Abstract
Background/Aims
Second-look endoscopy is performed to check for the possibility of post-endoscopic submucosal dissection (ESD) bleeding and to perform prophylactic hemostasis in most hospitals; however, there is little evidence about the efficacy of second-look endoscopy. We investigated whether second-look endoscopy after ESD is useful in the prevention of post-ESD bleeding.
Methods
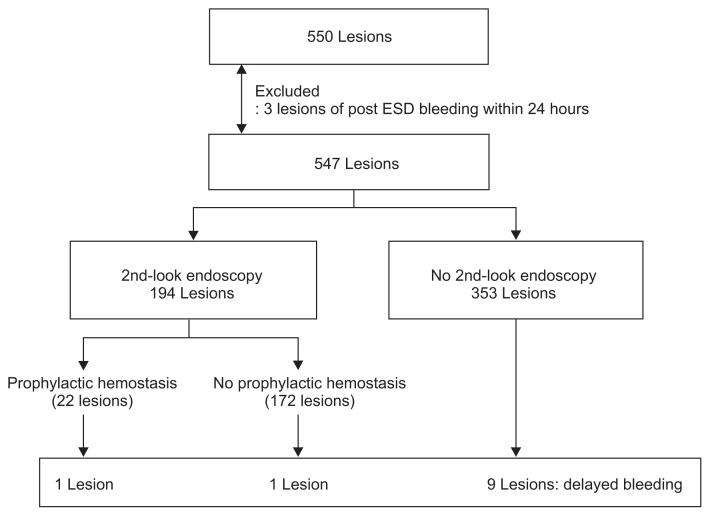
A total of 550 lesions with gastric epithelial neoplasms in 502 patients (372 men and 130 women) were treated with ESD between August 18, 2009 and August 18, 2010. After the exclusion of three lesions of post-ESD bleeding within 24 hours, 547 lesions (335 early gastric cancers and 212 gastric adenomas) were included for the final analysis.
Results
The occurrence rate of delayed post-ESD bleeding was not significantly different between the second-look group and the no second-look group (1% vs 2.5%, p>0.05). The only predictor of delayed bleeding was tumor size, regardless of second-look endoscopy after ESD (22.8±9.87 vs 15.1±10.47, p<0.05). There was no difference between the prophylactic hemostasis and nonprophylactic hemostasis groups, including the occurrence rate of delayed bleeding. In the second-look group with prophylactic hemostasis, the hospital stay was more prolonged than in the second-look group without prophylactic hemostasis, but there was no significant difference (p=0.08).
Conclusions
Second-look endoscopy to prevent delayed bleeding after ESD provides no significant medical benefits.
Keywords: Second-look endoscopy, Endoscopic submucosal dissection, Delayed bleeding, Prophylactic hemostasis
INTRODUCTION
Since its development in the late 1990s in Japan, endoscopic submucosal dissection (ESD) has increasingly been established as a promising procedure for epithelial neoplasm of the gastrointestinal (GI) tract.1,2 In contrast to endoscopic mucosal resection (EMR), ESD enables en bloc resection regardless of lesion size permitting a curative resection rate, and en bloc resection of early gastric cancer (EGC) is associated with a reduction in tumor recurrence.2–4
However, ESD demands advanced endoscopic techniques with lengthy procedure time. Besides, ESD is associated with a higher rate of complication such as perforation and bleeding compared to EMR.5–7
Post-ESD bleeding is one of the major concerns of endoscopists performing ESD. Although the frequency of complications in ESD has been decreased with improvements in technique and instrumentation, post-ESD bleeding has been reported in about 5% of cases.4,8,9 Endoscopists continue their efforts to decrease the rate of post-ESD bleeding. Takizawa et al.10 demonstrated that preventive coagulation of visibly exposed vessels on artificial ulcer decreased the post-ESD bleeding rate even if there was no evidence of bleeding at the end of ESD. Recently, post-ESD coagulation, regardless of the presence of bleeding at visible vessels on the resected area, is routinely performed.
In the same vein, a second-look endoscopy is routinely performed the next day or later after ESD in most hospitals to check for the possibility of post-ESD bleeding and to perform prophylactic hemostasis at the high risk lesion (such as the visible vessel) of rebleeding.11 However, there has been little evidence that second-look endoscopy is needed to prevent delayed post-ESD bleeding. A few previous studies were controversial and they did not make pure comparisons between the groups with or without the second-look endoscopy.11,12 Therefore, we conducted a retrospective study to evaluate whether a second look endoscopy was necessary for preventing delayed post-ESD bleeding.
MATERIALS AND METHODS
1. Subjects
A total of 550 lesions with a histologic diagnosis of gastric epithelial neoplasms (338 EGCs and 212 gastric adenomas) in 502 patients (372 men and 130 women) were treated with ESD at Samsung Medical Center between 18 August 2009 and 18 August 2010. Among them, 547 lesions (335 EGCs and 212 gastric adenomas) were included after exclusion of three lesions of post-ESD bleeding within 24 hours.
ESD was principally indicated for possible node negative EGCs according to the criteria of Soetikno et al.13 based on endoscopic findings including chromoendoscopy with biopsy and computed tomography findings. In cases of gastric adenomas, ESD was performed because lesions histologically diagnosed high grade dysplasia, may have contained foci of cancer, or patients strongly wanted the lesion to be resected. All ESDs were performed by two experienced endoscopists. Before ESD procedure, all patients provided written informed consent.
2. ESD procedure
The patients receiving anticoagulants or antiplatelets were instructed to stop taking them for 4 days or 1 week before the procedure. When there were more than two lesions in one stomach, the operator decided on a one stage or two stage procedure depending on the level of difficulty.
All ESD procedures were carried out under sedation with the intravenous administration of either midazolam combined with pethidine or propofol just before the procedure, and oxygen saturation and blood pressure were monitored.
ESD was performed using the following procedure. In brief, marking dots were made circumferentially at approximately 5 mm lateral to the margin of the lesion using a Needle knife (KD-1L-1, Olympus, Tokyo, Japan; Needle papillotome, MTW Endoscopy, Wesel, Germany) or a Dual knife (KD-650U; Olympus). After marking, a submucosal injection of saline mixed with epinephrine and indigocarmine was performed around the lesion to lift it off the muscle layer. If the tumor was located on the upper two-thirds of the stomach, a submucosal solution containing 10% glycerin was used. Then, an initial incision of mucosa was made with either a Needle knife or a Dual knife to allow insertion of the tip of the knife into the submucosa. After the initial incision, a circumferential mucosal incision was performed 5 mm outside the marking dots to separate the lesion from the surrounding nonneoplastic mucosa. This step was done using an electrosurgical knife such as the Needle knife, insulated-tipped (IT) knife (KD-610L; Olympus), or Dual knife with the use of an electrosurgical generator. After the circumferential incision, an additional submucosal injection was performed beneath the lesion. Finally, the submucosal connective tissue just beneath the lesion was directly dissected using an electrosurgical knife such as a Needle knife, an IT knife, or a Dual knife. The knife was chosen by preference of the operator. Hemostatic forceps (FD-410LR; Olympus) or hemoclips were used to control bleedings during resection. After dissection, visible vessels on the resected lesion were cauterized by Hemostatic forceps for the purpose of prophylactic hemostasis.
During and until 1 day after the procedure, a proton pump inhibitors (PPIs) or an histamine-2 receptor antagonists (H2RAs) were given via parental route and then standard dose of PPIs was prescribed for 4 weeks. The patients were allowed to take sips of water the day after the procedure and eat a light meal 2 days after if there was no problem.
A second-look endoscopy was performed 2 days after ESD before beginning meals only in patients who were enrolled in a prospective study for comparison between H2RAs and PPIs in the prevention of bleeding after ESD (this study was accepted in Hepatogastroenterology. In this study, there was no difference between the H2RAs and PPIs). If adherent clots or visible vessels with or without bleeding were observed during second look endoscopy, prophylactic hemostasis using Hemostatic forceps was done. The patient who underwent hemostasis during second-look endoscopy was allowed a light meal after being checked by endoscopy the next day.
3. Definitions
Post-ESD bleeding was defined as follows: if there was obvious hematemesis, melena, or hematochezia after ESD, or the level of hemoglobin decreased over 2.0 g/dL within 24 hours after ESD, which required endoscopic treatment.
Delayed bleeding was defined as post-ESD bleeding diagnosed later than 24 hours after ESD. Flow charts for inclusion in each analysis are shown in Fig. 1.
Fig. 1.
Flowchart showing the inclusion in the analysis of delayed bleeding.
ESD, endoscopic submucosal dissection.
4. Statistical analysis
The univariate analysis was performed for comparison of patient-related, tumor-related and procedure-related factors. Differences in the means of continuous data such as age, size, and procedure times were compared using Student t-tests. Categorical data such as sex, macroscopic type, and histologic type, were compared using the chi-square test and Fisher exact test.
p-values of <0.05 were considered statistically significant. If there was more than one predictor with a significant difference by univariate analysis, multivariate analysis by using a logistic regression model was planned.
RESULTS
1. Clinical and endoscopic characteristics in a second-look and no second-look group
A total of 547 lesions (335 EGCs and 212 gastric adenomas) in 499 patients (370 men and 129 women) were finally evaluated. A second-look endoscopy was performed in 194 lesions (second-look group). The baseline characteristics of subjects and lesions in both groups are summarized in Table 1. Mean age was 62.4±9.8 years (median age, 63 years; range, 32 to 84 years). In a second-look group, mean age was younger than no second-look group (58.8±8.8 vs 64.4±9.7, p<0.01). Patient-related factors such as sex, comorbidity, and use of anticoagulants or antiplatelet agents were not statistically significant between the second-look and the no second-look groups.
Table 1.
Characteristics of Patients and Lesions in Groups
| Characteristic | Total (547 lesions) | 2nd-look (194 lesions) | No 2nd-look (353 lesions) | p-value |
|---|---|---|---|---|
| Age | 0.000 | |||
| Median (range) | 63 (32–84) | 60 (32–75) | 66 (34–84) | |
| Mean±SD | 62.4±9.8 | 58.8±8.8 | 64.4±9.7 | |
| Male sex | 408 (74.6) | 148 (76.3) | 260 (73.7) | 0.539 |
| Comorbidities | ||||
| Hypertension (present) | 203 (37.1) | 66 (34.0) | 137 (38.8) | 0.309 |
| Diabetes mellitus (present) | 96 (17.6) | 33 (17.0) | 63 (17.8) | 0.907 |
| Heart disease (present) | 11 (2.0) | 1 (0.5) | 10 (2.8) | 0.107 |
| Anticoagulants/platelets (used) | 53 (9.7) | 19 (9.8) | 34 (9.6) | 1.000 |
| Histologic type | ||||
| Adenoma/adenocarcinoma | 212/335 | 68/126 | 144/209 | 0.200 |
| Location | ||||
| Upper/mid/lower third | 38/167/342 | 9/60/125 | 29/107/217 | 0.287 |
| AW/PW/LC/GC | 95/89/231/132 | 26/29/93/46 | 69/60/138/86 | 0.146 |
| Macroscopic type | ||||
| Elevated/flat or depressed | 411/136 | 137/57 | 274/79 | 0.079 |
| Size of the tumor, mm | 15.3±10.5 | 14.1±9.3 | 15.9±11.1 | 0.059 |
| Size of the resected specimen, mm | 43.2±13.3 | 42.7±11.6 | 43.5±14.2 | 0.489 |
| Resection style, en bloc/piecemeal | 523/24 | 191/3 | 332/21 | 0.016 |
| Procedure time, min | 66.0±39.1 | 57.6±32.5 | 70.7±41.6 | 0.000 |
| Delayed post-ESD bleeding | 11 (2.0) | 2 (1.0) | 9 (2.5) | 0.343 |
| Antiulcer medication after ESD, PPIs/H2RAs | 129/418 | 92/102 | 37/316 | 0.000 |
Data are presented as mean±SD or number (%).
SD, standard deviation; AW, anterior wall; PW, posterior wall; LC, lesser curvature; GC, great curvature; ESD, endoscopic submucosal dissection; PPIs, proton pump inhibitors; H2RAs, histamine-2 receptor antagonists.
The lesions were distributed similarly in both groups, most commonly in the lower third and lesser curvature side of the stomach. The histologic and macroscopic type were also not significantly different in both groups. The size of the lesions and resected specimens after ESD were measured in the longest axis. The size of the lesion in the second-look group was shorter than in the no second-look group (p=0.059). In the second-look group, en bloc resection rate was higher (98.5% vs 94.1%, p=0.016) and procedure time was also shorter than in the no second-look group (57.6±32.5 minutes vs 70.7±41.6 minutes, p<0.01). In the second-look group, PPIs were administered to more patients than in the no second-look group (p<0.01).
The hospitalization period for the second-look group was not delayed compared to the no second-look group (5.9±2.5 days vs 6.0±2.7 days, p=0.651).
2. Risk factors of delayed bleeding in a second-look and no second-look group
Delayed post-ESD bleeding occurred in 11 out of 547 lesions (2%). The occurrence rate of delayed post-ESD bleeding was not significantly different between the second-look group and the no second-look group (2/194 lesions [1%] vs 9/353 lesions [2.5%], p=0.343). All delayed bleeding was successfully managed with only endoscopic treatment. No delayed post-ESD bleeding was followed by rebleeding.
The univariate analysis of variables for delayed post-ESD bleeding is shown in Table 2. There were no significant differences between the delayed bleeding group and nondelayed bleeding group in age, sex, comorbidity, use of anticoagulants or anti-platelets, location of tumor, macroscopic type of tumor, en bloc resection rate, or procedure time. However, the size of the tumors was significantly larger in the delayed post-ESD bleeding group (22.8±9.9 vs 15.1±10.5, p<0.05). Ten out of 11 lesions in the delayed post-ESD bleeding group were larger than 15 mm in the size of the tumors (90.9% vs 41.6%, p=0.001). Also, the hospital stay was significantly longer in the delayed post-ESD bleeding group (10.2±5.8 vs 5.9±2.5, p=0.033). Furthermore, in the delayed bleeding group, there were no significant difference between the second-look group and the no second-look group in the patient and lesion-related factors.
Table 2.
Univariate Analysis of Predictors on Delayed Post-Endoscopic Submucosal Dissection Bleeding
| Occurred (11 lesions) | Not occurred (536 lesions) | p-value | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Total | 2nd-look (2 lesions) | No 2nd-look (9 lesions) | p-value | |||
| Age | 58.3±9.5 | 52.0±18.4 | 59.7±7.7 | 0.660 | 62.5±9.7 | 0.156 |
| Male sex | 9 (81.8) | 2 (100.0) | 7 (77.8) | 1.000 | 399 (74.4) | 0.738 |
| Comorbidities | ||||||
| Hypertension (present) | 4 (36.4) | 1 (50.0) | 3 (33.3) | 1.000 | 199 (37.1) | 1.000 |
| Diabetes mellitus (present) | 1 (9.1) | 0 | 1 (11.1) | 1.000 | 95 (17.7) | 0.699 |
| Heart disease (present) | 1 (9.1) | 0 | 1 (11.1) | 1.000 | 10 (1.9) | 0.202 |
| Anticoagulants/platelets (used) | 2 (18.2) | 1 (50.0) | 1 (11.1) | 0.345 | 51 (9.5) | 0.289 |
| Histologic type | ||||||
| Adenoma/adenocarcinoma | 4/7 | 0/2 | 4/5 | 0.491 | 208/328 | 1.000 |
| Location | ||||||
| Upper/mid/lower third | 0/2/9 | 0/1/1 | 0/1/8 | 0.345 | 38/165/333 | 0.366 |
| AW/PW/LC/GC | 1/2/6/2 | 0/0/2/0 | 1/2/4/2 | 0.565 | 94/87/225/130 | 0.793 |
| Macroscopic type | ||||||
| Elevated/flat or depressed | 7/4 | 1/1 | 6/3 | 1.000 | 404/132 | 0.479 |
| Size of the tumor, mm | 22.8±9.9 | 28.0±14.1 | 21.7±9.4 | 0.441 | 15.1±10.5 | 0.016 |
| ≥15 | 10 (90.9) | 2 (100.0) | 8 (88.9) | 1.000 | 223 (41.6) | 0.001 |
| ≥20 | 7 (63.6) | 1 (50.0) | 3 (66.7) | 1.000 | 136 (25.4) | 0.009 |
| Size of the resected specimen, mm | 48.0±13.7 | 50.0±14.1 | 47.6±13.4 | 0.832 | 43.1±13.3 | 0.226 |
| Resection style, en bloc/piecemeal | 11/0 | 2/0 | 9/0 | 1.000 | 512/24 | 1.000 |
| Procedure time, min | 64.3±19.8 | 77.5±13.4 | 61.3±20.3 | 0.321 | 66.1±39.4 | 0.879 |
| Antiulcer medication after ESD, PPIs/H2RAs | 2/9 | 1/1 | 1/8 | 0.345 | 127/409 | 1.000 |
Data are presented as mean±SD or number (%).
AW, anterior wall; PW, posterior wall; LC, lesser curvature; GC, great curvature; ESD, endoscopic submucosal dissection; PPIs, proton pump inhibitors; H2RAs, histamine-2 receptor antagonists.
In the second-look group with delayed post-ESD bleeding, one delayed bleeding occurred one day after prophylactic hemostasis for adherent hematoma on the ulcer base during the second-look endoscopy. The other occurred 10 days after second-look endoscopy without prophylactic hemostasis; however, this event developed after the patient underwent cardiovascular surgery 10 days after ESD.
In the no second-look group, nine delayed post-ESD bleeding were occurred on mean 3.4 days (median, 2 days; range, 1.5 to 12.0 days) after ESD. Bleeding findings, including one spurting, one oozing, two exposed vessels, and five adherent clots were observed.
3. Outcomes of prophylactic hemostasis in a second-look endoscopy
In the second-look group, 22 of the 194 lesions were performed prophylactic hemostasis for Forrest classification14 Ia to IIb lesions including four blood oozing, six visible vessels without any signs of hemorrhage, and 12 adherent clots or hematoma.
The characteristics of patients and lesion-related factors were not different between the prophylactic hemostasis group and the nonprophylactic hemostasis groups (Table 3).
Table 3.
Characteristics of the Prophylactic Hemostasis and Nonhemostasis Groups during Second-Look Endoscopy
| Hemostasis (22 lesions) | Nonhemostasis (172 lesions) | p-value | |
|---|---|---|---|
| Age | 55.5±10.3 | 59.2±8.6 | 0.062 |
| Male sex | 19 (86.4) | 129 (75.0) | 0.297 |
| Comorbidities | |||
| Hypertension (present) | 8 (36.4) | 58 (33.7) | 0.814 |
| Diabetes mellitus (present) | 6 (27.3) | 27 (15.7) | 0.223 |
| Heart disease (present) | 0 | 1 (0.6) | 1.000 |
| Anticoagulants/platelets (used) | 2 (9.1) | 17 (9.9) | 1.000 |
| Histologic type | |||
| Adenoma/adenocarcinoma | 5/17 | 63/109 | 0.241 |
| Location | |||
| Upper/mid/lower third | 1/4/17 | 8/56/108 | 0.379 |
| AW/PW/LC/GC | 2/1/16/3 | 24/28/77/43 | 0.094 |
| Macroscopic type | |||
| Elevated/flat or depressed | 14/8 | 123/49 | 0.462 |
| Size of the tumor, mm | 13.2±8.5 | 14.3±9.4 | 0.629 |
| Size of the resected specimen, mm | 41.5±10.0 | 42.8±11.8 | 0.613 |
| Resection style, en bloc/piecemeal | 22/0 | 169/3 | 1.000 |
| Procedure time, min | 59.5±30.9 | 57.4±32.8 | 0.772 |
| Anti-ulcer medication after ESD, PPIs/H2RAs | 10/12 | 82/90 | 1.000 |
Data are presented as mean±SD or number (%).
AW, anterior wall; PW, posterior wall; LC, lesser curvature; GC, great curvature; ESD, endoscopic submucosal dissection; PPIs, proton pump inhibitors; H2RAs, histamine-2 receptor antagonists.
The mean hospital stay was longer in patients with prophylactic hemostasis than in patients without prophylactic hemostasis, but there was no statistical significance (6.8±1.4 days vs 5.4±1.2 days, p=0.080) (Table 4).
Table 4.
Comparison of Hospital Stay according to Events
| Hospital stay, days | p-value | |
|---|---|---|
| 2nd-look endoscopy | 0.651 | |
| Yes | 5.9±2.5 | |
| No | 6.0±2.7 | |
| 2nd-look with hemostasis | 0.080 | |
| Yes | 6.8±1.4 | |
| No | 5.8±2.6 | |
| Delayed post-ESD bleeding | 0.033 | |
| Yes | 10.2±5.8 | |
| No | 5.9±2.5 |
Data are presented as mean±SD.
ESD, endoscopic submucosal dissection.
DISCUSSION
In the present study, we demonstrated that a second-look endoscopy after ESD did not reduce the delayed post-ESD bleeding even though prophylactic hemostasis for high risk lesions of rebleeding was performed during second-look endoscopy.
There were several studies about efficacy of routine second-look endoscopy for acute nonvariceal upper GI bleeding, and they still yielded conflicting results.15,16 Recently, meta-analysis data have suggested that routine second-look endoscopy with thermal coagulation reduced recurrent peptic ulcer bleeding, although these data failed to prove advantages in the clinical outcomes of patients with peptic ulcer bleeding.17,18 These results support the universal validity of second-look endoscopy after ESD for preventing delayed post-ESD bleeding. In addition, routinely performed preventive coagulation of visibly exposed vessels on artificial ulcer, regardless of the presence of bleeding at the end of ESD, could be provided for additional evidence of second-look endoscopy after ESD.10
Recently, a few studies were reported about the usefulness of second-look endoscopy after ESD for preventing delayed post-ESD bleeding. Goto et al.11 found that delayed bleeding occurred with similar probability between the conditions before and after second-look endoscopy and they suggested that a second-look endoscopy after ESD would contribute little to the prevention of delayed bleeding. In another study by Kim et al.,12 they reported that more delayed bleedings occurred before second-look endoscopy than after, and they suggested that a second-look endoscopy may have value for preventing delayed bleeding. Likewise, these studies showed conflicting results. However, the most important point in all of this is that they enrolled only patients who underwent second-look endoscopy and did not make a pure comparison between the groups with or without the second-look endoscopy. As a result, they compared only the rates of bleeding between before and after second look endoscopy.
In this study, we compared patients who underwent second-look endoscopy with patients who did not undergo second-look endoscopy. We also evaluated the outcomes between the groups with or without prophylactic hemostasis during second-look endoscopy. We found that the occurrence rate of delayed post-ESD bleeding was not different between the second-look group and the no second-look group. Furthermore, in the second-look group, delayed post-ESD bleeding occurred in both groups with or without prophylactic hemostasis. In a second-look group with prophylactic hemostasis, hospital stay tended to be prolonged more than it was with the second-look group without prophylactic hemostasis. In this study, the only predictor of delayed bleeding was tumor size, regardless of second-look endoscopy after ESD. Recently, Ryu et al.19 and Kim et al.20 reported prospective randomized controlled trials about the efficacy of second look endoscopy for prevention of delayed bleeding after ESD. They compared second look endoscopy group with non-second look endoscopy group, and showed that the second look endoscopy was not decreased delayed bleeding after ESD, even though the proportion of enrolled EGCs in their studies was relatively small and Ryu et al.19 enrolled relatively small number of cases. Furthermore, Kim et al.20 reported that a large tumor size was the only risk factor for delayed bleeding. It was similar with our study and previous studies about factors related to delayed bleeding following ESD.21 The present study supported the Kim et al.20 result.
This study had a few limitations. First, it was a retrospective study from a single center. However, the number of cases was not small compared with previous studies to evaluate the risk of post-ESD bleeding.8,11,22,23 In this study, only two experts performed ESD for gastric neoplasms, and the bias induced by endoscopists was relatively low. Second, patients who underwent a second-look endoscopy were enrolled in a prospective study for comparison between the H2RAs and PPIs in the prevention of bleeding after ESD (this study was accepted in Hepatogastroenterology). However, in this study, there was no difference between the H2RAs and PPIs in preventing bleeding from artificial ulcers after ESD. The effects of the H2RAs and PPIs in the prevention of bleeding after ESD remain controversial, although there have been several previous studies.23,24 In this study, there was also no difference between delayed bleeding group and the nondelayed bleeding group according to administration of PPIs or H2RAs after ESD (p=1.000).
In conclusion, we suggest that a second-look endoscopy to prevent delayed bleeding after ESD is useless because it could not reduce the rate of delayed bleeding even though prophylactic hemostasis was performed, repeat endoscopy after ESD make patients feel uncomfortable, and it can delay the time to start eating after ESD. However, further studies are needed to select the patients who is helpful to undergo a second look endoscopy for prevention of delayed bleeding after ESD.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666–2677. doi: 10.1007/s00464-011-1627-z. [DOI] [PubMed] [Google Scholar]
- 2.Chung IK, Lee JH, Lee SH, et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest Endosc. 2009;69:1228–1235. doi: 10.1016/j.gie.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 3.Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763–770. doi: 10.1016/j.gie.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Oka S, Tanaka S, Kaneko I, et al. Advantage of endoscopic sub-mucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 5.Toyonaga T, Man-i M, East JE, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000–1008. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oda I, Saito D, Tada M, et al. A multicenter retrospective study of endoscopic resection for early gastric cancer. Gastric Cancer. 2006;9:262–270. doi: 10.1007/s10120-006-0389-0. [DOI] [PubMed] [Google Scholar]
- 7.Miyahara K, Iwakiri R, Shimoda R, et al. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion. 2012;86:273–280. doi: 10.1159/000341422. [DOI] [PubMed] [Google Scholar]
- 8.Mukai S, Cho S, Kotachi T, et al. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012;2012:875323. doi: 10.1155/2012/875323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oda I, Gotoda T, Hamanaka H, et al. Endoscopic submucosal dissection for early gastric cancer: technical feasibility, operation time and complications from a large consecutive series. Dig Endosc. 2005;17:54–58. doi: 10.1111/j.1443-1661.2005.00459.x. [DOI] [Google Scholar]
- 10.Takizawa K, Oda I, Gotoda T, et al. Routine coagulation of visible vessels may prevent delayed bleeding after endoscopic submucosal dissection: an analysis of risk factors. Endoscopy. 2008;40:179–183. doi: 10.1055/s-2007-995530. [DOI] [PubMed] [Google Scholar]
- 11.Goto O, Fujishiro M, Kodashima S, et al. A second-look endoscopy after endoscopic submucosal dissection for gastric epithelial neoplasm may be unnecessary: a retrospective analysis of postendoscopic submucosal dissection bleeding. Gastrointest Endosc. 2010;71:241–248. doi: 10.1016/j.gie.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 12.Kim HH, Park SJ, Park MI, Moon W. Clinical impact of second-look endoscopy after endoscopic submucosal dissection of gastric neoplasms. Gut Liver. 2012;6:316–320. doi: 10.5009/gnl.2012.6.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soetikno R, Kaltenbach T, Yeh R, Gotoda T. Endoscopic mucosal resection for early cancers of the upper gastrointestinal tract. J Clin Oncol. 2005;23:4490–4498. doi: 10.1200/JCO.2005.19.935. [DOI] [PubMed] [Google Scholar]
- 14.Heldwein W, Schreiner J, Pedrazzoli J, Lehnert P. Is the Forrest classification a useful tool for planning endoscopic therapy of bleeding peptic ulcers? Endoscopy. 1989;21:258–262. doi: 10.1055/s-2007-1010729. [DOI] [PubMed] [Google Scholar]
- 15.Messmann H, Schaller P, Andus T, et al. Effect of programmed endoscopic follow-up examinations on the rebleeding rate of gastric or duodenal peptic ulcers treated by injection therapy: a prospective, randomized controlled trial. Endoscopy. 1998;30:583–589. doi: 10.1055/s-2007-1001360. [DOI] [PubMed] [Google Scholar]
- 16.Chiu PW, Lam CY, Lee SW, et al. Effect of scheduled second therapeutic endoscopy on peptic ulcer rebleeding: a prospective randomised trial. Gut. 2003;52:1403–1407. doi: 10.1136/gut.52.10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marmo R, Rotondano G, Bianco MA, Piscopo R, Prisco A, Cipolletta L. Outcome of endoscopic treatment for peptic ulcer bleeding: is a second look necessary? A meta-analysis. Gastrointest Endosc. 2003;57:62–67. doi: 10.1067/mge.2003.48. [DOI] [PubMed] [Google Scholar]
- 18.Tsoi KK, Chan HC, Chiu PW, Pau CY, Lau JY, Sung JJ. Second-look endoscopy with thermal coagulation or injections for peptic ulcer bleeding: a meta-analysis. J Gastroenterol Hepatol. 2010;25:8–13. doi: 10.1111/j.1440-1746.2009.06129.x. [DOI] [PubMed] [Google Scholar]
- 19.Ryu HY, Kim JW, Kim HS, et al. Second-look endoscopy is not associated with better clinical outcomes after gastric endoscopic submucosal dissection: a prospective, randomized, clinical trial analyzed on an as-treated basis. Gastrointest Endosc. 2013;78:285–294. doi: 10.1016/j.gie.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Kim JS, Chung MW, Chung CY, et al. The need for second-look endoscopy to prevent delayed bleeding after endoscopic submucosal dissection for gastric neoplasms: a prospective randomized trial. Gut Liver. 2014;8:480–486. doi: 10.5009/gnl13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HK, Cheung DY. The myth and truth about the usefulness of second-look endoscopy following endoscopic submucosal resection. Gut Liver. 2014;8:459–461. doi: 10.5009/gnl14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura M, Nishikawa J, Hamabe K, et al. Risk factors for delayed bleeding from endoscopic submucosal dissection of gastric neoplasms. Scand J Gastroenterol. 2012;47:1108–1114. doi: 10.3109/00365521.2012.699550. [DOI] [PubMed] [Google Scholar]
- 23.Uedo N, Takeuchi Y, Yamada T, et al. Effect of a proton pump inhibitor or an H2-receptor antagonist on prevention of bleeding from ulcer after endoscopic submucosal dissection of early gastric cancer: a prospective randomized controlled trial. Am J Gastroenterol. 2007;102:1610–1616. doi: 10.1111/j.1572-0241.2007.01197.x. [DOI] [PubMed] [Google Scholar]
- 24.Tomita T, Kim Y, Yamasaki T, et al. Prospective randomized controlled trial to compare the effects of omeprazole and famotidine in preventing delayed bleeding and promoting ulcer healing after endoscopic submucosal dissection. J Gastroenterol Hepatol. 2012;27:1441–1446. doi: 10.1111/j.1440-1746.2012.07144.x. [DOI] [PubMed] [Google Scholar]