Abstract
The distribution of the SNARE protein syntaxin-4 and the cation-chloride transporter NKCC were investigated in the outer plexiform layer of human retina using immunohistochemistry. Both proteins, which are proposed to be components of a GABA-mediated feedforward circuit from horizontal cells directly to bipolar cells, were enriched beneath S-cones. The expression pattern of syntaxin-4 was further analyzed in baboon and marmoset to determine if the synaptic specialization is common to primates. Syntaxin-4 was enriched beneath S-cones in both species which together with the human results indicates that this specialization may have evolved for the purpose of mediating unique color vision capacities that are exclusive to primates.
1. Introduction
Recently, we investigated the distribution of the soluble NSF-attachment protein receptor (SNARE) core complex protein syntaxin-4, the sodium-potassium-chloride transporter NKCC, and GABA receptors in the outer plexiform layer of macaque retina by immunohistochemical techniques [1, 2]. The question addressed was if primate horizontal cells may employ a feed-forward pathway from horizontal cells directly to cone bipolar cells. Syntaxin-4 has been shown to be expressed by horizontal cells in different mammals and it was used as an indicator for vesicular GABA release in horizontal cells [3–5]. In macaque monkeys, syntaxin-4 was found in HI and HII horizontal cells postsynaptic to all photoreceptor types, but highly enriched beneath short (S-) wavelength sensitive cones, co-localized with dendritic processes of HII horizontal cells. A concomitant enrichment of NKCC was observed beneath S-cones, pointing to a depolarizing effect of GABA [6, 7] in blue cone ON-bipolar cells. This pathway would allow excitatory signals, for example, yellow-OFF responses from long (L-) and middle (M-) wavelength sensitive cones, to travel via HII cells directly onto blue cone bipolar cells through sign-conserving synapses into the inner retina. Although the corresponding synaptic elements were expressed in mouse and ground squirrel retinas, enrichment at S-cones was not observed [1, 2], indicating the evolution of a primate-specific adjustment in support of enhanced processing of spectrally opponent signals.
Primate retina contains two types of horizontal cells like retinas of many other mammalian species (reviewed in [8]). However, the horizontal cells of primate retina stand out in terms of their cone connectivity pattern. The dendrites of HI horizontal cells contact L/M-cones but largely avoid contacts with S-cones (while the axon terminals are connected to rods). In contrast, HII cells form the majority of their synapses with S-cones via their dendrites and axon-like processes, but they form additional contacts with L- and M-cones [9–16]. It has been shown that HI cells provide cone opponent signals in the midget pathway in support of red-green color vision [17]. However, the role of HII cells is largely unknown. Taking their unique connectivity pattern into account, it seems natural that HII cells may play a role in the S-cone pathway and in S-cone opponent signaling. In line with this hypothesis, the receptive fields of S-cones exhibit a “yellow” surround, which is likely mediated by synaptic feedback from HII cells carrying L- and M-cone signals [18]. The feedback from horizontal cells onto cone terminals is achieved by either ephaptic feedback via connexin hemichannels, by pH changes in the synaptic cleft, or by a combination of both mechanisms (reviewed in [19, 20].
In addition, evidence exists for a direct feed-forward pathway between horizontal cells and bipolar cells. It is known that mammalian horizontal cells express the neurotransmitter gamma-aminobutyric acid (GABA), the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD), and the vesicular GABA-transporter vGAT (e.g.[5, 21–32]. Ultrastructural studies revealed synaptic contacts between horizontal and bipolar cell dendrites in cat and rabbit [33–35] and GABA receptors have been shown to be expressed on bipolar cell dendrites at the corresponding sites in the outer plexiform layer (OPL) [25, 36]. To preserve the proper synaptic sign of a GABAergic communication between horizontal cells and bipolar cells, ON bipolar cells express NKCC on their dendritic tips [7]. NKCC leads to an accumulation of chloride so that the ON bipolar cell depolarizes upon GABA release. Functional evidence for this excitatory feed-forward effect of GABA between horizontal cells and ON bipolar cells has been provided in experiments with adult mouse retina [6, 37, 38].
The question arises if different primate species indeed share the abovementioned specialization within the blue-yellow opponent circuitry, and at which point during evolution it occurred. In the present study, retinas of human, baboon, and marmoset were surveyed for their expression patterns of syntaxin-4 at S-cones. Immunohistochemical labeling revealed in all of the three different species that syntaxin-4 was enriched at S-cones in comparison to L/M-cones. Thus, our data further supports the hypothesis of a primate-specific, synaptic mechanism by which HII cells mediate color vision capacities that are unique to primates, and we conclude that this specialized feed-forward pathway emerged at a time during early primate evolution before the division of platyrrhines and catarrhines.
2. Methods
Tissue preparation
Human eyes were obtained through LifeSight (Seattle, WA). The donor was a 69 year old female with no known ocular disease. The face was iced within three hours of death, eyes were enucleated six hours post-mortem and kept on ice. Dissection for histological analyses of the retinas was performed 8 hours post-mortem. The tissue was fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB), pH 7.4, for 10 or 30 minutes at room temperature (RT).
Retinas of adult olive baboons (Papio anubis) were obtained through the Tissue Distribution Program of the National Primate Research Center at the University of Washington in accordance with guidelines for the care and use of animals at the University of Washington. After initial perfusion with carbogenated, bicarbonate-based Ames medium, the tissue was then transferred to the fixative for 20 minutes at RT.
Common marmosets (Callithrix jacchus) were tranquilized with ketamine and euthanized with an overdose of pentobarbital (80 – 150 mg/kg i.v.). Eyes were immediately removed and the posterior eyecups were immersion fixed in 4% PFA in PB for 15 minutes at RT. All procedures were approved by the local animal care committee and were in accordance with the law for animal experiments issued by the German government (Tierschutzgesetz).
Following fixation and washing in PB, retinas were cryoprotected in graded sucrose solutions (10, 20, 30% w/v), and stored in 30% sucrose at −20°C until use. Retinal pieces were sectioned vertically at 20 µm using a cryostat (Leica Microsystems).
Antibody characterization
All primary antibodies used in this study have been characterized in other studies of mammalian retina and the staining patterns observed here were consistent with previous reports. Polyclonal antibodies against syntaxin-4 (Millipore AB5330, immunogen corresponding to amino acids 2–23 of rat or mouse syntaxin-4) were raised in rabbit and used at a dilution of 1:500. Specificity of this antibody has been shown by Western blots of mouse retina and brain, where it recognized a single band at the appropriate molecular weight [4]. Immunostaining with syntaxin-4 shows the same staining pattern in the outer plexiform layer of guinea pig [5] mouse, rat, rabbit [3, 4] macaque, and ground squirrel ([1, 2] and this study). Preadsorption of the antibody with the corresponding control peptide blocked all staining in rabbit retina [3].
The monoclonal mouse NKCC (T4) antibodies used in this study were purchased through Developmental Studies Hybridoma Bank and are directed against both NKCC isoforms 1 and 2 (immunogen corresponding to the 38 kDa C-terminal fragment of human NKCC1 cotransporter [39] and have been shown to produce specific staining of a band above 140 kDa in Western blots of mouse, rat, rabbit, and ferret retinas and brain [7, 40]. NKCC staining has been shown before in macaque retina [1, 2, 7] and the overall staining pattern presented in our study (dilution 1:100) closely resembles immunohistochemical data from those earlier studies. However, immunostaining in mouse retina and control experiments in NKCC1-deficient mice yielded controversial results. [41] reported the immunostaining of NKCC-T4 in mouse retinas to be unspecific, whereas [42] have shown that all staining is abolished in NKCC1-deficient mice. Chloride accumulation – as the result of NKCC being present in the cell membrane – has been unequivocally localized to the dendrites of ON bipolar cells [6]. In addition, the effects were abolished by the NKCC-specific inhibitor bumetanide [6, 38]. Considering these unambiguous results at a functional level and the fact that the NKCC-T4 antibodies have been raised against primate NKCC, we conclude that despite the above mentioned discrepancy in mouse, the anatomical data obtained in macaque [1, 2, 7] and human (this study) are valid.
Goat polyclonal antibodies directed at the N-terminal peptide of human blue-sensitive opsin (Santa Cruz, sc-14363, dilution 1:100) used to label the corresponding cones including their pedicles in primates as well as other species [43, 44]. The specificity of the antibody was tested by the manufacturer with a Western blot analysis using mouse retinal extract, revealing a single band at the predicted size of 40 kDa. Rabbit polyclonal antibodies raised against human recombinant L/M-opsin (Millipore, AB5405, 1:100) were used for the same purpose. The antibody was used to label the corresponding cones in mouse [45] and macaque retina (manufacturer’s information).
Mouse monoclonal antibodies against the calcium-binding proteins calbindin (CaBP, 1:1000, Sigma C8666, raised against calbindin-D-28K from chicken gut) and parvalbumin (1:5000 Swant 235, raised against carp muscle parvalbumin) are well-established as horizontal cell markers in primate retina. CaBP labels HII horizontal cells (besides cone photoreceptors and two bipolar cell types), while parvalbumin labels both HI and HII horizontal cells [9, 46, 47]. The calbindin and parvalbumin antibodies which we have used here specifically stain the 45Ca-binding spot of calbindin-D (MW = 28,000, pI = 4.8) or of parvalbumin (MW 12,000 and IEF 4.9), respectively, in a two-dimensional immunoblot according to the information provided by the manufacturers.
Immunohistochemistry and confocal microscopy
Immunocytochemical labeling was performed using the indirect fluorescence method. Sections were incubated overnight at RT with primary antibodies diluted in blocking serum (5% normal donkey serum, 1% bovine serum albumin, and 0.5 or 1% Triton X-100 in PB). After washing in PB, secondary donkey antibodies conjugated to DyLight 488, 549 (Jackson ImmunoResearch) or Alexa 633 (Invitrogen) were applied for 1 hour RT at a dilution of 1:250 – 1:500 in blocking serum.
Images were taken using an Olympus FV1000 confocal microscope equipped with an argon and a HeNe laser. High-resolution scanning of image stacks was performed using an Olympus UPlanSApo 60×/1.35 oil immersion objective with at least 1024 × 1024 pixels and a z-axis increment of 0.3 µm. Brightness and contrast of the final images were adjusted using Adobe Photoshop.
3. Results
In a preceding study of macaque outer retina, syntaxin-4 has been shown to be expressed by HI and HII horizontal cells [1, 2]. It was clustered at the invaginating dendritic and axonal tips of these cells, postsynaptic to cones and rods, respectively. Beneath cone pedicles in more proximal regions of the OPL, syntaxin-4 was clustered in horizontal cells forming another dense immunoreactive layer per pedicle approximately at the level of desmosome-like junctions [48], where horizontal cells are known to express further synaptic proteins, including glutamate receptors and connexins [25, 43, 49].
Here, vertical cryostat sections of human retina were labeled with antibodies against syntaxin-4 and parvalbumin (Fig.1A–F). The calcium-binding protein parvalbumin was used as a marker for both HI and HII horizontal cells [9]. The syntaxin-4 staining pattern observed here was very similar to that in outer retinas of macaque and other mammals. Syntaxin-4 immunoreactive puncta showed complete overlap with parvalbumin, indicating that syntaxin-4 is expressed by horizontal cells. The majority of syntaxin-4 positive puncta were clustered at the dendritic horizontal cell processes beneath cone pedicles and at their axonal tips contacting rod spherules. In mouse [4] and macaque [1, 2] syntaxin-4 immunoreactivity at cone pedicles occurred as two distinct bands. The upper and lower band correspond to the level of invaginating dendritic tips of horizontal cells or the level of desmosome-like junctions between their dendrites beneath the cone pedicle, respectively (see also Fig. 2 in the current study). In addition, synatxin-4 was found in the dendritic shafts between these two layers. In human retina, this particular pattern of syntaxin-4 staining at cone pedicles was not readily observed, due to the slightly compromised tissue structure as a result of the constraints while working with human tissue (e.g. extended period of time between time of death and tissue dissection). Nevertheless, syntaxin-4 was found at the outermost region of the parvalbumin staining at putative cone pedicles and also well below that level (Fig. 1D–F).
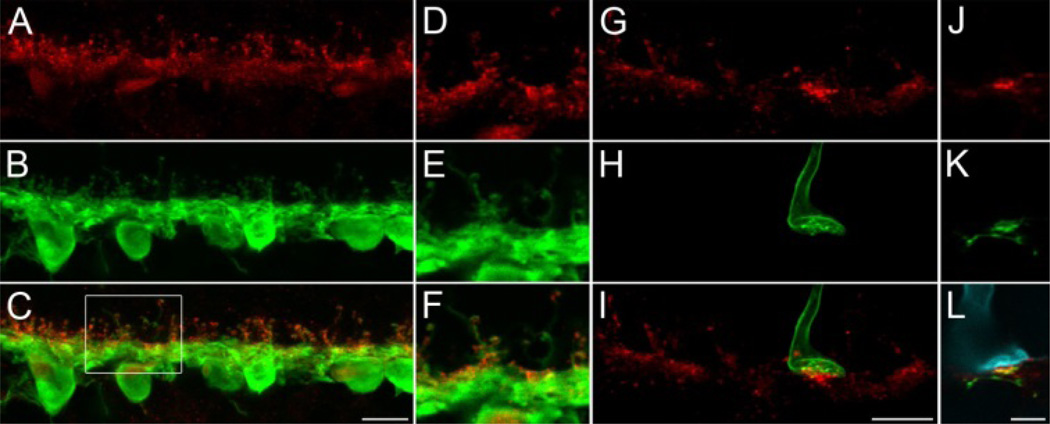
Fig. 1. Syntaxin-4 in human retina.
A–C: Maximum intensity projection of an image stack from vertical cryostat sections of the outer plexiform layer of human retina, double labeled against syntaxin-4 (red) and parvalbumin (green), a marker for HI and HII horizontal cells. D–E: Magnification of a single optical slice from the boxed region in C. Two putative cone pedicles (arrowheads) can be identified by clusters of syntaxin-4 staining. Syntaxin-4 is colocalized with horizontal cell dendrites and axon terminals. G–I: Double labeling of syntaxin-4 (red) and S-opsin (green). A projection of an image stack is shown which covers the full depth in z-direction of all four cone pedicles in the image (3 putative L-/M-cones indicated by arrowheads in G, 1 S-cone pedicle indicated by S-opsin labeling in H, I). Syntaxin-4 immunoreactive puncta appear brighter and denser at S-cones than at the remaining L-/M-cones. J–L: Single optical slice of a triple labeling of syntaxin-4 (red), calbindin (CaBP, a marker for HII horizontal cells, green), and S-opsin (cyan). Syntaxin-4 is colocalized with CaBP at S-cones. Scale bars: 10 µm in C, I; 5 µm in L.
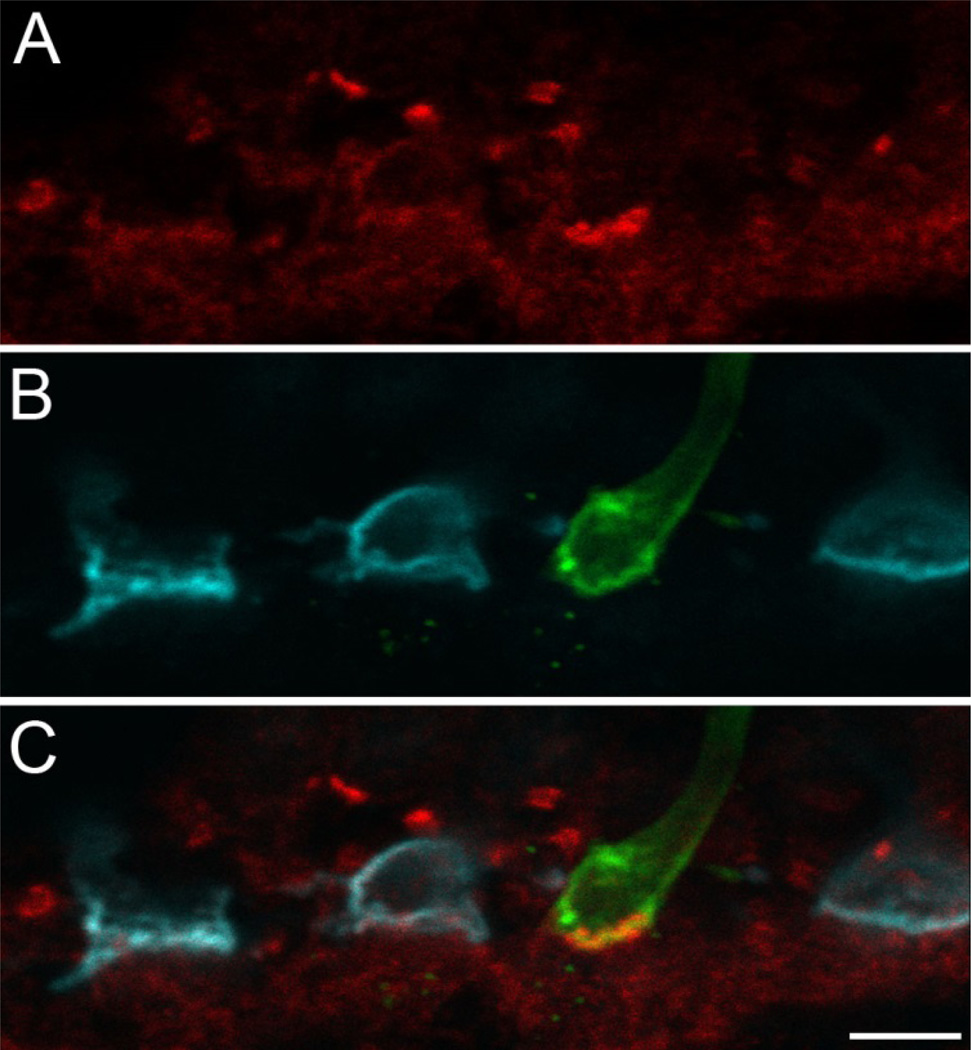
Fig. 2. NKCC in human retina.
Single optical slice of a triple labeling of syntaxin-4 (red), S-opsin (green), and M/L-opsin (cyan). Labeling of syntaxin-4 at an S-cone surrounded by three M/L-cones is shown. Bright, punctate staining can be observed at the S-cone pedicle (and at putative rod spherules in the distal OPL) while staining at M/L-cones appears weaker and more diffuse. Scale bar: 5 µm.
Further indicators of the bi-layered appearance of syntaxin-4 occurred in experiments where S-opsin was used as a marker for the corresponding cone pedicles (Fig. 1G–L). There, the syntaxin-4 labeled area showed a partial overlap with the pedicle base according to the expression in the invaginating horizontal dendritic tips while a large portion of syntaxin-4 immunoreactive puncta was also found beneath the pedicle base, resembling the aforementioned pattern of syntaxin-4 at cone pedicles in other species. Furthermore, these double labeling experiments with syntaxin-4 and S-opsin revealed that syntaxin-4 was enriched in the region beneath S-cone pedicles in comparison with the corresponding clusters at the remaining L-/M-cones (Fig. 1G–I), in line with our previous findings in macaque monkey.
In primate, antibodies against the calcium-binding protein calbindin (CaBP) label HII horizontal cells [9]. This marker was used in a triple labeling combined with syntaxin-4 and S-opsin (Fig. 1J–L). The brightly fluorescent syntaxin-4 puncta beneath the S-cone were colocalized with CaBP, which points to the proximal dendritic regions of HII horizontal cells as the source for enhanced syntaxin-4 expression at these sites. It should be noted that S-opsin negative cone pedicles were occasionally observed in some specimen, where the syntaxin-4 labeling was more intense than at other L-/M-cones or even at S-cones (data not shown). These aberrant clusters did not form regular patterns and sometimes occurred in patches, and they were not observed in any of the other primate species. Thus, it is likely a staining artifact, elicited by the aforementioned lack of routinely pristine human tissue for the purpose of immunolabeling synaptic components. However, the overall staining pattern and quality as well as the enrichment of syntaxin-4 S-cones in human is completely consistent with data from optimally treated macaque, baboon, and marmoset retinas and can therefore be considered as valid results.
Feed-forward signaling from horizontal cells to ON bipolar cells via GABA requires NKCC as an additional postsynaptic component to cause the adequate excitatory effect of this otherwise inhibitory neurotransmitter. Thus, vertical sections of human retina were triple labeled against S-opsin, L/M-opsin, and NKCC (Fig. 2). The experiments yielded an overall staining pattern of NKCC which was similar to previously published results from the OPL of macaque retina [7], where diffuse immunoreactivity was observed at most cone pedicles and a brighter, more punctate staining occurred at rods. Consistent with the idea of enhanced GABA release beneath S-cones, and in line with our data from macaque [1, 2], we found that the NKCC labeling at S-cone pedicles was stronger than at L/M-cones.
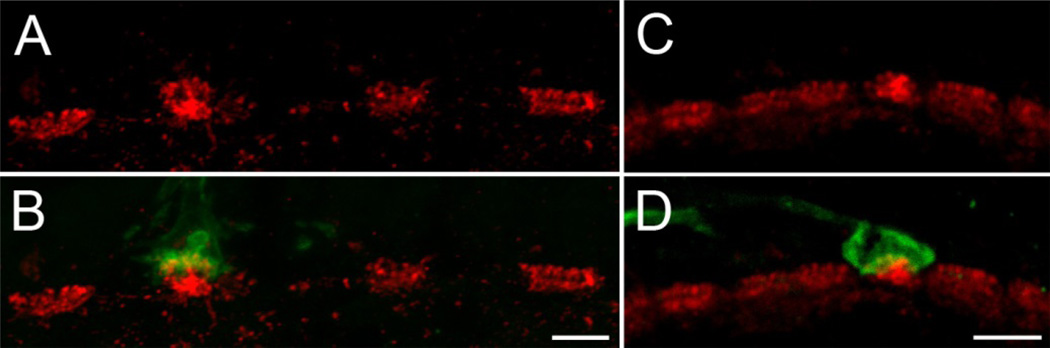
The expression pattern of syntaxin-4 was further investigated in the outer retinas of baboon and marmoset monkeys (Fig. 3), representative for Old and New World monkeys, respectively. Similar to what has been observed before, syntaxin-4 was primarily clustered in two bands at all cone pedicles and weak labeling was found at rod spherules. Again, the most prominent labeling was found beneath S-cones, where expression levels of syntaxin-4 were elevated.
Fig. 3. Syntaxin-4 in Old and New World monkeys.
Maximum intensity projections of image stacks from vertical cryostat sections of the outer plexiform layer of baboon (A, B) and marmoset (C, D) retinas are shown. Sections were double labeled against syntaxin-4 (red) and S-opsin (green). In both species syntaxin-4 immunoreactivity is enriched at S-cones. Scale bars: 5 µm
4. Discussion
The primate retina underwent several 'adjustments' through evolution in support of color vision. Some of these modifications from the classic blueprint of the mammalian retina are well known and include, for instance, the expression of a third cone opsin, the midget system, and cone-type selective wiring of the horizontal cells. These optimizations finally led to a sophisticated neuronal circuitry, specialized to create and processes cone opponent signals. The data presented here are concerned with a new component of primate-specific features in the early visual system, which is likely involved in the generation of color vision. This study provides evidence that the previously observed enrichment of syntaxin-4 beneath S-cone pedicles in macaque retina [1, 2] is a shared phenomenon between Old World monkeys as well as New World monkeys and humans. In addition, we show that NKCC expression is likewise enhanced beneath human S-cones.
Syntaxin-4 was used as an indicator for vesicular GABA release via horizontal cells [5]. Therefore, our data points to enhanced GABA release via HII horizontal cells beneath S-cones. In addition, NKCC expression was extraordinarily high at these sites in macaque [1, 2] and human retina (this study), attributable to a dense clustering of this protein in dendrites of blue cone bipolar cells. It is known that NKCC accumulates chloride in dendrites of ON cone bipolar cells, leading to an excitatory effect of the otherwise inhibitory neurotransmitter GABA [6, 37, 38].
Horizontal cells perform different tasks to directly control or modulate the responses and receptive field properties of cones and bipolar cells by both feedback and feed-forward mechanisms (reviewed in [50]. The enrichment of syntaxin-4 beneath S-cones indicates a primate-specific adjustment of the feed-forward component in the S-cone pathway: HII horizontal will release GABA when they get depolarized by the synaptic input from L-/M-cones at the offset of red or green light. The syntaxin-4 expression pattern suggests that GABA release is extraordinarily high beneath S-cones. Directly associated with this presynaptic specialization, blue cone bipolar cells appear to express high levels of NKCC [1, 2], and this study) therefore causing a strong excitatory effect of GABA. Thus, the blue cone bipolar cell is enabled to transmit not only the canonical “blue ON” signals, but also the corresponding “yellow OFF” opponent signals elicited by this newly proposed feed-forward input from HII cells. This response pattern lines up with the reported light responses in one of the output targets of blue cone bipolar cells, which is the small bistratified ganglion cell [51].
The fact that this specialization of the synaptic circuitry in the outer retina is found in New World monkeys, Old World monkeys, and humans but not in other mammals suggest that this phenomenon is unique to primates.
Spectrally opponent processing exists in other mammals (e.g. [52–56], however, among the mammals, red-green (RG) color vision is only found in primates. Moreover, the color processing associated with the ventral occipitotemporal cortex which is related to the conscious perception of hue is not present in other mammals. The midget ganglion cells are another primate innovation and ventral stream destinations are the major target of midget ganglion cell signals. Horizontal cells that are homologous to the HIIs are seen in other mammals; however, it is only in primates in which these cells combine S-cone and L/M cone signals. Above, we have noted how HII horizontal cells could provide circuitry for feedforward of L/M-OFF signals directly to blue-cone ON bipolar cells. If so, S-cone ON and OFF signals flowing in the other direction would also feedforward by the same circuitry to a subset of midget ganglion cells that have S-cones in their surround. In a dichromatic ancestor to modern primates this could have produced a midget ganglion cell based blue-yellow (BY) projection for the conscious perception of hue that is independent of the small bistratified ganglion cell blue-yellow system. Later, when primates evolved a third cone type, it would have split the midget BY system into separate BY and RG systems without the addition of new circuitry. This may explain many aspects of human conscious perception that have previously been mysterious [57].
Acknowledgments
We would like to thank Jing Huang for excellent technical assistance. We are grateful to Silke Haverkamp for providing marmoset tissue as well as to Juan Angueyra and the Tissue Distribution Program of the National Primate Research Center at the University of Washington (WaNPRC) for providing baboon tissue. WaNPRC is supported through NIH grant P51 OD010425. The T4 antibody developed by Christian Lytle was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. This study was further supported by NEI grants R01EY09303, R01EY01730, R01EY11850 P30EY001970, unrestricted funds from Research to Prevent Blindness, the Bishop Foundation and the Ray H. Hill foundation. J. Neitz is the Bishop Professor in Ophthalmology, and M. Neitz is the Ray H. Hill Professor in Ophthalmology.
References
- 1.Puller C, Haverkamp S, Neitz M, Neitz J. Synaptic elements for GABAergic feed-forward signaling between HII horizontal cells and blue cone bipolar cells are enriched beneath primate S-cones. PloS One submitted. 2013 doi: 10.1371/journal.pone.0088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puller C, Manookin MB, Neitz M, Neitz J. Syntaxin-4 Is Highly Enriched Beneath S-cone Pedicles In The Primate Retina. ARVO Meeting Abstracts. 2012;53:6323. [Google Scholar]
- 3.Hirano AA, Brandstatter JH, Vila A, Brecha NC. Robust syntaxin-4 immunoreactivity in mammalian horizontal cell processes. Vis Neurosci. 2007;24:489–502. doi: 10.1017/S0952523807070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H, Brecha NC. Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur J Neurosci. 2010;31:1388–1401. doi: 10.1111/j.1460-9568.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duebel J, Haverkamp S, Schleich W, Feng G, Augustine GJ, Kuner T, Euler T. Two-photon imaging reveals somatodendritic chloride gradient in retinal ON-type bipolar cells expressing the biosensor Clomeleon. Neuron. 2006;49:81–94. doi: 10.1016/j.neuron.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 7.Vardi N, Zhang LL, Payne JA, Sterling P. Evidence that different cation chloride cotransporters in retinal neurons allow opposite responses to GABA. J Neurosci. 2000;20:7657–7663. doi: 10.1523/JNEUROSCI.20-20-07657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peichl L. Morphology of Interneurons: Horizontal Cells. In: Dartt DA, editor. Encyclopedia of the Eye. Vol. 3. Oxford: Academic Press; 2010. [Google Scholar]
- 9.Wässle H, Dacey DM, Haun T, Haverkamp S, Grünert U, Boycott BB. The mosaic of horizontal cells in the macaque monkey retina: with a comment on biplexiform ganglion cells. Vis Neurosci. 2000;17:591–608. doi: 10.1017/s0952523800174097. [DOI] [PubMed] [Google Scholar]
- 10.Wässle H, Boycott BB, Röhrenbeck J. Horizontal Cells in the Monkey Retina: Cone connections and dendritic network. Eur J Neurosci. 1989;1:421–435. doi: 10.1111/j.1460-9568.1989.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 11.Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in primate retina: A golgi-light microscopic study of spectral connectivity. J. Comp. Neurol. 1994;343:387–405. doi: 10.1002/cne.903430305. [DOI] [PubMed] [Google Scholar]
- 12.Ahnelt P, Kolb H. Horizontal cells and cone photoreceptors in human retina: A golgi-electron microscopic study of spectral connectivity. J. Comp. Neurol. 1994;343:406–427. doi: 10.1002/cne.903430306. [DOI] [PubMed] [Google Scholar]
- 13.Dacey DM, Lee BB, Stafford DK, Pokorny J, Smith VC. Horizontal cells of the primate retina: cone specificity without spectral opponency. Science. 1996;271:656–659. doi: 10.1126/science.271.5249.656. [DOI] [PubMed] [Google Scholar]
- 14.Goodchild AK, Chan TL, Grünert U. Horizontal cell connections with short-wavelength-sensitive cones in macaque monkey retina. Vis. Neurosci. 1996;13:833–845. doi: 10.1017/s0952523800009093. [DOI] [PubMed] [Google Scholar]
- 15.Chan TL, Grünert U. Horizontal cell connections with short wavelength-sensitive cones in the retina: A comparison between New World and Old World primates. J. Comp. Neurol. 1998;393:196–209. [PubMed] [Google Scholar]
- 16.Kolb H, Mariani A, Gallego A. A second type of horizontal cell in the monkey retina. J. Comp. Neurol. 1980;189:31–44. doi: 10.1002/cne.901890103. [DOI] [PubMed] [Google Scholar]
- 17.Crook JD, Manookin MB, Packer OS, Dacey DM. Horizontal cell feedback without cone type-selective inhibition mediates "red-green" color opponency in midget ganglion cells of the primate retina. J Neurosci. 2011;31:1762–1772. doi: 10.1523/JNEUROSCI.4385-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packer OS, Verweij J, Li PH, Schnapf JL, Dacey DM. Blue-yellow opponency in primate S cone photoreceptors. J Neurosci. 2010;30:568–572. doi: 10.1523/JNEUROSCI.4738-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirasawa H, Yamada M, Kaneko A. Acidification of the synaptic cleft of cone photoreceptor terminal controls the amount of transmitter release, thereby forming the receptive field surround in the vertebrate retina. The journal of physiological sciences: JPS. 2012;62:359–375. doi: 10.1007/s12576-012-0220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klaassen LJ, Fahrenfort I, Kamermans M. Connexin hemichannel mediated ephaptic inhibition in the retina. Brain Res. 2012;1487:25–38. doi: 10.1016/j.brainres.2012.04.059. [DOI] [PubMed] [Google Scholar]
- 21.Guo C, Hirano AA, Stella SL, Jr, Bitzer M, Brecha NC. Guinea pig horizontal cells express GABA, the GABA-synthesizing enzyme GAD 65, and the GABA vesicular transporter. J Comp Neurol. 2010;518:1647–1669. doi: 10.1002/cne.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jellali A, Stussi-Garaud C, Gasnier B, Rendon A, Sahel JA, Dreyfus H, Picaud S. Cellular localization of the vesicular inhibitory amino acid transporter in the mouse and human retina. J Comp Neurol. 2002;449:76–87. doi: 10.1002/cne.10272. [DOI] [PubMed] [Google Scholar]
- 23.Kao Y, Lassova L, Bar-Yehuda T, Edwards R, Sterling P, Vardi N. Evidence that certain retinal bipolar cells use both glutamate and GABA. J Comp Neurol. 2004;478:207–218. doi: 10.1002/cne.20221. [DOI] [PubMed] [Google Scholar]
- 24.Kalloniatis M, Marc RE, Murry RF. Amino acid signatures in the primate retina. J. Neurosci. 1996;16:6807–6829. doi: 10.1523/JNEUROSCI.16-21-06807.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haverkamp S, Grünert U, Wässle H. The cone pedicle, a complex synapse in the retina. Neuron. 2000;27:85–95. doi: 10.1016/s0896-6273(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 26.Cueva JG, Haverkamp S, Reimer RJ, Edwards R, Wässle H, Brecha NC. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J Comp Neurol. 2002;445:227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirano AA, Brandstatter JH, Morgans CW, Brecha NC. SNAP25 expression in mammalian retinal horizontal cells. J Comp Neurol. 2011;519:972–988. doi: 10.1002/cne.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deniz S, Wersinger E, Schwab Y, Mura C, Erdelyi F, Szabo G, Rendon A, Sahel JA, Picaud S, Roux MJ. Mammalian retinal horizontal cells are unconventional GABAergic neurons. J Neurochem. 2011;116:350–362. doi: 10.1111/j.1471-4159.2010.07114.x. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann R, Heflin SJ, Hammond T, Lee B, Wang J, Gainetdinov RR, Caron MG, Eggers ED, Frishman LJ, McCall MA, Arshavsky VY. Rod vision is controlled by dopamine-dependent sensitization of rod bipolar cells by GABA. Neuron. 2011;72:101–110. doi: 10.1016/j.neuron.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wässle H, Chun MH. GABA-like immunoreactivity in the cat retina: Light microscopy. J. Comp. Neurol. 1989;279:43–54. doi: 10.1002/cne.902790105. [DOI] [PubMed] [Google Scholar]
- 31.Agardh E, Ehinger B, Wu J-Y. GABA and GAD-like immunoreactivity in the primate retina. Histochemistry. 1987;86:485–490. doi: 10.1007/BF00500621. [DOI] [PubMed] [Google Scholar]
- 32.Grünert U, Wässle H. GABA-like immunoreactivity in the macaque monkey retina: A light and electron microscopic study. J. Comp. Neurol. 1990;297:509–524. doi: 10.1002/cne.902970405. [DOI] [PubMed] [Google Scholar]
- 33.Dowling JE, Brown JE, Major D. Synapses of horizontal cells in rabbit and cat retinas. Science. 1966;153:1639–1641. doi: 10.1126/science.153.3744.1639. [DOI] [PubMed] [Google Scholar]
- 34.Kolb H. The organization of the outer plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1977;6:131–153. doi: 10.1007/BF01261502. [DOI] [PubMed] [Google Scholar]
- 35.Fisher SK, Boycott BB. Synaptic connections made by horizontal cells within the outer plexiform layer of the retina of the cat and the rabbit. Proc R Soc Lond B Biol Sci. 1974;186:317–331. doi: 10.1098/rspb.1974.0052. [DOI] [PubMed] [Google Scholar]
- 36.Vardi N, Sterling P. Subcellular localization of GABAA receptor on bipolar cells in macaque and human retina. Vision Res. 1994;34:1235–1246. doi: 10.1016/0042-6989(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 37.Varela C, Blanco R, De la Villa P. Depolarizing effect of GABA in rod bipolar cells of the mouse retina. Vision Res. 2005;45:2659–2667. doi: 10.1016/j.visres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 38.Chaffiol AJ, Cao Y, Ishii M, Ribelayga C, Mangel SC. Light/Dark Adaptive Regulation of GABAA Receptor and NKCC Expression and Activity Modulates Direct, GABA-mediated Horizontal Cell Signaling to ON-Cone Bipolar Cells. Invest. Ophthalmol. Vis. Sci. 2012;53:4306. [Google Scholar]
- 39.Lytle C, Xu JC, Biemesderfer D, Forbush B., 3rd Distribution and diversity of Na-K-Cl cotransport proteins: a study with monoclonal antibodies. Am J Physiol. 1995;269:C1496–C1505. doi: 10.1152/ajpcell.1995.269.6.C1496. [DOI] [PubMed] [Google Scholar]
- 40.Zhang LL, Fina ME, Vardi N. Regulation of KCC2 and NKCC during development: membrane insertion and differences between cell types. J Comp Neurol. 2006;499:132–143. doi: 10.1002/cne.21100. [DOI] [PubMed] [Google Scholar]
- 41.Zhang LL, Delpire E, Vardi N. NKCC1 does not accumulate chloride in developing retinal neurons. J Neurophysiol. 2007;98:266–277. doi: 10.1152/jn.00288.2007. [DOI] [PubMed] [Google Scholar]
- 42.Li B, McKernan K, Shen W. Spatial and temporal distribution patterns of Na-K-2Cl cotransporter in adult and developing mouse retinas. Vis Neurosci. 2008;25:109–123. doi: 10.1017/S0952523808080164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puller C, Haverkamp S. Cell-type-specific localization of protocadherin beta16 at AMPA and AMPA/Kainate receptor-containing synapses in the primate retina. J Comp Neurol. 2011;519:467–479. doi: 10.1002/cne.22528. [DOI] [PubMed] [Google Scholar]
- 44.Puller C, Ondreka K, Haverkamp S. Bipolar cells of the ground squirrel retina. J Comp Neurol. 2011;519:759–774. doi: 10.1002/cne.22546. [DOI] [PubMed] [Google Scholar]
- 45.Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH, Bech-Hansen NT, Reese BE. Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol. 2008;506:745–758. doi: 10.1002/cne.21526. [DOI] [PubMed] [Google Scholar]
- 46.Röhrenbeck J, Wässle H, Boycott BB. Horizontal cells in the monkey retina: immunocytochemical staining with antibodies against calcium binding proteins. Eur. J. Neurosci. 1989;1:407–420. doi: 10.1111/j.1460-9568.1989.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 47.Chiquet C, Dkhissi-Benyahya O, Cooper H. Calcium-binding protein distribution in the retina of strepsirhine and haplorhine primates. Brain Res Bull. 2005;68:185–194. doi: 10.1016/j.brainresbull.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Missotten L. The Ultrastructure of the Human Retina. Brussels: Editions Arscia S.A.; 1965. [Google Scholar]
- 49.Puller C, de Sevilla Müller LP, Janssen-Bienhold U, Haverkamp S. ZO-1 and the spatial organization of gap junctions and glutamate receptors in the outer plexiform layer of the mammalian retina. J Neurosci. 2009;29:6266–6275. doi: 10.1523/JNEUROSCI.5867-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thoreson WB, Mangel SC. Lateral interactions in the outer retina. Prog Retin Eye Res. 2012;31:407–441. doi: 10.1016/j.preteyeres.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dacey DM, Lee BB. The 'blue-on' opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature. 1994;367:731–735. doi: 10.1038/367731a0. [DOI] [PubMed] [Google Scholar]
- 52.Chen S, Li W. A color-coding amacrine cell may provide a blue-off signal in a mammalian retina. Nat Neurosci. 2012;15:954–956. doi: 10.1038/nn.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang L, Breuninger T, Euler T. Chromatic Coding from Cone-type Unselective Circuits in the Mouse Retina. Neuron. 2013;77:559–571. doi: 10.1016/j.neuron.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 54.Sher A, DeVries SH. A non-canonical pathway for mammalian blue-green color vision. Nat Neurosci. 2012;15:952–953. doi: 10.1038/nn.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekesten B, Gouras P. Cone inputs to murine striate cortex. BMC Neurosci. 2008;9:113. doi: 10.1186/1471-2202-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ekesten B, Gouras P. Cone and rod inputs to murine retinal ganglion cells: evidence of cone opsin specific channels. Vis Neurosci. 2005;22:893–903. doi: 10.1017/S0952523805226172. [DOI] [PubMed] [Google Scholar]
- 57.Schmidt BP, Neitz M, Neitz J. The neurobiological explanation for color appearance and hue perception. 2013 doi: 10.1364/JOSAA.31.00A195. submitted – this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]