Abstract
Hippocampal sclerosis (HpScl) is frequent in frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP), but it also occurs in dementia of the elderly with or without accompanying Alzheimer type pathology. HpScl has been hypothesized to be a neurodegenerative process given its association with TDP-43 pathology, but this is still controversial. TDP-43 pathology is found in Lewy body disease (LBD), but no study has focused on the pathologic and genetic characteristics of HpScl in LBD. We found HpScl in 5.2% of 669 LBD cases (289 transitional and 380 diffuse). Older age, higher Braak neurofibrillary tangle (NFT) stage, and presence of TDP-43 pathology were associated with HpScl. There was no difference in the frequency of HpScl between transitional and diffuse LBD, suggesting that Lewy related pathology appears to have no direct association with HpScl. All HpScl cases had TDP-43 pathology consistent with Type A pattern. HpScl cases harbored genetic variation in TMEM106B that has been previously associated with FTLD-TDP. Interestingly, the severity of TDP-43-positive fine neurites in CA1 sector, a possible pathologic precursor of HpScl, was associated with the TMEM106B variant. These results demonstrate HpScl in LBD is a TDP-43 proteinopathy and is similar to FTLD-TDP Type A. Furthermore, a subset of LBD cases without HpScl (“pre-HpScl”) had similar pathologic and genetic characteristics to typical HpScl, suggesting that the spectrum of HpScl pathology may be wider than previously thought. Some cases with many extracellular NFTs also had a similar profile. We suggest that HpScl is “masked” in these cases.
Keywords: Hippocampal sclerosis, Lewy body disease, Neuropathology, TDP-43, TMEM106B
INTRODUCTION
Hippocampal sclerosis (HpScl) in the elderly is a pathologic diagnosis characterized by selective neuronal loss and gliosis in CA1 sector and/or subiculum of the hippocampus [6,24]. The clinical manifestation is slowly progressive amnestic dysfunction. In many patients with dementia, HpScl coexists with other neurodegenerative disorders [31], including Alzheimer’s disease (AD), frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP), and Lewy body disease (LBD). The prevalence of HpScl in autopsy series of dementia ranges from 2.8% to 23.4% [31]. It is most frequently associated with FTLD-TDP and can be seen in approximately 70% of FTLD-TDP cases [11]. It is also relatively common in AD cases, and the reported prevalence is about 10%. Occasionally, it has been reported as the only pathologic explanation for dementia and is termed “pure-HpScl ” [1]. The prevalence of “pure-HpScl ” ranges from 0.4% to 4.6%.
The etiology of HpScl in the elderly is still unknown, but it has been hypothesized to be a neurodegenerative process given its association with TDP-43 pathology [2]. TDP-43 pathology was initially considered to be a specific marker for FTLD-TDP [26]; however, it is increasingly clear that TDP-43 pathology is also seen in some AD cases and in most AD cases with concurrent HpScl [2,5,21,24,27]. TDP-43 pathology was detected in 19% to 57% of AD cases [2,12] and about 80% of AD with HpScl cases [5]. While hypoxic damage causes a similar distribution of neuronal loss in the hippocampus, TDP-43 pathology is not detected in such cases. Recent studies have also shown genetic similarity between HpScl and FTLD-TDP. One of the most common genetic causes of FTLD-TDP is a mutation in the progranulin (GRN) gene, and most of these mutation carriers have HpScl [10,16,17]. In addition to causative mutations, two genetic risk variants have been identified as common risk factors for FTLD-TDP and HpScl in AD [5,21,29]. One is located in the 3’ untranslated region of GRN (rs5848), and the other is located in the uncharacterized transmembrane protein TMEM106B (rs1990622).
Lewy body disease (LBD) is pathologically characterized by Lewy bodies (LBs) and Lewy neurites, which are composed of α-synuclein [13,19]. LBD is accompanied by varying degrees of Alzheimer type pathology, including neurofibrillary tangles (NFTs) and senile plaques (SPs). LBD cases in this study had a range of clinical presentations including Parkinson’s disease, Parkinson’s disease with dementia, dementia with Lewy bodies (DLB), and other syndromes [14]. Recent studies have demonstrated that TDP-43 pathology is relatively common in LBD, with the reported frequency ranging from 18% to 60% [3,9,22,30]. The presence of TDP-43 pathology in LBD is associated with severity of tau pathology; however, the influence of α-synuclein pathology on the accumulation of TDP-43 pathology is poorly understood. As far as we know, there are no reports on the frequency and characteristics of HpScl in LBD.
The pathologic diagnosis of HpScl in the elderly can be difficult if abundant AD pathology coexists because NFTs also cause neuronal loss in similar regions of the hippocampus. In such cases, the diagnosis of HpScl is warranted only when neuronal loss is disproportionate to the number of extracellular NFTs. Given the high frequency of TDP-43 pathology in HpScl [2,24,25,27], TDP-43 immunohistochemistry can be helpful to the diagnosis of HpScl. Hatanpaa et al. reported [8] that TDP-43-positive fine neurites in the pyramidal layer of the hippocampus might be a precursor lesion of the HpScl in FTLD-TDP. Whether this also applies to TDP-43 pathology in HpScl in the elderly is not fully understood.
The aim of this study was to investigate pathologic and genetic characteristics of HpScl in LBD. In addition, we examined whether the severity and subtype of TDP-43 pathology differed between LBD cases with and without HpScl. The findings presented in this report contribute to increasing evidence that HpScl in the elderly might share disease processes with FTLD-TDP. Furthermore, results of immunohistochemical analysis of TDP-43 pathology indicate that the pathologic spectrum of HpScl might be wider than previously considered.
MATERIALS AND METHODS
Case material
The database of the brain bank at Mayo Clinic in Jacksonville was queried for consecutive cases received between 1997 to 2013 with a neuropathologic diagnosis of either limbic or diffuse LBD [13] in which slides were available for review and paraffin blocks were available for further research. There were 669 LBD cases: 380 diffuse and 289 transitional LBD. We excluded cases of incidental LBD, brainstem predominant LBD, amygdala predominant Lewy bodies in AD, and LBD cases harboring known pathogenic mutations. LBD found concurrent with other major neurodegenerative disorders, such as FTLD-TDP, progressive supranuclear palsy, corticobasal degeneration, Pick’s disease, and multiple system atrophy were excluded. 82% (547/669) of cases were classified as high- or intermediate-likelihood DLB based on the Third Consortium on DLB pathologic criteria [19]. Of the 669 LBD cases in our series, 248 (37%) overlapped with a recently published study investigating HpScl in the setting of advanced AD [21].
Neuropathologic methods
All cases had undergone neuropathologic assessment by a single neuropathologist (DWD), which included standardized tissue sampling and quantitative assessment of AD and Lewy pathology. Thioflavin-S fluorescent microscopy was used for the evaluation of SP and NFT densities in multiple brain regions, including 6 cortical sections, 4 sectors of the hippocampus, and 2 regions of the amygdala. NFTs were also assessed in the basal nucleus of Meynert and presence of SP was assessed in basal ganglia and cerebellum. The distribution of NFTs was used to assign a Braak NFT stage [4] and the distribution of SPs was used to assign a Thal amyloid-β (Aβ) phase, as previously described [20]. Immunohistochemistry was performed on all cases with α-synuclein antibody (NACP, 1:3000 rabbit polyclonal, Mayo Clinic antibody), and the LB densities in 5 cortical sections and the amygdala were assessed. The distribution of LBs was used to assign the subtypes of Lewy body pathology. In addition, immunohistochemistry with phosphorylated tau antibody (PHF-1, 1:1000 mouse monoclonal, gift from Dr. Peter Davies) was also performed in a subset of cases.
Diagnosis of HpScl
HpScl was assessed on hematoxylin-eosin stained sections. It was diagnosed when neuronal loss and gliosis was selective in the CA1 sector and/or subiculum of the hippocampus in the absence of other pathologic findings that could account for neuronal loss in this region. In cases with NFTs, neuronal loss had to be disproportionate to the density of extracellular NFTs.
TDP-43 immunohistochemistry
A 5-um-thick paraffin section of the hippocampus was screened with TDP-43 immunohistochemistry (MC2085 [32], 1:2500 rabbit polyclonal, from Leonard Petrucelli, Mayo Clinic, or with 10782-2-AP, 1:3000 rabbit polyclonal, Protein Tech Group, Chicago) in all cases using a DAKO Autostainer (Universal Staining System Carpinteria, California). In this study, qualitative and quantitative TDP-43 pathology was analyzed on sections that were immunostained with phosphorylated TDP-43 antibody (pS409/410, 1:5000 mouse monoclonal, Cosmo Bio Co., LTD).
All cases were subclassified based on the morphology and distribution of TDP-43 pathology in the hippocampus and adjacent cortex. In addition, a subset of the cases was studied with double-labeling immunohistochemistry with the combination of TDP-43 antibody (MC2085) and phosphorylated tau antibody (PHF-1) using previously published methods [15].
Criteria for subclassification
The Mackenzie scheme [18] was used to classify the cases with modification because it was established for FTLD-TDP and did not provide a means for classifying TDP-43 that occurs in the setting of AD and LBD. Type A is characterized by numerous neuronal cytoplasmic inclusions (NCIs) and dystrophic neurites (DNs) and occasional neuronal intranuclear inclusions (NIIs). Type B is characterized by numerous NCIs with minimal DNs and no NIIs. Type C is characterized by abundant DNs with minimal NCIs and no NIIs.
Of the 168 TDP-43-positive cases 101 were Type A and 43 cases had a pattern similar to Type B, but they also had variable numbers of NFTs with TDP-43 immunoreactivity. This NFT-like TDP-43 pathology was initially described in AD cases [2], but was not considered in FTLD-TDP scheme. We refer to this pattern of TDP-43 pathology as “Type B/NFT” in this study. The remaining 24 cases could not be assigned due to sparse TDP-43 pathology in the hippocampus and adjacent cortex. No case was assigned to Type C.
Semi-quantitative assessment of TDP-43 pathology
Semi-quantitative assessment of TDP-43 pathology was performed with pS409/410 antibody for all 35 HpScl cases and 32 randomly selected LBD cases without HpScl. We examined the presence and severity of TDP-43 pathology in the hippocampus, parahippocampal gyrus, inferior temporal cortex, mid-frontal cortex, amygdala, putamen, substantia nigra, and hypoglossal nucleus. Cytoplasmic lesions and neuritic lesions were scored separately using a 5 point grading scale (0= none, 0.5= rare, 1= mild, 2=moderate, 3=severe) by a single neuropathologist (N.A.) who was blinded to genetic data. Cytoplasmic lesions included NCIs and NFT-like TDP-43 pathology. Neuritic lesions included DNs and fine neurites in the pyramidal layer of the hippocampus as described by Hatanpaa [8]. The presence of NIIs was also assessed. In addition, the severity of fine neurites in the CA1 sector was assessed in all 101 Type A cases.
Genetic methods
For genotyping, genomic DNA was extracted from frozen brain tissue by standard procedures. Genotyping for GRN (SNP rs5848 C/T), TMEM106B (SNP rs1990622 T/C), and APOE alleles (SNP rs429358 T/C and rs7412 C/T) were assessed with Taqman SNP genotyping assays (Applied Biosystems, Carlsbad, CA) as previously reported [28,29]. Genotype calls were obtained with SDS v2.2.2 software (Applied Biosystems).
Stastical methods
SigmaPlot 12.0 (San Jose, CA) was used to analyze all statistical data and create graphs. A Kruskal-Wallis one-way analysis of variance on ranks or Mann-Whitney rank sum test was performed for group comparisons of continuous variables, as appropriate, and a post-hoc pairwise comparison was performed with Mann-Whitney rank sum test. A Chi-square test was performed for group comparisons of categorical data.
RESULTS
A summary of the demographic and neuropathologic characteristics of all LBD cases in this study is shown in Table 1. HpScl was detected in 35 of 669 cases (5.2%). The HpScl group had a significantly older median age at death than the Not HpScl group (81 vs. 78 years, P=0.010). In the HpScl group, 89% of cases were ≥ 75 years of age, while 68% of cases in the Not HpScl group were aged ≥ 75 years. There was no significant difference in the ratio of gender between the two groups (P=0.197).
Table 1.
Demographics, pathology and genetics of case material
| HpScl n=35 |
Not HpScl n=634 |
P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Male | 17 (49%) | 387 (61%) | 0.197 |
| Age at death, years | 81 (78, 85) | 78 (73, 84) | 0.010 |
| Post-mortem findings | |||
| Brain weight, grams | 1000 (880, 1140) | 1140 (1040, 1260) | <0.001 |
| Braak NFT stage | VI (III, VI) | IV (III, V) | <0.001 |
| Thal amyloid-β phase | 5 (4, 5) | 4 (3, 5) | 0.003 |
| Lewy body subtype | |||
| Diffuse | 21 (60%) | 359 (57%) | 0.828 |
| Limbic | 14 (40%) | 275 (43%) | |
| TDP-43 positivity | 35 (100%) | 133 (21%) | <0.001 |
| Type A | 35/35 (100%) | 66/133 (50%) | <0.001 |
| Type B/NFT | 0/35 (0%) | 43/133 (32%) | |
| Unclassified | 0/35 (0%) | 24/133 (18%) | |
| Genetic findings | |||
| TMEM106B, C allele carriers | 14/32 (44%) | 370/527 (70%) | 0.003 |
| GRN, T allele carriers | 21/32 (66%) | 277/527 (53%) | 0.209 |
| APOE, ε4 allele carriers | 21/32 (66%) | 293/538 (54%) | 0.293 |
All data is displayed as median (25th, 75th range), unless otherwise noted. TMEM106B rs1990622 C allele, GRN rs5848 T allele, and APOE ε4 allele carriers are displayed as number genotyped out of available cases.
AD and Lewy Pathology
The median brain weight was significantly less in the HpScl group than in the Not HpScl group (1000 vs. 1140g, P<0.001). The median Braak NFT stage and Thal Aβ phase were significantly greater in HpScl group than the Not HpScl group (NFT stage; VI vs. IV, P<0.001, Aβ phase; 5 vs.4, P=0.003). The prevalence of HpScl was significantly higher in the cases with more advanced Braak NFT stages (Table 2) and Thal Aβ phases. HpScl was detected in 9.1% of the cases with Braak NFT stages V–VI, while HpScl was detected in only 2.8% of those in stages 0-II. Similarly, HpScl was detected in 7.7% of the cases with a Thal Aβ phase of 4–5, while it was only detected in 2.7% of phase 0–1 cases. In contrast, the prevalence of HpScl was not associated with subtype of Lewy pathology (Table 1 and 2). The ratio of diffuse LBD was similar in the HpScl group and Not HpScl group (60% and 57%, P=0.828) (Table 1). HpScl was shown in 5.5% of diffuse LBD cases and 4.8% of limbic LBD cases (p=0.828) (Table 2).
Table 2.
Frequency of HpScl and TDP-43 pathology by Braak NFT stage and LBD subtype
| Braak NFT stage | ||||
|---|---|---|---|---|
| 0-II n=141 |
III-IV n=275 |
V-VI n=253 |
P-value | |
| HpScl | 4 (2.8%) | 8 (2.9%) | 23 (9.1%)* | 0.002 |
| TDP-43 positivity | 11 (7.8%) | 57 (21%)** | 100 (40%)** | <0.001 |
| LBD subtype | ||||
| Limbic n=289 |
Diffuse n=380 |
P-value | ||
| HpScl | 14 (4.8%) | 21 (5.5%) | 0.828 | |
| TDP-43 positivity | 63 (22%) | 105 (28%) | 0.102 | |
P<0.05
P<0.001 vs. Braak NFT stage 0-II.
The associations between HpScl, Braak NFT stage, Thal Aβ phase, and age at death were assessed by a multiple logistic regression model. Braak NFT stage and age at death were independently associated with HpScl (Braak NFT stage: OR 1.6, 95% confidence interval 1.08–2.32; age at death: OR 1.05, 95% confidence interval 1.004–1.100). In contrast, association of Aβ phase with HpScl was not significant in the multiple logistic model (P=0.940).
TDP-43 pathology
TDP-43 pathology was found in all of the HpScl cases (35/35), while it was found in only 21% of the cases without HpScl (133/634) (P<0.001) (Table 1). Of note, all HpScl cases exhibited Type A morphology and distribution. In contrast, the subtype of the Not HpScl group was heterogeneous. Type A was the most frequent subtype (50%), followed by Type B/NFT (32%). A subset (18%) of cases with TDP-43 pathology could not be classified because the density of lesions was too sparse. No cases of Type C were observed.
The overall prevalence of TDP-43 pathology was 25% (168/669). As previously reported [22], the presence of TDP-43 pathology was associated with higher Braak NFT stage (Table 2). For example, TDP-43 pathology was detected in 40% of cases with Braak stages V–VI, while it was detected in only 7.8% of cases with Braak stages 0-II. There was a trend for TDP-43 pathology to be more frequently in diffuse LBD than limbic LBD, but this was not significant (28% vs. 22%, P=0.102) (Table 2).
Given that a subset of the LBD cases with advanced AD pathology were included in a previous report [21], we analyzed characteristics of only the LBD cases not included in the previous series (Supplemental Table 1). When cases that overlapped were excluded , the demographic characteristics were similar, but Alzheimer type pathology measures (i.e., Braak NFT stage and Thal amyloid-β phase) differed since LBD cases with minimal AD pathology were not included in the previous report. In both series HpScl cases had TDP-43 pathology consistent with Type A pattern.
Semi-quantitative assessment of TDP-43 pathology
To clarify the characteristics of TDP-43 pathology in HpScl cases, we performed a semi-quantitative assessment of TDP-43 pathology using all 35 HpScl cases. In addition, we examined 32 randomly selected LBD cases without HpScl (Type A; 19 cases, Type B/NFT; 13 cases) for comparison purposes (Table 3).
Table 3.
Semi-quantitative assessment of TDP-43 pathology
| HpScl | Not HpScl | ||||||
|---|---|---|---|---|---|---|---|
| Type A n=35 |
Type A n=19 |
Type B/NFT n=13 |
P-value | ||||
| TDP-43 score |
Positivity | TDP-43 score |
Positivity | TDP-43 score |
Positivity | ||
| Neuronal cytoplasmic lesions | |||||||
| Dentate fascia | 2 (1, 3) | 35/35 (100%) |
1 (1, 1)** | 18/19 (95%) |
0.5 (0, 0.5)** | 9/13 (70%) |
<0.001 |
| CA1 | 0.5 (0.5, 1) | 30/35 (86%) |
0.5 (0.5, 1) | 15/19 (79%) |
1 (0.25, 1) | 10/13 (77%) |
0.619 |
| Subiculum | 1 (1, 2) | 35/35 (100%) |
1 (1, 2) | 16/19 (84%) |
1 (1, 2) | 13/13 (100%) |
0.550 |
| Parahippocampal gyrus | 2 (1, 3) | 35/35 (100%) |
2 (1, 3) | 17/19 (89%) |
1 (1, 2)* | 13/13 (100%) |
0.024 |
| Inferior temporal gyrus | 1 (0, 2) | 20/27 (74%) |
0.5 (0, 2) | 9/15 (60%) |
0 (0, 0.625)* | 3/10 (30%) |
0.047 |
| Amygdala | 2 (2, 3) | 33/33 (100%) |
3 (1, 3) | 19/19 (100%) |
3 (1.5, 3) | 13/13 (100%) |
0.930 |
| Putamen | 0 (0, 5) | 9/33 (27%) |
0 (0, 0)* | 0/19 (0%) |
0 (0, 0)* | 0/13 (0%) |
0.007 |
| Mid-frontal gyrus | 0 (0, 0) | 6/35 (17%) |
0 (0, 0) | 3/19 (16%) |
0 (0, 0) | 0/13 (0%) |
0.293 |
| Substantia nigra | 0 (0, 0) | 4/34 (12%) |
0 (0, 0) | 1/19 (5%) |
0 (0, 0) | 0/13 (0%) |
0.362 |
| Hypoglossal nucleus | 0 (0, 0) | 0/26 (0%) |
0 (0, 0) | 1/17 (6%) |
0 (0, 0) | 0/12 (0%) |
0.327 |
| Neuritic lesions | |||||||
| Dentate fascia | 0.5 (0, 0.5) | 24/35 (69%) |
0 (0, 0.5)* | 6/19 (32%) |
0 (0, 0)** | 1/13 (8%) |
<0.001 |
| CA1 | 2 (1, 3) | 35/35 (100%) |
1 (0.5, 2) | 19/19 (100%) |
0 (0, 0)** | 2/13 (15%) |
<0.001 |
| Subiculum | 1 (0.5, 2) | 35/35 (100%) |
1 (1, 2) | 18/19 (95%) |
0.5 (0, 0.5)** | 7/13 (54%) |
<0.001 |
| Parahippocampal gyrus | 2 (1, 3) | 35/35 (100%) |
1 (0.5, 3) | 17/19 (89%) |
0 (0, 0.75)** | 5/13 (40%) |
<0.001 |
| Inferior temporal gyrus | 0.5 (0, 2) | 20/27 (74%) |
0.5 (0, 1) | 8/18 (53%) |
0 (0, 0)** | 1/10 (10%) |
0.004 |
| Amygdala | 1 (1, 2) | 32/33 (97%) |
2 (0.5, 2) | 17/19 (89%) |
1 (0.25, 1)* | 10/13 (77%) |
0.020 |
| Putamen | 0 (0, 0.25) | 8/33 (24%) |
0 (0, 0)* | 0/19 (0%) |
0 (0, 0) | 0/13 (0%) |
0.013 |
| Mid-frontal gyrus | 0 (0, 0) | 6/35 (17%) |
0 (0, 0) | 3/19 (16%) |
0 (0, 0) | 0/13 (0%) |
0.290 |
| Substantia nigra | 0 (0, 0.5) | 15/34 (44%) |
0 (0, 0) | 4/19 (21%) |
0 (0, 0)* | 0/13 (0%) |
0.008 |
| Hypoglossal nucleus | 0 (0, 0) | 0/26 (0%) |
0 (0, 0) | 0/17 (0%) |
0 (0, 0) | 0/12 (0%) |
1.000 |
|
Neuronal nuclear inclusions |
n/a | 31/35 (89%) |
n/a | 11/19 (58%)* |
n/a | 1/13 (8%)** |
<0.001 |
TDP-43-positive cytoplasmic and neuritic lesions were scored separately using a 5 point grading scale (0= none, 0.5= rare, 1 = mild, 2=moderate, 3=severe). TDP-43 score shows median (25th, 75th range) in each brain region. Positivity is number TDP-43-positive out of available cases in each brain region. Abbreviations: n/a., not assessed.
p<0.05
P<0.001 vs. HpScl-Type A.
HpScl-Type A
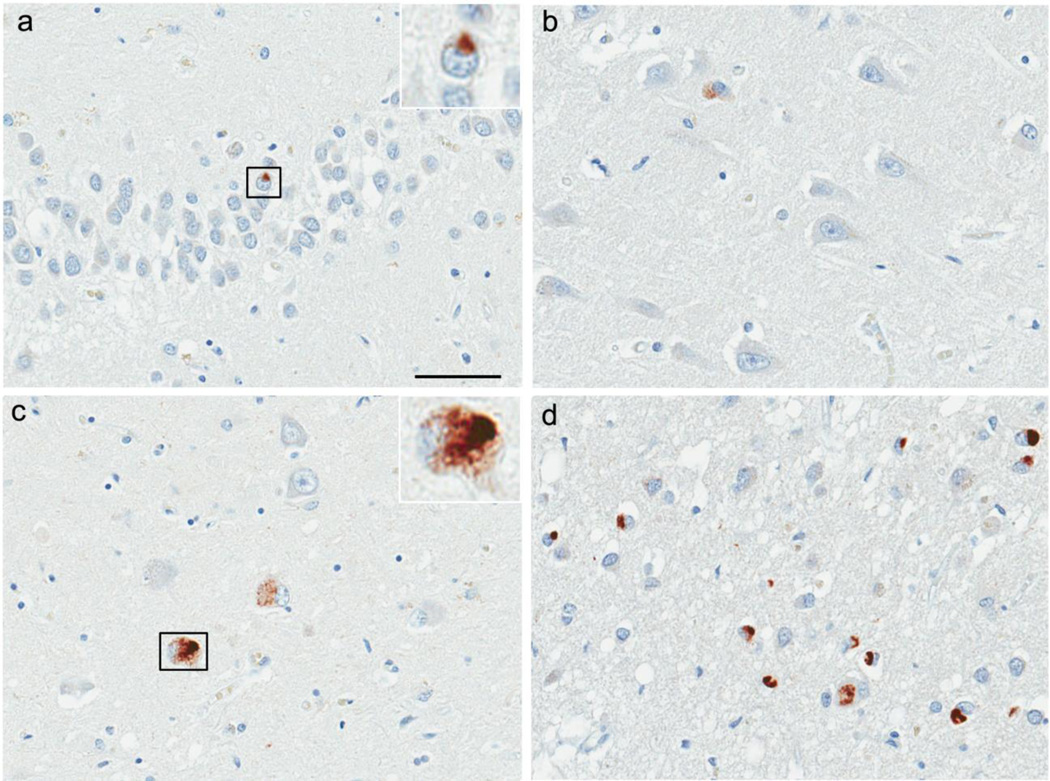
All cases had variable density of NCIs in the dentate fascia (Fig. 1a). Fine neurites in CA1 (Fig. 1b and c) and subiculum (Fig.1d), which have been suggested to be a pathologic precursor for HpScl in FTLD-TDP [8], were found in all cases, with significantly more in CA1 (P=0.027). In comparison, NCIs were sparse in CA1. All cases had a mixture of NCIs and short DNs in the parahippocampal gyrus (Fig. 1e). In the inferior temporal gyrus, 74% of the cases had similar pathology as the parahippocampal gyrus, but to a lesser extent (Fig. 1f). The amygdala was also affected in all cases; variable shapes of NCIs and short DNs were found (Fig. 1g). Extension of the pathology beyond the limbic and inferomedial temporal lobe was present in about 30% of the cases, but lesion density in these areas was sparse. TDP-43 pathology was found in 17% and 27% of the cases in the mid-frontal gyrus and putamen (Fig. 1h), respectively. In the substantia nigra (SN), sparse DNs and NCIs were found in 44% and 12% of the cases, respectively (Fig. 1i). No cases had TDP-43 pathology in the hypoglossal nucleus. NIIs were found in 89% of the cases, most frequently in the dentate fascia, CA1, and parahippocampal gyrus. In summary, the characteristic features of TDP-43 pathology in LBD cases with HpScl was consistent with FTLD-TDP Type A; however, the pathology was relatively restricted to limbic and inferomedial temporal lobe in about 70% of the cases.
Fig. 1.
Immunohistochemistry for TDP-43 in a hippocampal sclerosis case (Type A). Neuronal cytoplasmic inclusions (NCIs) in the dentate fascia (a). Fine neurites in the CA1 sector (b, c) and subiculum (d). Mixture of NCIs and dystrophic neurites (DNs) in the parahippocampal gyrus (e), inferior temporal gyrus (f), and amygdala (g). Inset in (f) shows a neuronal intranuclear inclusion with a lentiform shape. Sparse TDP-43 pathology in the putamen (h) and substantia nigra (i). Insets in (h) and (i) show a NCI and a DN, respectively. TDP-43 pS409/410 (a–i). Scale bars: a, b, d–I; 50um, C; 200um.
Not HpScl-Type A
In general, TDP-43 pathology was relatively mild in this group compared with HpScl-Type A. Significant differences were found in the dentate fascia and putamen, as well as in the presence of NIIs. There was a trend for the density of CA1 fine neurites to be less than in HpScl-Type A (P=0.097); however we also found that 42% of the cases in this group had moderate-to-severe CA1 fine neurites similar to HpScl cases. These findings suggest that a subset of the cases in the Not HpScl-Type A group had similar characteristics and severity of TDP-43 pathology to HpScl cases and may represent “pre-HpScl.”
Not HpScl-Type B/NFT
This group was clearly distinguished from HpScl-Type A due to sparse or no neuritic lesions in the hippocampus (Fig. 2a–c), parahippocampal gyrus (Fig. 2d), and inferior temporal gyrus. No cases had TDP-43 fine neurites in CA1 (Fig. 2b) or subiculum (Fig. 2c). In contrast, NCIs were frequent. The dentate fascia had NCIs in 70% of the cases, although they were sparse (Fig. 2a). All cases had NFT-like TDP-43 pathology in the subiculum with sparse or no NCIs (Fig. 2c). A mixture of NCIs and NFT-like TDP-43 pathology was found in the parahippocampal gyrus (Fig. 2d) and amygdala of all cases. The amygdala was the most severely affected region in this group. No case had TDP-43 pathology beyond the limbic and inferomedial temporal lobe. Double-labeling immunohistochemistry demonstrated that the NFT-like TDP43 pathology co-localized with tau pathology.
Fig. 2.
Immunohistochemistry for TDP-43 in the case without hippocampal sclerosis (Type B/NFT). Sparse neuronal cytoplasmic inclusions (NCIs) in the dentate fascia (a). Inset in (a) shows a NCI. TDP-43-positive fine neurites are not present in the CA1 sector (b) and subiculum (c). TDP-43 immunoreactivity is shown in some NFTs in the subiculum (c). Inset in (c) shows a NFT-like TDP-43 pathology. Mixture of NCIs and NFT-like TDP-43 pathology in the parahippocampal gyrus with sparse dystrophic neurites (d). TDP-43 pS409/410 (a–d). Scale bars: a–d; 50µm.
Genetic findings
We performed genetic analyses to ascertain whether the reported genetic risk variants for FTLD-TDP and HpScl in AD could also contribute to the development of HpScl in LBD. The TMEM106B rs1990622 variant, where the C allele correlates with an increase in GRN expression [7], is reported to be protective for FTLD-TDP and HpScl in AD [21,29]. The TMEM106B C allele was found significantly less often in the HpScl group than in the Not HpScl group (44% vs. 70%, P=0.003) (Table 1). 9.4% of the cases in the HpScl group were homozygous for the protective C allele; this was less than the Not HpScl group (23%). In comparison, the GRN rs5848 variant, where the T allele is associated with lower GRN expression, is reported to be a risk factor FTLD-TDP and HpScl in AD [5,21]. The frequency of the cases having the GRN T allele in the HpScl group was also higher than the Not HpScl group, but this was not statistically significant (66% vs.53%, P=0.209) (Table 1). In addition, we also examined APOE ε4 allele association with HpScl, but found no statistically significant differences between HpScl and Not HpScl groups (66% vs. 54%, P=0.293).
Excluding LBD cases that were included in a previous study of advanced AD [21] gave similar genetic findings (Supplemental Table 1) as in the entire LBD cohort. The frequency of the TMEM106B C allele in HpScl was lower than in Not HpScl (50% vs. 67%), although given the smaller sample size this did not reach statistical significance (P=0.288). The frequency of the cases having protective TMEM106B CC genotype was 7.1% and 22% in HpScl compared to Not HpScl, respectively.
A comparison of demographics and genetic characteristics by subtype of TDP-43 pathology is shown in Table 4. There were no differences in gender and age at death between the HpScl-Type A, Not HpScl-Type A, or Not HpScl-Type B/NFT groups. In contrast, genetic characteristics were different between the HpScl-Type A and the other groups. The TMEM106B C allele was found significantly less often in HpScl-Type A than in Not HpScl-Type A (44% vs. 76% P=0.005) and Not HpScl-Type B/NFT (44% vs. 74% P=0.027). The frequency of the cases having the GRN T allele in HpScl-Type A was also higher than in the others, but this was not statistically significant. The difference in the frequency of APOE ε4 allele was also not statistically significant between HpScl-Type A and the others.
Table 4.
Comparison of demographics and genetics by subtype of TDP-43 pathology
| HpScl | Not HpScl | P-value | ||
|---|---|---|---|---|
| Type A n=35 |
Type A n=66 |
Type B/NFT n=43 |
||
| Male | 17 (49%) | 32 (48%) | 19 (44%) | 0.893 |
| Age at death, years | 81 (78, 85) | 82 (77, 86) | 82 (77, 86) | 0.953 |
| TMEM106B, C allele carriers | 14/32 (44%) | 42/55 (76%)* | 25/34 (74%)* | 0.005 |
| GRN, T allele carriers | 21/32 (66%) | 32/55 (58%) | 19/34 (56%) | 0.697 |
| APOE, ε4 allele carriers | 21/32 (66%) | 42/58 (72%) | 21/36 (58%) | 0.367 |
Age at death is displayed as median (25th, 75th range). TMEM106B C allele, GRN T allele, and APOE ε4 allele carriers are displayed as number genotyped out of available cases.
p<0.05 vs. HpScl-Type A.
The genetic findings suggest that the TMEM106B variant strongly influences the development of HpScl in LBD, as was observed for HpScl in AD, while the influence of the GRN variant was weaker.
CA1 fine neurites and TMEM106B genotype
Given the pathologic findings that TDP-43-positive fine neurites might be pathologic precursor of HpScl, we hypothesized that (1) the severity of this pathology might be associated with the TMEM106B genotype in Type A cases and (2) Not HpScl-Type A cases with abundant CA1 fine neurites might have similar genetic characteristics as HpScl-Type A cases. To address this issue, we examined all Type A cases that we had access to, including 32 HpScl-Type A cases (Fig. 3a) and 55 Not HpScl-Type A cases (Fig. 3b and c). In addition, we divided the Not HpScl-Type A group into a High CA1 fine neurites group (Fig. 3b) and a Low CA1 fine neurites group (Fig. 3C). Cases with High fine CA1 neurites (n=30) had semiquantitative scores of 2 to 3, and cases with Low CA1 fine neurites (n=25) had scores 0 to1. Interestingly, the severity of CA1 fine neurites was significantly less in Type A cases having the protective CC genotype than in the cases having the TT genotype (p=0.002) and CT genotype (P=0.018) (Fig. 3d). Furthermore, Not HpScl-Type A cases with High fine CA1 neurites also had decreased frequency of the TMEM106B CC genotype (10%), which is similar to HpScl-Type A cases (9.4%) (Fig. 3e). These findings suggest that a subset of the LBD cases in the Not HpScl-Type A group had similar pathologic and genetic characteristics to HpScl cases. We considered these cases to be on the spectrum of HpScl pathology; however, they were not diagnosed as HpScl due to the absence of neuronal loss or the presence of many extracellular NFTs in the CA1 sector. We classified these cases as “pre-HpScl” if there was none to minimal neuronal loss or “masked HpScl if there was neuronal loss, but many extracellular NFTs (Fig. 4).
Fig. 3.
TDP-43-positive fine neurites in CA1 (a, b) were similar in HpScl-Type A (a) and Not HpScl-Type A High CA1 neurites (b) compared to Not HpScl-Type A Low CA1 neurites (c). CA1 fine neurites are significantly less in all Type A cases (a–c) with TMEM106B CC genotypes than in cases with TT (p=0.002) or CT genotype (p=0.018) (d). Not HpScl-Type A cases with High CA1 fine neurites had low frequency of TMEM106B CC genotype similar to HpScl-Type A cases (e). The boxes show median and 25th and 75th percentiles with whisker plots showing 10th and 90th percentiles. Scale bars: a–c; 50µm.
Fig. 4.
We propose a classification of hippocampal sclerosis in LBD based upon neuronal loss, TDP-43 pathology and extracellular NFTs in the CA1 sector (a). “Typical-HpScl “ has neuronal loss and TDP-43-positive fine neurites but no or relatively mild NFTs when compared with the degree of neuronal loss (b, e, h). “Pre-HpScl” has none to minimal neuronal loss or extracellular NFTs, but abundant TDP-43-positive fine neurites (c, f, i). “Masked HpScl” has neuronal loss and many extracellular NFTs but also abundant TDP-43-positive fine neurites (d, g, j). Arrows in (d) show extracellular NFTs. Hematoxylin & eosin (b–d), TDP-43 pS409/410 (e–g), Tau PHF-1 (h–j). Scale bars: b–j; 50um. NL=neuronal loss, eNFTs=extracellular neurofibrillary tangles, H&E=hematoxylin & eosin.
To determine if the spectrum of HpScl pathology also applies to non-LBD cases, we performed a validation study using 132 non-LBD cases without HpScl or advanced AD pathology (Braak NFT stage ≤III) by TDP-43 immunohistochemistry. The non-LBD validation cohort is described in Supplemental Methods. We found a single case that fit with “pre-HpScl” that had many TDP-43-positive fine neurites and absence of neuronal loss or extracellular NFTs in the CA1 sector (Supplemental Figure). This 86 year-old man pathologically had vascular ischemic dementia based on presence of multiple lacunar infarcts in the basal ganglia, thalamus, and cerebral white matter. TDP-43 pathology was consistent with Type A, and it was restricted to limbic and inferomedial temporal lobe. Braak NFT stage was II, Thal amyloid-β phase was 4, and there was no argyrophilic grain disease. There was no significant difference in the frequency of “pre-HpScl” between the validation cohort and LBD cohort with Braak NFT stage 0-III (0.76% vs. 2.7% P=0.354) (Supplemental Table 2).
DISCUSSION
In this study, HpScl was detected in 5.2% of the LBD cases. We demonstrated that HpScl in LBD is a TDP-43 proteinopathy associated with older age and greater AD pathology, but not with distribution of LBs. TDP-43 pathology was detected in all HpScl cases and 21% of the cases without HpScl. All HpScl cases had a pattern of TDP-43 pathology consistent with FTLD-TDP Type A, although the pathology was restricted to limbic and inferomedial temporal lobe in about 70% of the cases. Fine neurites in the CA1 sector and subiculum were found in all HpScl cases. Genetic analysis demonstrated the similarity with FTLD-TDP and HpScl. HpScl cases had the decreased protective allele in TMEM106B; however, the increased risk allele in GRN did not reach statistical significance. Our findings in LBD support the notion that HpScl in the elderly might share underlying disease mechanisms with FTLD-TDP and that there is a close relation between HpScl and FTLD-TDP Type A pathology.
Previous data regarding HpScl in LBD is limited. Yokota et al. [30] reported that none of their 56 LBD cases had HpScl. Their finding cannot be directly compared with our results due to the different pathologic characteristics of cases. In their study, 91% (51/56) of the cases had low Braak NFT stages (0 to IV). In our study only 2.9% of LBD cases with NFT stages 0-IV had HpScl, which may not be significantly different from their study. Of note, they also presented a case that had no HpScl, but abundant TDP-43-positive fine neurites in CA1 sector, which resembles our “pre-HpScl” cases. There was no difference in the frequency of HpScl between transitional and diffuse LBD cases (4.8% vs. 5.5%, P=0.828). In addition, the frequency of HpScl in our LBD cases with Braak NFT stages 0-II (2.8%) and NFT stages V–VI (9.1%) was similar to the reported prevalence of “pure-HpScl” [31] and HpScl in AD [5]. These results suggest that Lewy related pathology seems to have no direct association with HpScl.
The following findings support the notion that TDP-43-positive fine neurites might be a precursor lesion for HpScl [8]: 1) all HpScl cases had this pathology in the CA1 sector and subiculum, where selective neuronal loss occurs, and 2) the severity of CA1 fine neurites in Type A cases with and without HpScl was associated with the TMEM106B variant. We hypothesized that theTMEM106B CC genotype might potentially suppress the progression of fine neurites in the CA1 sector and subiculum, and thus prevent the development of HpScl. We found a subset of LBD cases, which were not diagnosed as having HpScl due to the absence of neuronal loss or the presence of many extracellular NFTs, but had abundant TDP-43-positive CA1 fine neurites and a low frequency of the TMEM106B CC genotype. It is plausible that these cases are on the spectrum of HpScl pathology. We propose a classification of HpScl in LBD based upon neuronal loss, TDP-43 pathology, and extracellular NFTs in the CA1 sector (Fig. 4). “Typical-HpScl” has neuronal loss and TDP-43-positive fine neurites, but no or relatively mild NFTs compared with the degree of neuronal loss. “Pre-HpScl” has none to minimal neuronal loss or extracellular NFTs, but it has abundant TDP-43-positive fine neurites. We propose for the first time that “masked HpScl” may represent HpScl as hidden by many extracellular NFTs, and only recognizable by presence of characteristic TDP-43-positive fine neurites.
In addition, we found a subject with “pre-HpScl” in a non-LBD validation cohort, suggesting that the concept about spectrum of HpScl pathology may also fit with the elderly patients outside the setting of LBD. We are unable to perform genetic studies due to the small size of the validation cohort. We encourage further studies using larger cohorts to clarify genetic associations. A recent genome-wide association study investigating HpScl as an endophenotype also validated the association with TMEM106B variant and HpScl in aged individuals [23]. We suggest that TDP-43 immunohistochemistry is needed to diagnose HpScl in the elderly because “pre-HpScl” and “masked-HpScl” cannot be detected by H&E staining. It should also be mentioned that TDP-43-positve fine neurites in the CA1 and subiculum are visible with the immunohistochemistry using non-phosphorylated TDP-43 antibodies (MC2085 and 10782-2-AP) as well as the phosphorylated TDP-43 antibody (pS409/410).
There are a few reports about TDP-43 subtype in LBD. Nakashima-Yasuda et al. reported [22] that the majority (∼95%) of TDP-43-positive cases were Type A, and fewer (∼5%) were Type B. Arai et al. also reported [3] that all TDP-43-positive cases were Type A if TDP-43 pathology progressed to the neocortex. They also demonstrated common biochemical features of LBD with Type A TDP-43 pathology and FTLD-TDP Type A based on the immunoblot pattern of phosphorylated C-terminal fragments of TDP-43. Our findings confirmed these findings that Type A is the most frequent subtype in LBD (60%, 101/168); however, the remaining cases were difficult to classify based on Mackenzie scheme for FTLD-TDP. We found that 26% (43/168) of the TDP-43-positive cases had a unique pattern of TDP-43 pathology; a mixture of NCIs and NFT-like TDP-43 pathology with no or sparse DNs in the hippocampus and adjacent cortex. These cases had only sparse NCIs in the dentate fascia and no TDP-43 pathology beyond the limbic and inferomedial temporal lobe, which is quite different from the distribution pattern of FTLD-TDP Type B, which has abundant NCIs in the dentate fascia and frequent pathology in the brainstem including hypoglossal nucleus [11]. Therefore, we called this pattern of TDP-43 pathology as Type B/NFT and distinguished it from Type B. We consider that a new classification criteria for TDP-43 subtype in AD and LBD is needed because the current scheme for FTLD-TDP does not fit with findings in AD and LBD.
In conclusion, HpScl in LBD is TDP-43 proteinopathy that is similar to FTLD-TDP-Type A. The presence of HpScl in LBD is associated with greater AD pathology, but not with the distribution of Lewy related pathology. We suggest that TDP-43 immunohistochemistry is needed to diagnosis HpScl in the elderly because TDP-43-positive fine neurites, which are suggested to be a hallmark of HpScl, are not visible with routine histologic methods. TDP-43 immunohistochemistry expands the spectrum of HpScl by revealing “pre-HpScl” in cases with minimal or no neuronal loss and “masked-HpScl” in cases with neuronal loss and severe NFT pathology. While the present findings lead to a more accurate description of HpScl in the elderly, particularly in LBD, further studies are needed to clarify the clinical significance of HpScl.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to all patients, family members, and caregivers who agreed to brain donation; without their donation these studies would have been impossible. We also acknowledge expert technical assistance of Linda Rousseau and Virginia Phillips for histology and Monica Castanedes-Casey for immunohistochemistry. This research was supported in part by a research grant from the Uehara Memorial Foundation, The Mangurian Foundation, and NIH grant P50NS72187, P50AG16574, and R01NS076471.
REFERENCES
- 1.Ala TA, Beh GO, Frey WH., 2nd Pure hippocampal sclerosis: a rare cause of dementia mimicking Alzheimer's disease. Neurology. 2000;54:843–848. doi: 10.1212/wnl.54.4.843. [DOI] [PubMed] [Google Scholar]
- 2.Amador-Ortiz C, Lin WL, Ahmed Z, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai T, Mackenzie IR, Hasegawa M, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta Neuropathologica. 2009;117:125–136. doi: 10.1007/s00401-008-0480-1. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 5.Dickson DW, Baker M, Rademakers R. Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly. Neuro-degenerative diseases. 2010;7:170–174. doi: 10.1159/000289231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickson DW, Davies P, Bevona C, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 7.Finch N, Carrasquillo MM, Baker M, et al. TMEM106B regulates progranulin levels and the penetrance of FTLD in GRN mutation carriers. Neurology. 2011;76:467–474. doi: 10.1212/WNL.0b013e31820a0e3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hatanpaa KJ, Bigio EH, Cairns NJ, et al. TAR DNA-binding protein 43 immunohistochemistry reveals extensive neuritic pathology in FTLD-U: a midwest-southwest consortium for FTLD study. Journal of neuropathology and experimental neurology. 2008;67:271–279. doi: 10.1097/NEN.0b013e31816a12a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Higashi S, Iseki E, Yamamoto R, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer's disease and dementia with Lewy bodies. Brain research. 2007;1184:284–294. doi: 10.1016/j.brainres.2007.09.048. [DOI] [PubMed] [Google Scholar]
- 10.Josephs KA, Ahmed Z, Katsuse O, et al. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66:142–151. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- 11.Josephs KA, Stroh A, Dugger B, Dickson DW. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–358. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephs KA, Whitwell JL, Weigand SD, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta Neuropathol. 2014 doi: 10.1007/s00401-014-1269-z. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosaka K. Diffuse Lewy body disease. Neuropathology. 2000;20(Suppl):S73–S78. doi: 10.1046/j.1440-1789.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 14.Kosaka K. Latest concept of Lewy body disease. Psychiatry and clinical neurosciences. 2014;68:391–394. doi: 10.1111/pcn.12179. [DOI] [PubMed] [Google Scholar]
- 15.Lin WL, Castanedes-Casey M, Dickson DW. Transactivation response DNA-binding protein 43 microvasculopathy in frontotemporal degeneration and familial Lewy body disease. J Neuropathol Exp Neurol. 2009;68:1167–1176. doi: 10.1097/NEN.0b013e3181baacec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackenzie IR. The neuropathology and clinical phenotype of FTD with progranulin mutations. Acta neuropathologica. 2007;114:49–54. doi: 10.1007/s00401-007-0223-8. [DOI] [PubMed] [Google Scholar]
- 17.Mackenzie IR, Baker M, Pickering-Brown S, et al. The neuropathology of frontotemporal lobar degeneration caused by mutations in the progranulin gene. Brain. 2006;129:3081–3090. doi: 10.1093/brain/awl271. [DOI] [PubMed] [Google Scholar]
- 18.Mackenzie IR, Neumann M, Baborie A, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 20.Murray ME, Bieniek KF, Banks Greenberg M, et al. Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol. 2013;126:545–554. doi: 10.1007/s00401-013-1161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murray ME, Cannon A, Graff-Radford NR, et al. Differential clinicopathologic and genetic features of late-onset amnestic dementias. Acta Neuropathol. 2014;128:411–421. doi: 10.1007/s00401-014-1302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakashima-Yasuda H, Uryu K, Robinson J, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta neuropathologica. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 23.Nelson PT, Estus S, Abner EL, et al. ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology. Acta neuropathologica. 2014;127:825–843. doi: 10.1007/s00401-014-1282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson PT, Schmitt FA, Lin Y, et al. Hippocampal sclerosis in advanced age: clinical and pathological features. Brain. 2011;134:1506–1518. doi: 10.1093/brain/awr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson PT, Smith CD, Abner EL, et al. Hippocampal sclerosis of aging, a prevalent and high-morbidity brain disease. Acta neuropathologica. 2013;126:161–177. doi: 10.1007/s00401-013-1154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 27.Pao WC, Dickson DW, Crook JE, Finch NA, Rademakers R, Graff-Radford NR. Hippocampal sclerosis in the elderly: genetic and pathologic findings, some mimicking Alzheimer disease clinically. Alzheimer Dis Assoc Disord. 2011;25:364–368. doi: 10.1097/WAD.0b013e31820f8f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rademakers R, Eriksen JL, Baker M, et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum Mol Genet. 2008;17:3631–3642. doi: 10.1093/hmg/ddn257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutherford NJ, Carrasquillo MM, Li M, et al. TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology. 2012;79:717–718. doi: 10.1212/WNL.0b013e318264e3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokota O, Davidson Y, Arai T, et al. Effect of topographical distribution of alpha-synuclein pathology on TDP-43 accumulation in Lewy body disease. Acta neuropathologica. 2010;120:789–801. doi: 10.1007/s00401-010-0731-9. [DOI] [PubMed] [Google Scholar]
- 31.Zarow C, Sitzer TE, Chui HC. Understanding hippocampal sclerosis in the elderly: epidemiology, characterization, and diagnostic issues. Curr Neurol Neurosci Rep. 2008;8:363–370. doi: 10.1007/s11910-008-0057-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang YJ, Xu YF, Cook C, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A. 2009;106:7607–7612. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.