Abstract
Background
Hospital readmissions are costly and associated with inferior patient outcomes. There is limited knowledge related to readmissions following esophagectomy for malignancy. Our aim was to determine the impact of readmission following esophagectomy on survival.
Methods
This cohort study utilizes Surveillance, Epidemiology, and End Results-Medicare data (2002–2009). Survival, length of stay (LOS), 30-day readmissions and discharge disposition were determined. Multivariate logistic regression models were created to examine risk factors associated with readmission.
Results
1,744 patients with esophageal cancer underwent esophagectomy. 80% (1390) of patients were male and mean age was 73 years. 71.8% (1251) of tumors were adenocarcinomas and 72.5% (1265) were distal esophageal tumors. 38% (667) of patients received induction therapy. Operative approach was transthoracic in 52.6% (918), transhiatal in 37.4% (653) and required complex reconstruction (intestinal interposition) in 9.9% (173). Stage distribution was: Stage I 35.3% (616), Stage II 32.5% (566), Stage III 27.9% (487) and Stage IV 2.3% (40). Median LOS was 13 days, hospital mortality was 9.3% (158) and 30-day readmission rate was 18.6% (212/1139 home discharges). 25.4% (443) were discharged to institutional care facilities. Overall survival was significantly worse for patients readmitted (p<0.0001, log-rank test). Risk factors for readmission were comorbidity score of 3+, urgent admission and urban residence.
Conclusions
Hospital readmissions following esophagectomy for cancer occur frequently and are associated with worse survival. Improved identification of patients at risk for readmission following esophagectomy can inform patient selection, discharge planning and outpatient monitoring. Optimization of such practices may lead to improved outcomes at reduced cost.
Keywords: Esophageal cancer, Esophageal surgery, operations, Surgery, complications, Outcomes
Introduction
Reshospitalizations place a significant burden on the healthcare system in our country. One report, by Jencks and colleagues, found that 19% of Medicare patients discharged from the hospital were readmitted within 30 days after discharge[1]. This was associated with an estimated cost of 17 billion dollars to our healthcare system in 2004. In addition, hospital readmissions have been associated with inferior long term survival following colectomy for colorectal cancer[2]. With the recent passage of the Patient Protection and Affordable Care Act, the Centers for Medicare and Medicaid Services (CMS) have placed an emphasis on reducing hospital readmission rates in order to improve the quality of health care in the United States[3]. Postoperative readmission rates have been examined for a variety of operations, including coronary artery bypass, pancreatic resections, colorectal resections, hip replacements, and abdominal aortic aneurysm repair, and have been reported to be as high as 21%[2,4–10].
An esophagectomy for esophageal cancer is a high risk surgical procedure, with large administrative datasets demonstrating mortality rates ranging from 7–28%[11–13]. Hospital readmissions following an esophagectomy for esophageal cancer are also a common occurrence, ranging from 5–25%[10,14,15]. However, there is limited knowledge related to risk factors and mortality following readmission following esophagectomy, or its impact on long term survival. In order to establish care processes that are designed to minimize preventable rehospitalizations, one must first understand what variables place an individual at risk for readmission to the hospital. The objective of this study was to examine the impact of hospital readmission following esophagectomy on survival and to determine risk factors for readmission after esophagectomy using the Surveillance, Epidemiology, and End Results (SEER)-Medicare linked database. We hypothesized that readmission following esophagectomy would be associated with a higher mortality rate.
Patients and Methods
We performed a retrospective cohort study using the SEER-Medicare linked database to assess the impact of hospital readmissions in patients with esophageal cancer treated with an esophagectomy between years 2002–2009. The SEER database is derived from 18 tumor registries, is maintained by the National Cancer Institute (NCI), and represents approximately 18% of United States population. Medicare beneficiaries within the registry have had their tumor records linked to all of their claims data. The quality, validity and generalizability of the SEER-Medicare data has been described previously [16]. Approval for the study was obtained from the Institutional Review Board of Emory University. We utilized the Medicare Denominator, Inpatient, Outpatient and Physician/Supplier files for this study.
Among all esophageal cancer patients from 2002 through 2009 in the SEER-Medicare dataset, the following sequential exclusions were made: patients less than 66 years old, patients treated with therapy other than surgery, and patients with partial fee-for-service or concurrent health maintenance organization enrollment, or both, 1 year before esophageal cancer surgery. Only full fee-for-service beneficiaries not enrolled in other insurance programs would have complete claims records, therefore, all other patients were excluded. Patients who were 65 years old at the time of diagnosis were excluded because they do not have Medicare claims data in the year before esophagectomy, which would preclude the determination of receipt of neoadjuvant chemotherapy and/or radiation, and the calculation of comorbidity scores.
Patient, disease and treatment information were available through the SEER registry and Medicare database. Specifically, Current Procedural Terminology (CPT) and International Classification of Diseases, 9th revision (ICD-9) codes were used to determine the surgical approach to esophagectomy (transthoracic versus transhiatal), patient comorbid medical conditions, and delivery of neoadjuvant chemotherapy and radiation (see Appendix 1 for specific Medicare billing codes, found at http://www.annalsthoracicsurgery.org/). Medicare claims within the Physician/Supplier and Outpatient files in the year before diagnosis were used to calculate a Klabunde-modified Charlson Comorbidity Index, which was then used for risk adjustment [17]. Chemotherapy and/or radiation administered within 4 months of esophagectomy was considered neoadjuvant therapy, as classified in prior publications using SEER-Medicare data [18]. For analysis of patient characteristics, indicators of low income or education were based on the lowest quartiles of median income and proportion with a high school education within a given zip code from Census Tract data. Tumor size, stage and histology were all based on information within 4 months of diagnosis in the SEER registry. All tumors were restaged to the American Joint Committee on Cancer (AJCC), 7th edition esophageal cancer staging system [19].
The primary outcome measure was hospital readmission with 30 days following discharge after esophagectomy. The denominator for analysis of hospital readmission was all patients discharged to home following esophageal resection for cancer. Patients discharged to an intermediate care facility (ICF) were not considered in the readmission analysis. Patients discharged to an ICF were not included in the readmission analysis, as it is difficult to determine what constitutes a hospital discharge or readmission, as patients are transferred from one inpatient care facility to another. Secondary outcomes were mortality and resource utilization following esophagectomy.
SAS Version 9.3 (Cary, NC) was used to perform all statistical analysis. Descriptive statistics are presented as counts with percentages, means with standard deviation, and/or median with interquartile range. Kaplan-Meier (KM) curves were generated that provide unadjusted survival estimates at postoperative points in time for patients who were and were not rehospitalized. Differences between strata were determined by log-rank tests. Binary logistic regression models were used to examine the association between patient demographic, clinical and treatment characteristics and hospital readmission following esophagectomy. Variables were selected a priori for inclusion in the multivariable analysis. All statistical tests were two-sided and used an α = 0.05 level of significance.
Results
1,744 patients in the SEER-Medicare dataset underwent esophageal resection for esophageal cancer between the years 2002 and 2009 and met inclusion criteria. The demographics and clinical details of patients at the time of hospital admission for esophagectomy are summarized in Table 1. These patients were predominantly elderly Caucasian males. The most common presentation of esophageal cancer was a distal esophageal adenocarcinoma. 38% (667/1,744) of patients received neoadjuvant chemotherapy and/or radiation. More than sixty percent of patients had a modified Charlson comorbidity score of zero. A transthoracic approach to esophagectomy was more common than transhiatal (52.6% vs. 37.4%). Complex reconstruction following esophagectomy (colonic or intestinal interposition, with or without microvascular anastomosis) was performed in 9.9%.
Table 1.
Patient and disease characteristics for esophagectomy patients
| Variable | n (%), mean ± SD |
|---|---|
| Male sex, n (%) | 1,390 (79.7%) |
| Age, mean ± SD, years | 73.0 ± 5.2 |
| Race | |
| Caucasian | 1,619 (92.8%) |
| African American | 84 (4.8%) |
| Other | 41 (2.4%) |
| Marital Status | |
| Married | 1,248 (71.6%) |
| Not Married | 496 (28.4%) |
| Education – Census Tract | |
| Lowest quartile | 384 (22.0%) |
| Poverty – Census Tract | |
| Lowest quartile | 130 (7.5%) |
| Comorbidity Score | |
| 0 | 1070 (61.4%) |
| 1 | 442 (25.3%) |
| 2 | 137 (7.9%) |
| 3+ | 95 (5.5%) |
| Type of Admission | |
| Elective | 1539 (88.3%) |
| Urgent | 122 (7.0%) |
| Emergency | 81 (4.6%) |
| Location of esophageal tumor | |
| Distal third | 1,265 (72.5%) |
| Middle third | 270 (15.5%) |
| Proximal third | 59 (3.4%) |
| Other | 150 (8.6%) |
| Operative approach | |
| Transthoracic | 918 (52.6%) |
| Transhiatal | 653 (37.4%) |
| Complex reconstruction | 173 (9.9%) |
| Neoadjuvant Therapy | |
| Chemotherapy Only | 80 (4.6%) |
| Radiation Only | 134 (7.7%) |
| Both Chemo and Radiation | 453 (26.0%) |
| Histology | |
| Adenocarcinoma | 1,251 (71.8%) |
| Squamous Cell | 426 (24.4%) |
| Other | 38 (2.2%) |
| Unknown | 29 (1.7%) |
| Grade | |
| Well to Moderate | 745 (42.7%) |
| Poor to Undifferientiated | 768 (44.0%) |
| Unknown | 231 (13.3%) |
| Stage | |
| I | 616 (35.3%) |
| II | 566 (32.5%) |
| III | 487 (27.9%) |
| IV | 40 (2.3%) |
| Unknown | 35 (2.0%) |
| Area of Residence | |
| Metropolitan | 1469 (84.2%) |
| Urban | 235 (13.5%) |
| Rural | 40 (2.3%) |
Postoperative outcomes in patients following esophagectomy for esophageal cancer are detailed in Table 2. Thirty day and in-hospital mortality rates were substantial, at 8.8% and 9.3%, respectively. Due to outliers causing skewed distributions, the mean length of stay (LOS) and intensive care unit (ICU) days were larger than the median values (18.1 vs. 13 and 10.6 vs. 6, respectively). Approximately one quarter of patients were discharged to an ICF (i.e. skilled nursing or rehabilitation facility). Out of 1,139 patients discharged to home following esophagectomy, 18.6% were readmitted to the hospital within 30 days of discharge and 31.3% within 90 days.
Table 2.
Postoperative outcomes in esophagectomy patients
| Variable | n (%), mean ± SD, or Median (IQR) |
|---|---|
| Discharge Disposition | |
| Home discharge | 1,139 (65.3%) |
| Intermediate care facility | 443 (25.4%) |
| Hospital Mortality | 162 (9.3%) |
| Length of Stay (LOS, days) | 18.1 ± 16.0 |
| LOS (days), median (IQR) | 13 (9 – 21) |
| Intensive Care Days | 10.6 ± 14.4 |
| Intensive Care Days, median (IQR) | 6 (2 – 13) |
| Readmission (out of 1,138 home discharges) | |
| Readmission within 30 days | 212 (18.6%) |
| Readmission within 90 days | 356 (31.3%) |
| Mortality (out of 1,691 had esophagectomy before 2010/01/01) | |
| 30 day mortality | 8.8% (148) |
| 90 day mortality | 17.9% (302) |
| 6 month mortality | 28.0% (473) |
| 1 year mortality | 43.94% (743) |
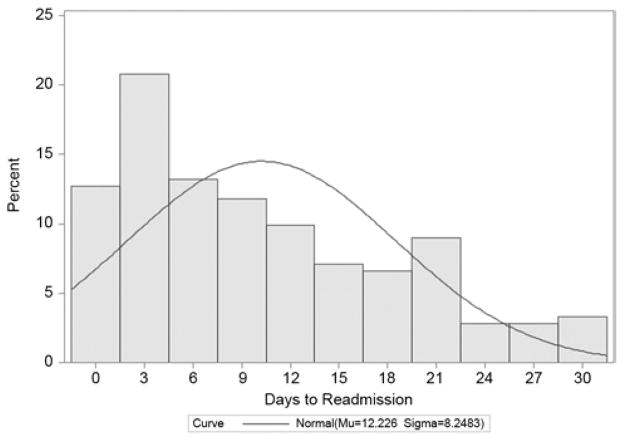
The proportion of hospital readmissions within 30 days of discharge, stratified by day of discharge, is shown in Figure 1. Mean time to hospital readmission in these patients was 12.5 days (standard deviation 8.2). Outcomes following hospital readmission within 30 days of esophagectomy are shown in Table 3. Over 80% of these readmissions were classified as “urgent or emergent”. Mortality occurred in 5.2% who were readmitted and another 18.9% were discharged to an ICF.
Figure 1.
Proportion of readmissions within 30 days of discharge, stratified by day after discharge.
Table 3.
Characteristics of 30 day hospital readmissions following esophagectomy in 212 patients
| Variable | n (%), mean ± SD, or Median (IQR) |
|---|---|
| Type of Admission | |
| Elective | 41 (19.3%) |
| Emergency | 108 (50.9%) |
| Urgent | 62 (29.3%) |
| Length of Stay (days) | 8.46 (9.94) |
| Length of Stay (days), median (IQR) | 5 (3 – 10) |
| Intensive Care Days | 2.54 (7.50) |
| Intensive Care Days, median (IQR) | 0 (0 – 2) |
| Discharge Disposition | |
| Home Discharge | 160 (75.5%) |
| Intermediate Care Facility | 40 (18.9%) |
| Hospital Mortality | 12 (5.2%) |
A multivariable binary logistic regression model was developed to examine variables associated with 30 day hospital readmission following esophagectomy. Variables were selected a priori for inclusion into the model. Results of the logistic regression model are shown in Table 4. A modified Charlson comorbidity score of 3+ was most strongly associated with 30 day hospital readmission. An urgent admission status and an urban (compared to metropolitan) area of residence were also significantly associated with readmissions, whereas African American race appeared to demonstrate a trend towards association with hospital readmission.
Table 4.
Multivariate analysis, risk factors for 30 day hospital readmission
| Variable | Odds Ratio | 95% Confidence Limits | P value | |
|---|---|---|---|---|
| Operative approach | ||||
| Transthoracic | 1 (Ref) | |||
| Transhiatal | 1.08 | 0.78 | 1.50 | 0.64 |
| Complex reconstruction | 0.92 | 0.52 | 1.61 | 0.77 |
| Neoadjuvant Therapy | ||||
| No | 1 (Ref) | |||
| Yes | 1.15 | 0.83 | 1.60 | 0.40 |
| Gender | ||||
| Male | 1 (Ref) | |||
| Female | 1.32 | 0.86 | 2.02 | 0.21 |
| Age | 1.01 | 0.98 | 1.04 | 0.67 |
| Race | ||||
| Caucasian | 1 (Ref) | |||
| African American | 1.98 | 0.90 | 4.34 | 0.09 |
| Other | 0.62 | 0.18 | 2.18 | 0.46 |
| Marital status | ||||
| Married | 1 (Ref) | |||
| Unmarried | 1.05 | 0.72 | 1.53 | 0.81 |
| Education | ||||
| Lowest 25% | 1.22 | 0.82 | 1.83 | 0.33 |
| Other | 1 (Ref) | |||
| Poverty Level | ||||
| Lowest 25% | 0.83 | 0.42 | 1.63 | 0.58 |
| Other | 1 (Ref) | |||
| Charlson Comorbidity Index | ||||
| 0 | 1 (Ref) | |||
| 1 | 1.10 | 0.76 | 1.58 | 0.62 |
| 2 | 1.22 | 0.65 | 2.29 | 0.54 |
| 3+ | 2.36 | 1.09 | 5.09 | 0.03 |
| Type of admission | ||||
| Elective | 1 (Ref) | |||
| Emergency | 1.28 | 0.59 | 2.82 | 0.53 |
| Urgent* | 1.88 | 1.07 | 3.32 | 0.03 |
| Location of tumor in esophagus | ||||
| Lower | 1 (Ref) | |||
| Middle | 1.00 | 0.59 | 1.70 | 0.99 |
| Upper | 1.20 | 0.51 | 2.83 | 0.68 |
| Other | 1.12 | 0.64 | 1.94 | 0.69 |
| Histology | ||||
| Adenocarcinoma | 1 (Ref) | |||
| Squamous Cell | 0.84 | 0.52 | 1.36 | 0.48 |
| Other/ Unknown | 1.08 | 0.49 | 2.39 | 0.86 |
| Grade | ||||
| Well / moderate | 1 (Ref) | |||
| Poor / undifferentiated | 1.24 | 0.88 | 1.74 | 0.23 |
| Unknown | 1.23 | 0.73 | 2.07 | 0.45 |
| Stage | ||||
| I | 1 (Ref) | |||
| II | 1.23 | 0.83 | 1.82 | 0.30 |
| III | 0.86 | 0.56 | 1.31 | 0.47 |
| IV | 0.56 | 0.16 | 2.03 | 0.38 |
| Unknown | 1.06 | 0.35 | 3.17 | 0.92 |
| Area of residence | ||||
| Metropolitan | 1 (Ref) | |||
| Urban | 1.59 | 1.06 | 2.39 | 0.02 |
| Rural | 2.26 | 0.92 | 5.55 | 0.08 |
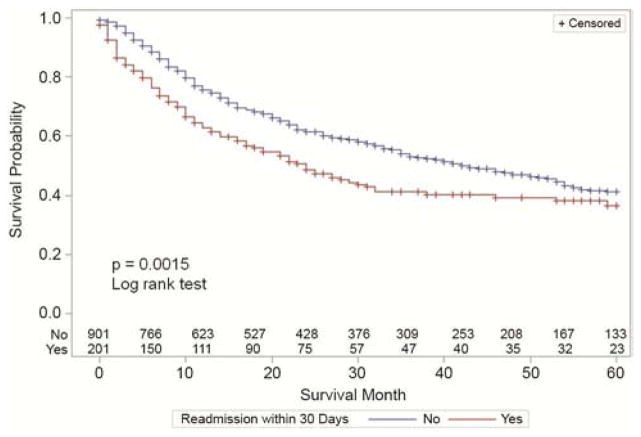
Finally, Kaplan-Meier survival curves were generated in order to compare survival in patients readmitted within 30 days following discharge versus those who were not. Again, only patients discharge to home were considered in this analysis, as it is difficult to define readmission for patients discharged to an intermediate care facility. Survival curves are shown in Figure 2. Overall survival was significantly worse for patients who were readmitted to the hospital within 30 days following discharge after esophagectomy.
Figure 2.
Kaplan-Meier overall survival curves for patients with readmitted to the hospital within 30 days following hospital readmission following esophagectomy, compared to those patient not readmitted to the hospital during that time period. A significant difference was noted between the survival curve (p=0.0015, log-rank test).
Comment
Hospital readmissions place a significant burden on the healthcare system, and are associated with billions of dollars of increased cost. This has led CMS and other payers to focus on identifying risk factors for readmission and putting care processes in place that are aimed at reducing readmission rates. We again demonstrate that hospital readmission after esophagectomy for esophageal cancer is common, occurring in 18% of patients within 30 days of discharge to home. Importantly, we show that hospital readmissions following esophagectomy are associated with inferior long term survival. No prior study has examined the relationship between readmission and long term survival following esophagectomy.
Variables associated with hospital readmission after esophagectomy in our study were a modified Charlson Comorbidity score of 3 or greater, urgent admission status, and an urban area of residence (as compared to metropolitan) and a complex reconstruction (colonic or small intestine interposition). In addition, African American race also demonstrated a trend toward being a risk factor for readmission. Schneider and colleagues have previously reported that a comorbidity score of 3 or greater is a risk factor for rehospitalization following colectomy [2]. An urgent, versus elective, admission status intuitively is a surrogate for complexity of care. Further, not living in a metropolitan area likely represents socio-economic actors that may predispose to hospital readmission. Whether other socio-economic factors, such as African American race, play a significant role in readmission after esophagectomy should be further evaluated. Perhaps more interestingly patient age, the operative approach (transhiatal vs. transthoracic), stage of disease, and the use of induction chemotherapy and/or radiation were not associated with readmission.
Several studies have looked at risk factors for readmission after a variety of other surgeries such as pancreatic, colorectal, coronary artery bypass, and orthopedic surgery [2,4–10]. Volume, medical comorbidities (such as COPD, heart failure, and chronic renal insufficiency), chronic steroid use, prolonged postoperative length of stay, malnutrition, postoperative complications, and the need for postoperative blood transfusion have all been associated with increased readmission rates. Not surprisingly, previous analysis of CMS data has shown that higher surgical volume and lower mortality rates are associated with lower readmission rates [4]. Unfortunately, there has been little research investigating readmission following esophagectomy. Previous studies examining outcomes after esophagectomy have reported readmission rates ranging from 5% to as high as 25% [10,14,15]. The observed readmission rate of 18% is consistent with these finds. Unlike studies examining other complex operations though, studies on esophagectomy have found no association between surgical volume and readmission rates [10,14,15,20].
We observed a 9.3% in hospital mortality rate following esophagectomy. This is in keeping with reported numbers from other studies using administrative data. In addition, 25.4% of patients in our series were discharged to some type of institutional care facility (skilled nursing home, rehabilitation facility etc.). When combined with an 18.6% 30 day rehospitalzation rate, a full 53.3% of patients suffered a morbid post-operative outcome. Clearly, opportunity for improvement in outcomes for esophagectomy exists. Data from board certified thoracic surgeons, as reported in the Society of Thoracic Surgeons (STS) General Thoracic Surgery Database (GTSD), demonstrates a much more acceptable in hospital mortality rate of 2.7% [21]. Data from single institutions also report excellent mortality rates for esophagectomy in the elderly population, as examined in this study, of 3.2% in persons age 70–79 [22].
Unfortunately, multiple barriers exist in regards to studying readmission rates after esophagectomy, such as low institutional volumes, readmission to different hospitals, as well as different payers. By using the SEER-Medicare linked database, we were able to capture a large volume of patients who underwent esophagectomy for esophageal cancer at many institutions. Furthermore, this allowed for analysis of a number of possible risk factors for readmission including patient demographics, comorbidities, cancer stage, operative approach, and the use of induction therapy while determining accurate readmission rates. The use of CMS data allows readmissions that occur at an institution other than the one at which the index operation was performed to be recorded and analyzed.
There are several limitations to this study. Foremost, this is a retrospective cohort study analyzing data from a national cancer registry linked to a large administrative dataset (CMS) and, therefore, subject to misclassification of data. This is particularly true for CMS data, which is collected for billing and not clinical purposes, and often lacks accuracy on clinical diagnosis. Also, because this is a Medicare population, the study is restricted to individuals aged 65 or older. Our study may not be representative of a younger cohort of patients, those with private insurance or those treated by board certified thoracic surgeons. In addition, specific clinical detail with regard to patient comorbid medical conditions and post-operative complications was not available in the analyzed datasets. Finally, data regarding hospital and surgeon volume was not examined, as it could not accurately be analyzed with our dataset.
In the future, linkage of robust clinical data from the STS-GTSD would allow for a more detailed analysis of specific patient risk factors for readmission following esophagectomy. Linkage to administrative databases from other payers would further allow for analysis of individuals younger than 65. Further, analysis of Medicare payments would allow for the estimation of the additional cost burden associated with hospital readmission following esophageal resection.
In summary, hospital readmission following esophagectomy for esophageal cancer occurs frequently and is associated with worse long term survival. Beginning in 2015, CMS will begin penalizing hospitals for readmission after certain surgeries [1]. Improved identification of patients at risk for hospital readmission following esophagectomy for esophageal cancer can inform patient selection, as well as guide discharge planning and outpatient monitoring strategies. The optimization of such practices may lead to improved outcomes at reduced costs.
Supplementary Material
Acknowledgments
This work is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Appendix 1. Medicare billing codes used to define type of esophagectomy and the administration of chemotherapy and radiation therapy
| Transhiatal esophagectomy | Billing codes |
|---|---|
| ICD-9 | 42.40–42.42 |
| HCPCS | 43107 |
| Transthoracic esophagectomy | |
| ICD-9 | 42.52, 42.5 |
| HCPCS | 43112, 43117, 43121, 43122 |
| Complex reconstruction (colonic or intestinal interposition, microvascular anastomosis) | |
| ICD-9 | 42.43, 42.55, 42.58, 42.62, 42.63, 42.65, 42.68, 43.5, 43.91, 43.99 |
| HCPCS | 43108, 43113, 43124 |
| Radiation therapy | Billing codes |
|---|---|
| ICD-9 | V58.0 V66.1 V67.1 92.20 92.21 92.22 92.23 92.24 92.26 92.27 9.28 |
| HCPCS | 31643 77300 77301 77305 77310 77315 77321 77326 77327 77328 77331 77332 77333 77334 77336 77370 77380 77381 77399 77401 77402 77403 77404 77406 77407 77408 77409 77411 77412 77413 77414 77416 77417 77418 77419 77420 77425 77427 77430 77431 77432 77470 77499 77520 77522 77523 77525 77750 77761 77762 77763 77781 77782 77783 77784 77799 C1716 C1717 C1718 C1719 C1720 C1790 C1791 C1792 C1793 C1794 C1795 C1796 C1797 C1798 C1799 C1800 C1801 C1802 C1803 C1804 C1805 C1806 C2616 G0126 G0173 |
| Chemotherapy | Billing codes |
|---|---|
| ICD-9 | V58.1 V66.2 V67.2 99.25 |
| HCPCS | 95549 96400 96404 96406 96410 96412 96414 96420 96420 96422 96423 96425 96440 96445 96450 96542 96545 C9017 J0182 J8510 J8530 J8560 J8610 J899 J9000 J9001 J9010 J9045 J9060 J9062 J9070 J9080 J9090 J9091 J9092 J9093 J9094 J9095 J9096 J9097 J9170 J9180 J9181 J9182 J9190 J9201 J9206 J9208 J9230 J9250 J9260 J9265 J9280 J9290 J9291 J9350 J9360 J9370 J9375 J9380 J9390 J9999 Q0083 Q0084 Q0085 Q0125 Q0127 Q0128 Q0129 S0178 S0182 S9329 S9330 S9331 |
Footnotes
Meeting Presentation: Society of Thoracic Surgeons Annual Meeting, Orlando, Florida. January 25, 2014
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–28. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Schneider EB, Hyder O, Brooke BS, et al. Patient readmission and mortality after colorectal surgery for colon cancer: impact of length of stay relative to other clinical factors. J Am Coll Surg. 2012;214(4):390–398. doi: 10.1016/j.jamcollsurg.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 3. [Accessed January 12, 2014];CMS Readmissions Reductions Program. Available at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html.
- 4.Tsai TC, Joynt KE, Orav EJ, et al. Variation in surgical-readmission rates and quality of hospital care. N Engl J Med. 2013;369:1134–42. doi: 10.1056/NEJMsa1303118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maniar HS, Bell JM, Moon MR, et al. Prospective evaluation of patients readmitted after cardiac surgery: Analysis of outcomes and identification of risk factors. J Thorac Cardiovasc Surg. 2013 doi: 10.1016/j.jtcvs.2013.10.066. [Epub] [DOI] [PubMed] [Google Scholar]
- 6.Price JD, Romeiser JL, Gnerre JM, et al. Risk analysis for readmission after coronary artery bypass surgery: developing a strategy to reduce readmissions. J Am Coll Surg. 2013;216(3):412–9. doi: 10.1016/j.jamcollsurg.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Kelly KN, Iannuzzi JC, Rickles AS, et al. Risk factors associated with 30-day postoperative readmissions in major gastrointestional resections. J Gastrointest Surg. 2014;18(1):35–44. doi: 10.1007/s11605-013-2354-7. [DOI] [PubMed] [Google Scholar]
- 8.Kent TS, Sachs TE, Callery MP, Vollmer CM. Readmission after major pancreatic resection: a necessary evil? J Am Coll Surg. 2011;213(4):515–23. doi: 10.1016/j.jamcollsurg.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Kassin MT, Owen RM, Perez SP, et al. Risk factors for 30-day hospital readmission among general surgery patients. J Am Coll Surg. 2012;215(3):322–30. doi: 10.1016/j.jamcollsurg.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodney PP, Stukel TA, Lucas FL, et al. Hospital volume, length of stay, and readmission rates in high-risk surgery. Ann Surg. 2003;238:161–7. doi: 10.1097/01.SLA.0000081094.66659.c3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Siewers AE, Finlayson EVA, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1129–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 12.Kohn GP, Galanko JA, Meyers MO, et al. National trends in esophageal surgery – are outcomes as good as we believe? J Gastrointest Surg. 2009;13:1900–12. doi: 10.1007/s11605-009-1008-2. [DOI] [PubMed] [Google Scholar]
- 13.Finks JF, Osbone NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varghese TK, Wood DE, Farjah F, et al. Variation in esophagectomy outcomes in hospitals meeting leapfrog volume outcome standards. Ann Thorac Surg. 2011;91:1003–10. doi: 10.1016/j.athoracsur.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Ferri LE, Mulder DS, et al. An enhanced recovery pathway decreases duration of stay after esophagectomy. Surgery. 2012;152(4):614–6. doi: 10.1016/j.surg.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(suppl):IV3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidty index using physician claims data. J Clin Epidemiol. 2000;53:1258–67. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 18.Abrams JA, Buono DL, Strauss J, et al. Esophagectomy compared to chemoradiation for early stage esophageal cancer in the elderly. Cancer. 2009;115(21):4924–4933. doi: 10.1002/cncr.24536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. [Google Scholar]
- 20.Auerbach AD, Maselli J, Carter J, et al. The relationship between case-volume, care quality, and outcomes of complex cancer surgery. J Am Coll Surg. 2010;211(5):601–8. doi: 10.1016/j.jamcollsurg.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright CD, Kucharczuk JC, O’Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg. 2009 Mar;137(3):587–95. doi: 10.1016/j.jtcvs.2008.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Tapias LF, Muniappan A, Wright CD, et al. Short and long-term outcomes after esophagectomy for cancer in elderly patients. Ann Thorac Surg. 2013 May;95(5):1741–8. doi: 10.1016/j.athoracsur.2013.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.