Abstract
Background/Aims
To analyze the effect of short-term supportive temporary partial enteral nutrition therapy for treating severe pediatric Crohn’s disease (CD).
Methods
We conducted a prospective, open-label study in pediatric patients with CD (n=78) from January 2007 to December 2011. The CD patients were divided into three groups according to disease severity (mild, moderate, and severe). Seventeen patients with severe CD received short-term partial enteral nutrition (SPEN) in addition to their general diet for 4 weeks after the induction of remission with medical treatment. This SPEN group was further divided into two groups by age (<13 years, ≥13 years). Nutritional parameters and Pediatric Crohn’s Disease Activity Index scores were analyzed at the initial enrollment and following 1 year of treatment for all groups.
Results
Nutritional status improved substantially after 1 year of treatment in the severe CD group. Nutritional status in the SPEN group improved considerably more than that in the non-SPEN group. Additionally, the <13-year-old group demonstrated better nutritional status improvement than the ≥13-year-old group.
Conclusions
SPEN may be effective in pediatric patients with severe CD for improving nutritional status and moderating disease severity.
Keywords: Crohn disease, Younger age, Partial enteral nutrition, Disease activity, Nutritional status
INTRODUCTION
The major symptoms of Crohn’s disease (CD), a type of inflammatory bowel disease (IBD), typically include abdominal pain, chronic diarrhea, and weight loss. More severe bowel complications, such as stricture, fistula, and perforation, might also be present. Nutritional deficiencies are also common due to a decrease in food intake despite an increase in daily caloric requirements.1,2
In pediatric patients, nutritional deficiencies might result in malnutrition and growth failure, which could interfere with disease recovery.3 Reports from clinical settings indicate that growth impairment due to nutritional deficiencies occurs in up to 88% of pediatric patients, prior to or following a CD diagnosis.1
Corticosteroids, 5-aminosalicylates, antibiotics, immune modulators, biological agents, and nutritional therapy are examples of the variety of treatment methods that are available for CD. Of these, corticosteroids are the most commonly used method for treating active CD due to its effectiveness in achieving clinical remission in approximately 80% of patients. Its use in children and adolescents is limited because of potential delays in growth and development and an increased risk of developing infections.4 The other modalities, particularly infliximab as a biological agent, are effective but can result in significant side effects, and their long-term effectiveness is limited.5–7 Nutritional therapy is effective for CD, can improve the patient’s nutritional status, and has been shown to improve serum albumin levels, body weight, and growth, including short-term growth.8 Therefore, nutritional therapy is the recommended treatment for pediatric patients with CD.9–12
Nutritional management can include the provision of nutrients and energy through enteral means. Options for the delivery of enteral nutrition include exclusive enteral nutrition (EEN), which supplies all of the essential nutrients and caloric requirements through an elemental or polymeric diet, and partial enteral nutrition (PEN), which uses a general diet supplemented with an elemental or polymeric formula to meet the patient’s caloric requirements.
Little is known about the specific mechanisms of enteral nutrition for treating IBD, although it has been suggested that enteral nutrition alters the normal intestinal flora, assists with recovery of the bowel, and results in a direct anti-inflammatory effect in the bowel.13 Furthermore, the use of EEN as first-line therapy may minimize side effects, improve growth and nutritional status,14,15 and be more effective than steroids in mucosal healing.13,16 And inducing remission, particularly in pediatric patients with CD,17 without resulting in a high occurrence of remission.14 EEN may also result in fewer episodes of CD recurrence and decreased serum inflammatory cytokine levels, when compared to a normal diet.18 The use of nutritional therapy in the induction of remission is up to the physician’s judgment; it is used as the early treatment regimen in 62% of patients in Europe and in only 4% of patients in the United States due to a lack of experience with and evidence supporting EEN use.19
Disadvantages of EEN include potential side effects, such as nausea, abdominal pain, flatulence, and diarrhea.9 In addition, patients are solely dependent on enteral nutrition for several weeks and are restricted from consuming a normal diet during that time. For these reasons, the use of EEN in pediatric patients with CD in clinical settings is limited, and PEN is considered to be more tolerable than EEN. However, the efficacy of PEN is thought to be poorer than that of EEN.20 This is difficult to state with certainty; the majority of research, to date, has focused on comparing the effects of EEN and steroids on improvement in disease severity or nutritional status during the induction and maintenance of remission. To our knowledge, there are no reports of the effect of short-term concomitant use of PEN after induction of remission.
Hence, in this study, we examined the effects of concomitant use of 4 weeks of supportive short-term partial enteral nutrition (SPEN) as an adjuvant therapy after induction of remission on nutritional status and CD severity.
MATERIALS AND METHODS
1. Pediatric Crohn’s disease activity index
The Pediatric Crohn’s Disease Activity Index (PCDAI) is the measurement of choice for disease severity in clinical trials of pediatric CD. PCDAI scores (range, 0 to 100 points) indicate the severity of disease. The PCDAI includes disease history three items (abdominal pain, the number of liquid stools, and general well-being), five physical examination items (abdominal examination, perirectal disease, extraintestinal manifestations, weight, and height), and three laboratory test items (hematocrit, albumin, and erythrocyte sedimentation rate). Each question has three scoring options (0, 5, or 10 points), except for the hematocrit and erythrocyte sedimentation rate items, which are scored as 0, 2.5, or 5 points. The disease history and physical examination items were completed during the first outpatient appointment. The clinical laboratory measurements were completed during the clinical examination. The total PCDAI scores were then calculated. The disease severity was classified as mild (12.5<PCDAI≤30), moderate (30<PCDAI≤45), or severe (PCDAI >45).21–23
2. Study design
The study was conducted from January 2007 to December 2011 at the Department of Pediatrics, Severance Hospital, Yonsei University College of Medicine, Seoul, and the Department of Pediatrics, Incheon St. Mary’s Hospital, The Catholic University of Korea College of Medicine, Incheon, Korea. The study was approved by the Institutional Review Board of each of medical institution, and it was conducted in compliance with the Declaration of Helsinki.
Patients with a new diagnosis or relapse of CD were assigned to the CD group (n=78). PCDAI scores and nutritional status were documented at initial enrollment and again after 1 year of treatment for the severe CD group.
The patients with CD were also divided into three groups according to the PCDAI scores: mild (n=25), moderate (n=19), and severe (n=34) groups. The patients with CD in the severe group were given the option of receiving SPEN, and 17 patients (50%) opted in. The remaining 17 patients were considered to be the non-SPEN group. The SPEN group was further divided into two age groups based on a fiducial age of 13 years, which is the cutoff for adolescence in Korea (n=7, <13 years; n=10, ≥13 years).
3. Enteral nutrition
Elemental and polymeric diets are equally effective for remission rates in CD.24–26 As a specific standard oral polymeric formula, Ensure® (Abbott Laboratories, Abbott Park, IL, USA) was used as the SPEN in this study. This formula consists of 14% protein, 54.5% carbohydrate, and 31.5% fat. Caloric density is 1.1 g/mL with a pH of 6.6 and an osmolarity of 330 mOsm/L. Ensure® liquid, in 250 mL, is composed of 8.8 g of milk with soy protein, 34.3 g of lactose-free carbohydrates, 8.8 g of corn oil, and 24 essential vitamins and minerals.
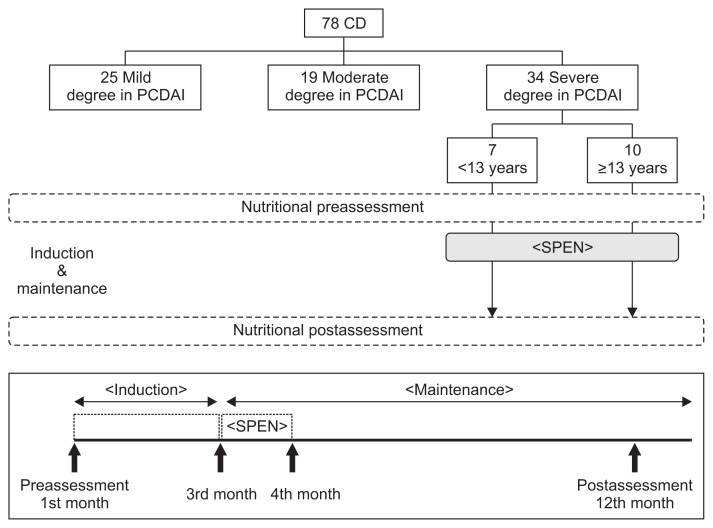
SPEN was provided orally to the 17 SPEN patients in the severe disease group. Total uptake was 20 kcal/kg27 in additional to a normal diet for 4 weeks commencing 8 weeks after the induction of remission using medical treatment (Fig. 1).
Fig. 1.
Study design for the use of supportive short-term partial enteral nutrition (SPEN) in 17 patients with severe Crohn’s disease (CD).
PCDAI, Pediatric Crohn’s Disease Activity Index.
4. Nutritional status
To assess nutritional status, the following measurements were collected: body weight (kg), height (cm), hemoglobin, transferrin saturation, ferritin, prealbumin, albumin, zinc, calcium, magnesium, phosphorous, vitamin A, vitamin B12, vitamin E, folate, and 25-OH-vitamin D.28
5. Statistical analysis
Statistical analysis was performed using SPSS version 20 (IBM, Armonk, NY, USA). Comparisons were conducted between nutritional status measurements at initial enrollment between the CD group and childhood functional abdominal pain (CFAP) group. PCDAI and nutritional status values were compared between initial enrollment and following 1 year of treatment for each CD disease severity group (mild, moderate, severe). The changes in PCDAI score and nutritional status values from initial enrollment to 1 year following treatment were calculated and compared between the SPEN and non-SPEN groups and between the SPEN age groups (<13 years and ≥13 years).
These comparisons were conducted using the paired t-test for continuous variables and the chi-square test for categorical variables. A p-value of <0.05 was considered statistically significant.
RESULTS
1. Comparison of nutritional status in patients with severe CD at initial enrollment and following 1 year of treatment
Within severe disease group, the values for nutritional status at initial enrollment and following 1 year of treatment are provided in Table 1. In the severe group, all nutritional values except only 25-OH-vitamin D were significantly improved after 1 year of treatment
Table 1.
Nutritional Status in Patients with Severe Crohn’s Disease at the Initial Enrollment and Following 1 Year of Treatment
| Severe group (n=34) | p-value | ||
|---|---|---|---|
|
| |||
| Enrollment | After 1 yr | ||
| Hemoglobin, g/dL | 9.4±2.7 | 12.4±2.1 | <0.001 |
| Transferrin, % | 10.8±5.4 | 24.8±8.4 | <0.001 |
| Ferritin, ng/mL | 34.5±27.8 | 149.2±38.4 | <0.001 |
| Prealbumin, mg/L | 132.6±87.2 | 227.5±101.2 | <0.001 |
| Albumin, g/dL | 3.4±2.9 | 4.2±0.8 | <0.001 |
| Zinc, μg/dL | 32.7±29.1 | 62.1±21.8 | <0.001 |
| Calcium, mg/dL | 8.6±2.1 | 9.2±1.1 | <0.001 |
| Magnesium, mmol/L | 0.7±0.2 | 0.9±0.3 | <0.001 |
| Phosphorous, mg/dL | 4.7±0.8 | 4.2±0.6 | <0.001 |
| Vitamin A, mg/L | 0.42±0.38 | 0.77±0.21 | <0.001 |
| Vitamin B12, pg/mL | 201.4±121.3 | 437.1±201.3 | <0.001 |
| Vitamin E, mg/L | 6.8±5.1 | 12.4±3.5 | <0.001 |
| Folate, ng/mL | 11.01±5.14 | 12.48±2.14 | 0.036 |
| 25-OH-vitamin D, ng/mL | 17.2±9.5 | 18.3±3.4 | NS |
Data are presented as mean±SD.
NS, not significant.
2. Comparison of nutritional status and PCDAI scores between the SPEN and non-SPEN groups
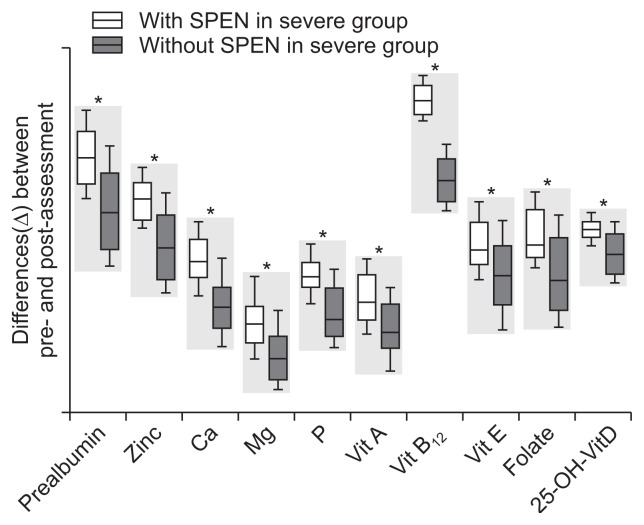
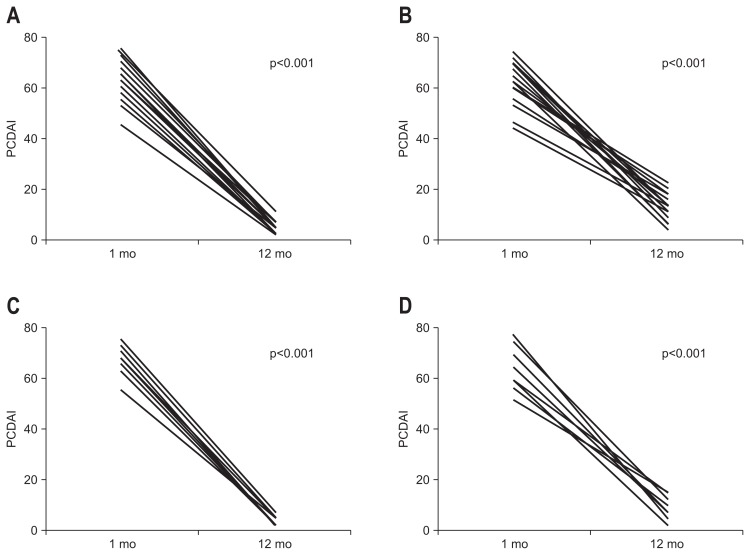
The differences (Δ) in nutritional status indicators between initial enrollment and after 1 year of treatment for the SPEN and non-SPEN groups are provided in Table 2 and Fig. 2. There were no significant differences in age, gender, PCDAI, disease location, behavior, and initial nutritional status between SPEN and non-SPEN group. The changes in nutritional status indicators were significantly higher in the SPEN group than in the non-SPEN group. The change in the PCDAI score was significantly higher in the SPEN group than in the non-SPEN group (Fig. 3A and B).
Table 2.
Differences (Δ) in Nutritional Status in Patients with Severe Crohn’s Disease at the Initial Enrollment and Following 1 Year of Treatment: Comparison between the Supportive Short-Term Partial Enteral Nutrition (SPEN) and Non-SPEN Groups
| Differences (Δ) between enrollment and 1 yr of treatment | Severe group | p-value | |
|---|---|---|---|
|
| |||
| SPEN (n=17) | Non-SPEN (n=17) | ||
| Age, yr | 14.1±3.8 | 13.8±4.1 | NS |
| Male | 9 (52.9) | 10 (58.8) | NS |
| Infliximab | 6 (35.3) | 6 (35.3) | NS |
| ΔBody weight, kg | 10.7±2.5 | 7.4±4.2 | <0.001 |
| ΔHeight, cm | 4.6±1.7 | 3.4±1.9 | 0.031 |
| ΔPCDAI | 62.7±16.4 | 50.2±17.1 | <0.001 |
| ΔHemoglobin, g/dL | 5.7±0.4 | 4.4±0.6 | 0.012 |
| ΔTransferrin saturation, % | 21.7±2.9 | 16.4±3.4 | 0.033 |
| ΔFerritin, ng/mL | 134.8±12.7 | 108.6±21.1 | 0.007 |
| ΔPrealbumin, mg/L | 117.6±30.4 | 82.7±42.1 | <0.001 |
| ΔAlbumin, g/dL | 0.9±0.2 | 0.7±0.4 | 0.041 |
| ΔZinc, μg/dL | 45.6±12.4 | 26.7±21.6 | <0.001 |
| ΔCalcium, mg/dL | 0.8±0.2 | 0.6±0.3 | 0.018 |
| ΔMagnesium, mmol/L | 0.3±0.1 | 0.2±0.1 | 0.049 |
| ΔPhosphorous, mg/dL | 0.6±0.1 | 0.5±0.2 | 0.038 |
| ΔVitamin A, mg/L | 0.48±0.17 | 0.32±0.21 | 0.007 |
| ΔVitamin B12, pg/mL | 297.4±27.1 | 197.6±42.8 | <0.001 |
| ΔVitamin E, mg/L | 7.2±1.4 | 6.3±3.1 | <0.001 |
| ΔFolate, ng/mL | 8.4±1.3 | 6.9±3.4 | <0.001 |
| Δ25-OH-vitamin D, ng/mL | 4.3±0.6 | 3.8±1.3 | <0.001 |
Data are presented as mean±SD or number (%).
NS, not significant; PCDAI, Pediatric Crohn’s Disease Activity Index.
Fig. 2.
Differences (Δ) in nutritional status in patients with severe Crohn’s disease at the initial enrollment and following 1 year of treatment between the supportive short-term partial enteral nutrition (SPEN) and non-SPEN groups.
*p<0.05.
Fig. 3.
Comparison of Pediatric Crohn’s Disease Activity Index (PCDAI) scores in patients with Crohn’s disease (CD) between initial enrollment and following 1 year of treatment. Comparisons were further made between patients with severe CD in the supportive short-term partial enteral nutrition (SPEN) (A) and non-SPEN (B) groups and between patients with severe CD receiving SPEN aged <13 years (C) and aged ≥13 years (D).
3. Comparison of nutritional status between the two age groups receiving SPEN
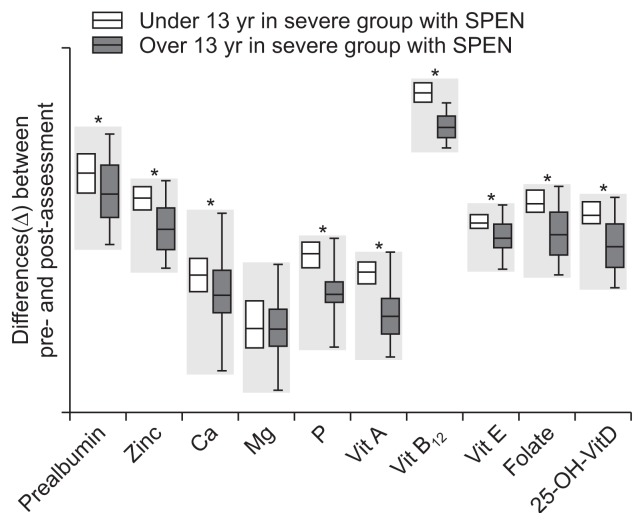
The differences (Δ) in nutritional status indicators between initial enrollment and after 1 year of treatment for the two age groups receiving SPEN are provided in Table 3 and Fig. 4. There were no significant differences between the two groups in age, sex, albumin levels, or magnesium levels. There were significantly greater changes in the nutritional value indicators in the patients aged <13 than in those aged ≥13 years. The change in the PCDAI score was also significantly higher in the younger group than in the older age group (Fig. 3C and D).
Table 3.
Differences (Δ) in Nutritional Status in Patients with Severe Crohn’s Disease Receiving Supportive Short-Term Partial Enteral Nutrition at the Initial Enrollment and Following 1 Year of Treatment: Comparison between Two Age Groups with a Fiducial Age of 13 Years
| Differences (Δ) between enrollment and 1 yr of treatment | Severe group with SPEN | p-value | |
|---|---|---|---|
|
| |||
| <13 yr (n=7) | ≥13 yr (n=10) | ||
| Age, yr | 10.4±2.7 | 16.1±3.3 | NS |
| Male | 4 (57.1) | 6 (60.0) | NS |
| ΔBody weight, kg | 9.6±2.1 | 7.5±3.4 | 0.017 |
| ΔHeight, cm | 5.9±2.1 | 3.2±2.1 | 0.01 |
| ΔPCDAI | 63.3±17.9 | 49.2±20.8 | <0.001 |
| ΔHemoglobin, g/dL | 5.9±0.4 | 5.6±0.2 | 0.017 |
| ΔTransferrin saturation, % | 26.8±1.3 | 24.8±3.4 | 0.032 |
| ΔFerritin, ng/mL | 134.8±12.7 | 127.4±13.6 | 0.018 |
| ΔPrealbumin, mg/L | 128.5±21.4 | 118.0±28.7 | 0.011 |
| ΔAlbumin, g/dL | 1.0±0.3 | 0.9±0.4 | NS |
| ΔZinc, μg/dL | 57.1±8.6 | 46.4±13.8 | <0.001 |
| ΔCalcium, mg/dL | 0.8±0.2 | 0.7±0.2 | 0.043 |
| ΔMagnesium, mmol/L | 0.3±0.1 | 0.3±0.1 | NS |
| ΔPhosphorous, mg/dL | 0.7±0.1 | 0.5±0.1 | 0.028 |
| ΔVitamin A, mg/L | 0.66±0.08 | 0.46±0.12 | <0.001 |
| ΔVitamin B12, pg/mL | 327.6±15.7 | 286.4±21.4 | <0.001 |
| ΔVitamin E, mg/L | 7.5±0.5 | 6.8±0.9 | <0.001 |
| ΔFolate, ng/mL | 9.6±0.7 | 7.7±2.1 | <0.001 |
| Δ25-OH-vitamin D, ng/mL | 5.1±0.4 | 4.2±0.8 | <0.001 |
Data are presented as mean±SD or number (%).
SPEN, supportive short-term partial enteral nutrition; NS, not significant; PCDAI, Pediatric Crohn’s Disease Activity Index.
Fig. 4.
Differences (Δ) in nutritional status in patients with severe Crohn’s disease receiving supportive short-term partial enteral nutrition (SPEN) at the initial enrollment and following 1 year of treatment, comparing two age groups with a fiducial age of 13 years.
*p<0.05.
DISCUSSION
Treatment goals for CD include improvements in the patient’s quality of life through induction and maintenance of disease remission. In addition, a quarter of CD patients are pediatric patients who are undergoing active growth and development, making it necessary to prevent failure to thrive, delayed puberty, osteoporosis, anemia, and vitamin deficiencies.
Similar to previous reports, nutritional status in the CD patients in the current study was poorer than that in the CFAP group at the initial enrollment. In addition to lower serum levels of vitamins, the levels of hemoglobin, ferritin, and albumin were also significantly lower in the CD group. Furthermore, following classification of our patients based on CD disease severity, it was evident that the nutritional status was poorer in the patients with more severe disease. Therefore, severe disease may play a greater role in the development of nutrition-related conditions in CD, such as failure to thrive, delayed puberty, anemia, and osteoporosis. Following 1 year of treatment, the patients in all groups demonstrated improvements in the nutritional parameters, and this was particularly true in the group with severe disease. This might be related to their poor nutritional status at initial enrollment.
Improvement in nutritional status was significantly higher in the group receiving SPEN than in those in the non-SPEN group after 1 year of treatment. Moreover, the improvement in PCDAI scores was also relatively high with the use of SPEN. There have been separate reports on the effect of EEN on the improvement of the disease itself29 and nutritional status.30,31 Our results indicate that SPEN, used as secondary therapy in the presence of conventional types of medical treatment, was effective in simultaneously improving nutritional status and disease severity. Improvement in nutritional status in the two age groups receiving SPEN, the <13 years group demonstrated better than the ≥13 years group. SPEN seems to be effective for younger group but further study is needed, as little is known about the specific mechanisms of enteral nutrition for treating IBD.
These results suggest that a 4-week course of SPEN, secondary to conventional treatment modalities, would be effective in improving nutritional status and controlling disease severity in pediatric patients with severe CD. SPEN may result in reduced complications and increased compliance. This may eventually assist with the maintenance of normal development in these patients and result in fewer medication side effects. In this way, SPEN might be a novel, alternative treatment modality in severe CD.
Further studies are warranted to determine which products and prescription of essential nutrients are appropriate in patients with CD presenting with altered metabolism, malabsorption, and decreased nutrient utilization. Long-term follow-up on the maintenance of remission in those receiving SPEN compared to those without SPEN should also be conducted. Moreover, because enteral nutrition has a steroid sparing effect, further studies are needed to investigate the reduction of steroids in the presence of SPEN.
Although this study had some limitations (open label study, number of patients was small, long follow-up interval), this results showed additional use of SPEN have significant effect on improving nutritional status, especially for young patient with severe CD. As this study is first to evaluate nutritional status after using SPEN, further studies are needed to find appropriate amount, duration, timing of SPEN.
In conclusion, our results demonstrate that the use of SPEN as an adjuvant therapy was tolerable and effective in improving the disease course and the nutritional status in pediatric patients with severe CD. Therefore, additional use of SPEN with conventional treatment modalities for pediatric patients presenting with severe CD can be new option.
ACKNOWLEDGEMENTS
The authors would like to thank all of the children with CD who participated in this study.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Kleinman RE, Baldassano RN, Caplan A, et al. Nutrition support for pediatric patients with inflammatory bowel disease: a clinical report of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39:15–27. doi: 10.1097/00005176-200407000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Shamir R. Nutritional aspects in inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2009;48(Suppl 2):S86–S88. doi: 10.1097/MPG.0b013e3181a15ca0. [DOI] [PubMed] [Google Scholar]
- 3.Song SM, Kim Y, Oh SH, Kim KM. Nutritional status and growth in Korean children with Crohn’s disease: a single-center study. Gut Liver. 2014;8:500–507. doi: 10.5009/gnl13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kappelman MD, Bousvaros A. Nutritional concerns in pediatric inflammatory bowel disease patients. Mol Nutr Food Res. 2008;52:867–874. doi: 10.1002/mnfr.200700156. [DOI] [PubMed] [Google Scholar]
- 5.Ordás I, Feagan BG, Sandborn WJ. Early use of immunosuppressives or TNF antagonists for the treatment of Crohn’s disease: time for a change. Gut. 2011;60:1754–1763. doi: 10.1136/gutjnl-2011-300934. [DOI] [PubMed] [Google Scholar]
- 6.Moustou AE, Matekovits A, Dessinioti C, Antoniou C, Sfikakis PP, Stratigos AJ. Cutaneous side effects of anti-tumor necrosis factor biologic therapy: a clinical review. J Am Acad Dermatol. 2009;61:486–504. doi: 10.1016/j.jaad.2008.10.060. [DOI] [PubMed] [Google Scholar]
- 7.De Bie CI, Hummel TZ, Kindermann A, et al. The duration of effect of infliximab maintenance treatment in paediatric Crohn’s disease is limited. Aliment Pharmacol Ther. 2011;33:243–250. doi: 10.1111/j.1365-2036.2010.04507.x. [DOI] [PubMed] [Google Scholar]
- 8.Knight C, El-Matary W, Spray C, Sandhu BK. Long-term outcome of nutritional therapy in paediatric Crohn’s disease. Clin Nutr. 2005;24:775–779. doi: 10.1016/j.clnu.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Borrelli O, Cordischi L, Cirulli M, et al. Polymeric diet alone versus corticosteroids in the treatment of active pediatric Crohn’s disease: a randomized controlled open-label trial. Clin Gastroenterol Hepatol. 2006;4:744–753. doi: 10.1016/j.cgh.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 10.El-Matary W. Enteral nutrition as a primary therapy of Crohn’s disease: the pediatric perspective. Nutr Clin Pract. 2009;24:91–97. doi: 10.1177/0884533608329660. [DOI] [PubMed] [Google Scholar]
- 11.Alastair F, Emma G, Emma P. Nutrition in inflammatory bowel disease. JPEN J Parenter Enteral Nutr. 2011;35:571–580. doi: 10.1177/0148607111413599. [DOI] [PubMed] [Google Scholar]
- 12.Critch J, Day AS, Otley A, et al. Use of enteral nutrition for the control of intestinal inflammation in pediatric Crohn disease. J Pediatr Gastroenterol Nutr. 2012;54:298–305. doi: 10.1097/MPG.0b013e318235b397. [DOI] [PubMed] [Google Scholar]
- 13.Fell JM, Paintin M, Arnaud-Battandier F, et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281–289. doi: 10.1046/j.1365-2036.2000.00707.x. [DOI] [PubMed] [Google Scholar]
- 14.Dziechciarz P, Horvath A, Shamir R, Szajewska H. Meta-analysis: enteral nutrition in active Crohn’s disease in children. Aliment Pharmacol Ther. 2007;26:795–806. doi: 10.1111/j.1365-2036.2007.03431.x. [DOI] [PubMed] [Google Scholar]
- 15.Rubio A, Pigneur B, Garnier-Lengliné H, et al. The efficacy of exclusive nutritional therapy in paediatric Crohn’s disease, comparing fractionated oral vs. continuous enteral feeding. Aliment Pharmacol Ther. 2011;33:1332–1339. doi: 10.1111/j.1365-2036.2011.04662.x. [DOI] [PubMed] [Google Scholar]
- 16.Alhagamhmad MH, Day AS, Lemberg DA, Leach ST. An update of the role of nutritional therapy in the management of Crohn’s disease. J Gastroenterol. 2012;47:872–882. doi: 10.1007/s00535-012-0617-9. [DOI] [PubMed] [Google Scholar]
- 17.Heuschkel RB, Menache CC, Megerian JT, Baird AE. Enteral nutrition and corticosteroids in the treatment of acute Crohn’s disease in children. J Pediatr Gastroenterol Nutr. 2000;31:8–15. doi: 10.1097/00005176-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto T, Nakahigashi M, Saniabadi AR, et al. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: a prospective study. Inflamm Bowel Dis. 2007;13:1493–1501. doi: 10.1002/ibd.20238. [DOI] [PubMed] [Google Scholar]
- 19.Stewart M, Day AS, Otley A. Physician attitudes and practices of enteral nutrition as primary treatment of paediatric Crohn disease in North America. J Pediatr Gastroenterol Nutr. 2011;52:38–42. doi: 10.1097/MPG.0b013e3181e2c724. [DOI] [PubMed] [Google Scholar]
- 20.Johnson T, Macdonald S, Hill SM, Thomas A, Murphy MS. Treatment of active Crohn’s disease in children using partial enteral nutrition with liquid formula: a randomised controlled trial. Gut. 2006;55:356–361. doi: 10.1136/gut.2004.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyams JS, Ferry GD, Mandel FS, et al. Development and validation of a pediatric Crohn’s disease activity index. J Pediatr Gastroenterol Nutr. 1991;12:439–447. doi: 10.1097/00005176-199105000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Otley A, Loonen H, Parekh N, Corey M, Sherman PM, Griffiths AM. Assessing activity of pediatric Crohn’s disease: which index to use? Gastroenterology. 1999;116:527–531. doi: 10.1016/S0016-5085(99)70173-3. [DOI] [PubMed] [Google Scholar]
- 23.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: child/adolescent. Gastroenterology. 2006;130:1527–1537. doi: 10.1053/j.gastro.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludvigsson JF, Krantz M, Bodin L, Stenhammar L, Lindquist B. Elemental versus polymeric enteral nutrition in paediatric Crohn’s disease: a multicentre randomized controlled trial. Acta Paediatr. 2004;93:327–335. doi: 10.1111/j.1651-2227.2004.tb02956.x. [DOI] [PubMed] [Google Scholar]
- 25.Grogan JL, Casson DH, Terry A, Burdge GC, El-Matary W, Dalzell AM. Enteral feeding therapy for newly diagnosed pediatric Crohn’s disease: a double-blind randomized controlled trial with two years follow-up. Inflamm Bowel Dis. 2012;18:246–253. doi: 10.1002/ibd.21690. [DOI] [PubMed] [Google Scholar]
- 26.Verma S, Brown S, Kirkwood B, Giaffer MH. Polymeric versus elemental diet as primary treatment in active Crohn’s disease: a randomized, double-blind trial. Am J Gastroenterol. 2000;95:735–739. doi: 10.1111/j.1572-0241.2000.01527.x. [DOI] [PubMed] [Google Scholar]
- 27.Hartman C, Berkowitz D, Weiss B, et al. Nutritional supplementation with polymeric diet enriched with transforming growth factor-beta 2 for children with Crohn’s disease. Isr Med Assoc J. 2008;10:503–507. [PubMed] [Google Scholar]
- 28.Conklin LS, Oliva-Hemker M. Nutritional considerations in pediatric inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2010;4:305–317. doi: 10.1586/egh.10.23. [DOI] [PubMed] [Google Scholar]
- 29.Sanderson IR, Udeen S, Davies PS, Savage MO, Walker-Smith JA. Remission induced by an elemental diet in small bowel Crohn’s disease. Arch Dis Child. 1987;62:123–127. doi: 10.1136/adc.62.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerasimidis K, Talwar D, Duncan A, et al. Impact of exclusive enteral nutrition on body composition and circulating micronutrients in plasma and erythrocytes of children with active Crohn’s disease. Inflamm Bowel Dis. 2012;18:1672–1681. doi: 10.1002/ibd.21916. [DOI] [PubMed] [Google Scholar]
- 31.Lambert B, Lemberg DA, Leach ST, Day AS. Longer-term outcomes of nutritional management of Crohn’s disease in children. Dig Dis Sci. 2012;57:2171–2177. doi: 10.1007/s10620-012-2232-2. [DOI] [PubMed] [Google Scholar]