Abstract
Background/Aims
Spinal metastases often severely limit the quality of life by causing severe pain and neurological deficits. The purpose of this study was to evaluate the palliative effect of radiotherapy (RT) for spinal metastases from hepatocellular carcinoma (HCC) and to identify factors predictive of survival in HCC patients with spinal metastases who received RT.
Methods
A retrospective analysis was performed on 192 patients with spinal metastases from HCC who received RT.
Results
Of 192 patients with spinal metastases from HCC, an overall pain response to palliative RT occurred in 187 patients (97.4%), with a complete pain response (CR) in 41 patients (21.4%) and a partial response in 151 patients (78.6%). A higher biologically effective dose (BED) and more advanced RT techniques were identified as predictive factors for a CR. The 1- and 2-year overall survival (OS) rates were 18.1% and 6.3%, respectively, and the median survival time was 4.5 months. A long OS was associated with good performance status, controlled primary HCC, absence of extrahepatic metastases, and a higher BED.
Conclusions
RT provided effective palliation for patients with painful spinal metastases from HCC. Our results provide information regarding pain control, survival outcomes, and predictive factors for the prognosis of HCC patients with spinal metastases treated with RT.
Keywords: Carcinoma, hepatocellular, Spinal metastases, Radiotherapy, Pain response, Predictive factors
INTRODUCTION
The development of various diagnostic tools and treatment modalities has improved the survival of hepatocellular carcinoma (HCC) patients. Consequently, bone metastases from HCC are diagnosed more frequently.1 Approximately 5% to 25% of HCC patients have bone metastases.2–5 The spine is the most frequent bone metastatic site in HCC, and spinal metastases have been reported to occur in approximately 50% to 75% of patients with bone metastases from HCC.2,5–7 Spinal metastases often severely limit the quality of life, causing severe pain and neurological deficits.
Radiotherapy (RT) can provide significant palliation of painful bone metastases in approximately 60% to 90% of patients, with up to 33% of patients achieving complete pain response (CR) at the irradiated site.8,9 At our institute, RT has been actively adopted in the management of bone metastases from HCC, particularly for those causing pain. We and others have previously reported that RT provides effective palliation for patients with painful bone metastases from HCC during the substantial median survival time.2,5,7,10
Previous studies have suggested that prognostic factors, including unfavorable primary tumor type, extrahepatic metastases, multiple bone metastases, and performance status, could predict overall survival (OS) in patients with spinal metastases from various solid tumors who receive RT.11,12 However, little is known about the predictive factors associated with pain response and survival in RT-treated patients with spinal metastases from HCC. Prediction of survival and pain response are important in determining proper management, including the optimal RT schedule for patients with symptomatic spinal metastases. The purpose of this study was to evaluate the palliative effect of RT for spinal metastases from HCC and to identify predictive factors of survival in RT-treated patients with spinal metastases from HCC.
MATERIALS AND METHODS
1. Patients
Between April 1992 and February 2012, 192 HCC patients underwent palliative RT for spinal metastases at 383 sites at our hospital. HCC diagnoses were confirmed radiologically (hyperattenuation in the arterial phase and washout in the late phase) using either contrast-enhanced computed tomography (CT) or magnetic resonance imaging (MRI).13 Ultrasound-guided tumor biopsies were performed in patients with inconclusive diagnostic imaging results. Pretreatment evaluation included medical history taking and physical examination, complete blood cell count, serum chemistries, liver function tests, serum α-fetoprotein (AFP) measurement, and diagnostic imaging studies. Liver function was estimated using the Child-Pugh classification, scored according to bilirubin and albumin serum levels, prothrombin time prolongation, presence or absence of ascites, and encephalopathy. Patients without new lesions or primary tumor progression on the follow-up enhanced CT and/or MRI after primary treatments against HCC were regarded as having controlled primary tumors. A newly detected hepatic nodule will be defined as new lesions when its longest diameter is at least 1 cm and the nodule shows the typical vascular pattern of HCC on dynamic imaging, that is, hypervascularization in the arterial phase with washout in the portal venous or late venous phase.14 Otherwise, patients were regarded as having uncontrolled primary tumors.
2. Diagnosis and evaluation of spinal metastases
Bone metastases were diagnosed using imaging studies plus the serum AFP level or biopsy and histological study. Characteristics of spinal metastases were evaluated using imaging studies including plain radiography, whole body bone scintigraphy, CT, MRI, and positron emission tomography. Tumor characteristics included multiplicity of spinal metastases, mass-type metastases, spinal cord compression, and pathological fracture secondary to metastatic lesions. Mass-type metastasis was defined as an expansile soft-tissue lesion with a clear boundary outside the spine. Spinal cord compression was defined as radiological tumor involvement of the spinal canal with severely impaired neurological function (American Spinal Injury Association impairment scale of A, B, or C).15 Only patients with pathologic fracture due to secondary malignancy were included in the study.
3. Pain assessment
Subjective pain response to treatment was assessed using the Brief Pain Inventory (BPI).16 The BPI assesses pain severity as “worst,” “least,” “average,” and “now” (current pain). In our study, the item “worst” was used to indicate pain severity. The numeric rating scale ranged from 0 to 10 (0, no pain and 10, pain as bad as can be imagined). Patients were interviewed by a physician before the start of RT, 2 weeks after RT, and every 3 months thereafter for 1 year. Pain response to treatment was defined according to the International Bone Metastases Consensus Working Party palliative RT endpoints.17 CR was defined as a zero pain score at the treated site with no concomitant increase in daily oral morphine equivalent analgesic intake. Partial response (PR) was defined as either pain reduction of 2 or more points below baseline at the treated site on a 0–10 scale without analgesic increase or analgesic reduction of 25% or more from baseline without an increase in pain. Pain progression (PP) was defined as pain increase of 2 or more points above baseline at the treated site with stable analgesic use or a stable pain score or 1 point above baseline with an increase of 25% or more in daily oral morphine equivalent. Patients not classified as having CR, PR, or PP were considered as having stable pain (SP).
4. Treatment
For spinal metastases, the radiation dose was prescribed to the mid-vertebral body. In the case of conventional 2-dimensional RT, radiation fields usually involved 1 normal vertebra above and below the metastatic lesion. The portion adjacent to the gross tumor volume (GTV) was included in the clinical target volume (CTV) in a radiation port using a 3-dimensional conformal RT (3D-CRT) or intensity-modulated radiation therapy (IMRT) planning system. For example, if the GTV involved the pedicle and lateral part of the vertebral body, the CTV encompassed the vertebral body, pedicle, transverse process, and lamina. The spinous process, contralateral pedicle, and lamina were excluded from the CTV. For postoperative treatment, the surgical bed was included from the CTV. Planning target volume (PTV) modification (0 to 1 cm) was allowed if the CTV extended to critical organs other than the normal spine. Total doses were recalculated and normalized to obtain biologically effective doses (BEDs) as the α/β ratio=10 Gy. The actual total dose was converted to BED as follows: BED=nd [1+d/(α/β)], where n is the number of fractions, and d, the dose per fraction. Calculated BED values ranged from 14.4 to 78 Gy10 with a median dose of 44.2 Gy10. Patients were immobilized in thermoplastic head-shoulder masks for the cervical spine. For the thoracic and lumbar spine, a customized total body vacuum bag was used.
In addition to RT, surgical decompression with or without instrumentation and systemic chemotherapy, including cisplatin, 5-fluorouracil, and sorafenib, and so on, were performed to relieve pain and prevent impending neurological deficit from spinal metastases. None of the patients received bisphosphonates to reduce the risk of skeletal-related events and the loss of bone mass.
5. Toxicity assessment
Radiation Therapy Oncology Group criteria were used to assess treatment-related toxicity. Acute reaction during RT was assessed from patient records, and the occurrence of radiation-induced myelopathy was identified as late toxicity.
6. Statistical analysis
Categorical variables are summarized using frequencies and percentages. To determine the association between pain response and covariates, univariate analysis was performed using the nonparametric Pearson chi-square or Fisher exact test. A receiver operating characteristic curve was used to select single cutoff value for various continuous variables. Likelihood ratio were used to select two contiguous cutoff values for the interval of a continuous variable. Multivariate analyses were performed using a stepwise logistic regression model. OS after diagnosis of spinal metastases was defined as the interval from the date of first observation of spinal metastases on CT or MRI to the date of death or last follow-up appointment. Survival was calculated using the Kaplan-Meier method. The log-rank test was used to examine the differences between survival curves for each potential predictive factor. Multivariate survival analyses were performed using a Cox proportional hazard model. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). A p-value of less than 0.05 was considered statistically significant.
RESULTS
1. Clinical features of spinal metastases
Patient, tumor, and treatment characteristics are summarized in Table 1. Of the 192 patients, 181 (94.3%) were followed up until death. During this time, 11 patients (5.7%) were lost to follow-up. The median follow-up duration was 4.2 months (range, 0.5 to 124.8 months). Forty-eight patients (25%) presented with synchronous metastases and 144 (75%) presented with meta-chronous metastases. The most commonly involved site on the spine was the lumbar vertebrae (48/192; 25%), followed by the thoracic vertebrae (46/192; 23.9%). Solitary spine metastasis was observed in 87 patients (45.7%), and ≥2 spine metastases were present in 105 patients (54.7%).
Table 1.
Patient, Tumor, and Treatment Characteristics (n=192)
| Characteristic | Value |
|---|---|
| Age, yr | 56 (20–82) |
| Gender | |
| Male | 157 (81.8) |
| Female | 35 (18.2) |
| ECOG performance status | |
| 0 | 16 (8.3) |
| 1 | 33 (17.2) |
| 2 | 55 (28.6) |
| 3 | 66 (34.4) |
| 4 | 22 (11.5) |
| AFP, ng/mL | 245.2 (1–120,000) |
| Child-Pugh classification | |
| A | 130 (67.7) |
| B | 42 (21.9) |
| C | 20 (10.4) |
| Primary HCC | |
| Controlled | 127 (66.1) |
| Uncontrolled | 65 (33.9) |
| Interval from diagnosis of primary tumor to spinal metastases, mo | 8.6 (0–127) |
| Baseline BPI score (pain severity) | 5.0 (2–10) |
| Extrahepatic metastases other than bone* | |
| Yes | 50 (26.0) |
| No | 142 (74.0) |
| Sites of spinal metastases | |
| Cervical | 26 (13.5) |
| Thoracic | 46 (24.0) |
| Lumbar | 48 (25.0) |
| Sacrum | 8 (4.2) |
| Combined (2 sites or more) | 64 (33.3) |
| Multiplicity of spinal metastases | |
| Yes | 105 (54.7) |
| No | 87 (45.3) |
| Mass-type metastases | |
| Yes | 46 (24.0) |
| No | 146 (76.0) |
| Spinal cord compression (ASIA scale A–C) | |
| Yes (A–C) | 25 (13.0) |
| No (D–E) | 167 (87.0) |
| Pathologic fracture | |
| Yes | 47 (24.4) |
| No | 145 (75.6) |
| BED, Gy10 | |
| ≤38 | 38 (19.8) |
| 39–53 | 132 (68.7) |
| >53 | 22 (11.5) |
| Treatment modalities | |
| RT alone | 140 (72.9) |
| RT+systemic chemotherapy† | 38 (19.8) |
| RT+surgical decompression | 12 (6.3) |
| RT+systemic chemotherapy†+surgical decompression | 2 (1.0) |
| RT technique | |
| Conventional | 107 (55.7) |
| 3D-CRT | 67 (34.9) |
| IMRT | 18 (9.4) |
| Interval from diagnosis of spinal metastases to start of RT, day | 7.0 (0–187) |
Data are presented as median (range) or number (%).
ECOG, Eastern Cooperative Oncology Group; AFP, α-fetoprotein; HCC, hepatocellular carcinoma; BPI, Brief Pain Inventory; ASIA, American Spinal Injury Association (A, no motor or sensory neurological function is preserved at or below sacral segments S4–5; B, incomplete sensory function but motor function is not preserved below the neurological level, extending through sacral segments S4–5; C, incomplete motor function but normal sensory function is preserved below the neurological level, and the majority of key muscles below the neurological level have a muscle power of grade <3; D, motor function is preserved below the neurological level, and the majority of key muscles below the neurological level have a muscle power of grade ≥3; E, normal motor and sensory function); BED, biologically effective dose; RT, radiotherapy; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy.
Including lung, lymph nodes, brain, and adrenal gland;
Including five patients who received sorafenib.
2. Pain response
Of the 192 patients with spinal metastases from HCC who received RT, 187 (97.4%), 41 (21.4%), 146 (78.6%), 3 (1.6%), and 2 (1.0%) showed overall pain response (CR+PR), CR, PR, SP, and PP, respectively (Table 2). Tumor and treatment characteristics were not associated with overall pain response. In univariate analysis, presence of pathologic fracture (p=0.013), BED (p<0.001), and RT technique (p=0.002) were identified as predictive factors for CR. Multivariate analysis revealed that predictive factors for CR were a higher BED (p=0.003) and a more advanced RT technique (3D-CRT or IMRT) (p=0.009) (Table 3).
Table 2.
Univariate Analysis to Identify Predictive Factors for the Pain Response
| Variable | No. of patients | Response (CR+PR)* | Nonresponse (SP+PP)* | p-value† | CR | Non-CR | p-value† |
|---|---|---|---|---|---|---|---|
| Multiplicity of spinal metastases | 0.388 | 0.739 | |||||
| Yes | 105 (54.7) | 104 (54.2) | 1 (0.5) | 17 (8.9) | 88 (45.8) | ||
| No | 87 (45.3) | 83 (43.2) | 4 (2.1) | 24 (12.5) | 63 (32.8) | ||
| Mass-type metastases | 0.080 | 0.734 | |||||
| Yes | 46 (24.0) | 42 (21.9) | 4 (2.1) | 9 (4.7) | 37 (19.3) | ||
| No | 146 (76.0) | 145 (75.5) | 1 (0.5) | 32 (16.7) | 114 (59.3) | ||
| Spinal cord compression (ASIA scale A–C) | 0.304 | 0.221 | |||||
| Yes (A–C) | 25 (13.0) | 21 (10.9) | 4 (2.1) | 3 (1.6) | 22 (11.4) | ||
| No (D–E) | 167 (87.0) | 166 (86.5) | 1 (0.5) | 38 (19.8) | 129 (67.2) | ||
| Pathologic fracture | 0.598 | 0.013 | |||||
| Yes | 47 (24.4) | 45 (23.4) | 2 (1.0) | 4 (2.1) | 43 (22.3) | ||
| No | 145 (75.6) | 142 (74.0) | 3 (1.6) | 37 (19.3) | 108 (56.3) | ||
| BED, Gy10 | 0.711 | <0.001 | |||||
| ≤38 | 38 (19.8) | 37 (19.3) | 1 (0.5) | 4 (2.1) | 34 (17.7) | ||
| 39–53 | 132 (68.7) | 128 (66.6) | 4 (2.1) | 24 (12.5) | 108 (56.2) | ||
| >53 | 22 (11.5) | 22 (11.5) | 0 | 13 (6.8) | 9 (4.7) | ||
| Treatment modalities | 0.335 | 0.093 | |||||
| RT alone | 140 (72.9) | 136 (71.9) | 4 (1.0) | 25 (13.0) | 115 (59.9) | ||
| RT+CTx | 38 (19.8) | 38 (19.8) | 0 | 13 (6.8) | 25 (13.0) | ||
| RT+S±CTx | 14 (7.3) | 13 (6.8) | 1 (0.5) | 3 (1.6) | 11 (5.7) | ||
| RT technique | 0.067 | 0.002 | |||||
| Conventional | 107 (55.7) | 102 (53.1) | 5 (2.6) | 14 (7.3) | 93 (48.4) | ||
| 3D-CRT or IMRT | 85 (44.3) | 85 (44.3) | 0 | 27 (14.1) | 58 (30.2) | ||
| Total | 192 (100.0) | 187 (97.4) | 5 (2.6) | 41 (21.4) | 151 (78.6) |
Data are presented as number (%).
CR, complete pain response; PR, partial response; SP, stable pain; PP, pain progression; ASIA, American Spinal Injury Association; BED, biologically effective dose; RT, radiotherapy; CTx, chemotherapy; S, surgery; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy.
CR, 41 patients (21.4%); PR, 146 patients (76.0%); SP, 3 patients (1.6%); PP, 2 patients (1.0%);
Determined using Pearson chi-square or Fisher exact test.
Table 3.
Multivariate Analysis to Identify Predictive Factors for a Complete Pain Response
| Variable | CR | ||
|---|---|---|---|
|
| |||
| OR | 95% CI | p-value* | |
| Pathologic fracture | 0.415 | 1.516–0.114 | 0.184 |
| BED, Gy10† | 0.003 | ||
| 39–53 | 0.555 | 1.726–0.179 | 0.309 |
| >53 | 0.296 | 0.659–0.133 | <0.001 |
| RT technique | 0.358 | 0.778–0.165 | 0.009 |
CR, complete pain response; OR, odds ratio; CI, confidence interval; BED, biologically effective dose; RT, radiotherapy.
Determined using stepwise logistic regression analysis;
Reference level of a BED was ≤38 Gy10.
3. Survival analysis
The OS rates at 1 and 2 years after spinal metastasis diagnosis were 18.1% and 6.3%, respectively, and the median survival time was 4.5 months (95% confidence interval, 3.7 to 5.3). Univariate analysis showed that good Eastern Cooperative Oncology Group (ECOG) performance status (p<0.001) and Child-Pugh classification (p=0.025), a controlled primary HCC (p<0.001), absence of extrahepatic metastases (p=0.012) and pathologic fracture (p=0.003), a higher BED (p<0.001), and occurrence of CR (p=0.001) were significantly associated with better survival. Multivariate analysis revealed that predictive factors for better survival were good ECOG performance status (p=0.001), presence of controlled primary HCC (p<0.001), absence of extrahepatic metastases (p=0.004), and a higher BED (p<0.001) (Table 4).
Table 4.
Univariate and Multivariate Analyses to Identify Predictive Factors for Overall Survival
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| No. of patients | Median survival±SE, mo | p-value* | Coefficient | HR | 95% CI | p-value† | |
| Age, yr | 0.492 | ||||||
| ≤55 | 86 | 4.8±0.5 | |||||
| >55 | 106 | 3.9±0.5 | |||||
| Gender | 0.430 | ||||||
| Male | 157 | 4.5±0.3 | |||||
| Female | 35 | 5.3±1.7 | |||||
| ECOG performance status | <0.001 | 0.639 | 1.895 | 1.302–2.757 | 0.001 | ||
| 0–2 | 104 | 5.7±0.3 | |||||
| 3–4 | 88 | 2.7±0.3 | |||||
| AFP, ng/mL | 0.143 | ||||||
| ≤200 | 98 | 4.7±0.5 | |||||
| >200 | 94 | 2.8±0.6 | |||||
| Child-Pugh classification | 0.025 | 0.259 | 1.165 | 0.693–1.755 | 0.164 | ||
| A, B | 172 | 4.5±0.4 | |||||
| C | 20 | 2.0±1.2 | |||||
| Primary HCC | <0.001 | 1.279 | 3.595 | 2.453–5.268 | <0.001 | ||
| Controlled | 127 | 6.2±0.5 | |||||
| Uncontrolled | 65 | 1.9±0.3 | |||||
| Interval from diagnosis of primary tumor to spinal metastases, mo | 0.966 | ||||||
| ≤9 | 100 | 4.2±0.5 | |||||
| >9 | 92 | 4.0±0.7 | |||||
| Baseline BPI score (pain severity) | 0.857 | ||||||
| ≤6 | 114 | 4.8±0.6 | |||||
| >6 | 78 | 4.2±0.5 | |||||
| Extrahepatic metastases other than bone | 0.012 | −0.560 | 0.571 | 0.391–0.835 | 0.004 | ||
| Yes | 50 | 2.8±0.6 | |||||
| No | 142 | 5.0±0.5 | |||||
| Sites of spinal metastases | 0.169 | ||||||
| Cervical | 26 | 2.5±1.3 | |||||
| Thoracic | 46 | 4.8±0.4 | |||||
| Lumbar | 48 | 5.7±1.4 | |||||
| Sacrum | 8 | 4.5±2.8 | |||||
| Combined (2 sites or more) | 64 | 3.7±0.6 | |||||
| Multiplicity of spinal metastases | 0.112 | ||||||
| Yes | 105 | 5.0±0.5 | |||||
| No | 87 | 3.9±0.7 | |||||
| Mass-type metastases | 0.577 | ||||||
| Yes | 46 | 4.7±1.7 | |||||
| No | 146 | 4.5±0.4 | |||||
| Spinal cord compression (ASIA scale A–C) | 0.839 | ||||||
| Yes (A–C) | 25 | 4.0±2.0 | |||||
| No (D–E) | 167 | 4.5±0.4 | |||||
| Pathologic fracture | 0.003 | −0.342 | 0.710 | 0.476–1.059 | 0.093 | ||
| Yes | 47 | 2.7±0.5 | |||||
| No | 145 | 5.0±0.3 | |||||
| BED, Gy10 | <0.001 | −0.624 | 0.536 | 0.383–0.751 | <0.001 | ||
| ≤38 | 38 | 2.4±0.6 | |||||
| 39–53 | 132 | 9.7±1.6 | |||||
| >53 | 22 | 15.2±4.2 | |||||
| Treatment modalities | 0.926 | ||||||
| RT alone | 140 | 3.9±0.6 | |||||
| RT+CTx | 38 | 4.0±1.3 | |||||
| RT+S±CTx | 14 | 5.3±0.6 | |||||
| RT technique | 0.110 | ||||||
| Conventional (2D) | 107 | 3.9±0.6 | |||||
| 3D-CRT or IMRT | 85 | 4.5±0.7 | |||||
| Pain response | 0.001 | 0.308 | 1.361 | 0.938–1.973 | 0.104 | ||
| CR | 41 | 7.2±1.8 | |||||
| Non-CR | 151 | 3.0±0.4 | |||||
SE, standard error; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; AFP, α-fetoprotein; HCC, hepatocellular carcinoma; BPI, Brief Pain Inventory; ASIA, American Spinal Injury Association; BED, biologically effective dose; RT, radiotherapy; CTx, chemotherapy; S, surgery; 2D, 2-dimensional; 3D-CRT, 3-dimensional conformal radiation therapy; IMRT, intensity-modulated radiation therapy; CR, complete pain response.
Determined using the log-rank test;
Determined using the Cox proportional hazard model.
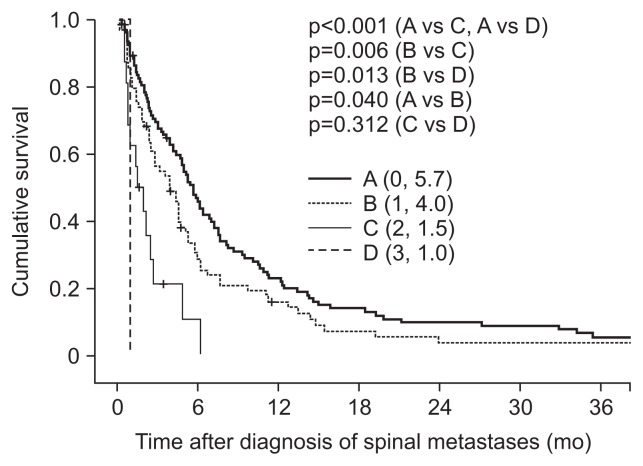
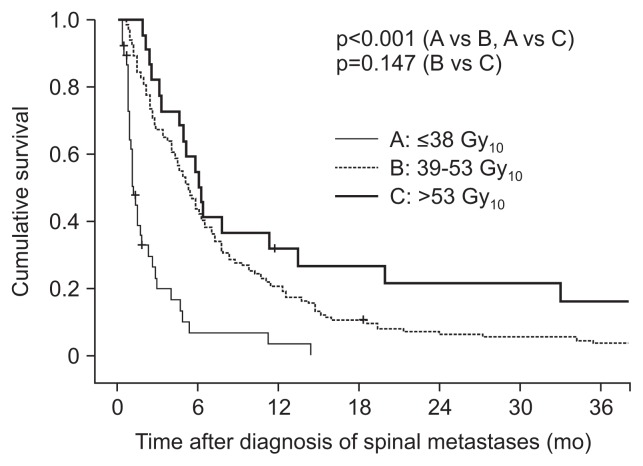
Patients were classified into four groups according to the number of unfavorable risk factors for OS: poor performance status (ECOG 3–4), presence of uncontrolled primary HCC, and presence of extrahepatic metastases. One hundred three patients were classified as group A (0 risk factors), 71 as group B (1 risk factor), 16 as group C (2 risk factors), and 2 as group D (3 risk factors). The median survival times were 5.7, 4.0, 1.5, and 1.0 months in groups A, B, C, and D, respectively (Fig. 1). Subgroup analyses revealed that median survival time was significantly different between groups A and B (p=0.040), groups A and C (p<0.001), groups A and D (p<0.001), groups B and C (p=0.006), and groups B and D (p=0.013), but not between groups C and D (p=0.312). Our findings suggested that a higher BED might be associated with long OS in HCC patients with spinal metastases (Fig. 2).
Fig. 1.
Stratified cumulative survival curves according to risk factor group. Risk factors were poor performance status (Eastern Cooperative Oncology Group 3–4), uncontrolled primary hepatocellular carcinoma, and the presence of extrahepatic metastases. Group A, 0 risk factors; group B, 1 risk factor; group C, 2 risk factors; group D, 3 risk factors. p-values were determined using the log-rank test.
Fig. 2.
Stratified cumulative survival curves according to biologically effective dose. p-values were determined using the log-rank test.
4. Radiation-induced toxicity
Two patients experienced grade 3 neutropenia and anemia. No other severe (grade 3–4) acute toxicity was observed. Radiation-induced myelopathy was not observed during the follow-up period for the entire study population.
DISCUSSION
The spine is the most common site of bone metastases from HCC. Spinal metastases frequently result in neurological deficit and severe pain, which affect functional capacity and quality of life.2,5–7 The treatment goals for spinal metastases are pain relief and bone stabilization. RT is a well-established effective treatment for alleviation of pain secondary to bone metastases from HCC.2,5,7,10
Metastatic bone pain is a multifactorial process. A relationship between tumor burden and overall pain response has not been established.10 The pain caused by bone metastases is unlikely to originate from the tumor burden but can originate not only from bone itself but also from nerve root compression or muscle spasm in the lesion area.18,19 Pain relief generally occurs after palliative RT with relatively low doses prior to the onset of radiological response. This early pain response may be related to a reduction in inflammatory cells, which release chemical pain mediators in the bone metastatic microenvironment.19,20 These reports support our result that patients who received RT showed overall pain response rate of 97.4%.
In the present study, we determined whether pain relief could be predicted using tumor characteristics and treatment modalities of spinal metastases from HCC. Tumor characteristics and treatment modalities were not significantly associated with overall pain response. Hence, all patients with painful spinal metastases from HCC, regardless of spinal assessment criteria in imaging studies, including presence of mass-type metastasis, pathologic fracture, and spinal cord compression, may obtain similar benefits from RT. However, higher CR rates were obtained with a higher BED or more advanced RT technique. Dose-response relationship studies have been mostly limited to comparing single-fraction versus multiple-fraction RT and have shown a higher rate of pain relapse with the single-fraction scheme.9,21,22 To date, the correlation between BED levels and CR has not been investigated. It is not clear why higher BEDs were associated with CR in our study. Considering that tumor cell-secreted inflammatory cytokines are a major source of bone pain,19,20 higher BED radiation may be more effective in suppressing cytokine production by tumor cells. Further studies are investigating the underlying mechanisms of RT-mediated pain relief for bone metastases. We found that CR was significantly associated with the RT technique. More advanced RT techniques such as 3D-CRT and IMRT enabled radiation of a higher BED to be targeted more accurately to metastatic lesions while lowering the dose to normal organs, including the spinal cord.
In our study, the 1-year and 2-year OS rates were 18.1% and 6.3%, respectively, and the median survival time was 4.5 months. ECOG performance status is a significant independent predictive factor for survival in many cancer types, including HCC. We found that good ECOG performance status was significantly associated with better survival in HCC patients with spinal metastases. The major cause of death in HCC patients with extrahepatic metastases, including bone metastases, is progression of the primary HCC lesion.7,23,24 This may be because liver function is strongly correlated with performance status. In our study, the presence of extrahepatic metastases other than in bone was found to be a significant negative prognostic factor for OS in HCC patients. However, most extrahepatic metastatic lesions were found not to be the direct cause of death in most affected HCC patients, with the exception of respiratory failure due to bilateral lung metastasis and cerebral hemorrhage due to brain metastasis.23 Hence, the presence of extrahepatic metastasis may be an indicator of primary HCC aggressiveness as a whole rather than an independent predictive factor.
In our study, we identified poor ECOG performance status (3 or 4), uncontrolled primary HCC, and the presence of extrahepatic metastases as risk factors for shorter overall survival in HCC patients with spinal metastasis who underwent RT. Patients with two or more risk factors had a significantly shorter median survival than those with no risk factors (≤1.5 months vs ≥5.7 months). Patients with no risk factors should be considered for intensive care, including treatments for intrahepatic or extrahepatic lesions. Dose-escalated RT (a minimum BED ≥39 Gy10) was also associated with prolonged OS in our study. The dose-escalated RT regimen appears to be safe and feasible for patients without any risk factors. Because higher BEDs were associated with higher CR rates in our study, poor performance status due to severe bone pain might be improved after dose-escalated RT, and the improved performance status might be associated with prolonged OS. But dose escalation might be more frequently administered to patients with good performance status for whom long-term survival was predicted. Therefore, this result should be carefully interpreted because all retrospective studies could always be exposed by a possibility of potential selection bias, and by confounding factors that are not easy to adjust for. The significance of dose escalation in palliative RT for spinal metastases from HCC should be further investigated in large-scale prospective studies.
In our study, among 192 HCC patients, 23 had additional treatments after local failure following initial RT. Thirteen patients received re-irradiation, two underwent surgical decompression, six received systemic chemotherapy, and two received combined treatment. In re-irradiation cases, the response to re-irradiation was not included in the primary outcome of the first RT. Late toxicity was not observed in any of the patients who received additional treatments. Furthermore, these patients had a longer median survival than patients who did not receive additional treatments (7.7 months vs 3.9 months). All patients who received additional treatment had good ECOG performance status (0, 1, or 2) and ≤1 risk factor, which suggested the necessity of additional treatments in HCC patients with initial RT failure.
In conclusion, RT provides effective palliation for patients with painful spinal metastases from HCC. In particular, RT with a higher BED may improve pain control and OS in these patients. The results of this study provide information regarding pain control, survival outcomes, and predictive factors for the prognosis of HCC patients with spinal metastases treated with RT.
ACKNOWLEDGEMENTS
This work was supported by a grant (0620390) from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Natsuizaka M, Omura T, Akaike T, et al. Clinical features of hepatocellular carcinoma with extrahepatic metastases. J Gastroenterol Hepatol. 2005;20:1781–1787. doi: 10.1111/j.1440-1746.2005.03919.x. [DOI] [PubMed] [Google Scholar]
- 2.Kaizu T, Karasawa K, Tanaka Y, et al. Radiotherapy for osseous metastases from hepatocellular carcinoma: a retrospective study of 57 patients. Am J Gastroenterol. 1998;93:2167–2171. doi: 10.1111/j.1572-0241.1998.00614.x. [DOI] [PubMed] [Google Scholar]
- 3.Katyal S, Oliver JH, 3rd, Peterson MS, Ferris JV, Carr BS, Baron RL. Extrahepatic metastases of hepatocellular carcinoma. Radiology. 2000;216:698–703. doi: 10.1148/radiology.216.3.r00se24698. [DOI] [PubMed] [Google Scholar]
- 4.Fukutomi M, Yokota M, Chuman H, et al. Increased incidence of bone metastases in hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2001;13:1083–1088. doi: 10.1097/00042737-200109000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Seong J, Koom WS, Park HC. Radiotherapy for painful bone metastases from hepatocellular carcinoma. Liver Int. 2005;25:261–265. doi: 10.1111/j.1478-3231.2005.01094.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim SU, Kim do Y, Park JY, et al. Hepatocellular carcinoma presenting with bone metastasis: clinical characteristics and prognostic factors. J Cancer Res Clin Oncol. 2008;134:1377–1384. doi: 10.1007/s00432-008-0410-6. [DOI] [PubMed] [Google Scholar]
- 7.He J, Zeng ZC, Tang ZY, et al. Clinical features and prognostic factors in patients with bone metastases from hepatocellular carcinoma receiving external beam radiotherapy. Cancer. 2009;115:2710–2720. doi: 10.1002/cncr.24300. [DOI] [PubMed] [Google Scholar]
- 8.Ratanatharathorn V, Powers WE, Moss WT, Perez CA. Bone metastasis: review and critical analysis of random allocation trials of local field treatment. Int J Radiat Oncol Biol Phys. 1999;44:1–18. doi: 10.1016/S0360-3016(98)00510-0. [DOI] [PubMed] [Google Scholar]
- 9.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–1436. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 10.Kim TG, Park HC, Lim DH, et al. Radiation therapy for bone metastases from hepatocellular carcinoma: effect of radiation dose escalation. J Korean Soc Ther Radiol Oncol. 2011;29:63–70. doi: 10.3857/jkstro.2011.29.2.63. [DOI] [Google Scholar]
- 11.van der Linden YM, Dijkstra SP, Vonk EJ, Marijnen CA, Leer JW Dutch Bone Metastasis Study Group. Prediction of survival in patients with metastases in the spinal column: results based on a randomized trial of radiotherapy. Cancer. 2005;103:320–328. doi: 10.1002/cncr.20756. [DOI] [PubMed] [Google Scholar]
- 12.Mizumoto M, Harada H, Asakura H, et al. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int J Radiat Oncol Biol Phys. 2011;79:208–213. doi: 10.1016/j.ijrobp.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 13.Torzilli G, Minagawa M, Takayama T, et al. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889–893. doi: 10.1002/hep.510300411. [DOI] [PubMed] [Google Scholar]
- 14.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 15.Ditunno JF, Jr, Young W, Donovan WH, Creasey G. The international standards booklet for neurological and functional classification of spinal cord injury. American Spinal Injury Association. Paraplegia. 1994;32:70–80. doi: 10.1038/sc.1994.13. [DOI] [PubMed] [Google Scholar]
- 16.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 17.Chow E, Wu JS, Hoskin P, Coia LR, Bentzen SM, Blitzer PH. International consensus on palliative radiotherapy endpoints for future clinical trials in bone metastases. Radiother Oncol. 2002;64:275–280. doi: 10.1016/S0167-8140(02)00170-6. [DOI] [PubMed] [Google Scholar]
- 18.Goblirsch MJ, Zwolak PP, Clohisy DR. Biology of bone cancer pain. Clin Cancer Res. 2006;12(20 Pt 2):6231s–6235s. doi: 10.1158/1078-0432.CCR-06-0682. [DOI] [PubMed] [Google Scholar]
- 19.Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/S0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- 20.Park HC, Seong J, An JH, Kim J, Kim UJ, Lee BW. Alteration of cancer pain-related signals by radiation: proteomic analysis in an animal model with cancer bone invasion. Int J Radiat Oncol Biol Phys. 2005;61:1523–1534. doi: 10.1016/j.ijrobp.2004.12.070. [DOI] [PubMed] [Google Scholar]
- 21.Wu JS, Wong R, Johnston M, Bezjak A, Whelan T Cancer Care Ontario Practice Guidelines Initiative Supportive Care Group. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594–605. doi: 10.1016/S0360-3016(02)04147-0. [DOI] [PubMed] [Google Scholar]
- 22.Sze WM, Shelley MD, Held I, Wilt TJ, Mason MD. Palliation of metastatic bone pain: single fraction versus multifraction radio-therapy--a systematic review of randomised trials. Clin Oncol (R Coll Radiol) 2003;15:345–352. doi: 10.1016/S0936-6555(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 23.Uchino K, Tateishi R, Shiina S, et al. Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer. 2011;117:4475–4483. doi: 10.1002/cncr.25960. [DOI] [PubMed] [Google Scholar]
- 24.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9. [DOI] [PubMed] [Google Scholar]