous studies comparing ERP findings between AIP and pancreatic cancer, a narrowed MPD longer than 3 cm, skipped MPD lesions, side branch derivation from the narrowed MPD, and upstream dilatation of the MPD less than 5 mm were all more frequent in AIP.3,6 However, several AIP cases were encountered in which differentiation from pancreatic cancer using ERP findings was difficult.
The present study examined ERCP findings focusing on the opening portion of the MPD and the distal portion of the CBD in 40 patients. The MPD within 1.5 cm from the orifice was maintained without irregular narrowing in 65% of AIP patients with irregular narrowing of the MPD in the pancreatic head. The maintained portion of the MPD showed a spindle or cystic shape. Distal CBD within 1.5 cm from the orifice was also maintained without stenosis in 78% of patients with stenosis of the lower bile duct. We termed these findings as MPD opening sign and distal CBD sign, respectively. Patients showing MPD opening sign also frequently showed distal CBD sign.
The characteristic histological pattern of AIP was designed as lymphoplasmacytic sclerosing pancreatitis with abundant infiltration of Ig4-positive plasma cells and lymphocytes, acinar atrophy, interlobular fibrosis and obliterative phlebitis in the pancreas.1,2 The pancreatic duct is narrowed by nonocclusive fibrosis with lymphoplasmacytic infiltration within the existing periductal elastic fiber layer, and the epithelium of the pancreatic duct is well preserved.1,2,13
Lymphoplasmacytic infiltration is detected around the major papilla continuing from an inflammatory pancreatic head;14 however, dense fibrosis is not seen in biopsy specimens taken from the major papilla of AIP patients. The opening portion of the MPD and the distal CBD are both located inside the duodenal wall and are surrounded by the sphincter of Oddi.15,16 The sphincter of Oddi consists of sphincter choledochus, sphincter pancreaticus, and sphincter ampullae.15,16 As radiological length of the sphincter pancreaticus in autopsy specimens is reported to be 11.4±4.1 mm,17 we defined MPD opening sign as the MPD within 1.5 cm from the orifice being maintained. Although the exact pathophysiological mechanisms underlying MPD opening sign and distal CBD sign remain unknown, we suspect that the opening portion of the MPD and the distal CBD are maintained in AIP patients, as lymphoplasmacytic infiltration occurs, but dense fibrosis does not, in the periductal portions of the MPD and CBD surrounded by the sphincter muscle. The major papilla has been reported to be swollen in 41% to 65%9–11 of AIP patients, resulting directly from an inflammatory pancreatic head; however, no relationship was identified between MPD opening sign and a swollen major duodenal papilla.
There are several limitations in this study. First is a retrospective design which was not subsequently tested in a blinded fashion in cohort of AIP patients. Secondly, comparative study of pancreatograms between AIP and pancreatic cancer could not be done, as pancreatograms of pancreatic head cancer involving the major papilla are rarely obtained. Thirdly, histological examination of the pancreatic head of AIP patients was not done comparing to cholangiopancreatograms. Forth is small sample size due to the rarity of the disease. Although it is preliminary, this is the first report about new characteristic features of ERCP in autoimmune pancreatitis.
In conclusion, the MPD and CBD adjacent to the major papilla are frequently preserved in patients with AIP involving the pancreatic head on ERCP. These signs are useful for diagnosing AIP on ERCP.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kamisawa T, Takuma K, Egawa N, Tsuruta K, Sasaki T. Autoimmune pancreatitis and IgG4-related sclerosing disease. Nat Rev Gastroenterol Hepatol. 2010;7:401–409. doi: 10.1038/nrgastro.2010.81. [DOI] [PubMed] [Google Scholar]
- 2.Kamisawa T, Chari ST, Lerch MM, Kim MH, Gress TM, Shimosegawa T. Recent advances in autoimmune pancreatitis: type 1 and type 2. Gut. 2013;62:1373–1380. doi: 10.1136/gutjnl-2012-304224. [DOI] [PubMed] [Google Scholar]
- 3.Kamisawa T, Imai M, Yui Chen P, et al. Strategy for differentiating autoimmune pancreatitis from pancreatic cancer. Pancreas. 2008;37:e62–e67. doi: 10.1097/MPA.0b013e318175e3a0. [DOI] [PubMed] [Google Scholar]
- 4.Nishino T, Oyama H, Toki F, Shiratori K. Differentiation between autoimmune pancreatitis and pancreatic carcinoma based on endoscopic retrograde cholangiopancreatography findings. J Gastroenterol. 2010;45:988–996. doi: 10.1007/s00535-010-0250-4. [DOI] [PubMed] [Google Scholar]
- 5.Sugumar A, Levy MJ, Kamisawa T, et al. Endoscopic retrograde pancreatography criteria to diagnose autoimmune pancreatitis: an international multicentre study. Gut. 2011;60:666–670. doi: 10.1136/gut.2010.207951. [DOI] [PubMed] [Google Scholar]
- 6.Takuma K, Kamisawa T, Tabata T, Inaba Y, Egawa N, Igarashi Y. Utility of pancreatography for diagnosing autoimmune pancreatitis. World J Gastroenterol. 2011;17:2332–2337. doi: 10.3748/wjg.v17.i18.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–358. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 8.Shimosegawa T Working Group Members of the Japan Pancreas Society; Research Committee for Intractable Pancreatic Disease by the Ministry of Labor, Health and Welfare of Japan. The amendment of the Clinical Diagnostic Criteria in Japan (JPS2011) in response to the proposal of the International Consensus of Diagnostic Criteria (ICDC) for autoimmune pancreatitis. Pancreas. 2012;41:1341–1342. doi: 10.1097/MPA.0b013e3182706ed5. [DOI] [PubMed] [Google Scholar]
- 9.Unno H, Saegusa H, Fukushima M, Hamano H. Usefulness of endoscopic observation of the main duodenal papilla in the diagnosis of sclerosing pancreatitis. Gastrointest Endosc. 2002;56:880–884. doi: 10.1016/S0016-5107(02)70364-7. [DOI] [PubMed] [Google Scholar]
- 10.Kubota K, Iida H, Fujisawa T, et al. Clinical significance of swollen duodenal papilla in autoimmune pancreatitis. Pancreas. 2007;35:e51–e60. doi: 10.1097/mpa.0b013e31812575b4. [DOI] [PubMed] [Google Scholar]
- 11.Kim MH, Moon SH, Kamisawa T. Major duodenal papilla in autoimmune pancreatitis. Dig Surg. 2010;27:110–114. doi: 10.1159/000286573. [DOI] [PubMed] [Google Scholar]
- 12.Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A. A new diagnostic endoscopic tool for autoimmune pancreatitis. Gastrointest Endosc. 2008;68:358–361. doi: 10.1016/j.gie.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Kamisawa T, Funata N, Hayashi Y, et al. Close relationship between autoimmune pancreatitis and multifocal fibrosclerosis. Gut. 2003;52:683–687. doi: 10.1136/gut.52.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamisawa T, Tu Y, Nakajima H, Egawa N, Tsuruta K, Okamoto A. Usefulness of biopsying the major duodenal papilla to diagnose autoimmune pancreatitis: a prospective study using IgG4-immunostaining. World J Gastroenterol. 2006;12:2031–2033. doi: 10.3748/wjg.v12.i13.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyden EA. The anatomy of the choledochoduodenal junction in man. Surg Gynecol Obstet. 1957;104:641–652. [PubMed] [Google Scholar]
- 16.Suda K, Miyano T, Hashimoto K. The choledocho-pancreaticoductal junction in infantile obstructive jaundice diseases. Acta Pathol Jpn. 1980;30:187–194. doi: 10.1111/j.1440-1827.1980.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 17.Flati G, Flati D, Porowska B, Ventura T, Catarci M, Carboni M. Surgical anatomy of the papilla of Vater and biliopancreatic ducts. Am Surg. 1994;60:712–718. [PubMed] [Google Scholar]