Abstract
Background
American Indian (AI) children experience the highest rates of early childhood caries (ECC) in the USA, yet no tool has been validated to measure the impact of ECC on their oral health-related quality of life (OHRQoL).
Objective
To validate a pediatric OHRQoL scale in a preschool, rural, reservation-based AI population.
Methods
In 2011 and 2012, we measured the OHRQoL of AI children attending Head Start in Navajo Nation with the 12-item preschool version of the pediatric oral health-related quality of life (POQL) scale administered to their parents/caregivers. Parents/caregivers also reported their children’s subjective oral health status (OHS) and oral health behavior adherence. Concurrently, calibrated dental examiners measured the children’s decayed, missing, and filled tooth surfaces (dmfs). Validation was assessed with internal reliability and convergent and divergent validity testing and exploratory factor analyses.
Results
We measured the outcomes in 928 caregiver-child dyads. All children were AI and in preschool [mean (SD) child age was 4.1 (0.5) years]. The majority of children had experienced decay [dmfs: 89 %, mean (SD): 21.5 (19.9)] and active decay [any ds: 70 %, mean (SD): 6.0 (8.3)]. The mean (SD) overall POQL score was 4.0 (9.0). The POQL scale demonstrated high internal consistency reliability (Cronbach alpha = 0.87). Convergent validity of the POQL scale was established with highly significant associations between POQL and caries experience, OHS, and adherence to oral health behaviors (all ps < 0.0001).
Conclusions
The POQL scale is a reliable and valid measure of OHRQoL in preschoolers from the Navajo Nation.
Keywords: Early childhood caries, Oral health-related quality of life, American Indian, Validation, Psychometrics, Severe early childhood caries
Introduction
Early childhood caries (ECC) in children aged 2–5 years increased between 1988–1994 and 1999–2004, with marked differences between ethnic groups [1–4]. The disease levels in American Indian/Alaska Native (AI/AN) children are by far the highest of any US demographic group. A 2012 Indian Health Service (IHS) oral health surveillance report indicated that 54 % of AI children age 12–71 months experienced ECC, with a mean decayed, missing, and filled teeth (dmft) measure of 3.5, exceeding that of their non-native counterparts by greater than three times (mean dmft = 1.2) (3). Children residing on Navajo Nation have the highest prevalence of decay observed in Indian Country: 85.9 % of Navajo children age 2–5 years have caries experience with a mean dmft of 6.5 (3).
Beyond objective clinical measures of dental decay, such as dmft, it is important to recognize and measure the impact of dental disease on subjective psychosocial characteristics, such as children’s social and role functioning, and to acknowledge the importance of the children’s experiences as a way to fully assess the consequences of dental disease. This facet of dental disease highlights the need to develop, validate, and utilize scales to measure oral health-related quality of life (OHRQoL). For the purposes of this paper, we defined OHRQoL as the functional and psychosocial impacts of dental disease on children, as perceived by their caregivers. Specifically, we examined the impact of oral health on events of everyday life that caregivers deem important to the individual child including their children’s social, emotional, physical, and role functioning [5].
Dental caries has impacted the OHRQoL in populations of various ages across the globe, including children [6, 7] and adults [8, 9]. Validated instruments used to measure OHRQoL in children exist [9–11]; however, few have been validated to measure OHRQoL in preschool children, and we could not find any published measures that have been validated in AI children. Recognizing that the experiences of AI families are unique, validation of a tool to specifically measure OHRQoL in young AI children is needed to better understand the impact of interventions on their oral health outcomes. One instrument, the preschool version of the pediatric oral health-related quality of life (POQL) scale, was developed and validated by Huntington and colleagues and specifically emphasizes the experiences and views of preschool-age children from low-income and minority populations [6]. The POQL scale includes 12 items and was developed and validated in an urban, primarily low-income population in Boston, MA. It measures OHRQoL across four functional domains: physical, role, social, and emotional functioning [6]. In its development and validation, the preschool version of the POQL was modified to account for age-specific developmental differences and showed strong sensitivity to detecting changes in OHRQoL. The POQL scale was recently used to measure OHRQoL in a pilot study sample of young AI children in the Northern Plains and demonstrated that worse POQL was associated with worse pediatric OHS and increased utilization of urgent dental services [10]. The psychometric properties of the scale (factor analytic structure, internal consistency reliability, and convergent and divergent validity) have not, however, been studied in a large sample of AI preschoolers.
Acknowledging the diversity across minority populations in the USA and the significant burden of dental disease in AI/AN children, we recognized the need to have a validated instrument to measure OHRQoL in AI preschool children. Our objective was to examine the reliability and validity of the Huntington POQL instrument in a sample of preschool AI children attending Head Start in the Navajo Nation.
Methods
Approvals
This study was approved by the Navajo Nation Human Research Review Board, governing bodies at tribal and local levels, the tribal departments of Head Start and Education, Head Start parent councils, and the University of Colorado Multiple Institutional Review Board. All adult participants provided written informed consent and parents provided written informed consent for their children before participating in the study activities.
Study sample
The analysis data set included baseline data from an ongoing large randomized trial (Clinicaltrials.gov NCT01116739) funded by the National Institute of Dental and Craniofacial Research (NIDCR: U54DE019259–03). The study involved the participation of the Navajo community in its development and implementation, with the aim of preventing ECC in AI preschool children in Head Start Centers on the Navajo Nation. The study was a cluster randomized clinical trial that recruited preschool children and their parents/caregivers from 52 Head Start classrooms across Navajo Nation. Eligible participants included children ages 3–5 at enrollment into Head Start and a parent or other adult primary caregiver (henceforth caregivers) for each child. Children younger than 3 years of age or without available adult participants and adults unable to understand English were excluded. A total of 1,016 caregiver-child dyads were enrolled in the clinical trial from participating Head Start Centers. With the goal of validating the POQL in an AI preschool population, we only included dyads who reported their children as AI. Children who were not reported as AI (n = 32) and/or had missing data for age (n = 2), OHS (n = 15), or dmfs (n = 34) were excluded from analyses, as were those with missing data for more than one-third of the POQL items (n = 21). Our final study sample of 928 dyads included 91.3 % of the originally recruited sample.
Data collection
Participating caregivers completed the baseline participant survey—the Basic Research Factors Questionnaire (BRFQ)—in 2011 or 2012. Survey data were collected via computer. Oral clinical assessments of enrolled children were completed concurrently.
Survey development
Basic research factors questionnaire (BRFQ)
The BRFQ was the product of the collaborative efforts of three oral health disparities centers developed with the support from: NIDCR U54DE019285, U54DE019275, and U54DE019259. The BRFQ contains a variety of oral health measures including the POQL as well as items assessing OHS, oral health behaviors, and socio-demographic characteristics.
Measures
Pediatric oral health-related quality of life (POQL) scale
We used the 12-item preschool version of the POQL instrument developed and validated by Huntington and colleagues to assess caregivers’ perceptions of the extent to which their children’s psychosocial well-being and functioning were negatively affected by oral health experiences [6]. The scale measure addresses the impact of oral health problems on three types of functioning: role functioning (missing school/day care), physical functioning (experiencing pain or having trouble eating), and emotional functioning (being angry/upset, worrying, or crying). Each item characterizes the impact of oral health experiences (events) on these three types of functioning by asking the frequency of the six events (e.g., ‘how often was your child in pain because of his or her teeth or mouth’). For children who had experienced the specified event, care-givers were asked to indicate the severity of the event, reporting ‘how bothered’ the child was by the experience (severity). As specified by the original scale developers, we calculated ‘impact scores’ by multiplying the frequency response (0–3) by the severity response (0–4). Impact scores were then summed and converted to a percent of the maximum possible score, resulting in an overall POQL score ranging from 0 to 100, with higher scores indicating worse OHRQoL.
Child oral health status (OHS)
The child’s OHS was subjectively measured using an item adapted from the 2007 National Survey of Children’s Health [11]. Caregivers were asked to ‘describe the health of your child’s teeth and mouth’ using the following categories: excellent, very good, good, fair, or poor. OHS was scored on a scale of 1 (excellent) to 5 (poor).
Adherent oral health behaviors
The oral health behavioral scale was established by the collaborating centers and included 12 items that measured reported influential oral health behaviors including minimizing exposure to fermentable carbohydrates (e.g., frequent sugary snacks, sleeping with a bottle at naptime or bedtime) and maximizing optimal oral health care (e.g., at least twice daily tooth brushing, use of fluoridated toothpaste, regular dental visits, consumption of fluoridated water) [12, 13]. For each item, responses were coded as adherent or non-adherent with current recommendations for good oral health behavior. For example, caregivers who reported that their participating child’s teeth were brushed at least twice a day were identified as adherent with recommended tooth brushing frequency. Caregivers who reported brushing that child’s teeth less frequently were coded as being non-adherent with that behavior. Oral health behavioral adherence represented the percentage of behaviors for which caregivers were adherent.
Oral health clinical assessments
Dental examiners (licensed dental hygienists) were calibrated annually for ECC clinical assessments utilizing the NIDCR and Pitts criteria (all inter-rater Kappa scores ≥0.80) [14, 15]. The dental examiners systematically measured decayed, missing-due-to-caries, and filled tooth surfaces (dmfs) using a direct light source and mouth mirror without probing or X-rays. Calibrated data recorders input the exam data into CARIN software developed with support from: US DHHS/NIH/NIDCR U54DE014251 and R21DE018650. These clinical assessments of the children were completed in 2011 and 2012 at the same study visit during which caregivers completed the BRFQ. We defined caries presence as having active decay (ds >0) and severe caries (S-ECC) as ≥1 dmf smooth surface(s) in primary anterior teeth or dmfs score ≥4 (age 3), ≥5 (age 4), ≥6 (age 5) [14].
Socio-demographic characteristics
Socio-demographic variables reported included the following: caregiver and child gender, race/ethnicity, and age; caregiver’s highest grade of formal schooling completed, employment status, household income; and child’s dental insurance status and source. Caregivers were asked to report all forms of dental insurance for their children.
Statistical analysis
We calculated descriptive statistics for caregiver and child characteristics including means and standard deviations for continuous variables and frequencies for categorical variables. The frequency of each response category for the scale items (event frequency and event severity) was tabulated and is reported in Table 2. To evaluate the internal consistency reliability of the POQL scale, we calculated a Cronbach alpha coefficient for the impact scores (report of event frequency multiplied by the report of event severity). To examine the underlying constructs of the POQL scale, we conducted an exploratory principal components factor analysis with Varimax rotation using the POQL impact scores. We calculated factor analysis solutions for a specified number of factors from one to six, with one factor for each POQL event.
Table 2.
Distribution of POQL item responses in a Navajo preschool population (n = 928)
| Event [n (%)]a | All of the time | Some of the time | Once in a while | Did not happen | Do not know or refused |
|---|---|---|---|---|---|
| Pain | 3 (0.3) | 34 (3.7) | 178 (19.2) | 700 (75.4) | 12 (1.3) |
| Angry/upset | 3 (0.3) | 23 (2.5) | 81 (8.7) | 811 (87.4) | 10 (1.1) |
| Cry | 5 (0.5) | 33 (3.6) | 119 (12.8) | 768 (82.8) | 3 (0.3) |
| Worried | 10 (1.1) | 33 (3.6) | 119 (12.8) | 744 (80.2) | 22 (2.6) |
| Trouble eating | 5 (0.5) | 40 (4.3) | 111 (12.0) | 762 (82.1) | 10 (1.1) |
| Missed school/day care | 1 (0.1) | 6 (0.7) | 51 (5.5) | 857 (92.4) | 13 (1.4) |
| Severity associated with event [n (%)] | Very bothered | Somewhat bothered | Bothered a little bit | Never bothered | Do not know or refused |
|---|---|---|---|---|---|
| Pain | 31 (3.3) | 41 (4.4) | 121 (13.0) | 22 (2.4) | 0 |
| Angry/upset | 8 (0.9) | 29 (3.1) | 61 (6.6) | 5 (0.5) | 4 (0.4) |
| Cry | 19 (2.1) | 32 (3.5) | 89 (9.6) | 15 (1.6) | 2 (0.2) |
| Worried | 12 (1.3) | 30 (3.2) | 97 (10.5) | 21 (2.3) | 2 (0.2) |
| Trouble eating | 17 (1.8) | 25 (2.7) | 102 (11.0) | 11 (1.2) | 1 (0.1) |
| Missed school/day care | 6 (0.7) | 12 (1.3) | 22 (2.4) | 16 (1.7) | 1 (0.1) |
‘In the past three months, did your child have ‘event’ because of his or her teeth or mouth?’ If a positive response, item is followed by a severity item: ‘how bothered was your child by the event?’
We conducted an ordinary least squares (OLS) regression analysis with POQL score as the dependent variable and dmfs, ds, oral health status, and oral health behavior adherence as independent variables in four separate convergent models and survey year, agency number, and single or multiple Head Start classrooms in the Head Start Center as independent variables in three separate divergent models. Three of the four convergent validity variables (dmfs, ds, and behavioral adherence) were used as continuous variables and OHS was a categorical variable (excellent-poor) with ‘excellent’ as a reference category. Two of the three divergent variables were used as dichotomous variables [survey year (2012 vs. 2011) and number of classrooms within a Head Start Center (>1 vs. 1)], and Head Start agency number was used as a categorical variable (1 to 5) with 5 as the reference category. These divergent variables were chosen as all other items in the BRFQ were hypothesized as being associated with caries experience and POQL, and we expected no association to be found between the divergent scales and POQL.
To further examine the relationship between the POQL scale items and ECC experience, we used unpaired t tests to compare mean ds in children who experienced a specific POQL event (e.g., experiencing pain) to children who had not experienced that event. Additionally, unpaired t tests were utilized to compare the mean POQL scores between children with with/without ECC, severe-ECC (S-ECC), ds, ms, fs, and dmfs >90 %. All analyses were conducted with SAS 9.3 (SAS Institute, Cary North Carolina).
Results
Sample characteristics
A total of 928 caregiver-child dyads were included in the final analytic cohort (Table 1). The mean (SD) caregiver age was 31.6 (9.2) years; the mean (SD) child age was 4.1 (0.5) years. The majority of caregivers were females (83.9 %), and their children were equally distributed by gender (49.4 % females). Nearly all caregivers reported themselves to be of AI descent (98.4 %), and all children were AI as a result of our inclusion criteria. The distribution of dmfs ranged from 0 to 88, with a mean (SD) of 21.5 (19.9) and a median of 16.0. The distribution of ds ranged from 0 to 68, with a mean (SD) of 6.0 (8.3) and a median of 3.0. The majority of caregivers reported positive OHS for their child (68.5 % responding excellent, very good, or good). The average (SD) of the behavioral adherence score was 51.2 % (22.3), indicating that on average caregivers were adherent with only half of recommended oral health behaviors.
Table 1.
Socio-demographic characteristics of Navajo parents/care-givers and their children’s dental experience and oral health status
| Caregiver characteristics (n = 928) | |
|---|---|
| Age (years) mean (SD) | 31.6 (9.2) |
| Gender (female) [n (%)] | 779 (83.9) |
| American Indian [n (%)] | 913 (98.4) |
| Formal education [n (%)] | |
| Less than high school | 140 (15.0) |
| High school diploma or GED | 343 (37.0) |
| Some college/technical or vocational school | 328 (35.3) |
| College degree or higher | 111 (12.0) |
| Not reported | 6 (0.7) |
| Employment [n (%)] | |
| Part-time or greater | 265 (28.6) |
| Student | 95 (10.2) |
| Homemaker | 208 (22.4) |
| Unemployed | 316 (34.1) |
| Other/not reported | 44 (4.7) |
| Income [n (%)] | |
| <$10,000 | 392 (42.2) |
| $10,000 to $19,999 | 161 (17.3) |
| $20,000 to $29,999 | 88 (9.5) |
| $30,000 to $39,999 | 65 (7.0) |
| $40,000 or higher | 87 (9.4) |
| Not reported | 134 (14.4) |
| Oral health behavior score [n (%)] | |
| Adherent | 51.2 (22.3) |
| Child characteristics (n = 928) | |
| Age [n (%)] | |
| 3 years | 387 (41.7) |
| 4 years | 517 (55.7) |
| 5 years | 24 (2.6) |
| Gender (female) [n (%)] | 458 (49.4) |
| Dental insurance [n (%)] | |
| IHS | 867 (93.4) |
| State children’s health insurance plan (SCHIP) | 7 (0.8) |
| Medicaid | 378 (40.7) |
| Private insurance | 43 (4.6) |
| Other | 56 (5.0) |
| Decayed, missing, and filled surfaces [mean (SD)] | 21.5 (19.9) |
| Median | 16.0 |
| Range | 0–88 |
| ds [mean (SD)] | 6.0 (8.3) |
| Median | 3 |
| Range | 0–68 |
| Caregiver-reported oral health status [n (%)] | |
| Excellent | 115 (12.4) |
| Very good | 195 (21.0) |
| Good | 326 (35.1) |
| Fair | 231 (24.9) |
| Poor | 61 (6.6) |
POQL
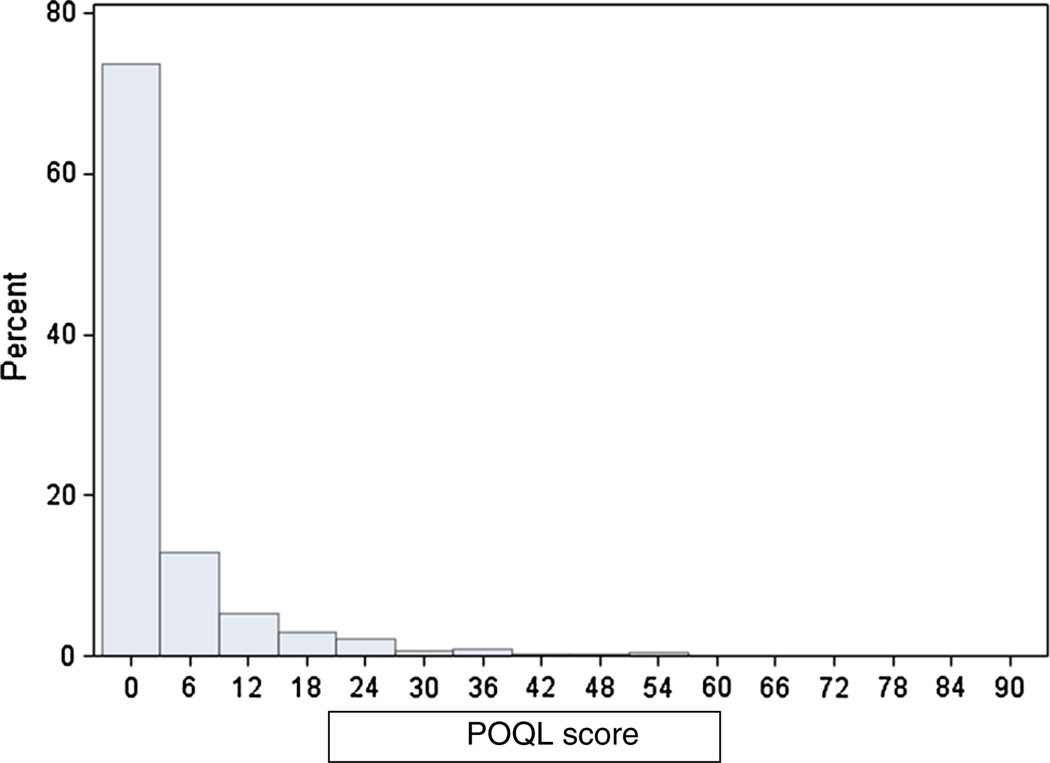
The overall distribution of POQL scores was positively skewed (Fig. 1). The mean (SD) overall POQL score was 4.0 (9.0), median was 0.0, first quartile was 0.0, and third quartile was 4.2. The distributions of response frequencies to the six events (pain, angry/upset, crying, worried, trouble eating, and missing school/day care) of the POQL instrument are presented in Table 2. A minority of care-givers reported that their children had experienced any of the POQL events, ranging from only 6.3 % (missed school) to 23.2 % (experienced pain). Of those children who were reported to have experienced these events, most were affected only ‘once in a while.’
Fig. 1.
Distribution of POQL scores in a Navajo preschool population (n = 928)
Scale reliability and factor analysis
The POQL scale had a standardized Cronbach alpha coefficient of 0.87, indicating good internal consistency reliability. Exploratory factor analysis suggested that the POQL items load onto one underlying factor, with factor loadings ranging from 0.64 to 0.87 and the single factor accounting for 61.9 % of total variance (Table 3). Of the multiple factor solutions we tested, the 3-factor solution corresponded most closely to the factor analysis findings by Huntington et al. [6], with separate factors for physical, emotional, and role functioning. This three-factor solution accounted for 82 % of the POQL variance.
Table 3.
Factor analysis results of the POQL in a Navajo preschool population* (n = 928)
| POQL events | Loadings: 1-factor solution | Loadings: 3-factor solution |
||
|---|---|---|---|---|
| Physical functioning | Emotional functioning | Role functioning | ||
| Pain | 0.85 | 0.85 | 0.28 | 0.16 |
| Angry or upset | 0.78 | 0.54 | 0.65 | 0.05 |
| Cry | 0.87 | 0.76 | 0.39 | 0.24 |
| Worried | 0.75 | 0.25 | 0.88 | 0.22 |
| Trouble eating | 0.81 | 0.76 | 0.22 | 0.31 |
| Missed school or day care | 0.64 | 0.26 | 0.17 | 0.94 |
| Total variance by Factor | 61.9 % | 38.4 % | 25.5 % | 18.2 % |
| Total variance by solutions | 61.9 % | 82.1 % | ||
Cronbach alpha correlations: correlations between each item and its target scale are shown in bold
Convergent Validity
In the linear regression analyses, overall POQL score was positively associated with each of the convergent measures: caries experience (dmfs), active decay (ds), care-giver-reported OHS, and adherence to oral health behaviors (all p < 0.0001) (Table 4). In independent models, the overall POQL score was higher (indicating worse OHR-QoL) by 0.12 point for each additional dmfs, 0.34 point higher for each additional ds, and 2.45 points higher for each unit of worsening OHS. Overall POQL score was lower (indicating better OHRQoL) with each 0.07 point decrease in POQL score for each percentage point increase in oral health behavior adherence (p < 0.0001). Divergent validity analyses indicated that POQL scores were not associated with survey year (p = 0.18), Head Start Agency number (p = 0.58), or number of classrooms in the Head Start Center (p = 0.84) (Table 4).
Table 4.
Ordinary least squares regression analysis with POQL as the dependent variable and each convergent or divergent measure as the independent variable in Navajo preschool children (n = 928)
| Parameter estimate (95 % CI) |
SE | p value | |
|---|---|---|---|
| Convergent models | |||
| dmfs* | 0.12 (0.09, 0.15) | 0.01 | <0.0001 |
| ds* | 0.34 (0.27, 0.41) | 0.03 | <0.0001 |
| Oral health status** | 2.45 (1.94, 2.95) | 0.26 | <0.0001 |
| Oral health behavior adherence* | −0.07 (−0.10, −0.05) | 0.01 | <0.0001 |
| Divergent models | |||
| Survey year (2012 vs. 2011) | −0.80 (−1.96, 0.37) | 0.59 | 0.18 |
| Agency number*** | 0.58 | ||
| 1 | 1.09 (−0.66, 2.84) | 0.89 | 0.22 |
| 2 | 1.25 (−0.65, 3.14) | 0.97 | 0.20 |
| 3 | 0.80 (−1.02, 2.63) | 0.93 | 0.39 |
| 4 | 0.11 (−1.73, 1.96) | 0.90 | 0.90 |
| Number of Head Start classrooms in each Head Start Center ([1 vs. 1) |
0.12 (−1.06, 1.30) | 0.60 | 0.84 |
Continuous variable
Categorical variable: 1 = Excellent, 2 = Very good, 3 = Good, 4 = Fair, 5 = Poor (excellent is reference group)
Categorical variable with five agencies: 5th agency is the reference group
Children who experienced each of the six events of the POQL measure (pain, angry or upset, crying, worrying, trouble eating, missing school/day care) had significantly more active decay (higher mean ds) compared to children who had not experienced any of these events (all ps < 0.0001 except for missed school/day care) (Table 5). Children with ECC, S-ECC, ds, ms, fs had significantly higher POQL scores than children without these respective caries experiences (see Table 6). Children missing teeth for caries had the highest POQL scores.
Table 5.
Comparison of mean number of decayed tooth surfaces (ds > 0) between Navajo preschool children with and without a POQL event (total n = 928)
| POQL event | With POQL event* |
Without POQL event** |
p value*** | ||
|---|---|---|---|---|---|
| n | Mean ds (SD) | n | Mean ds (SD) | ||
| Pain | 215 | 10.1 (11.7) | 713 | 4.8 (6.4) | <0.0001 |
| Angry/upset | 107 | 12.3 (14.9) | 821 | 5.1 (6.5) | <0.0001 |
| Cry | 157 | 10.5 (13.3) | 771 | 5.0 (6.4) | <0.0001 |
| Worried | 162 | 10.4 (13.1) | 766 | 5.0 (6.4) | <0.0001 |
| Trouble eating | 156 | 9.4 (12.1) | 772 | 5.3 (7.0) | <0.0001 |
| Missed school/day care | 58 | 8.0 (10.3) | 870 | 5.7 (7.9) | 0.11 |
With POQL event includes responses: all of the time, some of the time, once in a while
Without POQL event includes responses: did not happen, do not know, refused
Unpaired t test of difference in mean number of decayed surfaces by event reported versus not reported
Table 6.
POQL scores by caries experience in a Navajo preschool children (n = 928)
| Caries experience n (%) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ECC* |
S-ECC |
ds |
ms |
fs |
dmfs 90th percentile*** |
||||||||
| Yes 830 (89.4) |
No 98 (10.6) |
Yes 754 (81.3) |
No 174 (18.7) |
Yes 647 (69.7) |
No 281 (30.3) |
Yes 279 (30.1) |
No 649 (69.9) |
Yes 556 (59.9) |
No 556 (59.9) |
Yes 94 (10.1) |
No 834 (89.9) |
||
| POQL score mean (SD) | 4.4 (9.4) | 0.7 (2.6) | 4.7 (9.8) | 0.8 (2.5) | 4.7 (9.6) | 2.5 (7.5) | 6.9 (19.7) | 2.7 (7.1) | 4.7 (9.6) | 2.9 (8.0) | 7.3 (11.0) | 3.6 (8.7) | |
| p value** | <0.0001 | <0.0001 | 0.0002 | <0.0001 | 0.003 | 0.002 | |||||||
ECC includes children with any dmfs
Unpaired t test of mean score by group caries experience (Yes vs. No)
90th percentile was 52 dmfs or higher in this sample
Discussion
The preschool version of the Huntington et al. POQL scale appears to be a reliable and valid measure of OHRQoL in a Navajo preschool population. The scale showed excellent internal consistency as well as strong construct validity. The POQL overall scores and event frequencies were higher (worse) for children with worse dental disease (dmfs), active decay (ds), and worse caregiver-reported OHS relative to children with less dental disease, no active decay, and better OHS. The scale showed divergent validity with no association to year surveyed administered, Head Start Center, or number of classrooms in the Head Start Center. This evidence of scale validity is consistent with the findings of Huntington et al. [6] who developed the instrument and validated it in a low-income, minority population. Measures of internal consistency reliability were similar across the two studies, with Cronbach alpha values of 0.87 in the present analysis and 0.86 in the analysis by Huntington and colleagues. In addition, both studies found highly significant associations between POQL and caries experience and OHS. The validation of the POQL instrument in this sample is further strengthened by its association with mean dmfs and ds, and S-ECC, adding greater sensitivity to the analysis beyond just assessing an association with either caries present or absent.
To our knowledge, this is the first study to validate an OHRQoL measure in preschool, rural-dwelling, AI children. As such, the POQL provides an important tool for evaluating relevant outcomes in research targeting oral health disparities and the prevention of ECC.
There are notable differences between the sample used in this validation and the sample used by Huntington and colleagues. The Huntington et al. sample was composed of children 2–16 years of age, and our sample consisted of preschool children; however, our sample is similar to Huntington’s et al. subsample in which they tested the sensitivity to change of the preschool version of the POQL (3–5 years olds vs. 2–6 years old). Huntington et al.’s overall sample was composed primarily of white (56.2 %) and Hispanic (25.5 %) children who lived in an urban setting, compared to our study of Navajo preschoolers who lived in a rural setting. The caries experience was also different between the two samples. In our sample, 30.3 % of children were caries free (ds = 0) compared to 52.9 % caries-free children in the Huntington et al. preschool sample. Intriguingly, although our sample had a higher prevalence of active decay, their POQL scores were consistently lower when compared to the Huntington preschool sample, indicating better perceived OHRQoL. In our sample, caries-free children had a mean POQL score of 2.5 and children with caries (ds > 0) had a mean score of 4.7, compared to a mean score of 14.6 in the Huntington et al.’s. preschool sample. This difference persisted in the relationship between subjective OHS (care-giver-reported) and POQL. Children with positive OHS (good/very good/excellent) had a mean POQL of 2.4 in our sample, compared to 3.2 in the Huntington et al. sample, and those with negative OHS (fair/poor) had a mean POQL of 7.5 in our sample, compared to 11.0 in the Huntington et al. sample. Although the frequency of all of the scale events were reported less often in our sample than in the Huntington et al. sample, some patterns persisted across the samples including pain as the most frequently reported and missing school or day care as the least frequently reported event on the scale. It appears that even though the caries experience in this preschool, AI population was severe, their caregivers’ reported their perceived oral health ratings, including subjective psychological factors such as those measured by the POQL, as less severe. Perhaps this population’s long experience of extreme dental disease has habituated them to its consequences and increased their resilience to the events measured by the POQL. Also, since severe dental disease is so prevalent within this population, they may perceive severe dental disease as normal. Similar findings have been reported in other young AI populations [10]. Investigation of the validity of the POQL measure in other indigenous populations who experience similarly severe disease would expand our knowledge of its validity.
We have validated the use of the preschool version of the POQL in an AI preschool population who carry a disproportionate burden of oral health disease. Having a valid tool to measure the impact of oral health disease on the lives of these vulnerable AI children is needed to understand the impact of interventions aimed at improving their health outcomes. Population-based POQL scores are subjective, however, and although valid, scores may differ across populations due to variations in cultures, historical experiences, access to dental care, and/or place of residence (urban vs. rural). Use of the POQL scores to assess the impact of interventions within young AI populations is valid, as we expect disparities in OHRQoL to exist even within a relatively homogeneous group (same race/ethnicity, similar-aged children, and rural residence) given the disparities in dental experience and oral health status within the population. However, if population-based norms differ, as we suspect for this scale, using it to compare OHRQoL across populations may not be appropriate, and may not accurately reflect the disparities in OHRQoL associated with disparities in oral disease between populations. Also, increasing age has been found to be associated with higher POQL scores, even within a young population [10],and comparing POQL scores across populations of different ages may lead to a misunderstanding of their dental experiences.
ECC prevalence in this AI preschool population was notably high (81.3 % had S-ECC) and most children in the sample had many affected tooth surfaces (mean dmfs = 21.5/mean ds = 6.0). Given the severity of disease in this population, one would expect their OHRQoL to be impacted. Although we did not see numerically high POQL scores in this population (mean = 4.7 for children with active decay), associations of POQL with dmfs, ds, OHS, and oral health behavioral adherence were highly significant. A better understanding of the clinical significance of POQL scores would be valuable in interpreting their potential utility in the evaluation of oral health promotion programs for reducing ECC and ECC’s impact on health and well-being. The experiences of the AI population in which the POQL was validated may differ from those in other populations and may impact their resilience or perhaps perceptions of health—including oral health.
There are limitations to this study. Foremost, since few of the AI children in whom the POQL was validated were caries free, we may not have been able to accurately evaluate the performance of the POQL measure in these young AI children. Second, POQL is a subjective measurement that is vulnerable to recall and response biases and was used as a proxy for the impact of dental disease on children. We also compare our validation of the preschool version of the POQL to its validation in Huntington et al.’s preschool population, as well as in their overall pediatric population. Perception of POQL changes over time as children’s teeth and experiences also change, possibly making these comparisons less valid. Also, although we successfully met our objective and demonstrated the validity of the POQL in an AI preschool population, its use in older AI children or in other tribal populations residing in other regions warrants further validation.
Conclusion
The POQL scale is a reliable and valid measure of OHR-QoL in young AI children in the rural southwest, and its use as an outcome measure in oral health promotion interventions is acceptable. Due to the influence of differences in age, race/ethnicity, and access to dental services on POQL, we advise caution when comparing POQL scores between different racial/ethnic and cultural groups until further research on this scale has been completed.
Acknowledgments
Funding for the study was provided by the National Institute for Dental and Craniofacial Research (U54 DE019259–03, Albino). The findings and conclusions are those of the authors and do not necessarily represent the official position of the National Institutes of Health. We would like to thank the Navajo Tribe as well as the participants who gave so graciously of their time. We would like to thank Lucinda Bryant for her assistance with revising this manuscript and Carmen George and Nikola Toledo for their tireless work in Navajo Nation. Finally, we are grateful for the technical assistance provided by Michelle Henshaw and Sharron Rich at Boston University.
Abbreviations
- AI
American Indian
- AI/AN
American Indian/Alaska Native
- ECC
Early childhood caries
- dmfs
Decayed, missing, filled surfaces
- OHRQoL
Oral health-related quality of life
- OHS
Oral health status
- POQL
Pediatric oral health-related quality of life
Footnotes
This work does not report results of a clinical trial. The work described in this manuscript has not been published before; it is not under consideration for publication anywhere else; the manuscript has been approved by all co-authors, the Colorado Multiple Institutional Review Board, the National Institute of Dental and Craniofacial Research, and the Navajo Nation Human Research Review Board.
Conflict of interest None.
Contributor Information
Patricia A. Braun, Children’s Outcomes Research, Colorado Health Outcomes Programs, University of Colorado Anschutz Medical Campus, 13199 E. Montview Blvd. Suite 300 F443, Aurora, CO 80045, USA Patricia.braun@ucdenver.edu
Kimberly E. Lind, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA Department of Health Systems, Management and Policy, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
William G. Henderson, Children’s Outcomes Research, Colorado Health Outcomes Programs, University of Colorado Anschutz Medical Campus, 13199 E. Montview Blvd. Suite 300 F443, Aurora, CO 80045, USA Department of Biostatistics and Informatics, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Angela G. Brega, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
David O. Quissell, Department of Craniofacial Biology, School of Dental Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA
Judith Albino, Centers for American Indian and Alaska Native Health, Colorado School of Public Health, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
References
- 1.National Health and Nutrition Examination Survey (NHANES 1999–2002) [database on the Internet]. database. 2004 Available from: http://drc.hhs.gov/ [cited 4 March 2007]
- 2.National Health and Nutrition Examination Survey (NHANES III 1988–1994) [database on the Internet] United States Department of Health and Human Services. 1998 Available from: http://drc.hhs.gov/ [cited 4 March 2007]
- 3.Phipps KR, Ricks TL, Manz MC, Blahut P. Prevalence and severity of dental caries among American Indian and Alaska Native preschool children. Journal of Public Health Dentistry. 2012;72:208–215. doi: 10.1111/j.1752-7325.2012.00331.x. [DOI] [PubMed] [Google Scholar]
- 4.Dye BA, Tan S, Smith V, Lewis BG, Barker LK, Thornton-Evans G, et al. Trends in oral health status: United States, 1988–1994 and 1999–2004. In Statistics NCfH. Vital Health Statistics. 2007 [PubMed] [Google Scholar]
- 5.Locker D, Allen F. What do measures of ‘oral health-related quality of life’ measure? Community Dentistry and Oral Epidemiology. 2007;35:401–411. doi: 10.1111/j.1600-0528.2007.00418.x. [DOI] [PubMed] [Google Scholar]
- 6.Huntington NL, Spetter D, Jones JA, Rich SE, Garcia RI, Spiro A., 3rd Development and validation of a measure of pediatric oral health-related quality of life: the POQL. Journal of Public Health Dentistry. 2011;71:185–193. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GH, McGrath C, Yiu CK, King NM. A comparison of a generic and oral health-specific measure in assessing the impact of early childhood caries on quality of life. Community Dentistry and Oral Epidemiology. 2010;38:333–339. doi: 10.1111/j.1600-0528.2010.00543.x. [DOI] [PubMed] [Google Scholar]
- 8.Dahl KE, Wang NJ, Ohrn K. Does oral health matter in people’s daily life? Oral health-related quality of life in adults 35–47 years of age in Norway. International Journal of Dental Hygiene. 2012;10:15–21. doi: 10.1111/j.1601-5037.2011.00533.x. [DOI] [PubMed] [Google Scholar]
- 9.Crocombe LA, Broadbent JM, Thomson WM, Brennan DS, Poulton R. Impact of dental visiting trajectory patterns on clinical oral health and oral health-related quality of life. Journal of Public Health Dentistry. 2012;72:36–44. doi: 10.1111/j.1752-7325.2011.00281.x. [DOI] [PubMed] [Google Scholar]
- 10.Braun PA, Lind KE, Batliner T, Brega AG, Henderson WG, Nadeau K, et al. Caregiver reported oral health-related quality of life in young American Indian children. Journal of Immigrant and Minority Health/Center for Minority Public Health. 2013 doi: 10.1007/s10903-013-9870-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Child and adolescent health measurement initiative. [[cited 2012 May 20]];National Survey of Children’s Health. 2007 accessible at http://childhealthdataorg/learn/NSCH.
- 12.Brown LF. Research in dental health education and health promotion: a review of the literature. Health Education Quarterly. 1994;21:83–102. doi: 10.1177/109019819402100109. [DOI] [PubMed] [Google Scholar]
- 13.Hollister MC, Anema MG. Health behavior models and oral health: A review. Journal of Dental Hygiene. 2004;78:6. [PubMed] [Google Scholar]
- 14.Drury TF, Horowitz AM, Ismail AI, Maertens MP, Rozier RG, Selwitz RH. Diagnosing and reporting early childhood caries for research purposes. A report of a workshop sponsored by the National Institute of Dental and Craniofacial Research, the Health Resources and Services Administration, and the Health Care Financing Administration. Journal of Public Health Dentistry. 1999;59:192–197. doi: 10.1111/j.1752-7325.1999.tb03268.x. [DOI] [PubMed] [Google Scholar]
- 15.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–59. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]