Abstract
Aims
Clinical application of pudendal nerve (PN) afferent stimulation to restore bladder emptying in persons with neurological disorders requires increased stimulation-evoked voiding efficiencies (VEs). We tested the hypothesis that selective co-stimulation of multiple PN branches, either bilateral dorsal nerve of the penis (DNP) stimulation or selective stimulation of both the cranial sensory nerve (CSN) and DNP, will evoke larger reflex bladder contractions and result in higher VEs than stimulation of any single afferent pathway alone.
Methods
We measured the strength of bladder contractions, threshold volumes, and VEs produced by unilateral and bilateral stimulation of the DNP as well as singular and selective unilateral co-stimulation of the DNP and CSN in cats anesthetized with α-chloralose.
Results
Co-stimulation of afferent pathways generated significantly larger isovolumetric bladder contractions and evoked contractions at lower threshold volumes than individual stimulation. Co-stimulation of pudendal afferents also suppressed dyssynergic activity in the external anal sphincter produced by low frequency individual stimulation. VE was significantly improved with co-stimulation (172±6% of distention evoked volumes) over individual stimulation (141±6%).
Conclusions
Both types of co-stimulation evoked larger bladder contractions and increased VE over individual branch PN afferent stimulation and distention-evoked voiding. The decreased threshold volumes required for reflex bladder activation and increased VEs with co-stimulation support the use of stimulation of multiple individual stimulation-evoked reflexes to improve voiding efficiency.
Introduction
Spinal cord injury (SCI) causes lower urinary tract (LUT) dysfunction, including incontinence, retention, and detrusor-sphincter dyssynergia (DSD) (1), which leads to decreased quality of life (2). Intermittent catheterization and anticholinergic medications are used to treat LUT dysfunction but are associated with frequent urinary tract infections, urinary tract damage and side-effects (3). Voiding can be initiated by bladder tapping, Valsalva maneuvers, and distention (reflex), but the prevalence of DSD and associated poor voiding limit the utility of these approaches (3). Restoring LUT function in persons with neurological disease or injury, including SCI, remains a significant unmet need. Electrical stimulation is an alternative approach to restore LUT function, but existing approaches are not widely employed (4). Electrical stimulation of pudendal nerve (PN) afferents to cause reflex activation or inhibition of the bladder is an alternative approach to restore control of bladder function.
Electrical stimulation of the PN is capable of producing both excitation of the bladder and voiding, and inhibition of the bladder and continence (5, 6). Stimulation frequency directly influences the bladder response: low frequencies, 2-15 Hz, produce inhibition and continence while higher frequencies, 20-50 Hz, evoke excitation and sustained contractions (5, 7). Importantly, comparable inhibitory and excitatory reflexes have been demonstrated in persons with SCI (8-10), although the frequency tuning characteristics of these reflexes have not been defined (11).
More recent work differentiated the effects of stimulation of individual distal PN branches on bladder function. Selective electrical stimulation of the cranial sensory nerve (CSN) and dorsal genital nerve (DGN) branches each evoked sustained bladder contractions and generated increases in voiding efficiency (VE) compared to distention-evoked voiding. Similarly, in humans with SCI, two excitatory pudendo-vesical reflexes were evoked by selective stimulation of the proximal and distal urethra (12), presumably corresponding to the CSN and DGN.
However, VEs produced by PN stimulation in cats were limited to ~ 60% (13), likely as a result of the bladder volume dependence of the evoked reflexes. Bladder activation by pudendal afferent stimulation requires volumes of 70-80% of the threshold volume to elicit distension-evoked reflex contractions (7). This volume dependence does not result from length-tension properties of the detrusor (14), but rather from a neural mechanism mediated by superposition of pelvic and pudendal afferent activity (15). Presumably, as the bladder empties during pudendal afferent stimulation-evoked contractions, pelvic afferent activity is reduced (16), the superposed afferent activity declines, and the evoked contraction subsides, thereby limiting VE.
The purpose of this study was to test the hypothesis that stimulation of multiple PN afferents, either bilateral DGN stimulation or selective co-stimulation of both CSN and DGN, will evoke larger reflex bladder contractions and result in higher VEs than stimulation of any single afferent pathway alone. Producing efficient voiding with sustained bladder contractions, decreased outlet resistance, and lower residual volumes is imperative for eventual clinical application, as large residuals are linked to risk of bacteriuria (17).
Materials and Methods
Animal care and experimental procedures were approved by the Duke University Institutional Animal Care and Use Committee. Sexually intact, adult male cats (3.5-4.5 kg) were anesthetized with ketamine-HCl (35 mg·kg−1 im) and anesthesia was maintained with α-chloralose (65 mg·kg−1 iv, supplemented at 15 mg·kg−1). End-tidal CO2 was maintained between 3-4% with artificial respiration. Blood pressure was monitored with a catheter placed in the carotid artery and a solid-state pressure transducer. Body temperature was maintained at 38°C using a heating pad and intravenous fluids, 0.9% NaCl with 5% dextrose and 8.4 mg·L−1 NaHCO3, were administered (15 ml·kg−1·h−1).
A midline abdominal incision was made to expose the bladder, and a 3.5 Fr suprapubic catheter was inserted into the dome and secured with a purse string suture. Bladder pressure was measured with a solid-state pressure transducer (Deltran, Utah Medical) in series with the catheter and recorded. The urethra was occluded with a 3.5-5 Fr catheter during isovolumetric experiments and filling of the bladder. The threshold volume at which distension-evoked reflex contractions (DECs) occurred was found by filling the bladder with saline at 1-2 ml/min. External anal sphincter (EAS) electromyographic activity was recorded from two wire electrodes, amplified (1,000), filtered (10 Hz - 10 kHz), and recorded.
The PN was exposed through an incision between the base of the tail and the ischial tuberosity, transection of the gluteofemoralis, and dissection of the ischiorectal fossa. The sensory (SN) and rectal perineal (RP) branches of the PN were visible and further dissection of the distal portion of the SN branch revealed the CSN and dorsal nerve of the penis (DNP) branches (13).
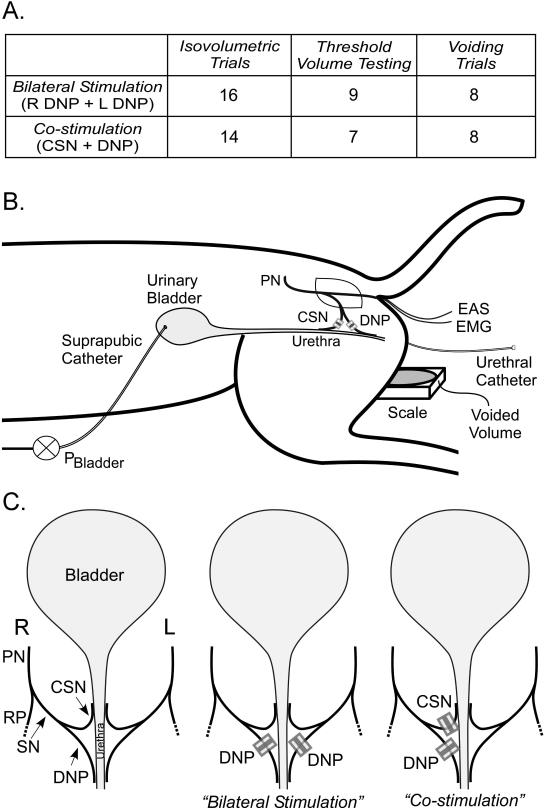
In co-stimulation experiments, the CSN and DNP were stimulated by unilateral implantation of cuff electrodes around each branch, and in bilateral stimulation experiments, cuff electrodes were placed around both right and left DNP branches (Figure 1). Monopolar electrodes were composed of platinum contacts embedded within silicone cuffs, and two asynchronous pulse generators (Pulsar 6bp, FHC Inc.) were used to deliver stimulation (0.1 ms cathodic stimulus pulses, for 30 s) between each cuff and subcutaneous 20-gauge needles. The optimal frequencies identified in previous studies (13) were used for unilateral stimulation of CSN (2 Hz) and DNP (33 Hz) or bilateral stimulation of DNP (33 Hz). Stimulation amplitude was 3 times the threshold (T) to produce a reflex EMG response in the EAS with 1 Hz stimulation.
Figure 1.
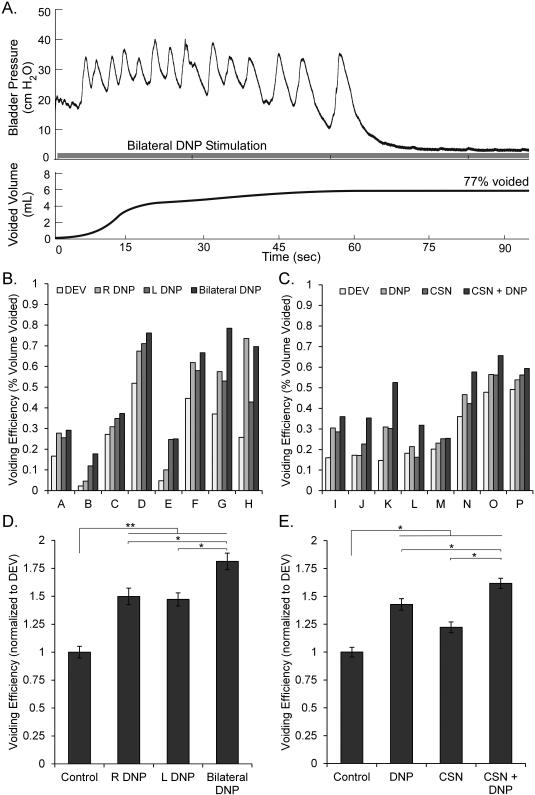
The effects of co-stimulation on isovolumetric bladder contractions, threshold volume, and VE were quantified (Figure 1A). Isovolumetric bladder contraction trials were conducted across 30 cats at approximately 80% of the threshold volume for DECs. Threshold volume experiments were conducted in 16 cats in which pudendal afferent stimulation generated reflex bladder contractions. The threshold volume to evoke bladder contractions with each stimulation type was determined by filling the bladder at 0.333 ml/min and delivering stimulation in randomized order every 30 seconds. Contractions were defined as increases in pressure greater than 25 cmH2O or 10 cmH2O above baseline. Voiding experiments were conducted in 16 cats in which pudendal afferent stimulation generated reflex bladder contractions. For each stimulation condition, the bladder was filled at 1-2 ml/min to 125% of the threshold volume for DECs. DNP stimulation at 10 Hz was used to inhibit bladder contractions and prevent voiding during the fill, if necessary (7). After reaching 125% of the threshold volume, the urethral catheter was removed and continuous stimulation was delivered. Measurements of volume voided and residual volume were used to determine VE:
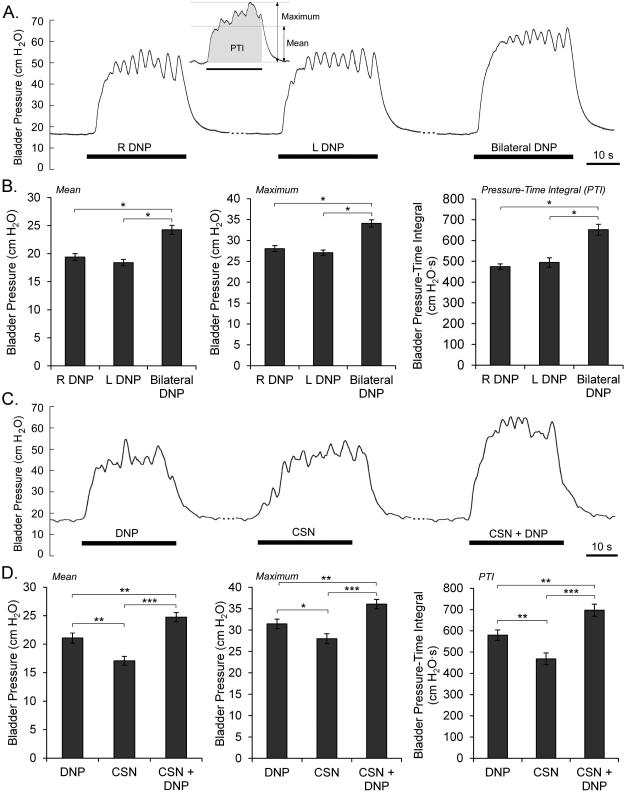
Bladder pressures during isovolumetric trials were quantified by mean and maximum pressure during stimulation minus the average of the baseline pressure during the 3 s before stimulation, as well as the pressure-time integral with respect to baseline (Figure 2A). The mean, maximum, and pressure-time integral were averaged across repeated trials within experiments and compared in an ANOVA with post hoc comparisons using Fisher’s Protected Least Significant Difference (PLSD) test. EAS recordings were stimulus-triggered averaged, rectified and integrated over the 20 ms following the stimulus artifact, and compared in an ANOVA with post hoc PLSD. Volume thresholds to evoke contractions with stimulation were normalized to the threshold volumes for DECs in each cat and analyzed by repeated measures ANOVA and post hoc PLSD. Stimulation-evoked VEs were normalized to distention evoked VEs for each experiment and analyzed with repeated measures ANOVA and post hoc PLSD.
Figure 2.
Results
The effects of bilateral DNP stimulation or selective unilateral co-stimulation of CSN and DNP on isovolumetric bladder contractions, threshold volumes, and VE were measured in anesthetized cats and compared to single nerve branch stimulation and distention-evoked reflexes.
Stimulation-Evoked Bladder Contractions
Robust bladder contractions were evoked in all cats by stimulation of the DNP (T= 0.43±0.04 mA, mean ± s.e.m.) or CSN (T= 0.44±0.05 mA) under isovolumetric conditions. Bilateral DNP stimulation evoked larger mean bladder pressures, maximum bladder pressures, and pressure-time integrals than either right or left unilateral stimulation (Figure 2A-B). There was no consistent laterality across animals, although laterality within animals was seen. In 12 cats where there were substantial differences between bladder contractions evoked by right or left unilateral stimulation, stimulation of the dominant side produced significantly larger mean bladder contractions (20±1.5 cmH2O) than the non-dominant side (14.9±1.4 cmH2O) (p=0.0229). Co-stimulation of CSN and DNP evoked larger mean pressures, maximum pressures and pressure-time integrals than individual stimulation of either CSN or DNP alone (Figure 2C-D).
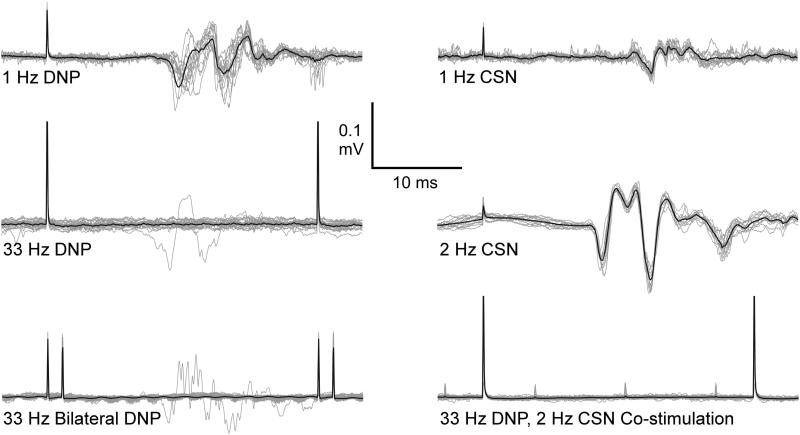
EAS Activation Varies with Stimulation Frequency and Spatial Pattern
Reflex activation of the EAS varied with stimulation frequency and pattern of co-stimulation (Figure 3). EAS responses (latency: 10-15 ms) were elicited in all cats with stimulation at 1 Hz of either DNP or CSN. Unilateral 33 Hz DNP stimulation evoked EAS responses that rapidly diminished after the first 1-2 pulses of the stimulation train (5, 7); while unilateral 2 Hz CSN stimulation evoked a consistent reflex response following each pulse. Similar to unilateral 33 Hz DNP stimulation, bilateral DNP stimulation and CSN and DNP co-stimulation evoked a reflex that diminished after the first 1-2 pulses. Rectified and integrated EAS responses during 2 Hz CSN stimulation were significantly larger than either 33 Hz DNP stimulation alone (19.5 times larger) or CSN and DNP co-stimulation (15.7 times larger) (p < 0.0006, n=10 CSN and DNP co-stimulation experiments).
Figure 3.
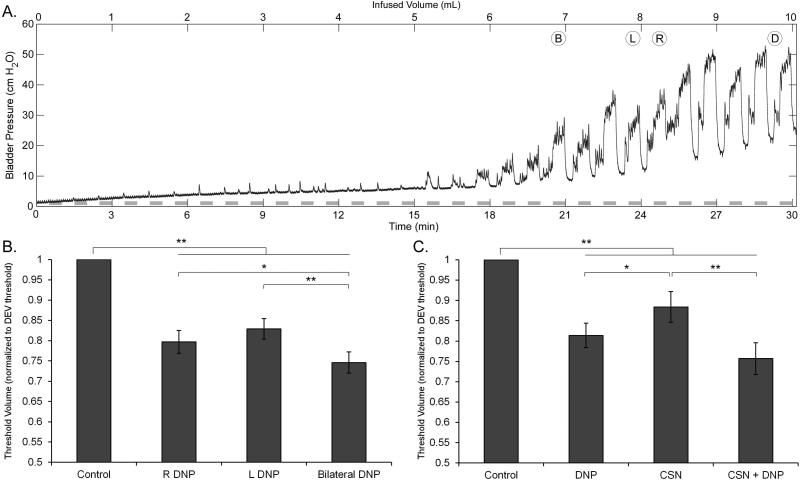
Effects of Stimulation on Threshold Volumes
Repeated randomized stimulation during slow bladder filling was used to determine volume thresholds to evoke sustained bladder contractions with each stimulation condition and for DECs (Figure 4A). Both unilateral and bilateral DNP stimulation evoked contractions at smaller bladder volumes than DECs, and bilateral DNP stimulation evoked contractions at smaller volumes than either right or left unilateral DNP stimulation alone (Figure 4B). Individual or selective co-stimulation of CSN and DNP evoked contractions at lower volumes than DECs, and co-stimulation evoked contractions at smaller bladder volumes than stimulation of either CSN or DNP alone, although the latter difference was not significant (Figure 4C). In two of seven cats, the threshold volume for selective co-stimulation of CSN and DNP was the same as the threshold volume for DNP stimulation alone, while it was lower in the remaining five.
Figure 4.
Effects of Stimulation on Voiding Efficiency
An example of bladder emptying with bilateral DNP stimulation is shown in Figure 5A. Voided volumes and VEs for both stimulation-evoked voiding and distention-evoked voiding varied across cats (Figure 5B), and VEs with stimulation were normalized by distension-evoked VEs to combine data across experiments. VE was significantly higher than distension-evoked voiding with either unilateral or bilateral DNP stimulation (159±7%, mean ± s.e.m.). Further, bilateral DNP stimulation increased VE over either right or left unilateral DNP stimulation alone in seven of eight cats, and produced significantly higher VEs (Figure 5C). Both individual and co-stimulation of CSN and DNP increased VE by 142±5% over distension-evoked voiding. Selective co-stimulation produced significantly larger VEs than individual stimulation of CSN or DNP alone (Figure 5E).
Figure 5.
Discussion
Electrical stimulation of PN afferents is a promising approach to restore control of bladder function. Although pudendal afferent stimulation generates robust reflex bladder contractions, residual bladder volumes following PN stimulation evoked voiding are too large for successful clinical translation. The results of this study indicate that selective co-stimulation of multiple PN afferents evokes larger bladder contractions, generates bladder contractions at smaller bladder volumes, and increases VE compared to stimulation of any single afferent pathway alone. Although statistically significant, the effect size of co-stimulation on voiding efficiency was limited from a functional perspective. The results may have been affected by the use of a spinal intact, anesthetized animal model, and additional experiments should be performed in persons with impaired voiding to determine the clinical promise of this approach.
Evoked Isovolumetric Bladder Contractions
Bilateral DNP stimulation and co-stimulation of CSN and DNP produced larger isovolumetric mean pressures, maximum pressures, and pressure-time integrals than isolated stimulation of individual PN branches. Co-stimulation did not result in superposition of responses from individual stimulation, suggesting a ceiling effect on bladder activation where co-stimulation cannot increase bladder contraction size beyond some maximum. Stimulation type during isovolumetric blocks was randomized, and the interaction between stimulation type and order was not significant for either bilateral or co-stimulation experiments. Thus, the larger bladder contractions evoked by co-stimulation were not dependent on the previous type of stimulation and did not result in carry-over effects to the next type of stimulation. All co-stimulation was interleaved in time, preventing simultaneous electrical stimulation of different branches. Therefore, the larger bladder contractions produced by co-stimulation are the consequence of a neural process resulting from PN afferent signals, facilitated by converging spinal cord inputs (18), rather than an increase in effective stimulation amplitude.
Suppression of Dyssynergic Activity
Neither 33 Hz bilateral DNP stimulation nor co-stimulation of CSN at 2 Hz and DNP at 33 Hz produced reflex activation of the EAS. Pudendal afferent stimulation has been shown to suppress activity in the skeletal muscles of the pelvic floor, and was also seen with co-stimulation. Electroneurogram (ENG) activity from the deep perineal branch of the pudendal nerve (innervates the EUS) was suppressed (19, 5) and intraurethral EMG recordings were similarly suppressed (7) during bladder contractions evoked by pudendal afferent stimulation in intact and spinal transected cats. Further, EMG from perineal muscles in persons with chronic SCI was suppressed during bladder contractions evoked by pudendal afferent stimulation (12).
We used the EAS electromyogram as a proxy for PN efferent reflex activity produced by stimulation, as previous studies showed strong congruence between EAS and EUS electromyograms in response to pudendal afferent stimulation (7, 13). However, differential activity in the EUS and EAS is well documented, and EAS activity may not reflect EUS activity or outlet pressure. The sphincters are capable of operating independently (20) and changes in activity seen in the EUS may not be seen in the EAS (21).
While 2 Hz CSN produced dyssynergic activation of the EAS, stimulation-evoked EAS activation was suppressed with co-stimulation of CSN and DNP, and PN afferent stimulation can be used reflexively to activate the bladder without activating PN efferents. This is an improvement over previous methods, such as sacral anterior root stimulation, which produce concomitant activation of bladder and sphincter (22). It remains to be determined whether DSD during bladder activation following chronic SCI is also suppressed by DGN stimulation.
Lower Threshold Volumes
Electrical stimulation of PN afferents evokes bladder contractions at approximately 80% of the threshold volume for DECs. The threshold volume dependence of the reflex is mediated by the convergence of pudendal and pelvic afferents in the sacral spinal cord (14, 15). Voiding efficiency produced by PN afferent stimulation appears to be limited by the volume threshold because as voiding occurs and the volume drops below the threshold volume, contractions cease. All types of pudendal afferent stimulation, including bilateral and co-stimulation, evoked bladder contractions at lower volumes than distension (23). The reduced volume threshold for stimulation-evoked contractions provides a potential mechanism for the increased VE with stimulation.
The threshold volume with bilateral DNP stimulation was lower than with unilateral DNP stimulation, presumably as a result of reinforced pudendal afferent signaling to the sacral spinal cord. CSN and DNP co-stimulation reduced the threshold volume to evoke robust bladder contractions compared to CSN stimulation alone, apparently due to DNP stimulation, as DNP stimulation evoked contractions at a lower volume than CSN stimulation. Therefore, any improvement in VE produced by co-stimulation over DNP stimulation alone is not solely attributable to a change in threshold volume. Rather, stimulation of CSN and DNP activates two separate reflex pathways (13, 23), which may explain why co-stimulation did not reduce threshold volume compared to DNP stimulation alone, but did improve VE. In contrast, bilateral DNP stimulation likely reinforced the pudendal afferent input in one of the reflexes in the spinal cord, producing lower threshold volumes and increased VE.
Enhanced Voiding Efficiency
Stimulation of either DNP or CSN increased VEs over distension-evoked voiding (13), and selective co-stimulation of either bilateral DNP or CSN and DNP further increased VE. Voiding efficiencies may have been adversely affected by anesthesia with α-chloralose, which is known to affect spinal reflexes and bladder activity (24), and the use of another anesthetic may lead to different results. Pelvic and pudendal afferents converge in the dorsal horn (18) and modulate the sacral spinal network that controls the pelvic efferent output of the sacral parasympathetic nucleus (25). Specifically, pudendal afferent fibers terminate in portions of the ipsilateral dorsal horn, intermediate zone, the dorsal gray commissure and contralateral dorsal horn (18). Bilateral stimulation may result in superposition of converging right and left pudendal afferent activity to produce reflex bladder contractions with lower levels of pelvic afferent signaling and thereby produce larger VEs than unilateral stimulation.
In contrast, the improved VE generated by co-stimulation of CSN and DNP over individual CSN or DNP stimulation is likely due to the activation of two separate reflex pathways (13, 23). The limited reduction in threshold volume with co-stimulation compared to DNP stimulation alone supports this mechanism.
Although not directly investigated, we can compare the VEs produced by bilateral stimulation and co-stimulation. Bilateral DNP stimulation improved VE more than co-stimulation of CSN and DNP. Additionally, in one cat where bilateral stimulation and co-stimulation evoked voiding was measured, bilateral DNP stimulation generated larger voided volumes than co-stimulation of DNP and CSN. This finding is supported by the significant decrease in threshold volume seen with bilateral stimulation, whereas co-stimulation did not reduce volume threshold compared to DNP stimulation.
Clinical Relevance
In a clinical setting, with a variety of causes of bladder dysfunction, ranging from neurological disease to traumatic injury, co-stimulation of multiple nerve branches may be more effective at controlling LUT symptoms, especially in the case of inadequate benefit from individual stimulation. In cases of complete SCI, spinal circuitry and reflexes below the injury are likely still intact. Studies evaluating stimulation of pudendal sensory pathways suggest that PN afferent stimulation is effective after transection of the spinal cord (6, 15), although efficient voiding may be limited due to DSD, and the impact of co-stimulation in persons with SCI remains to be determined in clinical studies. In cases such as these, bilateral DNP stimulation is more likely to be successful for evoking on-demand bladder contractions and increasing VE. Both branches of the dorsal genital nerve (DGN) travel close together near the distal urethra (26), and bilateral DNP stimulation could be implemented with a single, midline electrode. Percutaneous stimulation of the DGN produced a 50% or greater reduction in overactive bladder symptoms with DGN stimulation in 81% of subjects (27). Additionally, two independent pudendal-bladder reflex pathways are present in persons with SCI (12) and co-stimulation of these pathways may improve bladder activation and voiding. However, this will require surgical access (28) and implantation of a cuff electrode (29).
Conclusion
We quantified the effects of selective co-stimulation of multiple pudendal afferent pathways on bladder contractions and voiding in α-chloralose anesthetized cats. Both bilateral DNP stimulation and co-stimulation of CSN and DNP evoked larger bladder contractions and increased VE over single branch PN afferent stimulation and distention evoked voiding. Future development of neural prosthetics for restoration of bladder function should include these techniques of spatial patterning of pudendal afferent stimulation, particularly bilateral DNP stimulation which produced large on-demand bladder contractions coupled with suppression of pelvic floor reflex activity, to improve bladder control in persons with bladder dysfunction from neurological disease or injury.
Acknowledgements
The authors thank Gilda Mills for her technical assistance. This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS050514.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: Report from the standardisation sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21(2):167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- 3.Benevento BT, Sipski ML. Neurogenic bladder, neurogenic bowel, and sexual dysfunction in people with spinal cord injury. Phys Ther. 2002;82:601–612. [PubMed] [Google Scholar]
- 4.Gaunt RA, Prochazka A. Control of urinary bladder function with devices: successes and failures. In: Lynne CW, Canio P, editors. Prog Brain Res. Elsevier; 2006. pp. 163–94. [DOI] [PubMed] [Google Scholar]
- 5.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Frequency-dependent selection of reflexes by pudendal afferents in the cat. J Physiol. 2006;577(1):115–26. doi: 10.1113/jphysiol.2006.111815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tai C, Wang J, Wang X, de Groat WC, Roppolo JR. Bladder inhibition or voiding induced by pudendal nerve stimulation in chronic spinal cord injured cats. Neurourol Urodyn. 2007;26(4):570–7. doi: 10.1002/nau.20374. [DOI] [PubMed] [Google Scholar]
- 7.Woock J, Yoo P, Grill W. Activation and inhibition of the micturition reflex by penile afferents in the cat. Am J Physiol Regul Integr Comp Physiol. 2008;294(6):R1880–9. doi: 10.1152/ajpregu.00029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustafson KJ, Creasey GH, Grill WM. 2004;360(1-2):9–12. doi: 10.1016/j.neulet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Kirkham AP, Shah NC, Knight SL, Shah PJ, Craggs MD. The acute effects of continuous and conditional neuromodulation on the bladder in spinal cord injury. Spinal Cord. 2001;39(8):420–8. doi: 10.1038/sj.sc.3101177. [DOI] [PubMed] [Google Scholar]
- 10.Yoo P, Klein S, Grafstein N, Horvath E, Amundsen C, Webster G, et al. Pudendal nerve stimulation evokes reflex bladder contractions in persons with chronic spinal cord injury. Neurourol Urodyn. 2007;26(7):1020–3. doi: 10.1002/nau.20441. [DOI] [PubMed] [Google Scholar]
- 11.Possover M, Schurch B, Henleet KP. New strategies of pelvic nerves stimulation for recovery of pelvic visceral functions and locomotion in paraplegics. Neurourol Urodyn. 2010;29(8):499–503. doi: 10.1002/nau.20897. [DOI] [PubMed] [Google Scholar]
- 12.Yoo PB, Horvath EE, Amundsen CL, Webster GD, Grill WM. Multiple Pudendal Sensory Pathways Reflexly Modulate Bladder and Urethral Activity in Patients With Spinal Cord Injury. J Urol. 2011;185(2):737–43. doi: 10.1016/j.juro.2010.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoo P, Woock J, Grill W. Bladder activation by selective stimulation of pudendal nerve afferents in the cat. Exp Neurol. 2008;212(1):218–25. doi: 10.1016/j.expneurol.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boggs JW, Wenzel BJ, Gustafson KJ, Grill WM. Spinal Micturition Reflex Mediated by Afferents in the Deep Perineal Nerve. J Neurophysiol 2005. 2005;93(5):2688–97. doi: 10.1152/jn.00978.2004. [DOI] [PubMed] [Google Scholar]
- 15.Woock JP, Yoo PB, Grill WM. Mechanisms of reflex bladder activation by pudendal afferents. Am J Physiol Regul Integr Comp Physiol 2011. 2011;300(2):R398–R407. doi: 10.1152/ajpregu.00154.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Häbler HJ, Jänig W, Koltzenburg M. Myelinated primary afferents of the sacral spinal cord responding to slow filling and distension of the cat urinary bladder. J Physiol 1993 April 1. 1993;463(1):449–60. doi: 10.1113/jphysiol.1993.sp019604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Truzzi JCI, Almeida FMR, Nunes EC, Sadi MV. Residual Urinary Volume and Urinary Tract Infection—When are They Linked? J Urol. 2008;180(1):182–5. doi: 10.1016/j.juro.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 18.Thor KB, Morgan C, Nadelhaft I, Houston M, de Groat WC. Organization of afferent and efferent pathways in the pudendal nerve of the female cat. J Comp Neurol. 1989;288(2):263–79. doi: 10.1002/cne.902880206. [DOI] [PubMed] [Google Scholar]
- 19.Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neurosci Lett. 1998;244(3):137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- 20.Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation: mass contraction of the pelvic floor muscles. Int Urogynecol J. 1998;9(1):28–32. doi: 10.1007/BF01900538. [DOI] [PubMed] [Google Scholar]
- 21.Fedirchuk B, Shefchyk S. Membrane potential changes in sphincter motoneurons during micturition in the decerebrate cat. J Neurosci. 1993;13(7):3090–3094. doi: 10.1523/JNEUROSCI.13-07-03090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brindley GS. An implant to empty the bladder or close the urethra. J Neurol Neurosurg Psychiatry. 1977;40(4):358–69. doi: 10.1136/jnnp.40.4.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woock J, Yoo P, Grill W. Intraurethral stimulation evokes bladder responses via two distinct reflex pathways. J Urol. 2009;182(1):366–73. doi: 10.1016/j.juro.2009.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudy DC, Downie JW, McAndrew JD. alpha-Chloralose alters autonomic reflex function of the lower urinary tract. Am J Physiol Regul Integr Comp Physiol. 1991;261(6):R1560–R7. doi: 10.1152/ajpregu.1991.261.6.R1560. [DOI] [PubMed] [Google Scholar]
- 25.McMahon SB, Morrison JFB. Factors that determine the excitability of parasympathetic reflexes to the cat bladder. J Physiol 1982. 1982;322(1):35–43. doi: 10.1113/jphysiol.1982.sp014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaze A, Goldman H, Jones JS, Rackley R, Vasavada S, Gustafson KJ. Determining the Course of the Dorsal Nerve of the Clitoris. Urology. 2008;72(5):1040–3. doi: 10.1016/j.urology.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 27.Goldman H, Amundsen C, Mangel J, Grill J, Bennett M, Gustafson K, et al. Dorsal genital nerve stimulation for the treatment of overactive bladder symptoms. Neurourol Urodyn. 2008;27(6):499–503. doi: 10.1002/nau.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafson K, Zelkovic P, Feng A, Draper C, Bodner D, Grill W. Fascicular anatomy and surgical access of the human pudendal nerve. World Journal of Urology. 2005;23(6):411–418. doi: 10.1007/s00345-005-0032-4. [DOI] [PubMed] [Google Scholar]
- 29.Kent AR, Grill WM. Model-based analysis and design of nerve cuff electrodes for restoring bladder function by selective stimulation of the pudendal nerve. Journal of Neural Engineering. 2013;10(3):036010. doi: 10.1088/1741-2560/10/3/036010. [DOI] [PMC free article] [PubMed] [Google Scholar]