Abstract
Objective
Diabetes patients with limited resources often experience suboptimal care. Less is known about the role of effective health communication (HC) in caring for low income diabetes patients.
Methods
Ten health department clinics in TN participated in a trial evaluating a literacy-sensitive communication intervention. We assessed the quality of baseline HC and measured associations with diabetes outcomes. Assessments included: demographics, measures of HC, health literacy, self-care behaviors, self-efficacy, medication non-adherence, treatment satisfaction, and A1C. Unadjusted and adjusted multivariable regression models were used to test associations.
Results
Participants (N=411) were 49.7 ± 9.5 years, 61% female, uninsured (96%), with A1C 9.6 ± 2.1. In unadjusted analyses, better communication, was associated with lower medication non-adherence (OR 0.40-0.68, all p<0.05), higher treatment satisfaction (OR 1.76-1.96, all p<0.01), portion size reduction (OR 1.43, p<0.05), diabetes self-efficacy (OR 1.41, p<0.05), and lower A1C (β= −0.06, p<0.01). In adjusted analyses, communication quality remained associated with lower medication non-adherence (AOR 0.39-0.68, all p<0.05), and higher treatment satisfaction (AOR 1.90-2.21, all p<0.001).
Conclusions
Better communication between low-income patients and providers was independently associated with lower medication non-adherence and higher treatment satisfaction.
Practice Implications
Communication quality may be an important modifiable approach to improving diabetes care for vulnerable populations.
Keywords: diabetes, health communication, public health, primary care, provider education
1. Introduction
Approximately 26 million people in the U.S. have been diagnosed with diabetes, placing them at increased risk for the many untoward complications of poor control [1]. Often, minority diabetes patients and those with limited resources face disproportionate challenges such as greater barriers to access, poorer health outcomes, and increased burden of disease [2]. Many of these patients seek care in public healthcare settings, where despite strong evidence about the optimal treatment of diabetes, care often remains suboptimal [2-5]. Unfortunately, national efforts to achieve benchmarks in quality of care for these groups continue to fall short [6, 7].
Ineffective health communication between patients and providers in public healthcare settings may contribute to suboptimal care. Providers in these settings often report time constraints, challenges to continuity of care with patients, and greater limitations of staffing & resources, compared to private settings [8-10]. Additionally, patient factors such as limitations in health literacy and diabetes-specific numeracy (i.e. computational) skills may potentiate existing challenges to effective health communication when attempting to provide care for vulnerable populations [11, 12]. Low health literacy and diabetes numeracy are recognized barriers to adequate diabetes care [13, 14]. We have conducted several studies among both English and Spanish-speaking diabetes patients that have identified moderate to high prevalence of limited functional health literacy and numeracy skills [15-20]. We have also shown these limitations to be significantly associated with several diabetes-related factors such as poorer self-efficacy for self-management, less diabetes knowledge, worse medication adherence, and poorer glycemic control [15, 17-25]. Although patients with limited literacy and numeracy skills may experience poorer communication with their provider [26], less is known about the specific relationship between the patient-provider interaction and diabetes related outcomes; and current evidence has been shown to be of mixed quality as supported by a recent systematic review [13].
Academic and community partnerships may be an effective model for improving communication in healthcare and addressing disparities of diabetes care for underserved populations [27, 28]. The aims of this article therefore seek to address two specific research questions based on assessment of baseline data from a larger clinical trial occurring within the context of an academic-community partnership: 1) What is the perception of the quality of communication during clinical encounters by diabetes patients seeking care in a public health department setting?, and 2) What is the association among patients’ perception of the quality of communication and reports of self-care behaviors, treatment satisfaction, self-efficacy, and glycemic control?
2. Methods
2.1. Study Setting & Patients
In 2010 we established a partnership between an academic medical center and a regional health department in Tennessee whose state diabetes prevalence that year was high at 10.2% compared to the national average of 8.3% [1,29]. The PRIDE Study (Partnership to Improve Diabetes Education) is a prospective, cluster randomized-controlled trial designed to address health communication issues and develop a sustainable model for improving diabetes care in our region that includes both urban and rural settings [30].
Providers, including physicians, nurse practitioners, nurses, dieticians, and medical interpreters employed within 10 State Health Department Clinics were invited to participate, and clinics were randomly assigned to one of two conditions. Providers at five intervention sites were exposed to training in effective health communication including instruction on working with low health literacy populations, strategies for improving communication during clinical encounters (e.g. teach back, goal setting, reduction of jargon, motivational interviewing), and effective use of medical interpreters. In addition to evidence-based updates in diabetes care, these providers also received education on the use of a diabetes toolkit designed specifically for use among patients with limited literacy and numeracy skills [31]. The remaining five clinics were provided evidence-based updates in diabetes care and were given educational materials from the National Diabetes Education Program to share with patients. These five clinics did not receive any training in effective health communication.
Eligible patients at participating clinics included individuals with a diagnosis of Type 2 diabetes, between the ages of 18-85, English and/or Spanish-speaking, A1C ≥ 7.5%, and agreeing to the 2-year duration of the study. Patients were excluded for poor visual acuity (>20/50 on a pocket screener), clinically significant dementia/psychosis, or if they had a life expectancy less than 2 years. Providers that participated in the intervention or control site training sessions were incentivized with state-approved continuing education credits while patients received a cash remuneration of $20 following completion of baseline data collection. The Vanderbilt University and Tennessee State Health Department IRBs provided study approval prior to enrollment.
2.2. Main Measures
Patients were approached by bilingual research staff during regular clinic hours and by phone referral from clinic staff with informed consent obtained in the patient’s language of preference (English or Spanish). Baseline patient assessments included collection of demographic, anthropometric (height, weight, BMI), and clinical measures (blood pressure, A1C, lipid profile). Before the clinical encounter with a provider, each participant reported their current diabetes self-care behaviors including responses to a Personal Diabetes Questionnaire (PDQ-11) and the Adherence to Refills and Medications Scale (ARMS). The PDQ-11 is an eleven item version of an original 68-item scale [32] that assesses an individual’s current and planned nutritional and exercise behaviors. The ARMS is a validated 12-item measure that evaluates an individual’s level of medication non-adherence in the areas of medication taking and refill behaviors [33]. Psychometric assessment of the PDQ-11 indicated it is best to combine the first three items into a Poor Eating Behavior subscale (Cronbach’s alpha = 0.66) and items 4, 5, and 6 into a Use of Data to Modify Diet subscale (Cronbach’s alpha = 0.81). The remaining five items assess the frequency of meal skipping, portion control, physical activity, and stages of change for exercise & weight management and were treated as individual variables. Higher scores on the PDQ-11 indicate greater presence of the reported behavior and scores ≥ 16 on the ARMS reflect greater medication non-adherence. Health literacy was measured using the Short Test of Functional Health Literacy in Adults (s-TOFHLA) [34] and responses were dichotomized to adequate vs. less-than-adequate for scores ≥23 or ≤22 respectively. Diabetes treatment satisfaction and diabetes related self-efficacy were assessed using the Diabetes Treatment Satisfaction Questionnaire (DTSQ) and Perceived Diabetes Self-Management Scale (PDSMS) where higher scores indicate greater treatment satisfaction and self-efficacy respectively [35, 36].
Two measures of health communication were administered to each participant, one before and the other after the initial clinical encounter. The Interpersonal Processes of Care Survey (IPC-18), the “before” measure, has been validated in a multi-ethnic population and measures patients’ perception of provider communication on several dimensions [37]. We report the IPC-18 using three broad domains as recommended by Stewart et al. – 1) Communication includes the dimensions of “lack of clarity,” “elicitation of concerns,” and “explanation of results;” 2) Decision Making represents the dimension “working together;” and 3) Interpersonal Style includes the dimensions “compassionate” and “discriminated due to race/ethnicity” [38]. Questions referring to office staff were excluded to isolate patients’ perception of provider communication only. The Communication Assessment Tool (CAT) was administered after the encounter. The CAT measures perceptions of physician performance in the areas of communication and interpersonal skills and has been evaluated in a variety of care settings and among diverse patients [39].
2.3. Statistical Analysis
Patient characteristics were summarized using mean ± SD for continuous and ordinal variables, and proportions for categorical variables. Our main outcomes of interest were treatment satisfaction (DTSQ), medication non-adherence (ARMS), diabetes self-care behaviors (PDQ-11), self-efficacy (PDSMS), and glycemic control (A1C). We examined the independent association of these outcomes with each of the measured communication variables: IPC-18 domains (Communication, Decision Making, and Interpersonal Style) and CAT score. Responses for all communication variables were dichotomized to compare scores of 5 to scores < 5 so as to account for the tendency of values to cluster around positive responses (i.e. positive skew) and in congruence with previous analyses of these measures [40, 41]. Following log transformation, glycemic control (A1C) was analyzed using linear regression while all other outcomes were assessed using proportional odds logistic regression. Both unadjusted and adjusted associations were examined. To avoid overfitting, adjusted models included the following list of a priori defined variables: age, gender, race, ethnicity, health literacy status, education level, income, years since diagnosis, insurance (uninsured vs. some form of insurance), insulin use, and treatment assignment. Adjustment for treatment assignment was done due to the fact that providers at intervention cites had received some education on communication prior to completion of baseline data collection.
To address the issue of potential collinearity among covariates we computed a variance inflation factor (VIF) for each adjusted model. The maximum VIF value did not exceed a recommended threshold of 10 [42]. The effects of the main covariates on each outcome were reported as adjusted odds ratios (AOR) with 95% confidence intervals for ordinal outcomes and as change in log for A1C. Subjects with missing outcome or covariate values were excluded from the analyses. Findings with a 2-sided p-value < 0.05 were considered statistically significant. All statistical analyses were performed using statistical package R software version 2.15.0 (http://www.r-project.org).
3. Results
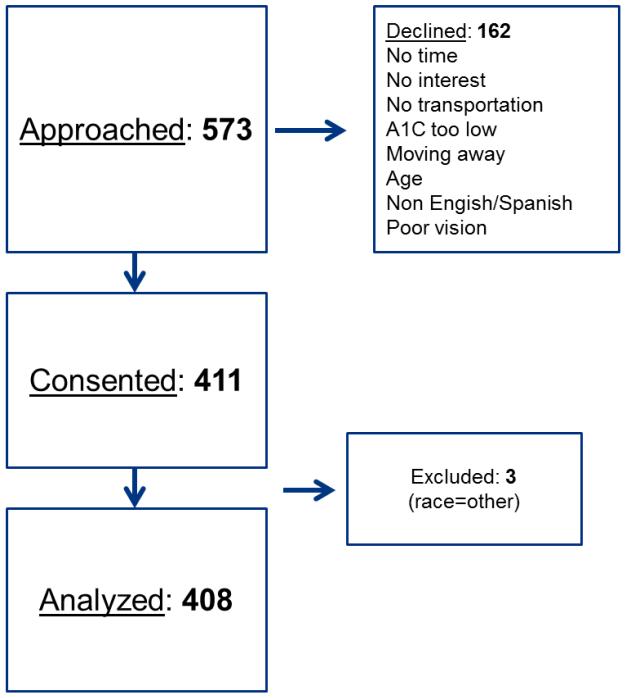
From July 2011 through August 2013, 573 patients were approached. One hundred and sixty-two patients either declined participation or were deemed ineligible; 411 patients were consented and enrolled. Three participants were excluded from this analysis as we focused on those individuals who self-identified as Non-Hispanic White, Non-Hispanic Black, or Hispanic/Latino, resulting in a final sample size of 408 participants (Figure 1). We observed that on average, participants were middle aged, predominantly female, had low annual income, and modest educational attainment. Nearly all participants were uninsured. The majority of participants were White but there was 37% minority representation (i.e. Black and/or Hispanic) in the sample. According to the s-TOFHLA, functional health literacy level was adequate in 83% of the sample. Assessment of diabetes-related characteristics revealed overall poor glycemic control, and over half of the sample was on insulin (nationally 17% of patients with diabetes were on insulin only in 2010) [1]. Most participants were overweight or obese.
Figure 1. Study Flow.
In unadjusted analyses (Table 2), higher communication, decision making, interpersonal style (i.e. IPC domains), and CAT scores were associated with near twice the odds of greater diabetes treatment satisfaction and near half the odds of higher medication non-adherence. Similarly, higher decision making scores were marginally associated with a 43% increase in the odds of greater portion size reduction and a 41% increase in the odds of greater self-efficacy for self-management. Higher interpersonal style score was also significantly associated with better glycemic control (A1C). No significant associations were observed for the other diabetes self-care variables as measured by the PDQ-11.
Table 2. Unadjusted & Adjusted Regression Modeling of Main Effects.
| Independent Communication Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Communication Score (IPC) | Decision Making Score (IPC) | Interpersonal Style Score (IPC) | Communication Assessment Test Score (CAT) |
|||||
|
|
||||||||
| Dependent Diabetes-related Outcome |
aUnadjusted OR (CI) |
aAdjusted OR (CI) |
aUnadjusted OR (CI) |
aAdjusted OR (CI) |
aUnadjusted OR (CI) |
aAdjusted OR (CI) |
aUnadjusted OR (CI) |
aAdjusted OR (CI) |
|
| ||||||||
| Treatment Satisfaction (DTSQ) n=385 |
1.76 (1.24, 2.51)† |
2.02 (1.38, 2.95)‡ |
1.96 (1.39, 2.76)‡ |
1.90 (1.31, 2.74)‡ |
1.92 (1.36, 2.74)‡ |
2.02 (1.39, 2.92)‡ |
1.8 (1.28, 2.54)‡ |
2.21 (1.53, 3.19)‡ |
|
| ||||||||
| Medication Non-adherence (ARMS) n=385 |
0.41 (0.29, 0.59)‡ |
0.45 (0.3, 0.66)‡ |
0.40 (0.28, 0.57)‡ |
0.39 (0.27, 0.57)‡ |
0.60 (0.42, 0.85)† |
0.66 (0.46, 0.95)* |
0.68 (0.48, 0.96)* |
0.68 (0.48, 0.98)* |
|
| ||||||||
| PDQ-11 Use of data to modify diet; n=385 |
0.92 (0.64, 1.31) |
0.81 (0.55, 1.20) |
0.92 (0.65, 1.3) |
0.78 (0.54, 1.13) |
0.73 (0.52, 1.04) |
0.64 (0.44, 0.92)* |
0.73 (0.52, 1.04) |
0.96 (0.67, 1.38) |
| Reduced portion size n=394 |
0.93 (0.65, 1.34) |
0.85 (0.58, 1.25) |
1.43 (1.01, 2.04)* |
1.23 (0.84, 1.79) |
1.04 (0.73, 1.48) |
0.94 (0.65, 1.36) |
0.95 (0.67, 1.35) |
0.79 (0.55, 1.15) |
|
| ||||||||
| Diabetes Self-efficacy (PDSMS) n=385 |
1.20 (0.84, 1.71) |
1.07 (0.73, 1.56) |
1.41 (1.0, 1.99)* |
1.41 (0.98, 2.03) |
1.01 (0.72, 1.43) |
0.99 (0.69, 1.42) |
1.21 (0.86, 1.69) |
1.05 (0.73, 1.5) |
|
| ||||||||
|
bUnadjusted β (CI) |
bAdjusted β (CI) |
bUnadjusted β (CI) |
bAdjusted β (CI) |
bUnadjusted β (CI) |
bAdjusted β (CI) |
bUnadjusted β (CI) |
bAdjusted β (CI) |
|
|
|
||||||||
| Glycemic Control (A1C) n=379 |
−0.02 (−0.06, 0.03) |
0.01 (−0.03, 0.05) |
−0.01 (−0.05, 0.03) |
0.00 (−0.04, 0.04) |
−0.06 (−0.10, − 0.02)† |
−0.04 (−0.08, 0.0) |
−0.01 (−0.05, 0.03) |
0.01 (−0.03, 0.05) |
Each model controlled age, race/ethnicity, gender, education, insurance, income, years since diagnosis, treatment assignment, literacy level, and insulin status
proportional odds logistic regression
linear regression of log transformed A1C.
p<0.05
p<0.01
p<0.001
In adjusted analyses that controlled for age, race/ethnicity, gender, education, insurance, income, years since diagnosis, treatment assignment, literacy level, and insulin status, several important associations remained significant. Higher communication, decision making, interpersonal style, and CAT scores remained significantly associated with twice the odds of reporting greater diabetes treatment satisfaction and near half the odds of greater medication non-adherence. Higher interpersonal style score was significantly associated with lower odds of using data to modify one’s diet. Finally, the previously observed associations between decision making score and portion size reduction, decision making score and self-efficacy for diabetes care, and interpersonal style score and glycemic control were reduced to non-significance [Table 2].
4. Discussion and Conclusion
4.1. Discussion
In this sample of predominantly uninsured, low-income, diabetes patients, we observed significant associations between patient’s perceptions of the quality of provider communication and several diabetes-related outcomes. Communication quality in this study was reflective of the provider’s ability to communicate clearly, effectively elicit patient concerns, explain results of laboratory and exam findings, involve the patient in decision making, spend adequate time with the patient, and demonstrate compassion & concern. Our study demonstrated that greater performance in these areas was significantly associated with higher diabetes treatment satisfaction and less medication non-adherence.
Treatment satisfaction and medication adherence clearly are important components of high value-based care for patients with chronic diseases, like diabetes. Lower satisfaction with treatment has been shown to be associated with diabetes complications and lower adherence to both medication and follow-up recommendations [43]. The reasons for medication non-adherence among diabetes patients are certainly multifactorial and often complex. Our findings lend support to those studies that have identified a positive association between provider communication quality and both objective and subjective reports of medication adherence. For example, among diabetes patients in a managed care setting, Ratanawongsa et al. found that when providers were rated lower in their ability to involve patients in decisions, understand their patients’ problems with treatment, and elicit confidence and trust, patients objectively were noted to have poorer medication refill adherence [44]. Similarly, Piette et al. reported that both general and diabetes-specific communication was positively associated with self-reported foot care, medication adherence, and diet and exercise behaviors among a diverse sample of patients primarily seen in a VA health care system [45].
To our knowledge, our study is among the first to identify similar associations between communication quality and diabetes outcomes among a predominantly low income, uninsured population seeking care in a public health setting, and provides insight into potential mechanisms for addressing disparities for vulnerable patients within these systems of care. It is important to note that racial/ethnic minorities in comparable practice settings have been shown to desire improved communication and support from their providers at similar rates compared to Whites [46, 47], yet providers are not always successful in meeting expectations for culturally competent care in general [48-50] and/or have been shown to potentially contribute directly to disparities of effective health communication [51]. These findings suggest that there remains a need for improvements in the patient-provider interaction during public health encounters for diabetes patients. This in fact is a major goal of our larger clinical trial that focuses on improving providers’ delivery of diabetes care in these settings [30].
Our analysis is subject to several limitations. Due to the cross-sectional design of the current set of analyses, we are unable to make inferences regarding causation among our observed associations. Also, despite successful recruitment of a diverse sample within a large public health system, we are cautious about generalizing our findings to other public health settings with different demographics. Our analyses were exploratory and no correction for multiple comparisons was performed. Further studies are needed to address each specific hypothesis of association, and to confirm reproducibility of our findings. Additionally, our sample size may have precluded the identification of associations between communication quality and other important diabetes factors such as diet/exercise behaviors and glycemic control. Finally, our study focuses on patient self-report of communication quality and self-care behaviors and is subject to social desirability biases.
4.2. Conclusion
Future evaluation of our program will provide additional knowledge and insight into the effects of improved provider communication on diabetes related outcomes. Overall, we have provided initial evidence that communication quality may be related to patient’s medication behaviors and overall satisfaction with care, supporting the role of effective health communication as a potentially key component of quality care for low income patients with diabetes.
4.3. Practice Implications
Safety-net, health department clinics often experience challenges of staffing and resources yet provide important access to care for low-income, underserved populations. Effective health communication in these settings may be an important component of high quality care for vulnerable populations. Targeted efforts such as ours to address disparities in diabetes care through improvements in the patient-provider interaction should be encouraged and supported.
Highlights.
Poor health communication (HC) may influence diabetes care for patients with limited resources
We examined the association of HC with select diabetes factors for patients in a safety-net system in TN
Predictors included self-reports of provider communication using validated measures
Better communication was associated with lower medication non-adherence and higher treatment satisfaction
Communication quality may be an important modifiable strategy for improving diabetes care for vulnerable populations
Table 1. Patient Characteristics and diabetes-related factors.
| Variable | Mean ± SD or n (%) |
|---|---|
|
| |
| N=408 | |
|
| |
| Demographics | |
|
| |
| Age | 49.7 ± 9.5 |
|
| |
| Gender | |
| Male | 160 (39%) |
| Female | 248 (61%) |
|
| |
| Race | |
| White | 257 (63%) |
| Black | 72 (18%) |
| Other | 79 (19%) |
|
| |
| Hispanic | |
| Yes | 97 (24%) |
| No | 311 (76%) |
|
| |
| Education | |
| Less than HS | 149 (37%) |
| HS grad/equivalent | 142 (35%) |
| Some college or beyond | 115 (28%) |
|
| |
| Health Literacy (s-TOFHLA) | |
| Adequate | 331 (83%) |
| Inadequate + Marginal (Limited) | 68 (17%) |
|
| |
| Household Income | |
| <$10,000 | 218 (54%) |
| $10,000-19,999 | 115 (28%) |
| >$20,000 | 71 (18%) |
|
| |
| Insurance Status | |
| Uninsured | 388 (96%) |
| Insured | 17 (4%) |
|
| |
| Diabetes Characteristics | |
|
| |
| A1C | 9.6 ± 2.1 |
|
| |
| Insulin use | |
| Yes | 242 (59%) |
|
| |
| Time since diagnosis, years | 9.0 ± 7.1 |
|
| |
| BMI (kg/m2) | 35.7 ± 8.9 |
|
| |
| LDL (mg/dl) | 102 ± 43 |
|
| |
| Blood Pressure | |
| Systolic | 133 ± 20 |
| Diastolic | 80 ± 10 |
|
| |
| Measures of Patient-Provider Interaction & Self-Care | |
|
| |
| IPC-18 (1-5) | |
| Communication | |
| Less than 5 | 264 (65%) |
| 5 | 144 (35%) |
| Decision Making | |
| Less than 5 | 231 (57%) |
| 5 | 177 (43%) |
| Interpersonal Style | |
| Less than 5 | 167 (41%) |
| 5 | 241 (59%) |
|
| |
| CAT Mean Score (1-5) | |
| Less than 5 | 210 (51%) |
| 5 | 198 (49%) |
|
| |
| Treatment Satisfaction (DTSQ) (0-36) | 28.1 ± 6.6 |
|
| |
| Medication Non-Adherence (ARMS) (12-48) | 17.2 ± 4.2 |
|
| |
| Self-efficacy (PDSMS) (8-40) | 16.6 ± 5.8 |
|
| |
| PDQ-11 | |
| Poor eating behavior (3-18) | 10.0 ± 3.4 |
| Use of data to modify diet (3-18) | 8.2 ± 4.7 |
| Skipped meals (%) | |
| Never | 196 (48%) |
| One or more times/month | 209 (52%) |
| Portion control (%) | |
| Never | 133 (33%) |
| One or more times/month | 271 (67%) |
| Physical activity (%) | |
| Very inactive-a little active | 174 (43%) |
| Moderate-very active | 231 (57%) |
| Weight management, stage of change | |
| No plan-starting next month | 217 (53%) |
| Started within 6 months-active ≥ 6 months | 187 (47%) |
| Exercise, stage of change | |
| No plan-starting next month | 237 (59%) |
| Started within 6 months-active ≥ 6 months | 167 (41%) |
Acknowledgements
The authors would like to thank the administration, staff, and patients at the Middle TN Department of Health system for their collaboration in the PRIDE program. We thank the staff at the Mayo Clinic Florida Office of Academic and Research Support for helpful input on later drafts. ROW is supported by a NIDDK Career Development Award (05DK092470). The project described was supported by the following grants: NIDDK 5R18 DK083264 and Vanderbilt University CTSA 5UL1TR000445. Study data were collected and managed using REDCap electronic data capture hosted at Vanderbilt University. Content of this manuscript was presented in poster format at the 36th Annual SGIM meeting in Denver, CO, April 25-27, 2013. ROW was the lead author of the manuscript. SE and AS provided statistical input. RLR and SK provided health communication expertise and mentorship to ROW. ROW, KAW, RLR, and SK provided substantive editorial input. All authors approved the final version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
No author has any conflicts of interest to report.
References
- 1.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. http://www.cdc.gov/diabetes/surveillance/index.htm. [Google Scholar]
- 2.Betancourt JR, Duong JV, Bondaryk MR. Strategies to reduce diabetes disparities: an update. Curr Diab Rep. 2012;12:762–8. doi: 10.1007/s11892-012-0324-1. [DOI] [PubMed] [Google Scholar]
- 3.Lanting LC, Joung IM, Mackenbach JP, Lamberts SW, Bootsma AH. Ethnic differences in mortality, end-stage complications, and quality of care among diabetic patients: a review. Diabetes Care. 2005;28:2280–8. doi: 10.2337/diacare.28.9.2280. [DOI] [PubMed] [Google Scholar]
- 4.Saydah S, Cowie C, Eberhardt MS, De Rekeneire N, Narayan KM. Race and ethnic differences in glycemic control among adults with diagnosed diabetes in the United States. Ethn Dis. 2007;17:529–35. [PubMed] [Google Scholar]
- 5.Andrulis DP. Access to care is the centerpiece in the elimination of socioeconomic disparities in health. Ann Intern Med. 1998;129:412–6. doi: 10.7326/0003-4819-129-5-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 6.Nelson A. Unequal treatment: confronting racial and ethnic disparities in health care. J Natl Med Assoc. 2002;94:666–8. [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services Agency for Healthcare Research and Quality: 2012 National Healthcare Disparities Report. U.S. Department of Health and Human Services Agency for Healthcare Research and Quality; Rockville, MD: 2013. [Google Scholar]
- 8.Linzer M, Manwell LB, Williams ES, et al. Working conditions in primary care: physician reactions and care quality. Ann Intern Med. 2009;151:28–36. doi: 10.7326/0003-4819-151-1-200907070-00006. [DOI] [PubMed] [Google Scholar]
- 9.Varkey AB, Manwell LB, Williams ES, et al. Separate and unequal: clinics where minority and nonminority patients receive primary care. Arch Intern Med. 2009;169:243–50. doi: 10.1001/archinternmed.2008.559. [DOI] [PubMed] [Google Scholar]
- 10.Davis MV, Cannon MM, Reese A, Lovette B, Porterfield DS. Public health case studies in diabetes prevention and control: innovation, partnerships, and funding. N C Med J. 2011;72:366–71. [PubMed] [Google Scholar]
- 11.Reyna VF, Nelson WL, Han PK, Dieckmann NF. How numeracy influences risk comprehension and medical decision making. Psychol Bull. 2009;135:943–73. doi: 10.1037/a0017327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sudore RL, Landefeld CS, Pérez-Stable EJ, Bibbins-Domingo K, Williams BA, Schillinger D. Unraveling the relationship between literacy, language proficiency, and patient-physician communication. Patient Educ Couns. 2009;75:398–402. doi: 10.1016/j.pec.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al Sayah F, Majumdar SR, Williams B, Robertson S, Johnson JA. Health literacy and health outcomes in diabetes: a systematic review. J Gen Intern Med. 2013;28:444–52. doi: 10.1007/s11606-012-2241-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boren SA. A review of health literacy and diabetes: opportunities for technology. J Diabetes Sci Technol. 2009;3:202–9. doi: 10.1177/193229680900300124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cavanaugh K, Huizinga MM, Wallston KA, et al. Association of numeracy and diabetes control. Ann Intern Med. 2008;148:737–46. doi: 10.7326/0003-4819-148-10-200805200-00006. [DOI] [PubMed] [Google Scholar]
- 16.Huizinga MM, Carlisle AJ, Cavanaugh KL, et al. Literacy, numeracy, and portion-size estimation skills. American journal of preventive medicine. 2009;36:324–8. doi: 10.1016/j.amepre.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osborn CY, Cavanaugh K, Wallston KA, White RO, Rothman RL. Diabetes numeracy: an overlooked factor in understanding racial disparities in glycemic control. Diabetes Care. 2009;32:1614–9. doi: 10.2337/dc09-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osborn CY, Cavanaugh K, Wallston KA, et al. Health literacy explains racial disparities in diabetes medication adherence. J Health Commun. 2011;16(Suppl 3):268–78. doi: 10.1080/10810730.2011.604388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White RO, 3rd, Osborn CY, Gebretsadik T, Kripalani S, Rothman RL. Development and validation of a Spanish diabetes-specific numeracy measure: DNT-15 Latino. Diabetes technology & therapeutics. 2011;13:893–8. doi: 10.1089/dia.2011.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White RO, Osborn CY, Gebretsadik T, Kripalani S, Rothman RL. Health literacy, physician trust, and diabetes-related self-care activities in Hispanics with limited resources. J Health Care Poor Underserved. 2013;24:1756–68. doi: 10.1353/hpu.2013.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavanaugh K, Wallston KA, Gebretsadik T, et al. Addressing literacy and numeracy to improve diabetes care: two randomized controlled trials. Diabetes Care. 2009;32:2149–55. doi: 10.2337/dc09-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborn CY, Cavanaugh K, Wallston KA, Rothman RL. Self-efficacy links health literacy and numeracy to glycemic control. J Health Commun. 2010;15(Suppl 2):146–58. doi: 10.1080/10810730.2010.499980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman RL, DeWalt DA, Malone R, et al. Influence of patient literacy on the effectiveness of a primary care-based diabetes disease management program. J Amer Med Assoc. 2004;292:1711–6. doi: 10.1001/jama.292.14.1711. [DOI] [PubMed] [Google Scholar]
- 24.Rothman RL, Malone R, Bryant B, et al. The Spoken Knowledge in Low Literacy in Diabetes scale: a diabetes knowledge scale for vulnerable patients. Diabetes Educ. 2005;31:215–24. doi: 10.1177/0145721705275002. [DOI] [PubMed] [Google Scholar]
- 25.White RO, Wolff K, Cavanaugh KL, Rothman R. Addressing Health Literacy and Numeracy to Improve Diabetes Education and Care. Diabetes Spectr. 2010;23:238–43. doi: 10.2337/diaspect.23.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schillinger D, Bindman A, Wang F, Stewart A, Piette J. Functional health literacy and the quality of physician-patient communication among diabetes patients. Patient Educ Couns. 2004;52:315–23. doi: 10.1016/S0738-3991(03)00107-1. [DOI] [PubMed] [Google Scholar]
- 27.Lesser J, Oscós-Sánchez MA. Community-academic research partnerships with vulnerable populations. Annu Rev Nurs Res. 2007;25:317–37. [PubMed] [Google Scholar]
- 28.Fancher TL, Keenan C, Meltvedt C, et al. An academic-community partnership to improve care for the underserved. Acad Med. 2011;86:252–8. doi: 10.1097/ACM.0b013e31820469ba. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System: Prevalence and Trends Data-Tennessee 2010 Diabetes. Office of Surveillance, Epidemiology, and Laboratory Services; 2010. [Google Scholar]
- 30.National Institute of Diabetes and Digestive and Kidney Disorders. Vanderbilt University . The Public Private Partnership Addressing Literacy-Numeracy to Improve Diabetes Care (PRIDE) National Library of Medicine (US); Bethesda, MD: 2011. Clinical-Trials.gov [Internet] NLM Identifier: NCT01344668. [Google Scholar]
- 31.Wolff K, Cavanaugh K, Malone R, et al. The Diabetes Literacy and Numeracy Education Toolkit (DLNET): materials to facilitate diabetes education and management in patients with low literacy and numeracy skills. Diabetes Educ. 2009;35:233–6. 8–41, 44–5. doi: 10.1177/0145721709331945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stetson B, Schlundt D, Rothschild C, Floyd JE, Rogers W, Mokshagundam SP. Development and validation of The Personal Diabetes Questionnaire (PDQ): a measure of diabetes self-care behaviors, perceptions and barriers. Diabetes Res Clin Pract. 2011;91:321–32. doi: 10.1016/j.diabres.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Kripalani S, Risser J, Gatti ME, Jacobson TA. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12:118–23. doi: 10.1111/j.1524-4733.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- 34.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38:33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 35.Bradley C. Diabetes treatment satisfaction questionnaire. Change version for use alongside status version provides appropriate solution where ceiling effects occur. Diabetes Care. 1999;22:530–2. doi: 10.2337/diacare.22.3.530. [DOI] [PubMed] [Google Scholar]
- 36.Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS) J Behav Med. 2007;30:395–401. doi: 10.1007/s10865-007-9110-y. [DOI] [PubMed] [Google Scholar]
- 37.Stewart AL, Nápoles-Springer A, Pérez-Stable EJ. Interpersonal processes of care in diverse populations. Milbank Q. 1999;77:305–39. 274. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stewart AL, Nápoles-Springer AM, Gregorich SE, Santoyo-Olsson J. Interpersonal processes of care survey: patient-reported measures for diverse groups. Health Serv Res. 2007;42:1235–56. doi: 10.1111/j.1475-6773.2006.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Makoul G, Krupat E, Chang CH. Measuring patient views of physician communication skills: development and testing of the Communication Assessment Tool. Patient Educ Couns. 2007;67:333–42. doi: 10.1016/j.pec.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez A, Schillinger D, Grumbach K, et al. Physician language ability and cultural competence. An exploratory study of communication with Spanish-speaking patients. J Gen Intern Med. 2004;19:167–74. doi: 10.1111/j.1525-1497.2004.30266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schenker Y, Stewart A, Na B, Whooley MA. Depressive symptoms and perceived doctor-patient communication in the Heart and Soul study. J Gen Intern Med. 2009;24:550–6. doi: 10.1007/s11606-009-0937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutner M, Nachtsheim C, Neter J. Applied Linear regression Models. 4th ed McGraw Hill; Irwin: 2004. [Google Scholar]
- 43.Biderman A, Noff E, Harris SB, Friedman N, Levy A. Treatment satisfaction of diabetic patients: what are the contributing factors? Fam Pract. 2009;26:102–8. doi: 10.1093/fampra/cmp007. [DOI] [PubMed] [Google Scholar]
- 44.Ratanawongsa N, Karter AJ, Parker MM, et al. Communication and medication refill adherence: the Diabetes Study of Northern California. J Amer Med Assoc Intern Med. 2013;173:210–8. doi: 10.1001/jamainternmed.2013.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18:624–33. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peek ME, Tang H, Cargill A, Chin MH. Are there racial differences in patients’ shared decision-making preferences and behaviors among patients with diabetes? Med Decis Making. 2011;31:422–31. doi: 10.1177/0272989X10384739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sarkar U, Piette JD, Gonzales R, et al. Preferences for self-management support: findings from a survey of diabetes patients in safety-net health systems. Patient Educ Couns. 2008;70:102–10. doi: 10.1016/j.pec.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kutob RM, Bormanis J, Crago M, Senf J, Gordon P, Shisslak CM. Assessing culturally competent diabetes care with unannounced standardized patients. Fam Med. 2013;45:400–8. [PubMed] [Google Scholar]
- 49.Gavin JR, Wright EE. Building cultural competency for improved diabetes care: African Americans and diabetes. J Fam Pract. 2007;56:S22–8. [PubMed] [Google Scholar]
- 50.Cabellero AE, Tenzer P. Building cultural competency for improved diabetes care: Latino Americans and diabetes. J Fam Pract. 2007;56:S7–13. [PubMed] [Google Scholar]
- 51.Johnson RL, Roter D, Powe NR, Cooper LA. Patient race/ethnicity and quality of patient-physician communication during medical visits. Am J Public Health. 2004;94:2084–90. doi: 10.2105/ajph.94.12.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]