Abstract
In multicellular organisms, effective communication between cells is a crucial part of cellular and tissue homeostasis. This communication mainly involves direct cell–cell contact as well as the secretion of molecules that bind to receptors at the recipient cells. However, a more recently characterized mode of intercellular communication—the release of membrane vesicles known as exosomes—has been the subject of increasing interest and intensive research over the past decade. Following the discovery of the exosome-mediated immune activation, the pathophysiological roles of exosomes have been recognized in different diseases, including cancer. In this review, we describe the biogenesis and main physical characteristics that define exosomes as a specific population of secreted vesicles, with a special focus on their role in oncogenic transformation and cancer progression.
Keywords: Extracellular vesicles, Tumor microenvironment, Signaling, Oncogenic reprogramming, Mesenchymal stem cells, Metastasis
Introduction
The term “exosomes” was first proposed in the early 1980s to refer to membrane vesicles that are released from neoplastic cell lines and are rich in 5′-nucleotidase activity [1]. Early reports then unraveled the endocytic origin of exosomes and focused on their role in the expulsion of cell surface proteins during the process of maturation of mammalian reticulocytes into erythrocytes [2–4]. Subsequent to exosomes discovery in reticulocytes, multiple cell types, including numerous tumor cell lines, have been shown to release exosomes in extracellular medium [5]. Exosomes are also found in abundance in many biological fluids such as blood, saliva, and urine [5]. The biological significance of exosomes is still not completely understood. However, whether secreted by cells into culture media, or by various organs and tissues into body fluids, exosomes have been suggested to mediate local and systemic intercellular communication by the transfer of various bioactive molecules thereby modulating physiological processes in the target cells and contributing to different aspects of physiology and disease. These nanoscale membrane structures represent natural vehicles in which cellular macromolecules, including lipids, proteins, and nucleic acids, can exit the donor cell, transit often over relatively long distances and exert their activities in another cell in an intact state [6–8].
Definition, biogenesis, and composition of exosomes
Exosomes are 30–100 nm phospholipid bilayer-enclosed vesicles that are secreted by the cells into the extracellular milieu [7]. They are generated through the endocytic pathway by intra-luminal invagination and budding from the limiting membrane of the late endosome giving rise to multivesicular body (MVB) with small cytosol-containing intraluminal vesicles (ILVs). The MVB is either sorted for cargo degradation by fusion with the lysosomes or sorted to fuse with the plasma membrane releasing the ILVs as exosomes into the extracellular space [7, 8]. The endocytic membrane trafficking is well coordinated and tightly controlled through several cytosolic regulatory and sorting mechanisms that dictate the number, composition and fate of ILVs and exosomes [7–10]. An important exosome biogenesis and cargo sorting mechanism involves the coordinated recruitment and utilization of ESCRT (endosomal sorting complex required for transport) machinery and associated proteins [9]. Other ESCRT-independent mechanisms have also been found to regulate biogenesis and secretion of exosomes [10].
Besides exosomes, cells are known to secrete a large variety of membrane vesicles of diverse size and composition into the extracellular space depending on the type and the current state of the cell [11]. Based on the mode of biogenesis and release, extracellular vesicles can be classified into three broad classes: (i) exosomes, (ii) ectosomes or shedding microvesicles that are derived directly from the plasma membrane through the outward budding and fission of membrane vesicles from the cell plasma membrane, and (iii) apoptotic bodies which are produced from cells undergoing cell death by apoptosis as a mechanism to prevent the leakage of potentially toxic cellular contents of dying cells [11–16] (Table 1). Exosomes are distinguished from other cell-derived vesicles by their origin, size, morphology, and composition. Although, in practice, other secreted vesicles might often be confused and/or co-isolated with exosomes; as the current purification protocols usually produce heterogeneous vesicle preparations, but vesicle type can often be verified based on the electron microscopic appearance and the presence of several (but not necessarily all) of the other listed features in Table 1 [11, 12].
Table 1.
Characteristics of different classes of extracellular vesicles
| Vesicle types | Origin | Size (nm) | Microscopic appearancea | Protein markers | Contents | Main references |
|---|---|---|---|---|---|---|
| Exosomes | Endolysosomal pathway; intra-luminal budding into late endosome and fusion of multivesicular body with cell membrane | 30–100 | Round-shaped, cup-shaped | Tetraspanins (CD63, CD9,CD81), alix and TSg101 | Cytosolic and membrane proteins including receptors and MHC molecules; mRNA, miRNA; lipid rafts | [11, 12] |
| Ectosomes, or shedding microvesicles | Cell membrane; outward budding | 50–1,000 | Round-shaped, irregular-shaped and electron-dense | Integrins, selectins, and CD40 ligand | Cytosolic and membrane proteins, including receptors; mRNA, miRNA | [13–15] |
| Apoptotic bodies | Cell membrane; outward blebbing of apoptotic cell membrane | 500–2,000 | Heterogeneous | Histones | Nuclear fragments, cell organelles | [11, 16] |
aElectron-microscopic appearance can be influenced by the fixation and preparation techniques
MHC major histocompatibility complex, TSg101 tumor susceptibility gene 101
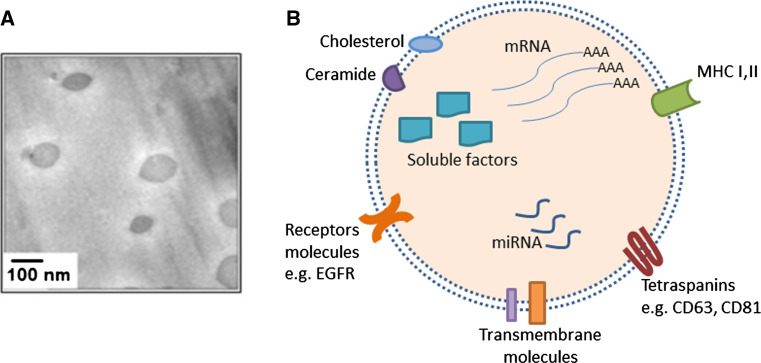
High-resolution electron microscopy coupled with advanced proteomic analysis of exosomes from different cells and disease models has provided deeper insights into the structural composition of exosomes [7] (Fig. 1). Due to their endosomal origin, all exosomes carry certain proteins, or protein families, including membrane transport and fusion proteins such as Rab GTPases, Annexins, heat shock proteins (Hsp70, Hsp 90), proteins involved in MVB biogenesis (Alix, TSG101), tetraspanins such as CD9, CD63, CD81, CD82 [5, 11, 17]. Exosomes also carry some cell-specific proteins depending on the cell of origin and the putative target function. For example, MHC class II and CD86 are abundant on exosomes from mature dendritic cells that stimulate CD4+ T cells [7, 18]. Lipidomic analysis showed that exosomes are enriched in lipid-rafts cholesterol [11, 19], the sphingolipid ceramide [10] and other lipid classes [20]. Besides proteins and lipids, exosomes contain nucleic acid cargo, most importantly mRNA and microRNA that can regulate protein expression and signaling pathways in recipient cells [21].
Fig. 1.
Exosome structure. a Morphologic characterization of exosomes by cryo-scanning electron microscope. Scale bar 100 nm. Micrograph adapted from Ref. [22]. b Schematic representation of an exosome with the main classes of “cargo” molecules
Exosomes in normal physiology
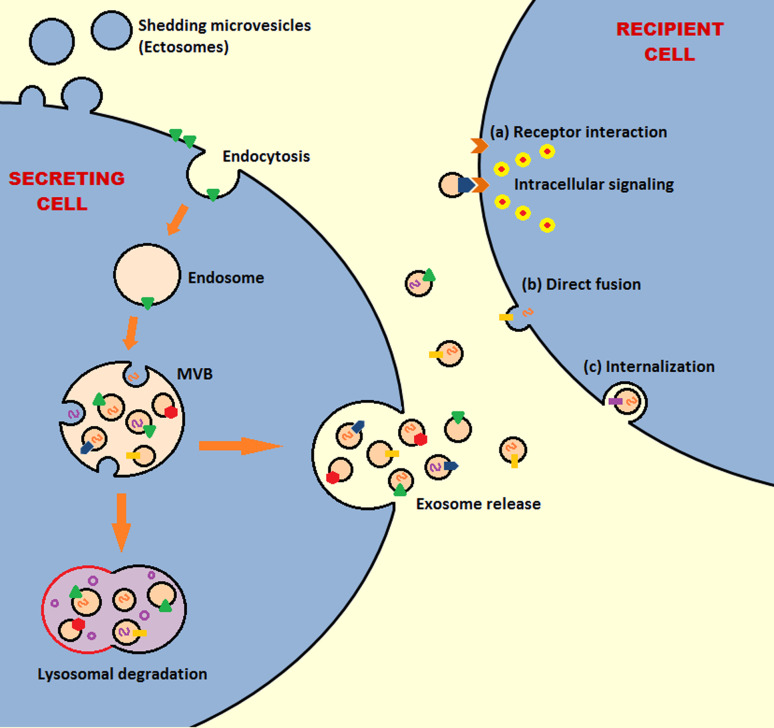
Exosomes could be considered miniature versions of the cell from which they originate. They contain cytosol and their membrane orientation is the same as that of the donor cell exposing the extracellular side of the cell membrane at their outer surface, which involves exposing various cell-specific membrane proteins/receptors that can be employed for specific interactions with recipient cells [8, 11]. Upon release, exosomes, with their large array of biologically active constituents, form a critical part of the cell’s microenvironment and are distributed to various locations where they interact with recipient cells [21, 22]. Several mechanisms have been hypothesized to describe the interactions of exosomes and recipient cells. Exosomes could fuse with the recipient cell membrane merging their membrane contents into the plasma membrane and delivering cytosolic contents to the recipient cell [21]. Alternatively, exosomes can activate cell surface receptors via protein and bioactive lipid ligands through receptor–ligand interactions and antigen presentation [8, 12]. Finally, exosomes may also be internalized by the recipient cells by mechanisms such as pinocytosis, phagocytosis or receptor-mediated endocytosis [23] (Fig. 2).
Fig. 2.
Schematic exosome biogenesis, release to the extracellular space, and interaction with target cell. Membrane proteins (polygons) and RNAs (curved symbols) are selectively incorporated into the intraluminal vesicles (ILVs). The multivesicular body (MVB) either fuses with lysosome or is destined to the cell membrane to release ILVs as exosomes into the extracellular space. Released exosomes can bind via specific receptors on the surface of recipient cell, fuse directly with the recipient cell membrane, or be internalized by the recipient cell
Although the functions of exosomes are not completely understood, research efforts in the past 20 years have unraveled important aspects of the biological significance of exosomes. Originally, exosomes were viewed as simply “garbage bags” or “inert cellular debris” allowing cells to dispose of the unwanted proteins in bags (vesicles) [23]. Early reports described exosomes as a major route for externalization of obsolete membrane proteins during reticulocytes maturation [24]. Exosomes were then discovered to be important conveyors of immune response by participating in antigen presentation and communication between immune cells, which has been intensively documented [7, 11]. Subsequently, as the exosome secretion is studied in more types of cells/tissues, more functions are being proposed/attributed to exosomes. For instance, studies have suggested a role for exosomes in coagulation [13, 25, 26], cell migration [27], cell phenotype modulation [28–30], inflammation [28–30], angiogenesis [34] and wound healing [35]. Multiple cell types have been reported to release exosomes in extracellular medium in vitro, including: hematopoietic cells (reticulocytes, T cells, B cells, macrophages, mast cells, dendritic cells, and platelets), intestinal epithelial cells, adipocytes, neuronal cells, fibroblasts, and numerous tumor cell lines [5, 36–38]. Exosomes are also found abundantly in vivo in many biological fluids including blood, urine, saliva, ascites, pleural effusions, synovial fluids, and breast milk [5]. Nevertheless, this widespread release of exosomes by cells is an indication of more biological roles that are yet to be discovered.
Exosomes in cancer
Cancers sustain on robust biological interaction networks that involve complex signaling systems that are maintained by constant mobilization of biological material across the membranes of cells [39]. Cancer cells actively secrete exosomes that constitute a critical part of tumor microenvironment and are released to the peripheral circulation and other biological fluids [40]. There is a growing interest by cancer researchers to elucidate the involvement of exosomes-mediated signaling in various tumor-related pathways. Current evidence suggests that exosomes participate in many key tumor-promoting processes. They can manipulate the local and systemic environment to aid in cancer growth and dissemination as well as programming the immune system to evade anti-tumor response [40].
Exosomes in cellular transformation
Exosomes released by neoplastic cells carry arrays of functional oncogenic molecules including proteins and microRNAs that could convey phenotypic transforming signals to non-malignant cells, including stem cells, which could have a great implication in tumor progression and metastasis [21, 41]. Indeed, studies have shown that exosomes play an important role in communication between cancer cells and mesenchymal stem cells (MSCs) and in the transformation and/or recruitment of MSCs to tumor sites [22, 42–45]. We recently demonstrated that prostate cancer cell-derived exosomes can induce neoplastic transformation of patient-derived adipose stem cells [22]. The neoplastic reprogramming of the patient-derived MSCs are associated with exosomal trafficking of oncogenic microRNAs (miR-125b, miR-130b and miR-155), K-ras and H-ras mRNAs and oncoproteins, such as Ras superfamily of GTPases Rab1a, Rab1b, and Rab11a [22]. The transformed MSCs undergo mesenchymal-to-epithelial transition (MET) and express neoplastic, vasculogenic and epithelial markers reminiscent of molecular features of prostate tumors in vivo [22]. Similarly, breast and ovarian cancer-derived exosomes induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts, thus contributing to the generation of tumor-associated myofibroblasts from MSCs in tumor stroma [42, 43]. In addition, cancer cells induced to express mesenchymal phenotype by epithelial-to-mesenchymal transition (EMT) release tissue factor-containing exosomes [44]. These exosomes are capable of transferring procoagulant activity to endothelium and thus influencing tumor-vascular interactions [44]. On the other hand, bone marrow mesenchymal stromal cells (BM-MSCs)-derived exosomes were reported to support multiple myeloma clone expansion and promote multiple myeloma (MM) formation in an animal model system [45].
The role of exosomes in tumor microenvironment and angiogenesis
Malignant tumors are complex structures that contain the transformed cells as well as the surrounding tumor stroma that consists of extracellular matrix, cancer-associated fibroblasts, cells of the immune system, endothelial cells, and tumor-associated vasculature. It is now evident that effective tumor growth requires a continuous cross-talk and interaction between cancer cells and other cellular components of tumor microenvironment [39]. Emerging evidence suggests that exosome secretion contributes to the tumor microenvironment in various ways by mediating intercellular communication. Exosomes are produced in abundance by all cells in the tumor microenvironment, leading to a network of complex interactions between the subsets of cells in the tumor niche [17, 40].
Exosomes from glioma and glioblastoma cells were shown to transfer oncogenic activity between cancer cells via horizontal propagation of active oncogenes and their associated phenotype among subsets of cancer cells [46]. The oncogenic mutant epidermal growth factor receptor EGFRvIII expressed by a subset of glioma cells is associated with aggressiveness and was found to be transferred in vesicles to adjacent tumor cells lacking this mutant receptor. This exosome-mediated transfer leads to the production of downstream anti-apoptotic and angiogenic mediators such as vascular endothelial growth factor (VEGF) in the recipient cells and results in morphological transformation and increase in anchorage-independent growth capacity of cells [46]. Colon cancer cells expressing mutant K-ras utilize their exosomes to transfer this oncogene to cells expressing the wild-type protein leading to enhanced growth and tumorigenicity [47]. Exosomes from prostate and other cancer cells can trigger the differentiation of fibroblast and mesenchymal stem cells within tumor stroma into myofibroblasts [43, 48]. This provides a favorable environment for tumor progression since the myofibroblasts are key source of matrix-remodeling proteins and participate in tumor angiogenesis [48]. Several studies have also demonstrated the angiogenic capability of cancer-derived exosomes in vitro and in vivo by carrying various angiogenic proteins [40, 46, 49]. Additionally, microRNAs in cancer-derived exosomes support tumor growth by binding and activating toll-like receptors (TLRs) in surrounding immune cells leading to a prometastatic inflammatory response in tumor microenvironment [50].
In solid tumors, a substantial fraction of the tumor cells is subjected to hypoxic conditions, which is an important feature of the tumor microenvironment [51]. Emerging evidence has suggested that hypoxia promotes aggressiveness and therapeutic resistance of cancers [51–53]; however, the precise underlying mechanisms remain unclear. A number of studies have indicated that hypoxic conditions enhance exosomes secretion in different tumor types [54, 55]. In the highly malignant glioblastoma system, hypoxia was shown to promote pro-angiogenic pathways through cancer-associated exosome-like vesicles secretion. The angiogenic effect of those vesicles was mediated through the protease-activated receptor 2 (PAR-2) signaling in endothelial cells [56]. Similarly, in other reports, hypoxia was found to stimulate different types of cancer cells to secrete exosomes with enhanced angiogenic and pro-metastatic potential, indicating that tumor cells may respond to hypoxia by secreting exosomes to modify their microenvironment, enhance angiogenesis and increase invasiveness; thereby, potentiating cancer aggressiveness [53, 57].
The role of exosomes in metastatic niche formation and cancer metastasis
Most cancer deaths are attributed to metastasis, which is generally refractory to any form of available therapies. Metastasis is a multistep process that involves the acquisition of migratory malignant phenotype that allows the cancer cells to invade the primary tumor microenvironment, then disseminate, and establish new growth at distant sites [58]. To metastasize, cancer cells must manipulate their microenvironment to optimize conditions for migration, implantation and growth both locally and at distant metastatic sites. This requires complex tumor-stromal interactions that involve various intercellular communication pathways [58]. Recent emerging evidence points to the importance of exosomes in various metastatic pathways owing to their ability to function as an escape route for metastasis-promoting proteins, microRNAs and other elements from the site of tumor to distant locations [22, 39, 59].
Exosomes secreted from primary tumors were found to contribute to the formation of metastatic niche, a permissive environment at the potential metastatic sites, by educating non-transformed cells in selected host tissues toward a pro-metastatic phenotype [59]. Exosomes from highly metastatic melanomas are able to permanently educate bone marrow progenitor cells (BMDCs) toward a pro-vasculogenic and pro-metastatic phenotype through upregulation of the MET oncoprotein, also known as hepatocyte growth factor receptor or SF receptor [60]. Melanoma-derived exosomes can also induce vascular leakiness at pre-metastatic sites. Additionally, homing of melanoma exosomes to sentinel lymph nodes imposes synchronized molecular signals that promote melanoma cell recruitment, extracellular matrix deposition, and vascular proliferation in the lymph nodes [61]. Further evidence supporting the involvement of tumor-secreted microvesicles in promoting invasion and metastasis includes the transfer of the extracellular matrix metalloproteinase inducer (EMMPRIN) in microvesicles from tumor cells, which stimulates expression of matrix metalloproteinase in fibroblasts and remodeling of the extracellular matrix [12, 62]. Interestingly, in addition to tumor-derived exosomes, studies have reported that stroma-derived exosomes can also support tumor metastasis; tumor-associated macrophages were reported to use exosomal secretion to shuttle invasion-potentiating microRNAs to breast cancer cells to promote their local invasiveness and metastasis [63]. Additionally, fibroblast-secreted exosomes were shown to stimulate breast cancer cells’ protrusive activity, motility, and invasion via Wnt-planar cell polarity (PCP) signaling pathway [64, 65].
Exosomes in cancer immunology
Evading destruction by the immune system is an important emerging hallmark for the development and progression of cancer. Cancer cells have evolved complex strategies to avoid being recognized by the immune system [8]. Those strategies involve an array of interactions between cancer cells and immune cells, including T- and B-lymphocytes, macrophages, and natural killer cells, leading to the suppression of their antitumor functions [39]. Exosomes derived from virtually any nucleated cell type, including cancer cells, display MHC class I molecules on their surface that could potentially induce CD8+ T cell activation [8, 11]. Initial reports suggested that exosomes released by tumor cells act as a source of tumor antigens for activating antigen-presenting cells (APCs), including dendritic cells, which in turn induce cytotoxic T lymphocyte-dependent antitumor response [66, 67]. However, extensive reports have suggested that tumor-derived exosomes might rather suppress tumor-specific and nonspecific immune responses of the host; for example, tumor-derived exosomes carrying Fas ligand, TRAIL or galectin 9 can promote T cell apoptosis [8, 68–70]. Tumor-derived exosomes can affect the APCs function by promoting the differentiation of monocytes to the immunosuppressive myeloid-derived suppressor cells (MDSCs) through the presence of prostaglandin E2 (PGE2), TGFβ, HSP72 and microRNAs in the exosomes [71–75]. MDSCs in turn support the function of regulatory T cells (Treg), which inhibit antitumor responses. In addition, tumor-derived extracellular vesicles can directly enhance regulatory T cells function and promote their expansion [76]. Other studies have demonstrated that tumor-derived microvesicles induce apoptosis of activated tumor-reactive T-lymphocytes, especially the CD8+ cells [76–78]. Conversely, activated T cell exosomes can promote tumor invasion and migration of some cancer cells via Fas signaling pathway [79]. Tumor-derived exosomes can also inhibit the differentiation and maturation of myeloid dendritic cells and macrophages through a TGFβ1-dependent mechanism, thereby exerting an immunosuppressive function [8, 80].
Cancer exosomes and the major signaling pathways
In the past few years, significance has been given to exosomes in the induction and modulation of cell-fate-inducing signaling networks that play a pivotal role in the initiation and sustenance of cancer [81]. The exosomal role in many pathways is not fully elucidated, but numerous reports have highlighted the impact of exosomal secretion on several cellular and intercellular signaling pathways. Some of those signaling pathways can modulate exosome secretion [81].
Transforming growth factor beta (TGF-β), which plays an important role in the promotion and maintenance of tumor stroma as well as the induction of EMT, is exported in exosomes of different types of cancer [48]. Tumor-derived exosomes have been shown to increase expression of TGF-β receptors I and II and induce TGFβ-related downstream signaling pathways in recipient cells. For example, breast cancer-derived exosomes activate the TGFβ receptor-mediated signaling pathway in mesenchymal stem cells promoting their differentiation to myofibroblasts, a key component of tumor stroma [42, 43]. Additionally, TGFβ1-rich exosomes can function in immune suppression by impairing the lymphocytes response to interleukin 2 [78].
Wnt-β-catenin signaling is another evolutionary conserved pathway that plays a crucial role in normal development and in cancer. Active Wnt proteins are secreted on exosomes that can modulate the activity of Wnt cellular signaling pathways [82, 83]. Two tetraspanins CD82 and CD9, abundantly expressed in exosomes, were found to regulate the Wnt signaling pathway through the exosomal discharge of β-catenin [84]. Additionally, fibroblast-derived exosomes were found to be loaded with autocrine Wnt-11 that was shown to stimulate breast cancer cells invasive behavior via the Wnt-planar cell polarity (PCP) signaling pathway [65].
The tumor suppressor P53 protein, which is a central player in cancer pathogenesis regulating different cell surveillance pathways, was found to regulate exosome secretion in cultured cells [84]. The rate of exosome secretion can be induced by p53 activation. P53 regulates the transcription of important genes such as TSAP6 that enhance exosome production. Conversely, suppression of TSAP6 in a mice model severely impairs the p53-mediated production of exosomes [85]. P53 also regulates the transcription of a set of genes that regulate the endosomal compartment and endosomal clearance of the EGFR receptor from the plasma membrane [86]. These studies have provided insights into the communications between endosomes, exosomes and p53-mediated transcriptional signaling [87].
Phosphatase and tensin homolog (PTEN) is one of the most frequently mutated/lost tumor suppressors in human cancers. It acts by regulating PI3 K–AKT signaling, cell growth and cell survival [88]. PTEN was found to be secreted in exosomes and exerts phosphatase activity in recipient cells, resulting in reduced cellular proliferation [89].
Notch signaling, a critical survival pathway that is deregulated in many cancers, was also found to be impacted by exosomes [89–91]. Interactions of pancreatic tumor-derived exosomes with target cells hamper the functioning of the Notch-1 survival pathway and activate apoptosis in a PTEN/GSK-3beta dependent manner [92].
A simplified summary of the main tumor-promoting molecules transported in exosomes and their biological effects is illustrated in Table 2.
Table 2.
Important tumor-promoting molecules transported by exosomes and their cancer-related effects
| Molecules carried in exosomes | Effects in cancer | References |
|---|---|---|
| TGF-β | Induce myofibroblastic phenotype in tumor stroma. Immune suppression | [42, 43, 78] |
| Wnt signaling components | Promote invasiveness | [65, 82] |
| PTEN | Reduce proliferation of recipient cells | [89] |
| EGFR | Angiogenesis-modulation via autocrine VEGF Production in Endothelial Cells. | [93] |
| PGE2, TGFβ, HSP72 and miRNAs | Immune suppression, monocyte differentiation to MDSCs | [71–75] |
| Fas ligand, TRAIL or galectin 9 | Immune suppression, apoptosis in activated T cells | [8] |
| EGFRvIII, mutant KRAS | Transfer of oncogenic proteins between cancer cells | [46, 47] |
| Tissue factor (TF) | Thrombosis, Angiogenesis | [44, 94] |
| EMMPRIN | Promote invasion, remodeling of the extracellular matrix | [62] |
| Tumor-specific antigens | Immunogenic antitumor effects | [67] |
TGF-β transforming growth factor beta, PTEN phosphatase and tensin homolog, EGFR epidermal growth factor receptor, PGE2 prostaglandin E2, HSP72 heat shock protein 72, TRAIL TNF-related apoptosis-inducing ligand
Conclusions
Understanding the communication codes sent by cancer cells is crucial for the development of successful cancer treatment. Exosomal signaling is emerging as a big player in cell–cell communication under many different entities, especially in cancer development and progression. The various pro-tumorigenic effects of exosomes are being increasingly reported. Some reports have suggested anti-tumor roles of exosomes, especially due to their potential to elicit anti-tumor immune response. However, the pro-tumorigenic potential of tumor-derived exosomes in cancer patients is supported by the observation that the level of circulating exosomes in cancer patients is higher than in normal individuals and increases with tumor progression [95]. Notably, cancer cells use their exosomes to transmit various detrimental effects onto their local and distant environment and the host immune system to facilitate their survival, growth, and metastasis. Given the relatively recent history of exosomal discovery and characterization, the exact mechanisms mediating the exosomal role in cancer are not yet fully elucidated. In this review, we described a direct role for tumor-derived exosomes in neoplastic transformation of tumor-tropic MSCs and some of the major cancer-related pathways impacted by exosomes, as reported in published literature. An important potential role of tumor-derived exosomes in drug resistance is now emerging, which further highlights the need for more work to better understand exosomes and to delineate these novel mechanisms [17, 96]. Nevertheless, further advances in exosomal analysis and elucidation of the intricacies of the exosomes biogenesis, secretion and uptake by recipient cells will provide us with the knowledge to interfere directly with exosome production and function in vivo and to further improve anti-cancer therapeutics. Being close replicas of their parental cells, relatively stable, and easily accessible from body fluids, exosomes have a great potential to become valuable tools in early diagnosis and targeted therapy of cancer.
References
- 1.Trams EG, Lauter CJ, Salem N, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim Biophys Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 2.Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J Biol Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 5.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Rak J, Guha A. Extracellular vesicles–vehicles that spread cancer genes. BioEssays. 2012;34:489–497. doi: 10.1002/bies.201100169. [DOI] [PubMed] [Google Scholar]
- 7.Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 8.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombo M, Moita C, van Niel G, Kowal J, Vigneron J, Benaroch P, Manel N, Moita LF, Thery C, Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 10.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 11.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 12.Ela S, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 13.Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood. 1999;94:3791–3799. [PubMed] [Google Scholar]
- 14.Stein JM, Luzio JP. Ectocytosis caused by sublytic autologous complement attack on human neutrophils. The sorting of endogenous plasma-membrane proteins and lipids into shed vesicles. Biochem J. 1991;274:381–386. doi: 10.1042/bj2740381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- 17.Azmi AS, Bao B, Sarkar FH. Exosomes in cancer development, metastasis, and drug resistance: a comprehensive review. Cancer Metastasis Rev. 2013;32:623–642. doi: 10.1007/s10555-013-9441-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood Cells Mol Dis. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J Biol Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 22.Abd Elmageed ZY, Yang Y, Thomas R, Ranjan M, Mondal D, Moroz K, Fang Z, Rezk BM, Moparty K, Sikka SC, Sartor O, Abdel-Mageed AB. Neoplastic reprogramming of patient-derived adipose stem cells by prostate cancer cell-associated exosomes. Stem Cells. 2014;32:983–997. doi: 10.1002/stem.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Jr, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 24.Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147:27–36. doi: 10.1002/jcp.1041470105. [DOI] [PubMed] [Google Scholar]
- 25.Al-Nedawi K, Szemraj J, Cierniewski CS. Mast cell-derived exosomes activate endothelial cells to secrete plasminogen activator inhibitor type 1. Arterioscler Thromb Vasc Biol. 2005;25:1744–1749. doi: 10.1161/01.ATV.0000172007.86541.76. [DOI] [PubMed] [Google Scholar]
- 26.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. Int J Cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 27.Kriebel PW, Barr VA, Rericha EC, Zhang G, Parent CA. Collective cell migration requires vesicular trafficking for chemoattractant delivery at the trailing edge. J Cell Biol. 2008;183:949–961. doi: 10.1083/jcb.200808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C. Exosome/microvesicle-mediated epigenetic reprogramming of cells. Am J Cancer Res. 2011;1:98–110. [PMC free article] [PubMed] [Google Scholar]
- 29.Jang YY, Collector MI, Baylin SB, Diehl AM, Sharkis SJ. Hematopoietic stem cells convert into liver cells within days without fusion. Nat Cell Biol. 2004;6:532–539. doi: 10.1038/ncb1132. [DOI] [PubMed] [Google Scholar]
- 30.Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 31.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald MK, Tian Y, Qureshi RA, Gormley M, Ertel A, Gao R, Aradillas Lopez E, Alexander GM, Sacan A, Fortina P, Ajit SK. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain. 2014 doi: 10.1016/j.pain.2014.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 35.Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, Han YP, Woodley DT, Li W. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol. 2008;28:3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, Hivroz C. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. J Immunol. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 37.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, Blot B, Haase G, Goldberg Y, Sadoul R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 38.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, Heyman M. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 39.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl) 2013;91:431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao D, Dai C, Peng S. Mechanism of the mesenchymal-epithelial transition and its relationship with metastatic tumor formation. Mol Cancer Res. 2011;9:1608–1620. doi: 10.1158/1541-7786.MCR-10-0568. [DOI] [PubMed] [Google Scholar]
- 42.Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol Oncol. 2011;123:379–386. doi: 10.1016/j.ygyno.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int J Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 44.Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J Biol Chem. 2012;287:43565–43572. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J Clin Invest. 2013;123:1542–1555. doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Al-Nedawi K, Meehan B, Micallef J, Lhotak V, May L, Guha A, Rak J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol. 2008;10:619–624. doi: 10.1038/ncb1725. [DOI] [PubMed] [Google Scholar]
- 47.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- 49.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabbri M, Paone A, Calore F, Galli R, Gaudio E, Santhanam R, Lovat F, Fadda P, Mao C, Nuovo GJ, Zanesi N, Crawford M, Ozer GH, Wernicke D, Alder H, Caligiuri MA, Nana-Sinkam P, Perrotti D, Croce CM. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc Natl Acad Sci USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casazza A, Di Conza G, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2014;33:1743–1754. doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 52.Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim Biophys Acta. 2012;1826:272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc Natl Acad Sci USA. 2013;110:7312–7317. doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci USA. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramteke A, Ting H, Agarwal C, Mateen S, Somasagara R, Hussain A, Graner M, Frederick B, Agarwal R, Deep G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol Carcinog. 2013 doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 59.Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peinado H, Aleckovic M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, Garcia-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883–891. doi: 10.1038/nm.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 62.Sidhu SS, Mengistab AT, Tauscher AN, LaVail J, Basbaum C. The microvesicle as a vehicle for EMMPRIN in tumor-stromal interactions. Oncogene. 2004;23:956–963. doi: 10.1038/sj.onc.1207070. [DOI] [PubMed] [Google Scholar]
- 63.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luga V, Wrana JL. Tumor-stroma interaction: revealing fibroblast-secreted exosomes as potent regulators of Wnt-planar cell polarity signaling in cancer metastasis. Cancer Res. 2013;73:6843–6847. doi: 10.1158/0008-5472.CAN-13-1791. [DOI] [PubMed] [Google Scholar]
- 65.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 66.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 67.Andre F, Schartz NE, Movassagh M, Flament C, Pautier P, Morice P, Pomel C, Lhomme C, Escudier B, Le Chevalier T, Tursz T, Amigorena S, Raposo G, Angevin E, Zitvogel L. Malignant effusions and immunogenic tumour-derived exosomes. Lancet. 2002;360:295–305. doi: 10.1016/S0140-6736(02)09552-1. [DOI] [PubMed] [Google Scholar]
- 68.Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L, Stringaro A, Molinari A, Arancia G, Gentile M, Parmiani G, Fais S. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303–1316. doi: 10.1084/jem.20011624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huber V, Fais S, Iero M, Lugini L, Canese P, Squarcina P, Zaccheddu A, Colone M, Arancia G, Gentile M, Seregni E, Valenti R, Ballabio G, Belli F, Leo E, Parmiani G, Rivoltini L. Human colorectal cancer cells induce T-cell death through release of proapoptotic microvesicles: role in immune escape. Gastroenterology. 2005;128:1796–1804. doi: 10.1053/j.gastro.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 70.Klibi J, Niki T, Riedel A, Pioche-Durieu C, Souquere S, Rubinstein E, Le Moulec S, Guigay J, Hirashima M, Guemira F, Adhikary D, Mautner J, Busson P. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood. 2009;113:1957–1966. doi: 10.1182/blood-2008-02-142596. [DOI] [PubMed] [Google Scholar]
- 71.Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L. Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res. 2007;67:2912–2915. doi: 10.1158/0008-5472.CAN-07-0520. [DOI] [PubMed] [Google Scholar]
- 72.Xiang X, Poliakov A, Liu C, Liu Y, Deng ZB, Wang J, Cheng Z, Shah SV, Wang GJ, Zhang L, Grizzle WE, Mobley J, Zhang HG. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124:2621–2633. doi: 10.1002/ijc.24249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, Corbelli A, Fais S, Parmiani G, Rivoltini L. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. 2006;66:9290–9298. doi: 10.1158/0008-5472.CAN-06-1819. [DOI] [PubMed] [Google Scholar]
- 75.Xiang X, Liu Y, Zhuang X, Zhang S, Michalek S, Taylor DD, Grizzle W, Zhang HG. TLR2-mediated expansion of MDSCs is dependent on the source of tumor exosomes. Am J Pathol. 2010;177:1606–1610. doi: 10.2353/ajpath.2010.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wieckowski EU, Visus C, Szajnik M, Szczepanski MJ, Storkus WJ, Whiteside TL. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8 + T lymphocytes. J Immunol. 2009;183:3720–3730. doi: 10.4049/jimmunol.0900970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim JW, Wieckowski E, Taylor DD, Reichert TE, Watkins S, Whiteside TL. Fas ligand-positive membranous vesicles isolated from sera of patients with oral cancer induce apoptosis of activated T lymphocytes. Clin Cancer Res. 2005;11:1010–1020. [PubMed] [Google Scholar]
- 78.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 79.Cai Z, Yang F, Yu L, Yu Z, Jiang L, Wang Q, Yang Y, Wang L, Cao X, Wang J. Activated T cell exosomes promote tumor invasion via Fas signaling pathway. J Immunol. 2012;188:5954–5961. doi: 10.4049/jimmunol.1103466. [DOI] [PubMed] [Google Scholar]
- 80.Yu S, Liu C, Su K, Wang J, Liu Y, Zhang L, Li C, Cong Y, Kimberly R, Grizzle WE, Falkson C, Zhang HG. Tumor exosomes inhibit differentiation of bone marrow dendritic cells. J Immunol. 2007;178:6867–6875. doi: 10.4049/jimmunol.178.11.6867. [DOI] [PubMed] [Google Scholar]
- 81.Wendler F, Bota-Rabassedas N, Franch-Marro X. Cancer becomes wasteful: emerging roles of exosomes in cell-fate determination. J Extracell Vesicles. 2013;2:24. doi: 10.3402/jev.v2i0.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 83.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 85.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 86.Yu X, Riley T, Levine AJ. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 2009;276:2201–2212. doi: 10.1111/j.1742-4658.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 87.Hupalowska A, Miaczynska M. The new faces of endocytosis in signaling. Traffic. 2012;13:9–18. doi: 10.1111/j.1600-0854.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 88.Wrighton KH. Tumour suppressors: role of nuclear PTEN revealed. Nat Rev Cancer. 2011;11:154. doi: 10.1038/nrc3028. [DOI] [PubMed] [Google Scholar]
- 89.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci Signal. 2012;5:ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 90.Beloribi S, Ristorcelli E, Breuzard G, Silvy F, Bertrand-Michel J, Beraud E, Verine A, Lombardo D. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells. PLoS ONE. 2012;7:e47480. doi: 10.1371/journal.pone.0047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sharghi-Namini S, Tan E, Ong LL, Ge R, Asada HH. Dll4-containing exosomes induce capillary sprout retraction in a 3D microenvironment. Sci Rep. 2014;4:4031. doi: 10.1038/srep04031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int J Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 93.Al-Nedawi K, Meehan B, Kerbel RS, Allison AC, Rak J. Endothelial expression of autocrine VEGF upon the uptake of tumor-derived microvesicles containing oncogenic EGFR. Proc Natl Acad Sci USA. 2009;106:3794–3799. doi: 10.1073/pnas.0804543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 95.Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol. 2011;2011:1–11. doi: 10.1155/2011/842849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS ONE. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]