Abstract
Purpose
The marrow composition throughout the body is heterogeneous and changes with age. Due to the heterogeneity, invasive biopsies of the iliac crest do not truly represent the complete physiological status, impeding the clinical effectiveness of this method. Therefore, we aim to provide verification for an in vivo imaging technique using co-registered histologic examinations for assessment of marrow adiposity.
Methods
Five recently expired (i.e. < 24 hours) human cadavers were scanned with a dual source CT (DECT) scanner in order to measure marrow fat in the lumbar vertebrae. These donors were also imaged using water-fat MRI (wfMRI) which was used to estimate the fraction of yellow marrow. After imaging, lumbar columns were excised and the superior and inferior aspects of 21 vertebrae were removed. The remaining center section was processed for histological examination to find the ratio of adipocyte volume per tissue volume (AV/TV).
Results
Results of DECT and wfMRI had a high correlation (r = 0.88). AV/TV ranged from 0.18 to 0.75 with a mean (SD) of 0.36 (0.18). Inter-evaluator reliability for AV/TV was r > 0.984. There were similar correlations between AV/TV and the imaging modalities, DECT-derived MF and wfMRI (r = 0.802 and 0.772, respectively).
Conclusions
A high MF variation was seen among the 25 vertebrae imaged. Both DECT and wfMRI have a good correlation with the histologic adipocyte proportion and can be used to measure MF. This makes longitudinal studies possible without painful, less-effective, invasive biopsies
Keywords: DECT, Water-Fat MRI, marrow verification, imaging co-registration
INTRODUCTION
Cancellous bone of a single vertebral body is heterogeneous, with density varying by as much as 20% [1]. Common in vivo methods of assessing cancellous BMD (e.g. DXA, Quantitative Computed Tomography) integrate the density of calcified tissue with marrow density. However, marrow composition can vary from higher density hematopoietic red marrow to less dense adipocytic yellow marrow. Increased marrow fat can also alter microstructure heterogeneity of the trabecular matrix and has also been shown to influence vertebral failure patterns [2–4]. Most bone histopathology studies were limited in focus to studying osseous structures [5–7] or specific pathologic abnormalities in cancellous bone [8–10]. To our knowledge, there has been no study performed to correlate in vivo imaging of bone and bone marrow with coregistered histologic quantification of marrow fat.
We present here a study on the correlation between histologic examination of marrow fat fraction and clinical imaging by means of Dual Energy Computed Tomography (DECT) and Magnetic Resonance Imaging (MRI). DECT has the ability to identify marrow composition through use of basis material decomposition techniques [11]. We have already developed water-fat MRI in a previous study to measure the fat fraction signal in bone marrow [12].
METHOD AND MATERIALS
1. Subjects
Donors were accepted into this study as part of a larger investigation into the heterogeneity of cancellous bone. Approval for study was given by the Anatomy Bequest Program at the University of Minnesota. The five donors were female with a mean age of 56.8 ± 8.2 years. 4 of the 5 subjects were diagnosed with cancer and last treated with radiation or chemotherapy within the past 6 months. The diagnoses included cancer of the cervix, breast, and epiglottis and Chronic Lymphocytic Leukemia. Potential subjects were excluded with a history of knee, hip, or shoulder replacement, obesity, or were ever treated with a bisphosphonate. Specific skeletal sites with diagnosed metastatic disease were avoided. Donors were received by the University of Minnesota Anatomy Bequest Program.
2. Imaging
Within 24 hours postmortem, fully intact bodies were brought to the Radiology department at the University of Minnesota Medical Center, Fairview in Minneapolis, MN. Each body was first imaged using a Siemens Somatom Definition Flash DECT scanner (Siemens Medical Solutions USA, Inc., Malvern, PA, USA). The scan utilized Dual source CT at energies of 80kVp and at 140kVp with an additional tin filter for accurate image co-registration. Each donor was scanned cephalic caudal with 1mm slices for both energies. Lack of acceptable resolution resulted with only 20 lumbar vertebra imaged with MRI. Vertebral body excision also reduced the number of vertebral bodies examined by both imaging modalities and H&E examination to 17 (Table 1).
Table 1.
Donor demographics and lumbar vertebrae selection
| Lumbar Vertebrae Examined* | ||||||
|---|---|---|---|---|---|---|
| Donor | Age | Body Mass Index |
Disease | Excised | DECT | MRI |
| 1 | 53 | 22.9 | Cervical Ca | L2-L4 | All 5 | L2-L5 |
| 2 | 47 | 20.7 | Breast Ca | L1-L4 | All 5 | All 5 |
| 3 | 62 | 22.8 | Epiglottic Supraglottic Tumor | All 5 | All 5 | L5 |
| 4 | 68 | 23.5 | CLL | All 5 | All 5 | All 5 |
| 5 | 54 | 22.1 | Hepatitis | L1-L4 | All 5 | All 5 |
| Average: | 56.8 | 22.4 | Count: | 21 | 25 | 20 |
17 vertebral bodies were imaged with DECT, MRI, and H&E histology
For DECT, yellow marrow volume fractions (YMF) were calculated from basis material composition estimates for bone ROIs relative to the manufacturer-reported basis material compositions for the CT calibration phantom. Average basis material composition (ρH2O, ρK2HPO4) was estimated for an ROI placed within matching regions of in CT data sets acquired at 80 kVp and 140 kVp using the following equations:
| (Equation 1) |
| (Equation 2) |
| (Equation 3) |
where CT80 and CT140 are the average CT values within the defined ROIs and βH2O,80, βH2O,140, βK2HPO4,80, βK2HPO4,140 are calibration slopes determined from CT calibration phantom measurements.
These basis material compositions were interpreted relative to water and a theoretical prediction of the basis material composition of yellow marrow obtained from energy-dependent mass attenuation coefficients calculated using an openly available tool (XCOM) [13, 14], atomic composition data from ICRU Publication 46 and a density of 0.93 g/cm3 [15]. Patient measurements were projected onto the line in the basis material space connecting the basis material composition of yellow marrow and that of water using a minimum distance projection, P, parameterized such that the position P=100% corresponded to yellow marrow and the position P=0% corresponded to water. Since red bone marrow is more dense than water, values of P can range from −66% to 100%. The CT-derived estimate of MF was then normalized to the MRI scale without affecting correlation using: YMF = 0.6 * P + 0.396.
After DECT imaging, the donor was imaged on a 3 Tesla MRI scanner (Tim TRIO, Siemens Medical Solutions, Malvern, PA, USA) using a three-point Dixon technique [16–18] as used previously for a in vivo pelvic study [12]. Images were acquired with the donor in supine position using the vendor’s spine array receive coil in combination with an anterior body matrix coil centered over the lumbar spine. After localizer scans, a series of 3D gradient echo images were acquired with TR = 9 ms, flip = 1°, matrix 448×266, 256 slices, 1.5 mm slice thickness, acquisition time 225 s. Three consecutive images were acquired with TE = 2, 3, and 4 ms. The raw image data were reconstructed offline in Matlab (MathWorks, Natick MA) using the method of Berglund et al. [18], as implemented in the ISMRM Fat-Water Toolbox [19], and using a 9-peak model for the lipid spectral peaks [20]. The water and fat images produced by the reconstruction were used to calculate the signal fat fraction (sFF), defined as sFF=fat/(fat+water). The sFF maps were exported in DICOM format for analysis. ROIs were recorded in order to ensure accurate co-registration with DECT imaging.
3. Excision, registration technique
After clinical imaging, vertebral samples were removed by the University of Minnesota Bequest program staff. Vertebral bodies were separated from the pedicles using a diamond tipped bone saw and placed in formalin. 21 individual Lumbar vertebrae were then cleaned of soft tissue to expose cortical bone.
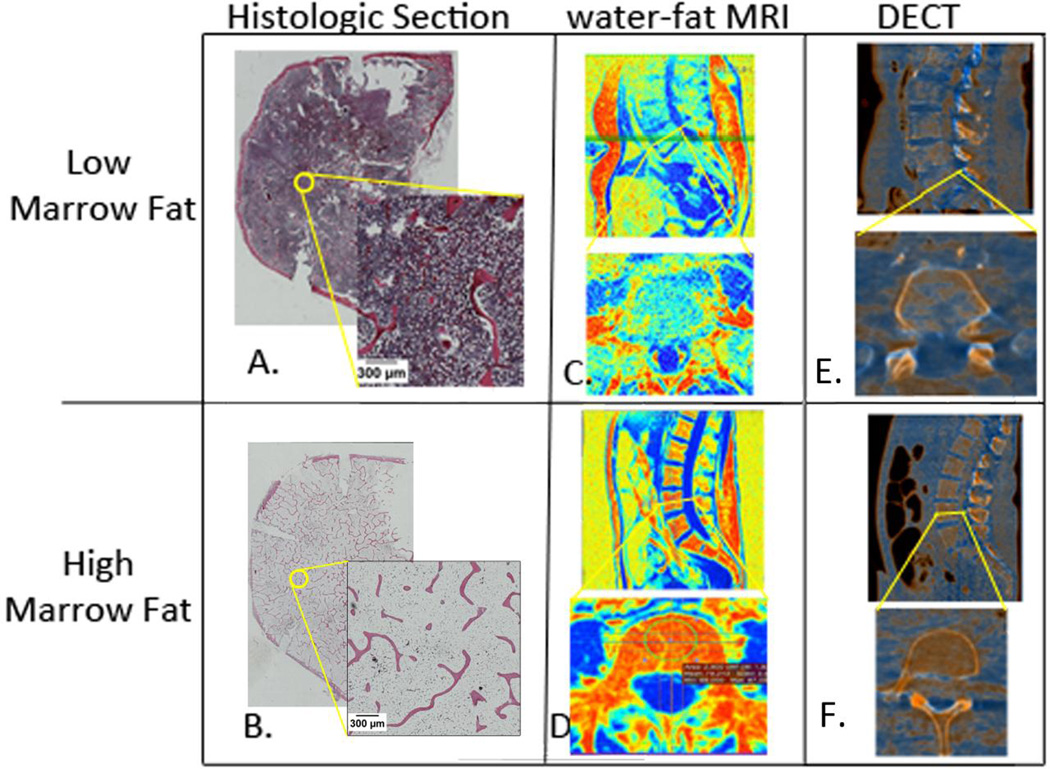
The vertebral bodies were cut with a DELTA PEC bench band saw (Chang Type, Taiwan) to remove superior and inferior aspects and keep a 5mm section. Taking a histologic section from the center of the vertebral body ensured that the AV/TV was taken from a representative section of the imaging ROI. These sections were decalcified (Formical-2000, Decal Chemical Corp., New York, USA) (20x volume per sample) until the cortical bone was completely demineralized. The sections were cut mid-sagittally to make left and right halves. Each vertebral half was then embedded in paraffin wax and stained by the H&E staining method. Eight vertebral body midsections were processed whole instead of being cut in half so that matching ROIs could analyzed (Figure 1).
Figure 1.
Multi-Modality Co-registration between histologic examination and in vivo imaging. Two vertebral bodies analyzed: top row is representative case of lower marrow fat and bottom row is higher marrow fat. A. & B. axial section of whole vertebral body with H&E staining. Void spaces of adipocytes were contoured by threshold and artifacts were removed at the discretion of the investigator. C. & D. water-fat MRI images of lumbar vertebrae and example of ROI placement. E. & F. DECT images reconstructed to highlight marrow fat intensities. For imaging modalities, red represents high marrow fat concentration.
4. Histology image tile procedure
A full digital image of each vertebrae half was produced by image tiling with a Nikon AZ100M Macro Fluorescence Scope (Nikon Corporation, Tokyo, Japan) with a color camera at 4x magnification. The cellular components of the produced images were then analyzed using ImageJ [21]. The scale was calibrated to 512 pixels/mm and the images were converted to an 8-bit gray scale. An approximately 2 mm diameter region of interest in each vertebra half was selected and areas of high artifact were avoided. A threshold was applied to identify adipocytes as void spaces remaining from the H&E staining process. Non-adipocytic void spaces, commonly seen as artifacts in H&E staining, were excluded from this selection process at the discretion of each user. Adipocyte volume per tissue volume (AV/TV) was calculated for each half. The AV/TVs for both halves were averaged together.
In order to test intra-sample variability, histologic examinations were also performed at 0.5cm superior and 0.5cm inferior to the middle in three vertebral bodies. Also, two investigators examined the histology images for the marrow fat ratio in order to test inter-user variability using the intraclass correlation coefficient.
RESULTS
DECT-derived yellow marrow fraction estimations were calculated for all 25 lumbar vertebrae in the five donors. YMF ranged from 0.07 to 0.91 for the lowest and highest fat signal, respectively. For wfMRI-derived signal fat fraction, values could only be determined for 20 lumbar vertebrae; one subjects had poor signal to noise ratio above L5 and another had a poor signal to analyze L1. Values ranged from 0.22 for less fatty marrow to 0.79 for the highest sFF.
The AV/TV of 21 lumbar vertebrae ranged from 0.18 to 0.75 with a mean (SD) of 0.36 (0.18). Vertebral bodies taken from the same cadaver showed much less diversity as seen by standard deviations of AV/TV between 0.03 and 0.07. For the three vertebrae that were analyzed at three levels within 1 cm, the average coefficient of variation between the sections was 0.08. There was a high correlation between the two users as seen by an intraclass correlation coefficient of r = 0.984.
17 vertebral bodies were scanned with both DECT and wfMRI and then processed for H&E examination. There were moderate correlations between the AV/TV and the results of DECT and wfMRI (r = 0.80 and 0.77, respectively) (Figure 2a). A higher correlation was seen between the two imaging modalities with similar ROIs than with histology (r = 0.88) (Figure 2b).
Figure 2.
Marrow fat estimation from histology and in vivo imaging modalities. A) 17 vertebral bodies were imaged with DECT and MRI and then processed for H&E examination. Results of the imaging are compared with histologic adipocyte volume per tissue volume (AV/TV). B) Correlation between the marrow fat estimation results from two non-invasive imaging techniques.
DISCUSSION
Current clinical practice of marrow fat assessment typically involves an invasive and painful bone marrow biopsy of the posterior iliac crest. However, this method does not give an accurate picture of the heterogeneity that is present in bone marrow throughout the body. For the first time, AV/TV quantifications taken from vertebral bodies have been correlated with results from dual energy CT (DECT) and wfMRI modalities. Both DECT and wfMRI were able to estimate yellow marrow fat fractions as validated by AV/TV in 17 coregistered samples.
Although bone marrow adipocyte parameters have been used to quantify marrow fat from trephine biopsies of the iliac crest [22–25], it is not commonly used on H&E stains of intact vertebral body cross-sections. In our small sample size, we found that inter-subject AV/TV can vary by nearly 60%, and even different intra-subject sites varied by as much as 40%. This high variability could be due to the diverse treatment effects each individual received prior to death or just a reflection of the true heterogeneous makeup of bone marrow within individuals. The technique used to estimate AV/TV is highly reproducible if proper instruction is given for estimating adipocyte grayscale threshold.
1. Clinical implications
With the increased commercial availability of dual energy CT scanners and recent proof of acceptable dose exposure [14], DECT is becoming more prevalent in hospitals and treatment centers. The ability to measure marrow fat at any cancellous bone site could provide a pathway for studies to monitor changes, in vivo, using DECT. It may also be useful in radiation therapy treatment planning where targeting of active bone marrow would reduce the radiation treatment exposure to healthy tissue. DECT can easily be adapted in treatment facilities (e.g. radiation oncology departments) where whole body PET/CTs are commonplace and single energy CT imaging is commonly performed to monitor cancer survivors.
2. Limitations
The subjects in this study were all given cytotoxic treatments within the last 6 months before death which is known to affect the bone marrow composition. For donors who underwent radiation therapy, treatment fields and specific absorbed dose were not known for each skeletal site. Also, investigations into the true variation in the average human body would need this examination repeated with a more controlled population. However, this study was focused on validating marrow fat fraction estimation from in vivo imaging techniques. Decomposition processes may introduce some bias into the data. However, these processes are likely to be negligible, as bone marrow from recently deceased individuals is commonly transplanted to immunocompromised patients.
3. Conclusion
In conclusion, cancellous bone regions were examined by histology and co-registered in vivo clinical imaging. Marrow fat fraction intensities taken from imaging were validated with histologic quantification of adipocyte prevalence. This may be able to monitor bone health of individuals who have undergone cytotoxic treatment, especially the cancer survivor.
Acknowledgments
This work was supported by the National Institute of Health grants (1R01CA154491-01; RO3 AR055333-02,1K12-HD055887-01), the by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number 8UL1TR000114-02, and Cancer Center Support Grant P30 CA77398. Other grant support includes the Minnesota Medical Foundation and seed grants from the university (Academic Health Center, Grant in Aid, Masonic Cancer Center breast cancer research). Susanta K Hui is a scholar of the BIRCWH (Building Interdisciplinary Careers in Women's Health) program. This work was supported by Japan Society for the Promotion of Science Core to Core Program (23003).
The authors wish to thank Angie McArthur and her staff at the University of Minnesota Anatomy Bequest Program as well as the women who donated their bodies for the advancement of education and research. We would also like to thank Colleen Foster of the BioNet Histology and Immunohistochemistry Laboratory at the University of Minnesota.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banse X, Devogelaer JP, Munting E, Delloye C, Cornu O, Grynpas M. Inhomogeneity of human vertebral cancellous bone: systematic density and structure patterns inside the vertebral body. Bone. 2001;28:563–571. doi: 10.1016/s8756-3282(01)00425-2. [DOI] [PubMed] [Google Scholar]
- 2.Wegrzyn J, Roux JP, Arlot ME, Boutroy S, Vilayphiou N, Guyen O, Delmas PD, Chapurlat R, Bouxsein ML. Role of trabecular microarchitecture and its heterogeneity parameters in the mechanical behavior of ex vivo human L3 vertebrae. J Bone Miner Res. 2010;25:2324–2331. doi: 10.1002/jbmr.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hayashi T, Chen H, Miyamoto K, Zhou X, Hara T, Yokoyama R, Kanematsu M, Hoshi H, Fujita H. Analysis of bone mineral density distribution at trabecular bones in thoracic and lumbar vertebrae using X-ray CT images. J Bone Miner Metab. 2011;29:174–185. doi: 10.1007/s00774-010-0204-1. [DOI] [PubMed] [Google Scholar]
- 4.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr. 2009;19:109–124. doi: 10.1615/critreveukargeneexpr.v19.i2.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engelke K, Graeff W, Meiss L, Hahn M, Delling G. High spatial resolution imaging of bone mineral using computed microtomography. Comparison with microradiography and undecalcified histologic sections. Invest Radiol. 1993;28:341–349. doi: 10.1097/00004424-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Bromley RG, Dockum NL, Arnold JS, Jee WS. Quantitative histological study of human lumbar vertebrae. J Gerontol. 1966;21:537–543. doi: 10.1093/geronj/21.4.537. [DOI] [PubMed] [Google Scholar]
- 7.Grote HJ, Amling M, Vogel M, Hahn M, Posl M, Delling G. Intervertebral variation in trabecular microarchitecture throughout the normal spine in relation to age. Bone. 1995;16:301–308. doi: 10.1016/8756-3282(94)00042-5. [DOI] [PubMed] [Google Scholar]
- 8.Bassett LW, Mirra JM, Cracchiolo A, 3rd, Gold RH. Ischemic necrosis of the femoral head. Correlation of magnetic resonance imaging and histologic sections. Clin Orthop Relat Res. 1987:181–187. [PubMed] [Google Scholar]
- 9.Zanetti M, Bruder E, Romero J, Hodler J. Bone marrow edema pattern in osteoarthritic knees: correlation between MR imaging and histologic findings. Radiology. 2000;215:835–840. doi: 10.1148/radiology.215.3.r00jn05835. [DOI] [PubMed] [Google Scholar]
- 10.Saadat E, Jobke B, Chu B, Lu Y, Cheng J, Li X, Ries MD, Majumdar S, Link TM. Diagnostic performance of in vivo 3-T MRI for articular cartilage abnormalities in human osteoarthritic knees using histology as standard of reference. Eur Radiol. 2008;18:2292–2302. doi: 10.1007/s00330-008-0989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodsitt MM, Hoover P, Veldee MS, Hsueh SL. The composition of bone marrow for a dual-energy quantitative computed tomography technique. A cadaver and computer simulation study. Invest Radiol. 1994;29:695–704. doi: 10.1097/00004424-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Bolan PJ, Arentsen L, Sueblinvong T, Zhang Y, Moeller S, Carter JS, Downs LS, Ghebre R, Yee D, Froelich J, Hui S. Water-fat MRI for assessing changes in bone marrow composition due to radiation and chemotherapy in gynecologic cancer patients. J Magn Reson Imaging. 2013;38:1578–1584. doi: 10.1002/jmri.24071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger MJ, Hubbell J, Seltzer S, Chang J, Coursey J, Sukumar R, Zucker D. XCOM: Photon cross sections database. NIST Standard Reference Database. 1998;8:87–3597. [Google Scholar]
- 14.Johnson TR, Krauss B, Sedlmair M, Grasruck M, Bruder H, Morhard D, Fink C, Weckbach S, Lenhard M, Schmidt B. Material differentiation by dual energy CT: initial experience. European radiology. 2007;17:1510–1517. doi: 10.1007/s00330-006-0517-6. [DOI] [PubMed] [Google Scholar]
- 15.Photon E. Proton and Neutron Interaction Data for Body Tissues. ICRU Report. 1992;46 [Google Scholar]
- 16.Dixon WT. Simple proton spectroscopic imaging. Radiology. 1984;153:189–194. doi: 10.1148/radiology.153.1.6089263. [DOI] [PubMed] [Google Scholar]
- 17.Glover GH, Schneider E. Three-point Dixon technique for true water/fat decomposition with B0 inhomogeneity correction. Magn Reson Med. 1991;18:371–383. doi: 10.1002/mrm.1910180211. [DOI] [PubMed] [Google Scholar]
- 18.Berglund J, Johansson L, Ahlstrom H, Kullberg J. Three-point Dixon method enables whole-body water and fat imaging of obese subjects. Magn Reson Med. 2010;63:1659–1668. doi: 10.1002/mrm.22385. [DOI] [PubMed] [Google Scholar]
- 19.Hu HH, Bornert P, Hernando D, Kellman P, Ma J, Reeder S, Sirlin C. ISMRM workshop on fat-water separation: insights, applications and progress in MRI. Magn Reson Med. 2012;68:378–388. doi: 10.1002/mrm.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton G, Yokoo T, Bydder M, Cruite I, Schroeder ME, Sirlin CB, Middleton MS. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011;24:784–790. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Rasband WS. ImageJ. Bethesda, Maryland, USA: U.S. National Institutes of Health; 1997–2011. [Google Scholar]
- 22.Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int. 2008;19:1323–1330. doi: 10.1007/s00198-008-0574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen A, Dempster DW, Recker RR, Lappe JM, Zhou H, Zwahlen A, Muller R, Zhao B, Guo X, Lang T, Saeed I, Liu XS, Guo XE, Cremers S, Rosen CJ, Stein EM, Nickolas TL, McMahon DJ, Young P, Shane E. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98:2562–2572. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen A, Dempster DW, Stein EM, Nickolas TL, Zhou H, McMahon DJ, Muller R, Kohler T, Zwahlen A, Lappe JM, Young P, Recker RR, Shane E. Increased marrow adiposity in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2012;97:2782–2791. doi: 10.1210/jc.2012-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiele J, Hoeppner B, Zankovich R, Fischer R. Histomorphometry of bone marrow biopsies in primary osteomyelofibrosis/-sclerosis (agnogenic myeloid metaplasia)--correlations between clinical and morphological features. Virchows Arch A Pathol Anat Histopathol. 1989;415:191–202. doi: 10.1007/BF00724905. [DOI] [PubMed] [Google Scholar]