Abstract
The discovery of an inner mucus layer normally impervious to bacteria has changed our way of understanding the interaction between commensal bacteria and the host epithelial cells. This inner colon mucus layer is rapidly renewed and converted into the outer mucus layer by host controlled endogenous proteolytic processing. The mucus characteristics esteem from the properties of the main protein component of these layers, the MUC2 mucin. This forms an enormously large net-like structure that builds the laminated inner mucus layer that largely acts as a size exclusion filter excluding bacteria. In the absence of MUC2 mucin, there is no inner mucus layer and bacteria reach the epithelial cell surface, penetrate the crypts and are also found inside epithelial cells, something that leads to severe inflammation. Other mouse models that spontaneously develop colitis due to different defects, like an absent ion channel (Nhe3) or immunological mediators (Tlr5, IL-10), all also have a defective inner colon mucus layer. Human patients with active ulcerative colitis have this layer penetrable to bacteria and beads the size of bacteria. Some of the ulcerative colitis patients in remission have a normal mucus layer whereas others have a penetrable inner mucus layer. Together, this suggests that the inner mucus layer and its integrity is important for the protection of the colon epithelium and inhibiting activation of the immune system as in ulcerative colitis.
Keywords: Mucus, Mucin, Colon, Ulcerative colitis
Colon
The large intestine is the home of 1013-14 commensal bacteria that normally live in friendly coexistence with us [1]. The recent progress in DNA sequencing methodology has made it possible to get more insight into this community as only a minority of all bacteria have been possible to cultivate in vitro. The human microbiota is dominated by members of the Fermicutes and Bacteroidetes families [2] and the number of species is in the 1,000 range. The number of genes outnumber the humans at least a 100-fold. How all these bacteria can live in our intestine without causing an overt immune reaction has been long debated and assumed to depend on immune cells being able to distinguish harmful bacteria from our commensals. This explanation seemed unlikely and it was not until 2008 that a more logic explanation was published when we discovered that there is an inner mucus layer of colon separating bacteria from epithelium [3, 4].
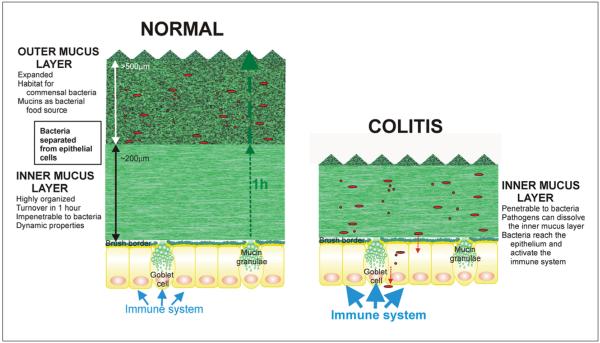
The colon mucus system is based on an ingenious two-layered design (fig. 1). The inner mucus layer formed by the goblet cells makes an anchored layer that excludes bacteria. This layer is then converted into an outer layer where the commensal bacteria can enter and thrive by using the numerous glycans provided by the mucins [3, 5]. The system is identical in the germ-free animal, although less developed, showing that it is the host endogenous protease activities that control the transition from the inner to the outer mucus layer [3].
Fig. 1.
Simplified model of normal and colitic distal colon mucus layers.
MUC2 Mucin and Colon Mucus Layers
The mucus scaffold is formed by the MUC2 mucin. This is a large glycoprotein with two central mucin domains where a high number of proline, threonine and serine amino acids (PTS sequence) are densely decorated with O-linked glycans as to form long, stiff and extended rod-like structures called a mucin domain [6]. Together, these mucin domains are almost 0.5 μm long. In contrast to this central domain, the N- and C-terminals are highly condensed disulfide-bond stabilized globular structures comprising about 1,200 and 800 amino acids, respectively [7]. The mass of a fully glycosylated MUC2 mucin monomer is about 2.5 MDa.
There is more complexity in the MUC2 mucin as secreted from the goblet cells. During the intracellular bio-synthesis, the C-terminus forms disulfide-bond mediated dimers and the N-terminal trimers [8, 9]. We have presented a model for how this MUC2 polymer can be packed in the goblet cells granulae and allow a more that 1,000-fold expansion upon release without entangling [7]. When the MUC2 polymers have been expanded they will form net-like planar sheets built by MUC2 dimers [7]. Important for the birth of these mucin sheets is the ion milieu where sufficient levels of bicarbonate are necessary to precipitate calcium and generate a sufficiently high pH [7, 10].
The continuous release of MUC2 sheets, largely from the surface goblet cells, will interact with the already present inner mucus layer [11]. In this way, a laminated inner mucus layer will be formed [7]. This layer is about 50 μm in mice and is renewed in 1–2 h as measured in the distal colon of live animals [3, 11, 12]. It is thicker in humans, around 200 μm [12].
This inner mucus layer is devoid of bacteria. The reason for this is largely physical exclusion based on size. Conclusions based on a number of experiments show that beads the size of bacteria are similarly excluded as bacteria [13]. However, smaller molecules can easily pass. On the outer side of the inner mucus layer the MUC2 mucin is processed in unknown ways by host proteases allowing the mucin to expand 3–4 times in volume and by this increasing the pore sizes and allowing bacteria to enter [3].
Colitis Models
If the inner mucus layer is now separating the commensal bacteria from the epithelium, what happens if this inner layer is absent or defect? This knowledge was gathered from mice lacking the MUC2 mucin or with MUC2 mutations [3, 14, 15]. These mouse models develop severe inflammation with bloody diarrhea, weight loss, rectal prolapse, and for the mice lacking Muc2 also colon cancer after 3–6 months, directly reminding of ulcerative colitis.
The most commonly used experimental model of colitis in rodents is the dextran sodium sulfate (DSS) model where the animals drink water supplemented with 3–5% DSS [16, 17]. In this model, infiltration of immune cells is observed around day 3 and an overt inflammation around day 5. When the inner mucus layer of these mice was analyzed, this was penetrated by bacteria as soon as DSS was expected to reach the colon [18]. Direct application of DSS to mucus formed on explants further showed that the beads the size of bacteria, in contrast to controls, immediately passed the inner mucus layer. This suggests that the DSS models disrupt the inner mucus layer in such a way that bacteria can penetrate. If this is continued for a couple of days it triggers an immune response.
We have further analyzed tissue sections from a number of known mouse strains that develop spontaneous colitis. These were selected to span different mechanisms for colitis development [12]. The colitis models discussed are all dependent on the presence of commensal bacteria as antibiotic treatments lower or relieve the inflammation in the Core1−/−, Nhe3−/−, Tlr5−/−, and IL-10−/− mice [19-22]. Mice deficient in certain ion channels have been observed to develop spontaneous colitis. The Nhe3 (Slc29a3) channel is an exchanger for protons (out) and sodium (in). When mice lacking this were analyzed, bacteria were observed in the inner mucus layer. That ion channels are important for proper mucus formation is also suggested from studies of mice lacking the CFTR channel defect in cystic fibrosis [10]. In this case pathological mucus was observed in the small intestine, but not in the large intestine.
As ulcerative colitis is believed to be an immunological disease, it was of major importance to address mucus properties in this context [23, 24]. Mice lacking Tlr5 can either develop obesity or colitis, and when the colitic mice were analyzed these also turned out to have an inner penetrable mucus [12, 25]. The first animal model of colitis was the IL-10−/− mice [26]. In our animal house, these mice only develop minor inflammation, but they still have a mucus that is penetrable to bacteria and beads [12]. Interestingly, the inner mucus layer thickness, both in vivo and ex vivo, is thicker, suggesting that it is more important with properties than thickness.
Mucus Degradation
The MUC2 mucin protein core is covered with glycans to such an extent that the mature MUC2 is more than 80% glycans [5]. A major function of these glycans is to protect the protein core from being degraded by proteases. These glycans cannot be degraded by intestinal host enzymes, but well by bacterial ones. Commensal bacteria typically produce exoglycosidases that remove one sugar moiety at a time, and as these glycans are extended and complicated it is suggested that the degradation of these O-glycans will take time. A mouse model lacking the glycosyltransferase that adds galactose to the N-acetylgalactosamine attached to the mucin protein core to make the for mucin typical core 1 glycan has been generated [19]. When this was analyzed the inner mucus layer was not organized and bacteria could penetrate close to the epithelial cells. This suggests that mucin glycans must be sufficiently complex as a way to slow down the mucin degradation.
Another potential way of disrupting the inner mucus layer could be by specific proteases capable of cleaving the MUC2 mucin in such a way that its polymeric network falls apart. Such a protease was found to be secreted by the colonic parasite Entamoeba histolytica [27]. A specific cleavage at almost the same site in MUC2 was more recently described for the bacteria Porphyromonas gingivalis, a mouth pathogen [28]. In both cases the cleavage will dissolve the mucus gel, but the cleavage site can be protected by a specific MUC2 glycosylation.
Interestingly, none of the screened commensal bacteria of Lactobacilli and Bacteroidetes family secreted any proteases that affected the MUC2 mucin [29]. Together, it is likely that commensal bacteria need to have sufficiently long and complex mucin glycans to digest the mucin sufficiently slow. There will probably be some more aggressive bacteria that can degrade the mucin protein faster. A balance between newly released mucus and its degradation is the main factor for maintaining an inner mucus layer.
Ulcerative Colitis
Together, all our models suggest that bacteria in contact with the epithelium are a crucial step in the early development of colon inflammation. The reason for bacteria reaching the epithelium is a failure of the inner mucus layer to keep the bacteria away. Do these models have bearing on the human disease ulcerative colitis?
As routine colonoscopy has to be performed on laxated patients where the bacterial amounts are limited and the mucus layer difficult to preserve, we studied the mucus quality formed on sigmoid biopsies [12]. Patients with active ulcerative colitis (Mayo visual score 1–3) had an inner mucus layer that was penetrable to beads the size of bacteria. Control patients had an inner mucus layer that separated the beads from the epithelium by 200–400 μm. This was also the case for a majority of the patients with ulcerative colitis in remission (Mayo visual score 0). However, a few patients in this small study (3/17) showed an inner mucus layer that was penetrable.
Previous studies have also suggested that there are more bacteria in contact with the epithelium in ulcerative colitis [30]. Together, our observations so far suggest that there is a relation between ulcerative colitis, defects in the inner mucus layer, contact of bacteria with the epithelium, and the development of inflammation.
A New Model for Ulcerative Colitis Pathogenesis
The results discussed here suggest an important role of the inner colon mucus layer in protecting the epithelium from contact with bacteria. It is suggested that colon can tolerate some bacteria reaching the epithelium as is also suggested by the presence of a group of non-reacting phagocytic macrophages in the lamina propria [31]. However, a sufficiently large exposure of bacteria to the epithelium and the underlying immune system will trigger a strong immune reaction and inflammation. The immune system can influence the secretion of mucins and the properties of the inner mucus layer, and as already shown it is likely that there are more, but poorly understood couplings between the immune system and the inner mucus layer.
Figure 1 shows some of the important features of the normal colon inner mucus layer and how this can be affected in colitis where its capacity to keep the bacteria at a distance is insufficient.
Ulcerative colitis is caused by many mechanisms, something that has created today’s situation where there is no unifying model for why classic ulcerative colitis has a similar symptomatology. However, our suggestion of the properties of the inner colon mucus layer as a unifying model for the pathogenesis of ulcerative colitis will provide novel understanding and inspire to new research on this important and serious disease [4].
Acknowledgements
This work was supported by the Swedish Research Council, The Swedish Cancer Foundation, The Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgren’s University Hospital (LUA-ALF), Wilhelm and Martina Lundgren’s Foundation, Söderbergs Stiftelser, Assar Gabrielson Foundation, The Sahlgrenska Academy, National Institute of Allergy and Infectious Diseases (U01AI095473, the content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH), and The Swedish Foundation for Strategic Research – The Mucus-Bacteria-Colitis Center (MBC) of the Innate Immunity Program.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kuro-kawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Dore J, Weissenbach J, Ehrlich SD, Bork P. Entero-types of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johansson MEV, Phillipson M, Petersson J, Holm L, Velcich A, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johansson MEV, Sjovall H, Hansson GC. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol. 2013;10:352–361. doi: 10.1038/nrgastro.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johansson MEV, Holmen Larsson JM, Hansson GC. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proc Natl Acad Sci USA. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johansson MEV, Ambort D, Pelaseyed T, Schutte A, Gustafsson J, Ermund A, Subramani D, Holmen-Larsson J, Thomsson K, Bergstrom J, van der Post S, Rodriguez-Pineiro A, Sjovall H, Backstrom M, Hansson GC. Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci. 2011;68:3535–3641. doi: 10.1007/s00018-011-0822-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ambort D, Johansson MEV, Gustafsson JK, Nilsson H, Ermund A, Johansson BR, Kock P, Hebert H, Hansson GC. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godl K, Johansson MEV, Karlsson H, Morgelin M, Lidell ME, Olson FJ, Gum JR, Kim YS, Hansson GC. The N-termini of the MUC2 mucin form trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–47256. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- 9.Lidell ME, Johansson MEV, Mörgelin M, Asker N, Gum JR, Kim YS, Hansson GC. The recombinant C-terminus of the human MUC2 mucin forms dimers in CHO cells and heterodimers with full-length MUC2 in LS 174T cells. Biochem J. 2003;372:335–345. doi: 10.1042/BJ20030003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, Hebert H, Sjovall H, Hansson GC. Bicarbonate and functional CFTR channel is required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johansson MEV. Fast renewal of the distal colonic mucus layers by the surface goblet cells as measured by in vivo labeling of mucin glycoproteins. PLoS One. 2012;7:e41009. doi: 10.1371/journal.pone.0041009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson MEV, Gustafsson JK, Holmen-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA, Gewirtz AT, Sjövall H, Hansson GC. Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and in patients with ulcerative colitis. Gut. 2013 doi: 10.1136/gutjnl-2012-303207. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafsson JK, Ermund A, Johansson MEV, Schutte A, Hansson GC, Sjovall H. An ex vivo method for studying mucus formation, properties and thickness in human colonic biopsies and mouse small and large intestinal explants. Am J Physiol Gastrointest Liver Physiol. 2012;302:G430–G438. doi: 10.1152/ajpgi.00405.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Velcich A, Yang WC, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295:1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 15.Van der Sluis M, De Koning BAE, De Bruijn ACJM, Velcich A, Meijerink JPP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AWC. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 16.Isao O, Shigeru H, Masahiro Y, Toshifumi O, Yoshio I, Rintaro N. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 17.Axelsson L, Landström E, Goldschmidt T, Grönberg A, Bylund-Fellenius A. Dextran sulfate sodium (DSS) induced experimental colitis in immunodeficient mice: Effects in CD4+-cell depleted, athymic and NK-cell depleted SCID mice. Inflamm Res. 1996;45:181–191. doi: 10.1007/BF02285159. [DOI] [PubMed] [Google Scholar]
- 18.Johansson MEV, Gustafsson JK, Sjoberg KE, Pettersson J, Holm L, Sjovall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5:e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu J, Wei B, Wen T, Johansson MEV, Xiaowei Liu X, Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, McDaniel M, Sferra TJ, Turner J, Chen H, Hansson GC, Braun J, Xia L. Loss of intestinal core 1-derived O-glycans causes spontaneous colitis. J Clin Invest. 2011;121:1657–1666. doi: 10.1172/JCI45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijay-Kumar M, Sanders CJ, Taylor RT, Kumar A, Aitken JD, Sitaraman SV, Neish AS, Uematsu S, Akira S, Williams IR, Gewirtz AT. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laubitz D, Larmonier CB, Bai A, Midura-Kiela MT, Lipko MA, Thurston RD, Kiela PR, Ghishan FK. Colonic gene expression profile in NHE3-deficient mice: evidence for spontaneous distal colitis. Am J Physiol Gastrointest Liver Physiol. 2008;295:G63–G77. doi: 10.1152/ajpgi.90207.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- 24.Schirbel A, Fiocchi C. Inflammatory bowel disease: established and evolving considerations on its etiopathogenesis and therapy. J Dig Dis. 2010;11:266–276. doi: 10.1111/j.1751-2980.2010.00449.x. [DOI] [PubMed] [Google Scholar]
- 25.Carvalho FA, Koren O, Goodrich JK, Johansson MEV, Nalbantoglu I, Aitken JD, Su Y, Chassaing B, Walters WA, Gonzlez A, Clemente JC, Cullender TC, Barnich N, Darfeuille-Michaud A, Vijay-Kumar M, Knight R, Ley RE, Gewirtz AT. Transient inability to manage proteobacteria promotes chronic gut inflammation in TLR5-deficient mice. Cell Host Microbe. 2012;12:139–152. doi: 10.1016/j.chom.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn R, Löhler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 27.Lidell ME, Moncada DM, Chadee K, Hansson GC. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal part and dissolves the protective colonic mucus gel. Proc Nat Acad Sci USA. 2006;103:9298–9393. doi: 10.1073/pnas.0600623103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Post S, Subramani DB, Backstrom M, Johansson MEV, Vester-Christensen MB, Mandel U, Bennett EP, Clausen H, Dahlén G, Sroka A, Potempa J, Hansson GC. Site-specific O-glycosylation on the MUC2 mucin inhibits cleavage by the Porphyromonas gingivalis secreted cysteine protease (RgpB) J Biol Chem. 2013;288:14636–14646. doi: 10.1074/jbc.M113.459479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subramani DB, Johansson MEV, Dahlén G, Hansson GC. Lactobacillus and Bifidobacterium species do not secrete proteases that can cleave the MUC2 mucin protein core which organizes the colon mucus layers. Benef Microbes. 2010;1:343–350. doi: 10.3920/BM2010.0039. [DOI] [PubMed] [Google Scholar]
- 30.Swidsinski A, Loening-Baucke V, Theissig F, Engelhardt H, Bengmark S, Koch S, Lochs H, Dorffel Y. Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut. 2007;56:343–350. doi: 10.1136/gut.2006.098160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]