Abstract
Virulence of the human pathogen, V. vulnificus, is associated with encapsulation, serum complement resistance, and genotype. The C-genotype of this bacterium is correlated (>90%) with virulence and with isolation source (clinical settings). E-genotype strains are highly correlated with environmental isolation (93%) but appear less virulent. In this study, we characterized the importance of genotype, encapsulation, serum complement, and in situ exposure to estuarine water on the survival of the two genotypes in human serum. Results confirmed the superior ability of C-genotype strains to survive exposure to human serum, as well as the significance of complement, and revealed that lack of capsule allowed serum killing of both C- and E-genotypes. Cells incubated in situ responded similarly to cells incubated in vitro with the exception of E-environmental strains. Interestingly, our studies found that those cells of the E-genotype, typically considered non-pathogenic, which were isolated from wound infections demonstrated serum survival similar to that of virulent, C-genotype, strains.
Keywords: in situ incubation, wound infections, capsule
1. Introduction
Vibrio vulnificus exists in estuaries across the globe, and is associated with a variety of aquatic organisms, particularly bivalves such as the eastern oyster, Crassostrea virginica. Ingestion of raw or undercooked oysters carrying V. vulnificus can lead to rapid and severe septicemia, multi-organ failure, necrotizing fasciitis, and in some cases death [1,2,3]. Indeed, V. vulnificus accounts for ~95% of all seafood related deaths in the U.S. and has the highest case fatality rate (>50%) of any foodborne pathogen [4,5].
While most bacterial pathogens display a single mode of transmission, V. vulnificus has an alternate route of infection via introduction into an open wound [6]. Wound infections typically result from a wound inflicted by handling shellfish or other recreational activities in coastal environments and carry a mortality rate of ca. 25% [2].
Not all strains of this bacterium are equally pathogenic; of the three known biotypes of V. vulnificus, biotype 1 represents the majority of strains that can result in human disease upon exposure. This biotype can be further subdivided into two genotypes, aptly named for their most common sources of isolation. “C” (clinical) genotypes are typically pathogenic, and 93% of isolates from clinical settings are of this genotype [7]. Conversely, “E” (environmental) genotypes are generally non-pathogenic, and 90% percent are isolated from environmental settings, including oysters, sediment, and water samples [7]. These two genotypes can be distinguished using a simple and rapid polymerase chain reaction, in which one of two virulence correlated alleles, vcgE or vcgC, is amplified [8].
Although C- and E-genotypes significantly correlate with isolation source, it is becoming increasingly apparent that a subset of E-genotypes has acquired the ability to cause human disease, with most isolates being implicated in wound infections. This is of particular medical concern considering the documented increase in the number of V. vulnificus wound infections and subsequent deaths as a result of climate change [9].
The pathogenicity of this bacterium is believed to arise from several putative virulence factors, including the production of iron-binding siderophores, exoenzymes, and capsular polysaccharides, as well as the ability to survive exposure to human serum [1,10,11,12]. While most of the proposed virulence factors remain unconfirmed, the production of capsular polysaccharide (CPS) is highly correlated with pathogenicity [1,10,13,14,15]. In fact, previous research has shown that the LD50 of the encapsulated, “opaque” strains is as low as a single cell [13] whereas non-encapsulated, “translucent” strains of V. vulnificus display little or no virulence [13,14].
Our lab and others have previously reported on the ability of some V. vulnificus strains to survive exposure to human serum [12,16,17,18]. Both the C- and E- genotypes of V. vulnificus typically produce CPS, a trait known to aid in defense against serum, yet our lab has found the E-genotypes to show greater susceptibility when exposed to human serum than C-genotypes [16]. A variety of suggestions for the mechanisms of this phenomenon have been offered, the most significant of which may be differences in expression of the siderophore gene, viuB or capsule switching. Previous research from our lab indicated that C-genotype cells, all of which were viuB positive, showed significantly higher serum survival than E-genotype strains, most of which lacked this siderophore-encoding gene [16]. Our studies have also indicated that E-genotype cells more frequently revert to the non-encapsulated, translucent phenotype than cells of the C-genotype [19], possibly rendering them more susceptible to the bactericidal effects of serum.
The intent of the present study was to further characterize the degree of serum resistance demonstrated by the two genotypes of V. vulnificus, with particular attention to clinically isolated E-genotypes. We also investigated the role of capsule in serum survival, employing strains that have undergone a permanent mutation in the wzb gene within the CPS operon. A unique aspect of this study involved exposing cells to environmental conditions to induce the natural physiology of V. vulnificus cells prior to introduction into human serum. This aspect was accomplished by subjecting the cells to 24 h of in situ incubation in estuarine water using membrane diffusion chambers.
2. Results and Discussion
2.1. Role of Genotype and Isolation Source in Serum Survival
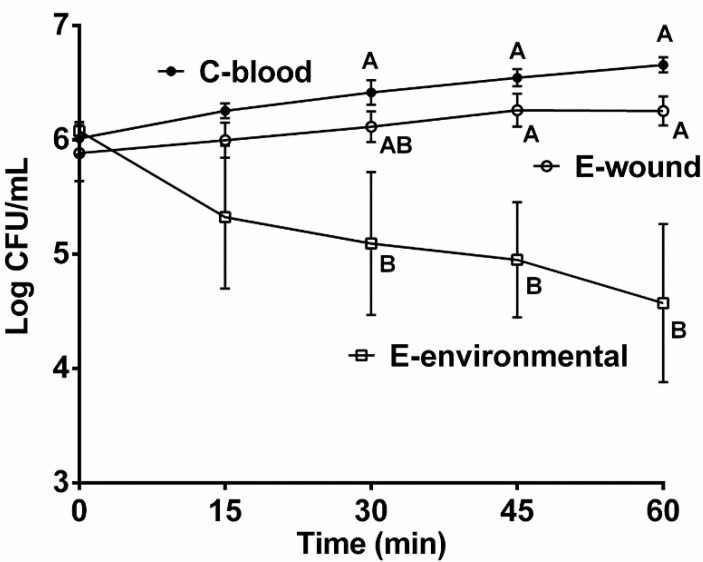
Examination of four clinically isolated C-genotype and four environmentally isolated E-genotype strains revealed a significant difference in their ability to survive in human serum (Figure 1). While C-genotype strains exhibited total survival and even growth, exposure to serum was inhibitory to E-genotype growth, supporting results previously reported [16]. This difference was particularly evident after 60 min of incubation.
Figure 1.
Role of genotype and isolation source on survival in human serum. Survival of clinically isolated C-genotypes (MO6-24, CMCP6, C7184, YJ016; closed circles), E-genotype wound isolates (E64MW, LSU2098, LSU1657, LSU549; open circles) and environmentally isolated E-genotypes (JY1305, JY1701, ENV1, SS108-A3A; open squares), cells exposed to human serum for 60 min. Error bars represent the standard error of the mean for four strains with three replicates each. Different letters indicate statistically significant differences (two-way ANOVA).
While C-genotypes predominate in septicemia cases, a considerable number of E-genotypes have been isolated from wound infections. This finding has prompted further interest into E-genotype strains, i.e., do all E-genotypes have the ability to cause wound infections or is there a subset of E-genotypes that have unique virulence factors or share virulence factors with C-genotypes? Additionally, V. vulnificus wound infections associated with recreational water activities have become more prevalent in both the U.S. and Europe likely as a result of warming water temperatures [9,20,21,22]. We therefore examined if E-genotype strains isolated from wound infections differ in human serum sensitivity compared to non-clinical, environmentally isolated E-genotypes. Unlike environmental E-genotypes, wound isolates resisted the bacteriocidal effects of human serum at a rate similar to that of C-genotypes (Figure 1). Interestingly, a recent comparative genome analysis [23] revealed that the E-genotype wound isolate, E64MW, shares 43 genes with three C-genotype blood isolates (CMCP6, YJ016, and M06-25) which were absent in the two non-clinical E-genotypes (JY1305 and JY1701). From these results we suggest that a subgroup of E-genotypes have acquired mechanisms to successfully colonize and infect the human host although further investigation is required to more fully understand the relationship between C-genotypes and this subset of E-genotypes.
2.2. Role of Capsule in Serum Survival
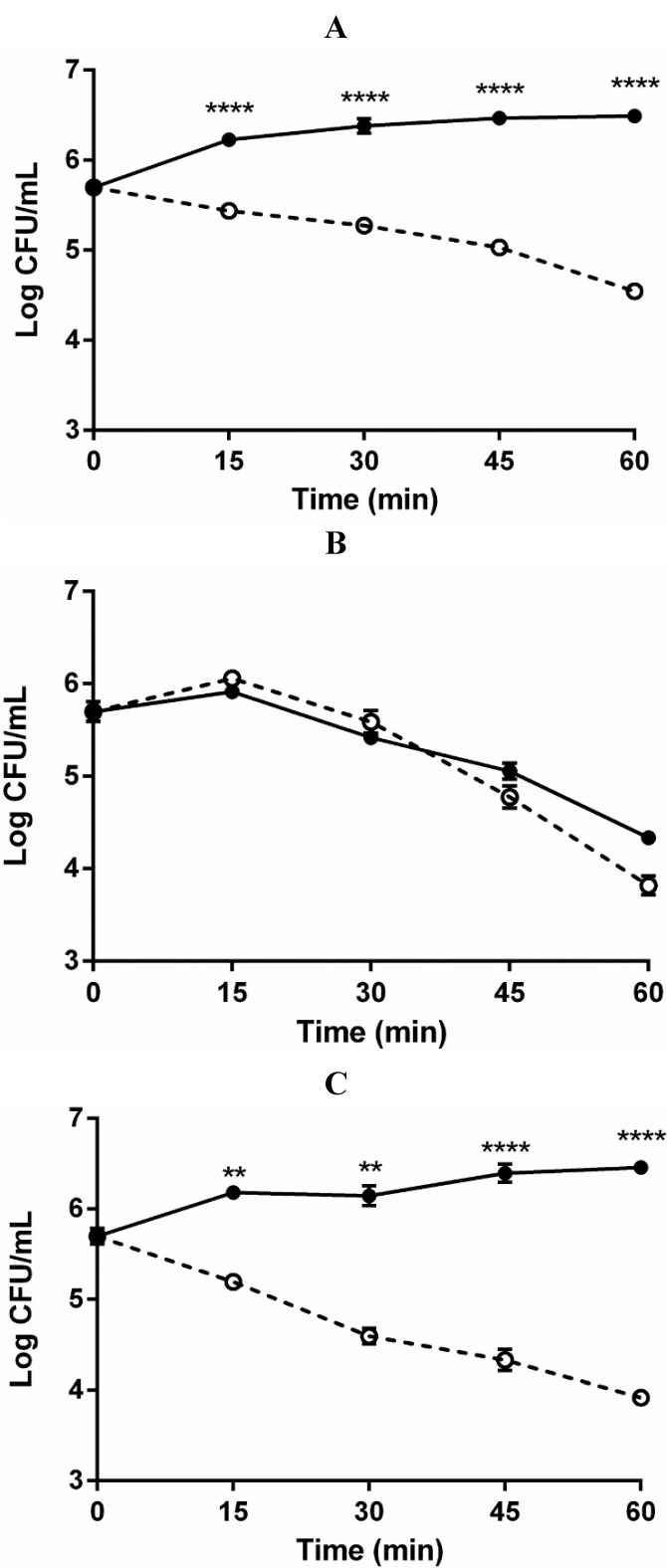
We also analyzed the effect of capsule on serum survival by both C- and E-genotypes. All capsular polysaccharide mutants employed appeared phenotypically identical and possess genetic determinants affecting the functionality of the group 1 CPS operon which directs CPS biosynthesis and transport [14,24,25]. In contrast to the encapsulated clinical strains (C and E genotypes) which exhibited population growth, the non-encapsulated strains of both genotypes underwent more than a 1-log decrease in culturability within 60 min (Figure 2).
Figure 2.
Role of capsular polysaccharide in survival of V. vulnificus exposed to human serum. (A) Survival of opaque and translucent clinical C-genotype (C7184/Op, closed circles; C7184/Tr, open circles); (B) Opaque and translucent environmental E-genotype (JY1701/Op, closed circles; JY1701/Tr, open circles); (C) Opaque and translucent clinical E-genotype (LSU1657, closed circles; LSU1657/Tr, open circles) exposed to human serum for up to 1 hour. Error bars represent the standard error of the mean for three replicates per strain. Two-Way ANOVA with Bonferroni post hoc test (** p < 0.01; **** p < 0.0001).
Culturability of the non-encapsulated environmental E-genotype strain decreased nearly 2-logs. Using the standard method for visual determination of encapsulation [13,15], none of the non-encapsulated mutants exhibited capsule when plated whereas all parent strains produced the “opaque”, encapsulated colonial phenotype. Thus, the presence of CPS appears to play a significant role in serum survival regardless of genotype or clinical/environmental source (Figure 2). This is likely due to the resistance that negatively charged CPS imparts against antimicrobial components present in human serum, or to shifts in osmolarity [26].
2.3. Role of Complement in Serum Survival
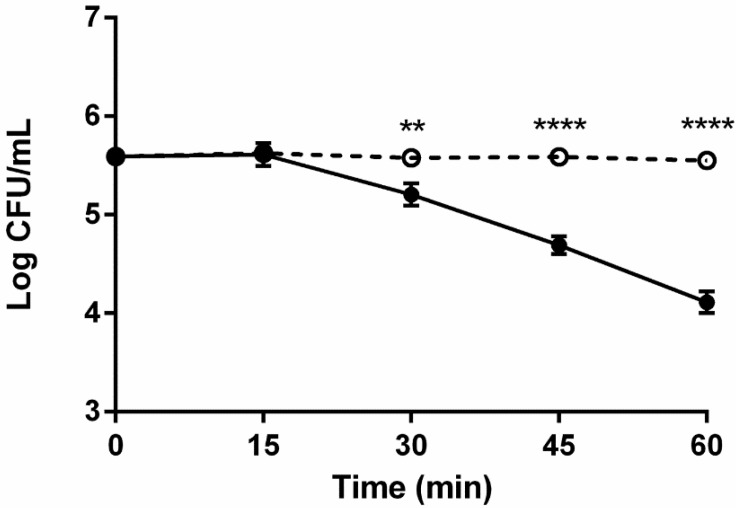
Inactivated serum allowed for more than 1-log greater survival of non-encapsulated strains compared to survival in active serum (Figure 3). These results indicate that inactivation of bacteriocidal components, including complement proteins, allows for survival and even growth of non-encapsulated strains, regardless of genotype.
Figure 3.
Role of complement in bactericidal activity of serum against translucent strains of V. vulnificus. Effect of complement active serum on translucent strains (C7184/Tr; LSU1657/Tr; and JY1701/Tr) in complement active (closed circles) or complement inactive (open circles) serum. Error bars represent the standard error of the mean for three replicates per strain. Asterisks indicate statistically significant differences. Two-Way ANOVA with Bonferroni post hoc test (** p < 0.01; **** p < 0.0001).
2.4. Effect of in situ Incubation on Serum Survival
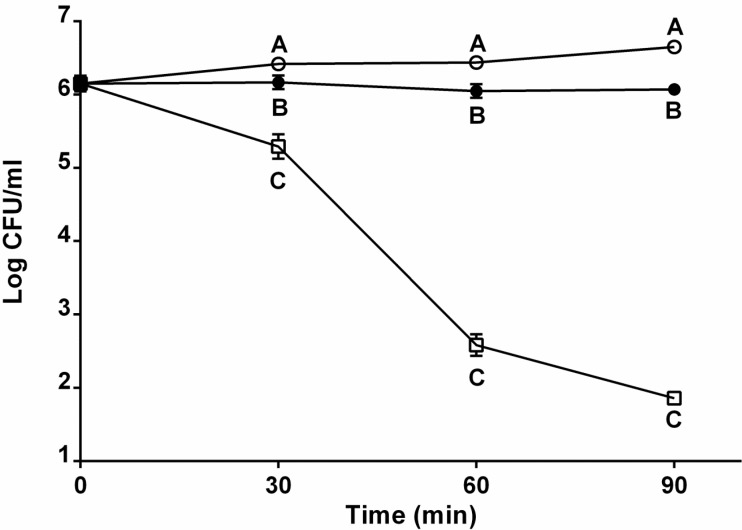
To determine if cells of V. vulnificus present in the natural environment respond to human serum in a manner similar to what we observed for in vitro grown cells, we exposed cells to in situ conditions by placing them in an estuary for 24 h prior to their exposure to human serum. This was accomplished employing membrane diffusion chambers that house the cells while allowing them to be exposed to salinity, temperature, nutrient, viruses and other dissolved matter fluctuations naturally present in such environments. As seen with our in vitro studies, both clinically isolated C-genotype and wound E-genotype isolates maintained serum resistance (Figure 4) whereas environmental E-genotype strains were more susceptible to serum relative to survival in vitro (Figure 1 and Figure 4).
Figure 4.
Serum survival of V. vulnificus genotypes and isolate types following in situ incubation. Survival of clinically isolated C-genotypes (CMCP6, YJ016; open circles), clinically isolated E-genotypes (LSU2098, E64MW; closed circles), and environmentally isolated E-genotypes (JY1305, ENV1; squares) in human serum for up to 90 min after incubation in situ (estuarine waters). Error bars represent the standard error of the mean for two strains and three replicates. Different letters indicate statistically significant differences (two-way ANOVA).
Thus, little difference in survival was detected between cells incubated in situ and in vitro prior to exposure to serum, with the exception of E-environmental strains which displayed a weaker resistance to human serum following in situ incubation. This finding suggests that the environmental conditions cells experience in estuarine waters, such as fluctuating salinity, temperature, and nutrient availability, may alter the potential for serum survivability. Differences in expression of the hemolysin, vvhA, had previously been observed by our lab for cells of the two V. vulnificus genotypes incubated in situ [27].
3. Materials and Methods
3.1. Bacterial Strains and Growth Conditions
Table 1 lists the strains that were used in this study along with genotype, source of isolation, and capsule phenotype. The translucent strains used in this study, originally isolated and identified by our lab, underwent a spontaneous mutation of the wzb gene resulting in permanent loss of its ability to produce capsular polysaccharide. These strains are referred to as TR2 strains as previously described [24] and were genetically confirmed to lack the wzb gene via PCR while still possessing the flanking wza and wzb genes.
Table 1.
List of V. vulnificus strains used in this study including their respective genotypes, source of isolation, and colony opacities (capsule presence).
| Strain Name | Genotype | Isolation Source | Opacity |
|---|---|---|---|
| CMCP6 | C | Human Blood | Opaque |
| YJ016 | C | Human Blood | Opaque |
| MO6-24 | C | Human Blood | Opaque |
| C7184 | C | Human Blood | Opaque |
| C7184/Tra | C | Spontaneous CPS mutant | Translucent |
| LSU2098 | E | Human wound | Opaque |
| E64MW | E | Human wound | Opaque |
| LSU549 | E | Human wound | Opaque |
| LSU1657 | E | Human wound | Opaque |
| LSU1657/Tra | E | Spontaneous CPS mutant | Translucent |
| ENV1 | E | Water | Opaque |
| SS108-A3A | E | Oyster | Opaque |
| JY1305 | E | Oyster | Opaque |
| JY1701 | E | Oyster | Opaque |
| JY1701/Tra | E | Spontaneous CPS mutant | Translucent |
a Isogenic CPS mutants lacking the wzb gene of the CPS operon, referred to as a TR2 genotype by Chatzidaki-Livanis et al. [24].
Bacterial cultures were taken from freezer stocks and grown overnight in heart infusion (HI) broth at 30 °C with shaking. For in vitro studies, overnight cultures were diluted in fresh HI broth at a 1/100 (v/v) ratio and grown to logarithmic phase (OD610 0.15–0.25).
3.2. In Situ Incubations
Membrane diffusion chambers used in this study were originally designed by McFeters and Stuart [28,29]. These consist of two 76 mm, 0.2 µm hydrophilic polycarbonate filters (Midland Scientific Inc.; cat# 1220891) sandwiched between three doughnut shaped sections of Plexiglas. Using this apparatus, 25 mL of bacterial culture at a final cell concentration of ca. 104−5 CFU/mL was aseptically injected into each autoclaved chamber which were then deployed into Calico Creek, an estuarine water body located in Beaufort, North Carolina. Water temperatures at the site of deployment were typically 20–25 °C with salinities of 15–34 ppt. After 24 h of incubation, the chambers were retrieved and the cells aseptically removed for serum studies.
3.3. Human Serum Exposure
Pooled male human serum (MP Biomedicals, Santa Ana, CA, USA) was used for all studies. To determine the significance of bacteriocidal components, including the complement cascade, in serum sensitivity, serum was heat-inactivated by incubation of the serum at 56°C for 30 min. In vitro studies were adapted from Bogard and Oliver [16]. To achieve a final cell concentration of ca. 105−6 cells/mL serum, log-phase cells (12 µL) were inoculated into 788 µL serum, or in the case of in situ incubation, 100 µL of cells were added to 1 mL of serum. In all cases cells were incubated in serum at 37°C for up to 2 h, with culturability assessed at 15 or 30 min time intervals by serially diluting into PBS followed by plating onto HI agar to determine CFU/mL after 24 h incubation at 30°C.
3.4. Statistical Analysis
Each experiment was performed with at least three replicates per strain. Log transformed data were analyzed using GraphPad Prism (v. 5.0; GraphPad Software Inc. San Diego, CA, USA). Statistical analyses were performed using one and two-way analyses of variance (ANOVA) followed by Bonferroni post hoc test for multiple comparisons. Significance was determined using a 95% confidence interval.
4. Conclusions
The findings reported here support previous studies and demonstrate that genotype and capsular polysaccharide significantly impact V. vulnificus serum survivability and likely pathogenicity, and are therefore important virulence determinants for this organism. However, E-genotype strains isolated from wound infections were found to exhibit serum resistance similar to that of the clinically isolated C-genotypes, and this resistance was maintained regardless of in vitro or in situ incubation prior to exposure to serum. Thus, while genotype largely correlates with source of isolation, further genetic distinctions within E-genotypes are necessary to predict pathogenic potential.
Acknowledgments
This study was supported by the Cooperative State Research, Education and Extension Service, U.S. Department of Agriculture, under Award No. 2007-35201-18381.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the U.S. Department of Agriculture.
Author Contributions
James Oliver conceived and supervised the studies. Heather Ryan initiated the research as a portion of her Honors in Biology thesis. Tiffany Williams and Mesrop Ayrapetyandeveloped the mutants and significantly expanded on the studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jones M.K., Oliver J.D. Vibrio vulnificus: Disease and pathogenesis. Infect. Immun. 2009;77:1723–1733. doi: 10.1128/IAI.01046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliver J.D. Vibrio vulnificus: Death on the half shell. A personal journey with the pathogen and its ecology. Microb. Ecol. 2012;793:793–799. doi: 10.1007/s00248-012-0140-9. [DOI] [PubMed] [Google Scholar]
- 3.Oliver J.D. Vibrio vulnificus. In: Thompson F.L., Austin B., Swing J., editors. The Biology of Vibrios. American Society for Microbiology; Washington, DC, USA: 2006. pp. 349–366. [Google Scholar]
- 4.Feldhusen F. The role of seafood in bacterial foodborne diseases. Microb. Infect. 2000;2:1651–1660. doi: 10.1016/S1286-4579(00)01321-6. [DOI] [PubMed] [Google Scholar]
- 5.Oliver J.D., Kaper J. Vibrio species. In: Doyle M.P., Beuchat L.R., editors. Food Microbiology: Fundamentals and Frontiers. 3rd ed. American Society for Microbiology; Washington, DC, USA: 2007. pp. 343–379. [Google Scholar]
- 6.Oliver J.D. Wound infections caused by Vibrio vulnificus and other marine bacteria. Epidemiol. Infect. 2005;133:383–391. doi: 10.1017/S0950268805003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosche T.M., Yano Y., Oliver J.D. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 2005;49:381–389. doi: 10.1111/j.1348-0421.2005.tb03731.x. [DOI] [PubMed] [Google Scholar]
- 8.Warner E., Oliver J.D. Multiplex PCR assay for detection and simultaneous differentiation of genotypes of Vibrio vulnificus biotype 1. Foodborne Pathog. Dis. 2008;5:691–693. doi: 10.1089/fpd.2008.0120. [DOI] [PubMed] [Google Scholar]
- 9.Baker-Austin C., Trinanes J.A., Taylor N.G.H., Hartnell R., Siitonen A., Martinez-Urtaza J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Chang. 2013;3:73–77. doi: 10.1038/nclimate1628. [DOI] [Google Scholar]
- 10.Gulig P.A., Bourdage K.L., Starks A.M. Molecular pathogenesis of Vibrio vulnificus. J. Microbiol. 2005;43:118–131. [PubMed] [Google Scholar]
- 11.Kim H.Y., Ayrapetyan M., Oliver J.D. Survival of Vibrio vulnificus genotypes in male and female serum, and production of siderophores in human serum and seawater. Foodborne Pathog. Dis. 2014;11:119–125. doi: 10.1089/fpd.2013.1581. [DOI] [PubMed] [Google Scholar]
- 12.Simpson L., Oliver J. Ability of Vibrio vulnificus to obtain iron from transferrin and other iron-binding proteins. Curr. Microbiol. 1987;15:155–157. doi: 10.1007/BF01577265. [DOI] [Google Scholar]
- 13.Simpson L.M., White V.K., Zane S.F., Oliver J.D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect. Immun. 1987;55:269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright A.C., Simpson L.M., Oliver J.D., Morris J.G., Jr. Phenotypic evaluation of acapsular transposon mutants of Vibrio vulnificus. Infect. Immun. 1990;58:1769–1773. doi: 10.1128/iai.58.6.1769-1773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida S., Ogawa M., Mizuguchi Y. Relation of capsular materials and colony opacity to virulence of Vibrio vulnificus. Infect. Immun. 1985;47:446–451. doi: 10.1128/iai.47.2.446-451.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogard R.W., Oliver J.D. Role of iron in human serum resistance of the clinical and environmental Vibrio vulnificus genotypes. Appl. Environ. Microbiol. 2007;73:7501–7505. doi: 10.1128/AEM.01551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jarecki A. Master’s Thesis. University of North Carolina at Charlotte; Charlotte, NC, USA: 1995. The Role of Starvation in the Resistance of Vibrio vulnificus to the Bacteriocidal Activity of Human Serum. [Google Scholar]
- 18.Linkous D.A. Master’s Thesis. University of North Carolina at Charlotte; Charlotte, NC, USA: 1998. Comparison of Virulence among Vibrio vulnificus Strains of Varying Capsular and LPS Serotypes. [Google Scholar]
- 19.Hilton T., Rosche T., Froelich B., Smith B., Oliver J.D. Capsular polysaccharide phase variation in Vibrio vulnificus. Appl. Environ. Microbiol. 2006;72:6986–6993. doi: 10.1128/AEM.00544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Urtaza J., Bowers J.C., Trinanes J., DePaola A. Climate anomalies and the increasing risk of Vibrio parahaemolyticus and Vibrio vulnificus illnesses. Food Res. Int. 2010;43:1780–1790. doi: 10.1016/j.foodres.2010.04.001. [DOI] [Google Scholar]
- 21.Torres. L., Escobar S., Lopez A.I., Marco M.L., Pobo V. Wound infection due to Vibrio vulnificus in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2002;21:537–538. doi: 10.1007/s10096-002-0767-4. [DOI] [PubMed] [Google Scholar]
- 22.Weis K.E., Hammond R.M., Hutchinson R., Blackmore C.G. Vibrio illness in Florida, 1998–2007. Epidemiol. Infect. 2011;139:591–598. doi: 10.1017/S0950268810001354. [DOI] [PubMed] [Google Scholar]
- 23.Morrison S.S., Williams T., Cain A., Froelich B., Taylor C., Baker-Austin C., Verner-Jeffreys D., Hartnell R., Oliver J.D., Gibas C.J. Pyrosequencing-based comparative genome analysis of Vibrio vulnificus environmental isolates. PLoS One. 2012;7:e37553. doi: 10.1371/journal.pone.0037553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chatzidaki-Livanis M., Jones M.K., Wright A.C. Genetic variation in the Vibrio vulnificus group 1 capsular polysaccharide operon. J. Bacteriol. 2006;188:1987–1998. doi: 10.1128/JB.188.5.1987-1998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright A.C., Powell J.L., Kaper J.B., Morris J.G., Jr. Identification of a group 1-like capsular polysaccharide operon for Vibrio vulnificus. Infect. Immun. 2001;69:6893–6901. doi: 10.1128/IAI.69.11.6893-6901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts I.S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Ann. Rev. Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 27.Smith B.E., Oliver J.D. In situ gene expression by Vibrio vulnificus. Appl. Environ. Microbiol. 2006;72:2244–2246. doi: 10.1128/AEM.72.3.2244-2246.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliermans C.B., Gorden R.W. Modification of membrane diffusion chambers for deep-water studies. Appl. Environ. Microbiol. 1977;33:207–210. doi: 10.1128/aem.33.1.207-210.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McFeters G.A., Stuart D.G. Survival of coliform bacteria in natural waters: Field and laboratory studies with membrane-filter chambers. Appl. Microbiol. 1972;24:805–811. doi: 10.1128/am.24.5.805-811.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]