Abstract
Mice are the small animal model of choice in biomedical research due to the low cost and availability of genetically engineered lines. However, the devices utilized in current mouse models of implant-associated bone infection have been limited to intramedullary or trans-cortical pins, which are not amenable to treatments involving extensive debridement of a full-thickness bone loss and placement of a segmental antibiotic spacer. To overcome these limitations, we developed a clinically faithful model that utilizes a locking fracture fixation plate to enable debridement of an infected segmental bone defect (full-thickness osteotomy) during a revision surgery, and investigated the therapeutic effects of placing an antibiotic-laden spacer in the segmental bone defect.
To first determine the ideal time point for revision following infection, a 0.7 mm osteotomy in the femoral mid-shaft was stabilized with a radiolucent PEEK fixation plate. The defect was inoculated with bioluminescent Staphylococcus aureus, and the infection was monitored over 14 days by bioluminescent imaging (BLI). Osteolysis and reactive bone formation were assessed by X-ray and micro-computed tomography (micro-CT). The active bacterial infection peaked by 5 days post-inoculation, however the stability of the implant fixation became compromised by 10–14 days post-inoculation due to osteolysis around the screws. Thus, day 7 was defined as the ideal time point to perform the revision surgery.
During the revision surgery, the infected tissue was debrided and the osteotomy was widened to 3 mm to place a poly-methyl methacrylate spacer, with or without vancomycin. Half of the groups also received systemic vancomycin for the remaining 21 days of the study. The viable bacteria remaining at the end of the study were measured using colony forming unit assays. Volumetric bone changes (osteolysis and reactive bone formation) were directly measured using micro-CT image analysis. Mice that were treated with local or systemic vancomycin did not display gross pathology at the end of the study. While localized vancomycin delivery alone tended to decrease the bacterial burden and osteolysis, these effects were only significant when combined with systemic antibiotic therapy.
This novel mouse model replicates key features of implant-associated osteomyelitis that make treatment extremely difficult, such as biofilm formation and osteolysis, and imitates the clinical practice of placing an antibiotic-laden spacer after infected tissue debridement. In addition, the model demonstrates the limitations of current PMMA spacers and could be an invaluable tool for evaluating alternative antimicrobial treatments for implant-associated bone infection.
Keywords: Bone infection, osteomyelitis, mouse, revision, antibiotics, PMMA
1. Introduction
Implant-related bone infections (osteomyelitis or OM) occur in 5% of the 2 million fracture fixation cases annually in the United States and cost $15,000-$50,000 per incident [1, 2]. Despite aggressive prophylactic strategies, the risk of infection establishment in open fracture cases can be as high as 50% [3]. Implant-associated OM can be extremely challenging and costly to treat considering that it can lead to high patient morbidity due to multiple revision surgeries, which involve aggressive debridement of the bone and soft tissue, possible exchange of the implants, as well as long courses of systemic antibiotics.
Antibiotic-laden poly-methyl methacrylate (PMMA) beads or spacers are often placed into the debridement site to manage dead space and augment the systemic antibiotics with high local doses [4, 5]. PMMA is not biodegradable, however, which results in failure to release most of the antibiotics and necessitates a second surgery to remove the spacers. Furthermore, many antibiotics are incompatible with PMMA because they are heat-labile and cannot withstand the exothermic polymerization reaction or they impair PMMA polymerization [4, 6]. Therefore, the surgeon’s choices of compatible antibiotics are limited, and significant research efforts are focused on exploring alternative biomaterials for local antibiotic delivery as well as bone regeneration in an infected non-union. To date, most of these novel materials and techniques have only been characterized in vitro.
Studies that have evaluated local antibiotic delivery in small animal models primarily evaluated prophylactic intervention rather than treatment of a well established infection [7, 8], or used small models that cannot readily accommodate debridement and placement of antibiotic-laden spacers [9]. Large animal models that could replicate clinical scenarios of established infections are costly and typically require strict ethical justifications, which limit their use in exploratory research [10]. Further, they are less conducive for longitudinal evaluation using techniques such as micro-computed tomography or bioluminescent imaging due to resolution and feasibility limitations. The ideal small animal model to study post-infection treatments would utilize a fixation device that maintains stability after infection establishment and can readily accommodate an antibiotic-laden biomaterial spacer in a bone defect following extensive debridement. The limited in vivo evaluation of novel antibiotic spacers and dual-purpose (infection management and bone regeneration) scaffolds may be attributable to the unavailability of such an animal model [11, 12].
Chen and colleagues simulated established implant-associated osteomyelitis in the rat using internal plate fixation of a segmental bone defect with debridement following two weeks after inoculation [13]. One limitation of this model is that it begins with a large segmental bone defect that is created during the inoculation surgery and additional bone is not excised during the debridement, which is often necessary in chronic osteomyelitis cases due to bone necrosis and the presence of sequestra [14]. As an alternative to the rat, the smaller size and lower cost of the mouse would more readily enable quantitative in vivo imaging and high throughput studies for screening new potential therapies. Further, the genetic manipulability of the mouse is a powerful tool that could be leveraged to further elucidate the etiological mechanisms of implant-associated osteomyelitis as well as the host’s immune response. More recently, locking fracture fixation plates have been developed for mice [15]. These devices hold great potential for enabling a variety of important research pursuits related to bone trauma in the mouse model, including implant-associated infection.
We hypothesized that a mouse model with an established bone infection in association with a fracture fixation plate, which can accommodate an antibiotic spacer after infected tissue debridement, could recapitulate the clinical features of implant-associated osteomyelitis. Further, this mouse model would enable investigation of local and systemic antibiotic therapies through both longitudinal and end-point quantification of the infection and changes to the bone. We first performed a study to characterize the time course of the infection and determine the relevant revision time point. Next, we characterized the responsiveness of the established infection model to treatment with local and systemic vancomycin therapy following extensive surgical debridement.
2. Materials and Methods
2.1 Determination of the ideal time point for revision following infection
2.1.1 Animals and surgical procedures
All animal studies were performed in accordance with protocols approved by the University of Rochester’s Committee on Animal Resources. Female BALB/cJ mice were purchased from Jackson Research Labs (Bar Harbor, ME) at 13–15 weeks of age and acclimated for one week prior to surgery. The mice were sedated and anesthetized with xylazine (12 mg/kg) and ketamine (130 mg/kg) injected intraperitoneally. The right femur was exposed by a direct lateral approach and a 6-hole radiolucent polyether ether ketone (PEEK) plate with a 40 nm titanium coating was installed across the anterolateral surface using 4 titanium screws (RISystem; Davos, Switzerland), which were placed in the four outermost screw holes of the plate. The use of a PEEK plate with a titanium coating was intended to enable longitudinal micro-CT imaging without beam hardening and metal artifacts, while facilitating cellular and bacterial interaction similar to metallic hardware. To prevent screw pull-out from the bone in the event of septic loosening from osteolysis, the fixation was reinforced in a cerclage-fashion by tying a 5-0 nylon monofilament suture around the bone and plate in between each pair of proximal and distal screws. A 0.7 mm transverse osteotomy was cut through the femoral mid-diaphysis using a 0.67 mm wire gigli saw and a cutting guide (RISystem; Davos, Switzerland). A 1 mm radius semi-circle of fibrillar collagen sheet (Kensey Nash; Exton, PA), which was uninfected or soaked for at least 2 hours in an overnight culture of bioluminescent methicillin-sensitive S. aureus (Xen36; PerkinElmer, Inc.; Waltham, MA), was placed into the defect. The viable bacterial load on each collagen sheet was determined to be 8.0 ± 2.9 × 104 CFU by sonicating the sheets and plating dilutions of the suspensions (n=8); however, this may be an underestimate based on the ability of S. aureus to strongly adhere to collagen [16]. The muscle and skin were closed with 5-0 nylon monofilament sutures. Buprenorphine was administered subcutaneously (0.1 mg/kg) to manage pain every 6–12 hours, beginning at the time of sedation, for up to 3 days following surgery. The bacterial infection was monitored over 14 days by bioluminescent imaging (BLI), while osteolysis and reactive bone formation were assessed by planar X-ray as previously described [17]. At the end of the 14 days, the bones were formalin-fixed and imaged by micro-computed tomography (micro-CT) after removing the fixation plate and screws. The fixation hardware was prepared for scanning electron microscopy (SEM), while the bones were prepared for decalcified histology.
2.1.2 Bioluminescent imaging of the active bacterial infection
The Xen36 bacterial infection was evaluated longitudinally through BLI using an IVIS Spectrum imaging system (PerkinElmer, Inc.; Waltham, MA) with a five-minute dorsal exposure. The average luminescent radiance was quantified through the Living Image software (PerkinElmer, Inc.; Waltham, MA) by using a fixed-size region of interest (ROI) around the right thigh of each mouse.
2.1.3 Radiographic imaging of osteolysis
To monitor changes to the bone, X-rays (LX-60 X-ray Cabinet, Faxitron Bioptics LLC; Tucson, AZ) were taken at 26 kV with an exposure time of 5 seconds. After removing the plate and screws, the femurs were imaged by micro-CT (VivaCT 40, Scanco Medical; Bassersdorf, Switzerland) with a 10.5 μm isotropic voxel size using an integration time of 300 ms, energy of 55 kV, and intensity of 145 μA. 3D renderings of the micro-CT scans were visualized using OsiriX software (Pixmeo; Geneva, Switzerland) and qualitative analysis of osteolysis and osteogenesis was performed as previously described [18].
2.1.4 Decalcified histology and scanning electron microscopy of implants
Decalcified histology was performed as previously described [19]. Briefly, the femurs were fixed in 10% neutral buffered formalin, decalcified in 10% EDTA, and embedded in paraffin. The 5 μm sections were stained for tartrate-resistant acid phosphatase (TRAP) to examine osteoclast activity, Gram stained to assess bacterial colonization, or stained with alcian blue/hematoxylin/orange G (ABH/Orange G) to distinguish cells and new bone formation.
The plate and screws from each mouse were fixed in 2.5% glutaraldehyde/4% paraformaldehyde in 0.1M Cacodylate buffer, post-fixed in 1% osmium tetroxide, dehydrated, and gold sputter coated. The samples were imaged by SEM (LEO 982 FE-SEM, Carl Zeiss SMT; Thornwood, NY) for qualitative assessment of bacterial colonization and biofilm formation on the implant surfaces.
2.2 Evaluation of vancomycin treatment after the revision surgery
2.2.1 Animals, surgical procedures, and antibiotic therapy
During the inoculation surgery described above, the screws were placed in the most proximal, most distal, and central 2 holes of the fixation plate (Fig. S1). The change in screw placement relative to the preliminary study was decided based on results regarding osteolysis around the screws. For this treatment study, we adapted the strategy of placing new screws in new holes (Fig. S1) to minimize the chance of septic loosening during the treatment period. The infection was allowed to establish for 7 days post-inoculation. Prior to the revision surgery, PMMA bone cement (SmartSet® MV Bone Cement; DePuy Synthes, Warsaw, IN) spacers were prepared according to the manufacturer’s instructions as a placebo (PBO-PMMA) or mixed with 5 wt% vancomycin (0.5 mg per murine spacer; Vanco-PMMA) and formed in a mold to make 1.8 × 3 mm cylinders. The in vitro release kinetics of vancomycin from the PMMA spacers (Fig. S2) were characterized by placing the scaffolds into 1 mL of PBS and replacing the solution at each time point. The vancomycin concentration was measured using a spectrophotometer (Synergy Mx Microplate Reader, BioTek Instruments Inc.; Winooski, VT) at an absorbance wavelength of 280 nm.
At the time of revision, any pathologic tissue was debrided away, the cerclage sutures were removed, and new screws were placed into the middle proximal and distal holes of the plate. The 2 most central screws were removed and bone debridement was achieved by widening the osteotomy to 3 mm using a 0.22 mm wire gigli saw and cutting guide (Fig. S1). The defect was thoroughly irrigated with sterile phosphate buffered saline (PBS) and the plate fixation was reinforced with new cerclage sutures between the distal and proximal pairs of screws. A PBO-PMMA or Vanco-PMMA spacer was tied into the defect using a 5-0 braided nylon suture. The surgeon was blinded to the treatment group by another person who placed the PBO-PMMA or Vanco-PMMA into numbered vials that corresponded to the randomly assigned mice. The muscle and skin were closed and the mice were allowed to heal for an additional 21 days. Select groups also received systemic vancomycin beginning at the revision surgery (110 mg/kg twice daily subcutaneously (SC Vanco)) [20] until sacrifice. One hundred microliters of a 25.5 mg/mL vancomycin in PBS solution was dispensed at each dosage. The mice on average weighed 23 g with a range of 21–25 g. The systemic dosing was chosen based on a similar pharmacokinetic profile in mice to that observed in humans receiving 1 g of vancomycin every 12 h [21–23]. The mice were randomly assigned to the treatments after ranking the day 5 BLI values, such that each group would have a similar distribution of BLI-based infection severities. The sample size was at least 7 per group, except for the placebo group that did not receive antibiotics (PBO-PMMA, n=4) due to ethical and animal health concerns. Upon tissue harvest, the bone, soft tissue, fixation hardware, and PMMA implants were placed into separate vials with 1 mL of sterile PBS and frozen at -80°C for CFU analysis.
2.2.2 Micro-CT analysis of osteolysis and reactive bone formation
One day after the revision surgery (day 8) and on the final day of the study (day 28), the right femur of each mouse was scanned in vivo by micro-CT with a 17.5 μm isotropic voxel size using an integration time of 300 ms, energy of 55 kV, and intensity of 145 μA. 3D reconstruction and quantitative analysis of the micro-CT images was performed using Amira software (Amira 5.4.5, FEI Visualization Sciences Group; Burlington, MA). For each mouse, the micro-CT images that were collected on day 8 were co-registered with the images that were collected on day 28 to directly measure the volumetric bone changes. The proximal ROI and distal ROI encompassed the bone from the edge of the 3 mm osteotomy to the nearest screw on the proximal and distal sides of the defect, respectively. The images were binarized using a global bone mineral density threshold of 435 mg HA/cm3 and subtracted from one another to measure the volumes of bone resorption and reactive bone formation, which were normalized to the original bone volume at day 8.
2.2.3 Measurement of bacterial colonization of the tissue and implants
To measure the viable bacterial load remaining in the bone and soft tissue, each sample was homogenized in 3 mL of sterile PBS using a T10 basic ULTRA-TURRAX® disperser (IKA Works, Inc.; Wilmington, NC). Residual bacteria were dislodged from the fixation hardware and PMMA implants by sonicating the samples in 1 mL of sterile PBS for 3 minutes at 35 kHz (VWR Symphony Ultrasonic Cleaner; VWR Intl.; Radnor, PA) and then vortexed. The tissue homogenates and sonicated suspensions were spread on Luria agar plates in 10-fold dilutions and incubated overnight at 37°C for CFU counts, which were normalized to the mass of each collected sample. Normalized CFU data were transformed using log10(1+X) before statistical analysis.
2.3 Data analysis
PBO-PMMA was compared against Vanco-PMMA, with or without SC Vanco, in a two-way ANOVA. When the interaction term was significant, Sidak’s post-hoc test was used to compare PBO-PMMA vs. Vanco-PMMA and PBO-PMMA + SC Vanco vs. Vanco-PMMA + SC Vanco. Correlations were examined using the nonparametric Spearman correlation by ranks. Bonferonni’s correction was applied to control the family-wise error rate for the multiple two-way ANOVA’s conducted at each time point over the BLI time course and for the multiple pairs of metrics that were examined for correlations. Prism software was used for all statistical tests (GraphPad Software Inc.; La Jolla, CA). Differences were considered significant for the multiplicity-adjusted p < 0.05.
3. Results
3.1 Determination of the ideal time point for revision following infection
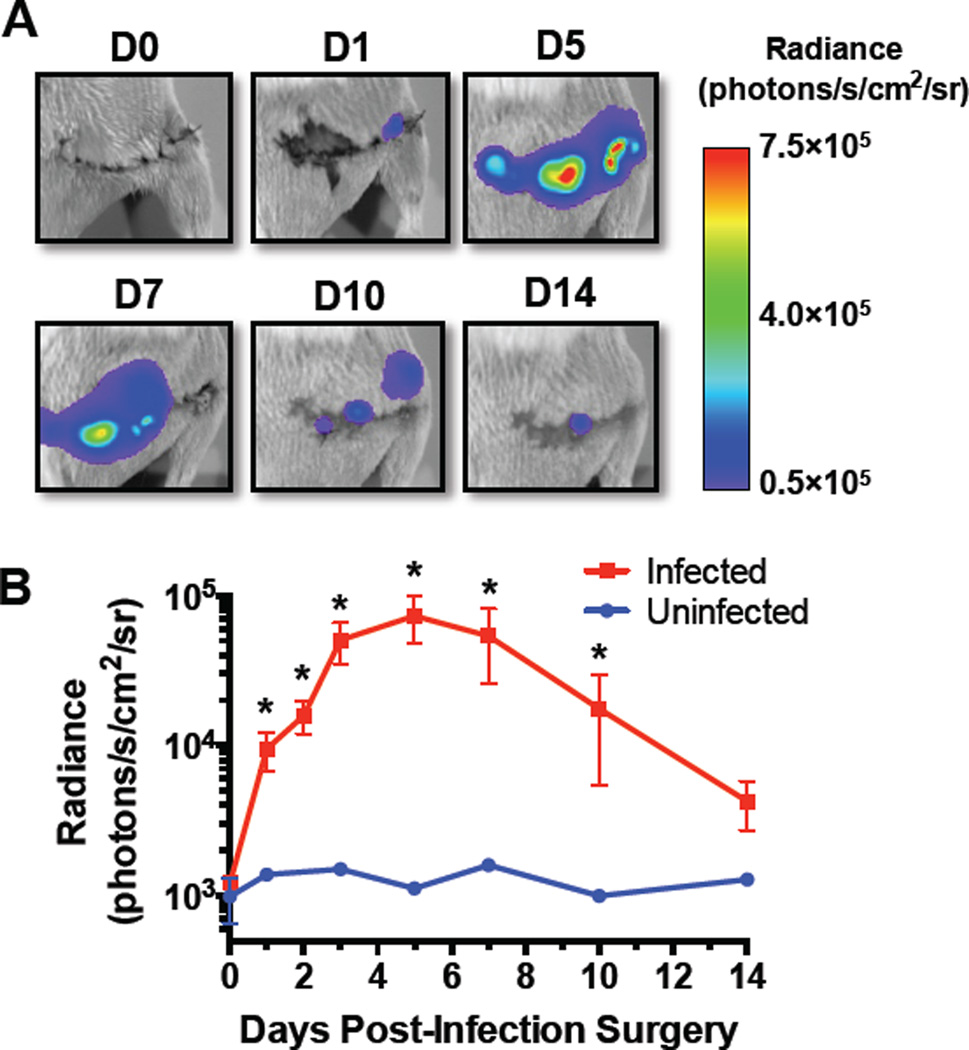
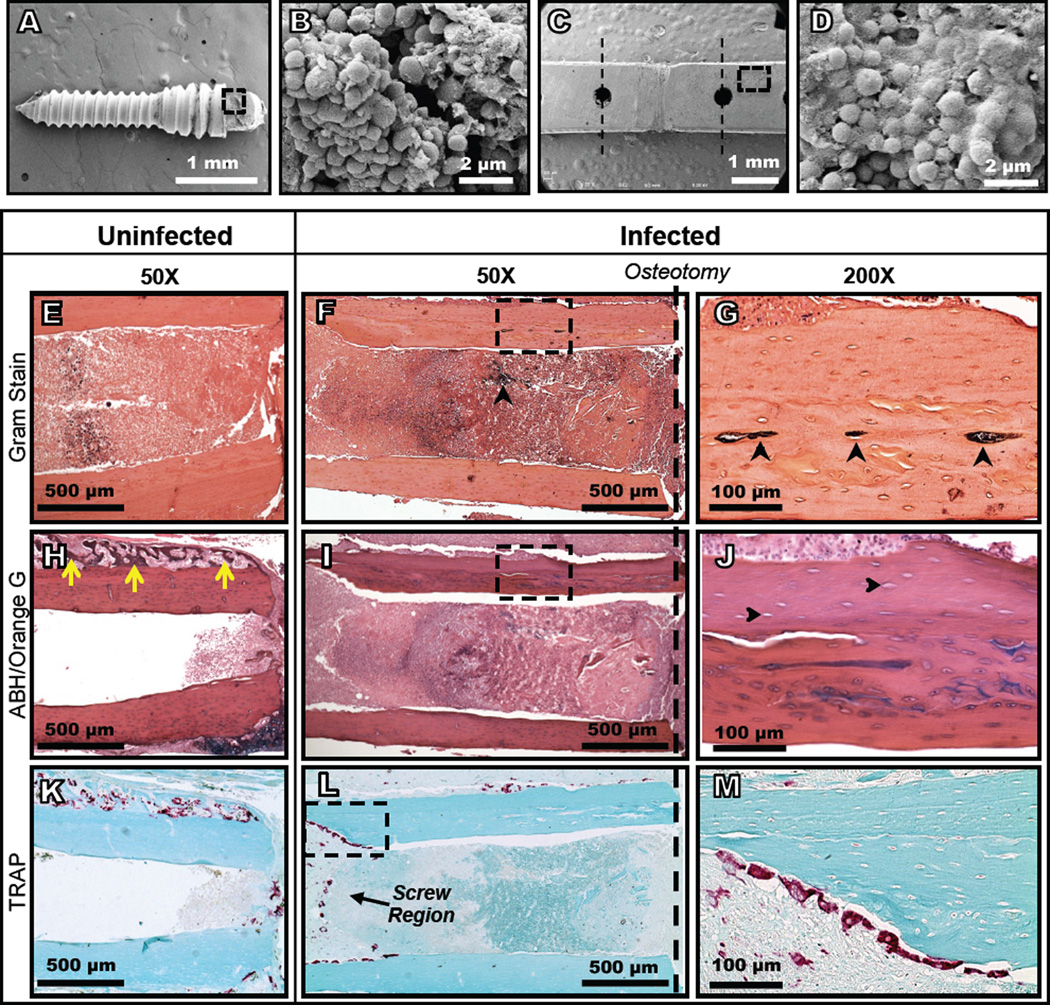
The pathogen burden, as measured by BLI, increased to a peak by 5 days post-inoculation and then declined through day 14 (Fig. 1). None of the mice died during the study time course. The infected mice did not lose body weight compared to the uninfected mice and the bioluminescence signal was only observed within the thigh, which suggests that the mice had an isolated surgical site infection and were otherwise healthy. Large bacterial clusters and biofilm formation were observed on the plates and screws using SEM (Fig. 2A-D). While Gram staining failed to detect positive staining in the uninfected control femurs (Fig. 2E and Fig. S3A,B), bacteria were readily found embedded within necrotic cortical bone of infected mice (Fig. 2F,G). Another major difference was the large amount of new reactive bone that formed on the periosteal surface of the control (uninfected) femurs (Fig. 2H), and the absence of this osteogenic response on the infected femurs (Fig. 2I,J). Finally, TRAP staining revealed that the majority of the osteoclasts in the uninfected femurs were remodeling the new reactive bone (Fig. 2K), while most of the osteoclasts in the infected femurs were resorbing cortical bone adjacent to the screws (Fig. 2L,M).
Figure 1.
In vivo bioluminescence imaging (BLI) demonstrates the active bacterial infection peaking by 5 days post-inoculation. (A) Representative BLI heat maps of S. aureus colonization demonstrate that the bacteria dispersed throughout the thigh. (B) Average radiance values measured from fixed-size ROIs surrounding the thigh increased for the first 5 days before declining through day 14.* denotes multiplicity-adjusted p<0.05 vs. uninfected by repeated measures 2-way ANOVA of log-transformed data with Sidak’s test for multiple comparisons.
Figure 2.
Bacterial colonization of the bone, fixation plates, and screws as well as active osteolysis and bone necrosis are evident in micrographs of samples from infected mice 14 days after inoculation. Bacteria and biofilm were found on the screws (A, B) and the plates (C, D) beyond the 3 mm debridement zone (dashed lines) in scanning electron micrographs. Gram stained histological sections (E-G) demonstrate bacterial colonization (black arrows) within the bones of infected mice (F, G). Alcian blue/hematoxylin/orange G stained histology in an adjacent section revealed robust new woven bone formation on the periosteal surface of uninfected femurs (yellow arrows in H), which was absent on the infected femurs (I, J). The empty osteocyte lacunae in the mature bone of infected mice (J; black arrows), which did not occur in uninfected mice (H and Supplemental Figure 3C,D), suggest necrosis induced by the infection. Osteoclast activity in uninfected mice was limited to remodeling of the newly formed bone along the periosteal surface (K), while active bone resorption in the vicinity of the screws was observed in TRAP stained sections of the infected mice (L, M).
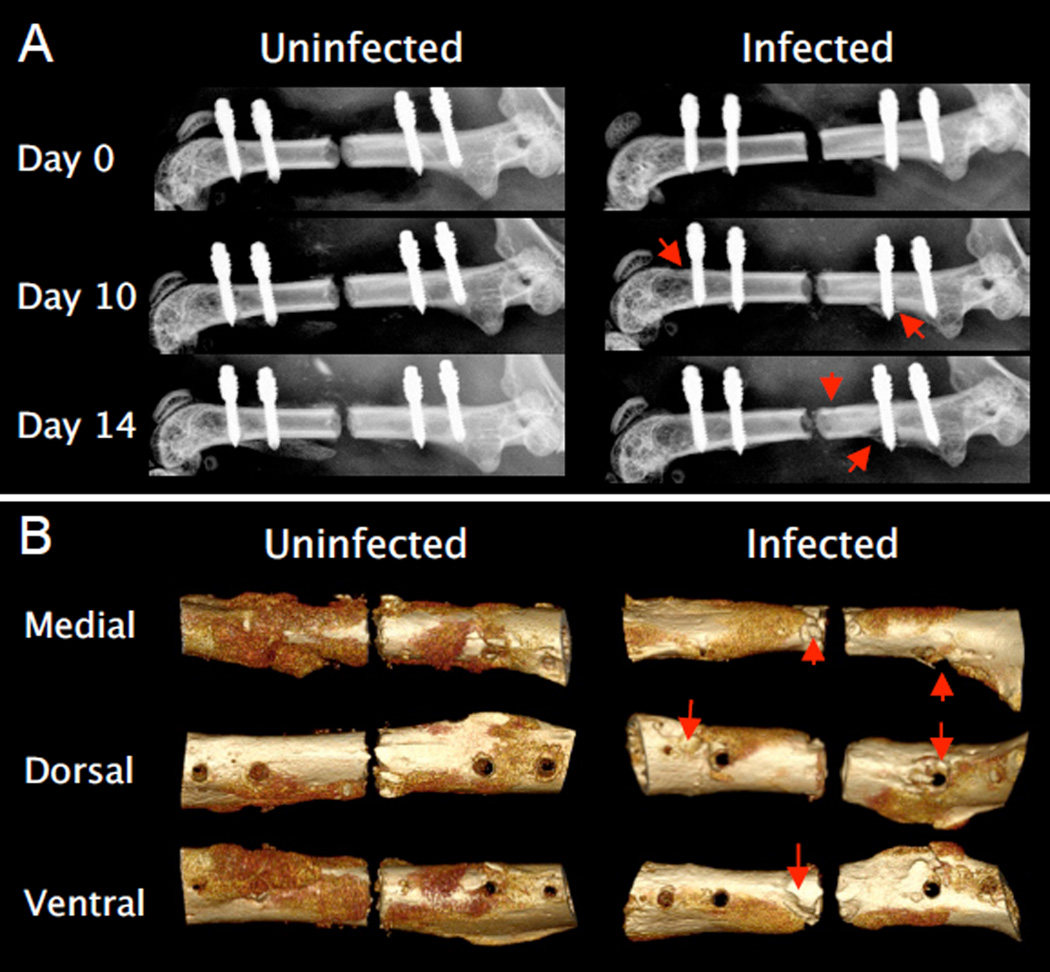
Radiographic assessment of the instrumented femurs revealed substantial osteolysis by day 10 post-inoculation in the infected mice, which progressed out to day 14, and raised concerns about potential implant loosening at these later time points (Fig. 3). Additionally, one infected mouse sustained a fracture through the most distal screw hole between days 10 and 14, which likely resulted from dramatic thinning of the cortex in this region. Based on these results and the potential confounding effects of implant loosening, day 7 post-inoculation was chosen as the ideal time point to perform the revision surgery, considering that the active bacterial burden peaked by day 5 and the implant stability was not compromised by osteolysis at this time point.
Figure 3.
Radiographic evidence of increased osteolysis and decreased periosteal bone formation in infected versus uninfected femurs. Osteolysis (arrows), particularly around the screws, began developing by day 10 as seen in the X-rays (A). Three dimensional micro-CT renderings from day 14 reaffirm the extensive osteolysis around the screws and other regions of the cortex (B). Also note the robust periosteal new bone formation on the medial and ventral sides of the uninfected femur, which is markedly decreased on the infected femur.
3.2 Treatment of the infection with tissue debridement and vancomycin therapy
3.2.1 Effects of local and systemic vancomycin on the bacterial infection
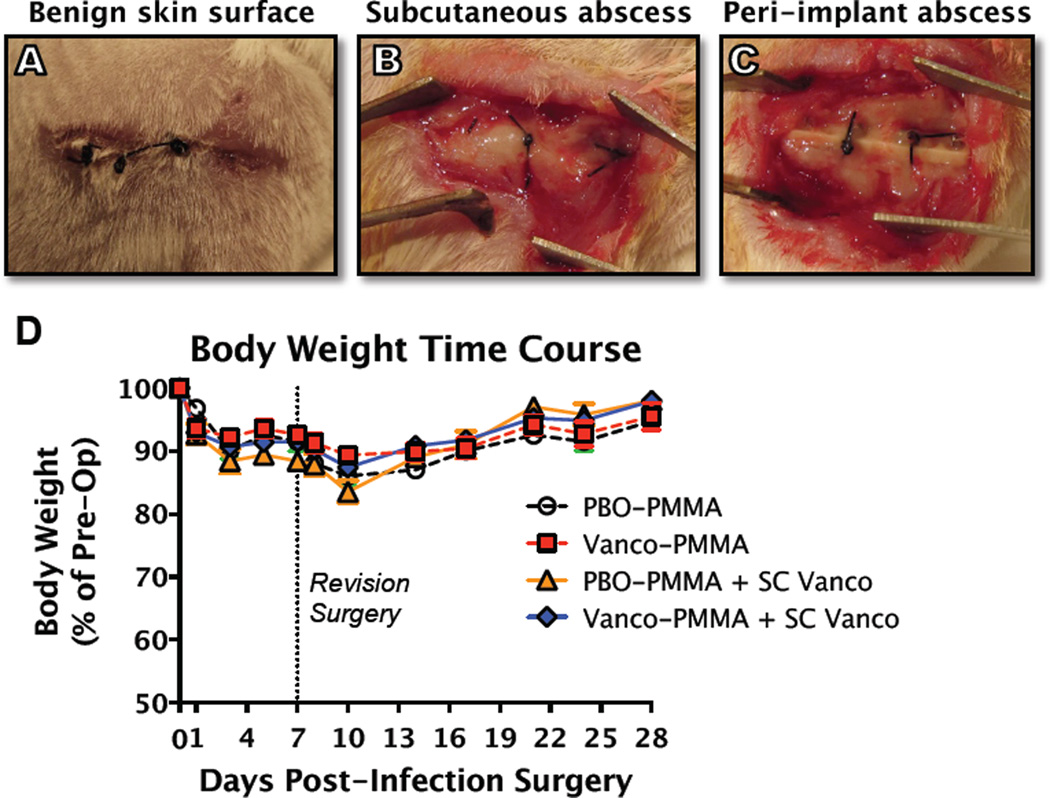
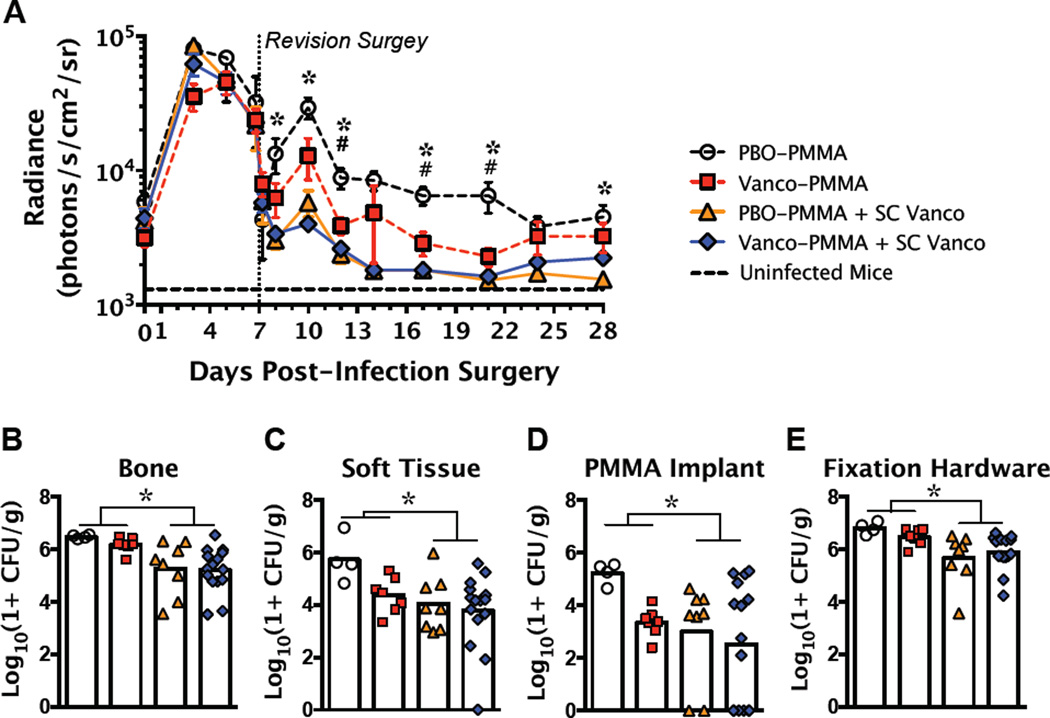
Gross assessment of the infected mice at the time of revision revealed that the skin surface was largely benign, but substantial subcutaneous and peri-implant abscesses had formed (Fig. 4A-C). None of the mice died or lost significant body weight due to the infection over the full 28 days, even when not treated with antibiotics (PBO-PMMA group; Fig. 4D), suggesting that they remained systemically aseptic. Infected tissue debridement during the revision surgery greatly reduced the bacterial burden, as demonstrated by pre-op and post-op BLI measurements (Fig. 5A). SC Vanco treatment significantly reduced the bacterial infection beginning by day 8 (1 day post-revision) and maintained significant BLI reduction through day 28. Among the mice that did not receive SC Vanco therapy, Vanco-PMMA also significantly reduced the active infection from day 12 through day 21 (Fig. 5A). No significant differences in BLI values were observed between the PBO-PMMA + SC Vanco and Vanco-PMMA + SC Vanco groups. Upon tissue collection at the end of the study, all clinical signs of the infection were absent in the mice that received local or systemic vancomycin. Minimal subcutaneous and peri-implant abscesses were present in two of the four PBO-PMMA mice. By the end of the study, SC Vanco therapy significantly reduced the viable bacteria within the bone and soft tissue as well as on the PMMA implants and fixation hardware, as measured by CFU assays (Fig. 5B-E). Vanco-PMMA treatment tended to reduce the bacterial CFU counts among the mice that did not receive SC Vanco, but the differences were not statistically significant. Despite the significant reductions in bacterial colonization, none of the bones and only one soft tissue sample from the Vanco-PMMA + SC Vanco group were found to be bacteria culture negative. Interestingly, most of the vancomycin-laden PMMA implants and all of the fixation plates and screws, even in the Vanco-PMMA + SC Vanco group, were highly colonized by bacteria at the end of the study (Fig. 5D,E). Further, the BLI values early in the treatment time course (day 12) were significantly correlated with the total bacterial CFU at the end of the study (r=0.49, p=0.004). The total bacterial CFU was also significantly correlated with the BLI measurements on day 28 (r=0.56, p=0.001), but were not significantly correlated with any BLI time points before the revision surgery.
Figure 4.
Modest gross pathology was observed at the time of the revision surgery (7 days post-infection), as the mice typically presented with a benign skin surface (A) and substantial subcutaneous (B) and peri-implant (C) abscesses. The mice did not lose body weight due to the infection, even without antibiotic treatment (PBO-PMMA), which suggests that the infection was contained to the surgical site. The sample size was n = 7–15 per group, except for PBO-PMMA, where n=4.
Figure 5.
Local and systemic vancomycin treatment each significantly reduced the bacterial burden. (A) Significant reductions in bacterial load by SC Vanco treatment and Vanco-PMMA treatment were observed through in vivo BLI. SC Vanco significantly reduced bacterial CFU counts in the bone (B) and soft tissue (C) as well as on the PMMA implants (D) and fixation hardware (E). Vanco-PMMA treatment tended to reduce the bacterial counts compared to PBO-PMMA in mice that did not receive SC Vanco, but differences were not significant. * denotes multiplicity-adjusted p<0.05 for the effect of SC Vanco by 2-way ANOVA. # denotes multiplicity-adjusted p<0.05 for PBO-PMMA vs. Vanco-PMMA after 2-way ANOVA, using Sidak’s test for multiple comparisons. n = 7–15 per group, except for PBO-PMMA, where n=4.
3.2.2 Effects of local and systemic vacomycin on osteolysis
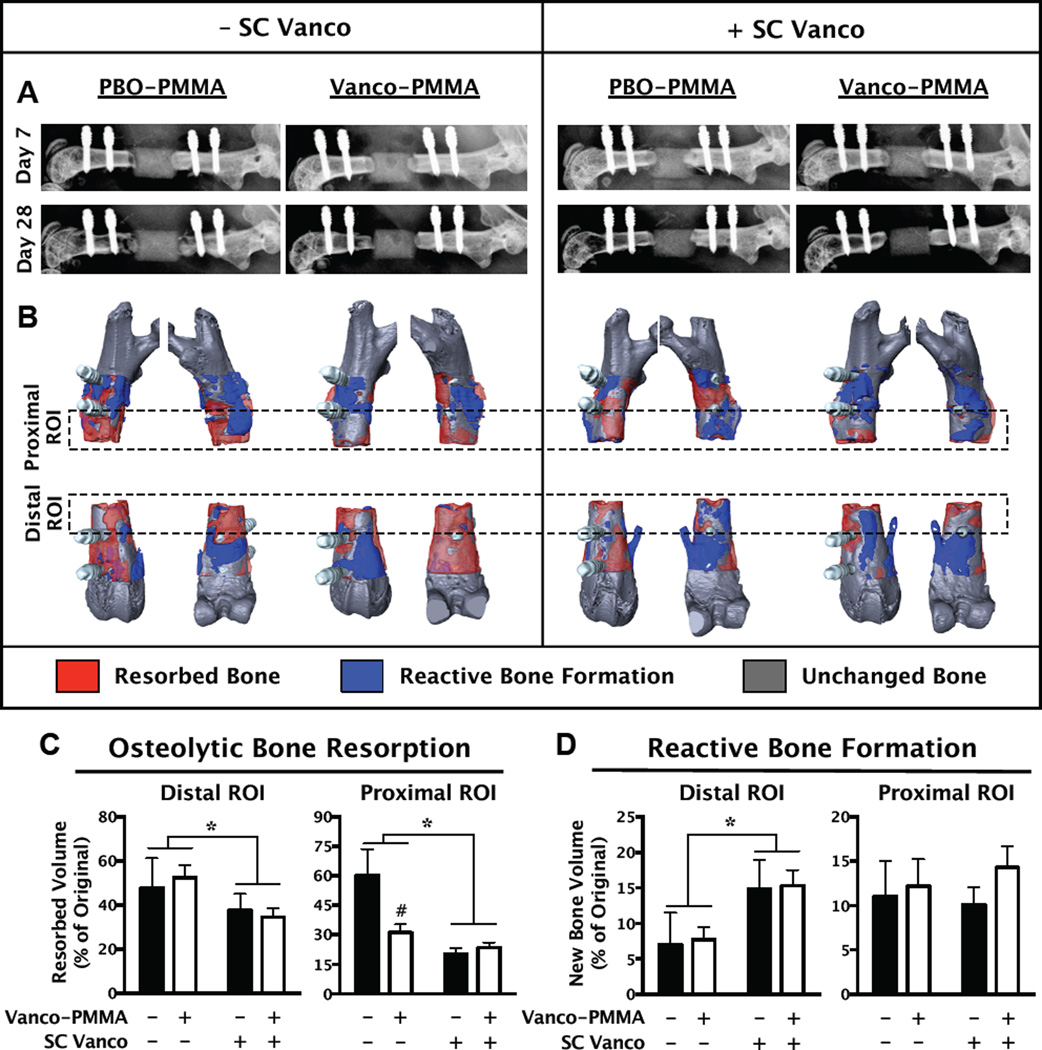
Dramatic changes to the bone were observed through longitudinal X-rays (Fig. 6A) and 3D renderings of the micro-CT scans (Fig. 6B). Severe osteolysis predominantly occurred distal to the defect site. The resorbed bone volume was significantly reduced with SC Vanco therapy as well as by Vanco-PMMA treatment among the mice that did not receive SC Vanco (Fig. 6C). The reactive bone formation on the distal side was also increased in the mice that received SC Vanco (Fig. 6D). Stability of the plate fixation was maintained in all mice that received either local or systemic vancomycin therapy, but stability was not maintained in the placebo-treated mice (PBO-PMMA), based on displacements of the bone relative to the screws in the micro-CT scans.
Figure 6.
Local and systemic vancomycin treatment each significantly reduced the osteolytic bone resorption that was induced by the infection. Reductions in osteolysis were observed in groups treated with Vanco-PMMA or SC Vanco through X-rays (A) and 3D renderings of micro-CT scans (B). The micro-CT scans from day 8 and day 28 were overlaid, binarized, and subtracted from one another to directly assess the volumetric bone changes. The resorbed bone volume, which tended to be more extensive on the distal side, was significantly reduced with SC Vanco treatment (C). On the proximal side, Vanco-PMMA also significantly reduced osteolysis between the groups that did not receive SC Vanco. Distal bone formation was significantly increased by SC Vanco treatment (D). * denotes p<0.05 for the effect of SC Vanco by 2-way ANOVA. # denotes multiplicity-adjusted p<0.05 for PBO-PMMA vs. Vanco-PMMA after 2-way ANOVA with a significant interactive effect, using Sidak’s test for multiple comparisons. n = 7–15 per group, except for PBO-PMMA, where n=4.
4. Discussion
This study establishes a novel mouse model of implant-associated osteomyelitis with a locking fracture fixation plate, and investigates the efficacy of revision with debridement and antibiotic eluting PMMA spacers that are typically used clinically. Many characteristics of this model are consistent with the clinical and physiological salient features of human implant-associated osteomyelitis, including abscess formation, biofilm on the implants, bone necrosis, involucra, and osteolysis that can lead to septic implant loosening when not managed by antibiotics. Using the mouse as a model readily enabled longitudinal quantification of the infection management through BLI, which was further corroborated by significant correlations with the bacterial CFU counts at the end of the study.
Previously, mouse models of implant-associated osteomyelitis have been limited to intramedullary [9, 20, 24] or trans-cortical pins [17, 25]. These models are beneficial for studying the general etiology of osteomyelitis and biofilm formation [24] or systemic treatment strategies such as various antibiotic regimens [20] and immunization [26]. These pin implants are useful models for studying periprosthetic infection, but may be less relevant to trauma-related studies where plate stabilization of a bone defect is employed. Further, these pin models are not amenable to extensive surgical debridement followed by placement of an antibiotic-laden segmental spacer to study local antibiotic therapy or bone healing in a septic non-union. Implementing the locking fixation plate was a critical step in overcoming these limitations for two reasons. First, it enables the infection to be studied in the presence of a well-stabilized bone trauma (osteotomy). Second, the plate can accommodate a revision surgery with extensive tissue debridement and placement of an antibiotic-laden spacer. Further, the radiolucency of the PEEK plate enables the transient micro-CT imaging and measurement of osteolysis and reactive bone formation.
During revision procedures, surgeons may either retain or exchange the orthopaedic implant. Clinical treatment algorithms will often recommend retention of the implant if it is mechanically stable, the patient is being treated within 10–21 days of presenting infection symptoms, and the infection site has undergone aggressive surgical debridement [1, 27]. This mouse model is consistent with the descriptions of a case for implant retention considering that the revision is taking place 7 days post-inoculation, the fixation plate is mechanically stable at the time of revision, and an extensive debridement of both the bone and soft tissue is performed.
In the preliminary study to determine the ideal time point for revision after infection, we observed that the BLI values increased to a peak by 3–5 days post-inoculation before declining through day 14. A similar trend was observed in a tibial trans-cortical pin model [17] and a femoral intramedullary pin model [24] that used various bioluminescent S. aureus strains. This decline is primarily attributable to the host’s immune defenses eliminating the planktonic bacteria. S. aureus can form biofilms, however, and also become internalized within host cells, such as osteoblasts [28], to evade the immune response. While in biofilm, the bacteria may transition to a more quiescent state [29] in which the bioluminescent emissions would be diminished with the metabolic dormancy. The debridement and initiation of antibiotic therapy may have released bacteria that were in biofilm or induced proliferation, as evidenced by the increased BLI signal from day 8 to day 10 (Fig. 5A). The use of BLI to evaluate antibiotic therapies in this model may be limited to early times after revision surgery (Days 7–14). Beyond this time, any therapies that can suppress planktonic colonies may be indistinguishable, as the BLI signal may not correlate with the bacteria in biofilm.
While antibiotic-laden PMMA spacers are used clinically, the limited and unpredictable antibiotic release raises skepticism about the efficacy of this local delivery approach [30, 31]. Therefore, patients are also typically placed on parenteral and oral antibiotic regimens to augment the localized delivery. Consistent with these clinical practices, treating the mice systemically with vancomycin significantly improved the outcomes by reducing the bacterial burden as well as osteolysis. The contributions of vancomycin delivered via PMMA were much less dramatic, but tended to show improvements over the PBO-PMMA group. No significant differences were observed between the PBO-PMMA + SC Vanco and Vanco-PMMA + SC Vanco treated mice, which indicates that the local vancomycin delivery did not augment the systemic therapy. The higher BLI values at early time points (days 8 and 10) in the Vanco-PMMA treated group compared to the PBO-PMMA + SC Vanco group (Fig. 5A) suggest that the vancomycin may elute from the PMMA too slowly and not reach high enough concentrations. Alternatively, this may be explained by the inefficient diffusion of vancomycin away from the PMMA depot and into the surrounding tissues to reach all of the sites of bacterial contamination at sufficient concentrations. This result reaffirms the poor delivery efficiency of PMMA as well as the uncertainty in the required elution kinetics. This could potentially be improved by increasing the antibiotic concentration within the PMMA or by increasing the surface area by using beads instead of a spacer; but there are currently no standards of practice for surgeons mixing antibiotics into PMMA to use as a reference point. Additionally, the sustained vancomycin elution from the PMMA was clearly insufficient considering that the bacteria colonized the Vanco-PMMA spacers by the end of the study. Similar problems of antibiotic-laden PMMA colonization have been encountered in two-stage exchange arthroplasties in humans [32, 33].
These data demonstrate that significant differences in treatment outcomes resulting from local and systemic antibiotics could be detected in this infection model. The finding that no mice were bacteria culture negative at the end of the study, despite the lack of clinical symptoms, indicates that there is room for significant improvements by alternative antimicrobial therapies in future studies. Vancomycin was chosen for these initial studies because it has low tissue toxicity and is one of the most common clinically used antibiotics in hand-mixed PMMA beads or spacers behind gentamicin and tobramycin. It is also effective against methicillin-resistant S. aureus (MRSA), which is a significant clinical problem in osteomyelitis cases [5]. Although a methicillin-sensitive strain of S. aureus (MSSA) was used in this study, the minimum inhibitory concentration of vancomycin is not necessarily different between MSSA and MRSA strains [34]. The retention of the biofilm-ridden plate and screws in this mouse model is clinically relevant, but also creates a formidable challenge for the antimicrobial therapy and may be a primary factor in the failed antibiotic treatment observed herein. Vancomycin is known to penetrate Staphylococcal biofilms, but may not kill the bacteria despite the potential to achieve very high concentrations [35, 36]. This lack of efficacy may be attributable to the slower diffusion of vancomycin through the biofilm, giving the bacteria more time to adapt [36], or to the metabolic quiescence of some bacteria in the biofilm [29]. Alternatively, rifampin in combination with another antibiotic, like vancomycin, is commonly regarded as a critical treatment strategy against implant-associated Staphylococcal infections [1, 37]. This is based on the notion that rifampin is efficacious against Staphylococci in biofilm [38, 39]; however, rifampin is incompatible with PMMA spacers because this antibiotic impairs the polymerization of PMMA [6]. Future studies of alternative antimicrobial therapies in this mouse model with implant retention will likely require successful treatment of the biofilm in order to realize complete eradication of the infection.
While the mouse possesses many advantages as outlined above, the small size may place additional design constraints on novel local delivery strategies that would be unnecessary in larger animals. Additionally, the smaller tissue size of the localized infection in mice limits the required diffusion distance of antibiotics. This could potentially inflate the apparent efficacy of an experimental therapy if the same bioavailability is unachievable in a larger animal or human. The sample size of the group without antibiotic treatment (PBO-PMMA) was limited to 4 mice due to ethical and animal health concerns. Extensive osteolysis and consistent septic loosening of the fixation hardware were observed in this group. The variance within the PBO-PMMA group was low in most metrics, however, so this data still provides an important reference point for the outcomes of untreated mice, without confounding the statistical differences observed. Characteristics of the model presented herein depend on many potential variables that are related to the infection, surgical techniques, and treatment strategies, which may possess complex interactions. Therefore, it is critical that any future studies that adapt this model also carefully design the appropriate control groups.
The value of this infection model is that it presents with many of the important characteristics that make treatment of implant-associated osteomyelitis extremely challenging, including biofilm formation on the implants and extensive osteolysis. This study reaffirms the limited benefits of antibiotic delivery via PMMA and these data provide an important baseline for comparison with other antibiotics or new delivery techniques in future studies. This mouse model will be an invaluable tool in the assessment of novel biomaterial spacers for local antibiotic delivery and may provide new insights to the biofilm-related disease mechanisms of implant-associated osteomyelitis.
Supplementary Material
Figure S1. Schematic of the infection-revision model and the evaluation time course. Images from each surgery and post-op X-rays demonstrate the procedural techniques. Note the use of sutures tied around the bone and plate in between each set of screws to improve mechanical stability of the fixation plate in cases of insufficient antibiotic treatment, which can result in dramatic osteolysis and implant loosening.
Figure S2. Less than 15% of the vancomycin loaded into the PMMA spacers is released during the first 6 days in vitro. The mouse-sized (1.8 mm diameter × 3 mm) PMMA spacers, loaded with 5 wt% vancomycin, were placed into 1 mL PBS. At each time point, the concentration of vancomycin in the PBS was measured by the optical density at 280 nm using a spectrophotometer.
Figure S3. Positive gram staining and empty osteocyte lacunae were not present in uninfected mice. Dark staining in Gram-stained sections from uninfected mice was artifact only (A-B). Bone necrosis, as indicated by empty osteocyte lacunae, in the infected mice is not attributable to the osteotomy considering that necrosis was not observed in the uninfected mice (C-D).
Acknowledgements
The Authors would like to thank Romano Matthys (RISystem) for custom designing the titanium-coated PEEK fixation plates, Michael Thullen (University of Rochester) for his assistance with micro-CT, Karen Bentley (University of Rochester) for her expertise in scanning electron microscopy, and Sarah Mack (University of Rochester) for her assistance with histology. This study was supported by the AOTrauma Research- CPP on Bone Infection and NIAMS/NIH grant P30AR061307. Jason Inzana is supported by an NSF graduate research fellowship (NSF Award DGE-1419118). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Science Foundation, National Institutes of Health, or AOTrauma.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
Contributor Information
Jason A. Inzana, Email: jason.inzana@rochester.edu.
Edward M. Schwarz, Email: edward_schwarz@urmc.rochester.edu.
Stephen L. Kates, Email: stephen_kates@urmc.rochester.edu.
Hani A. Awad, Email: hani_awad@urmc.rochester.edu.
References
- 1.Darouiche RO. Treatment of infections associated with surgical implants. The New England journal of medicine. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Patil S, Montgomery R. Management of complex tibial and femoral nonunion using the Ilizarov technique, and its cost implications. J Bone Joint Surg Br. 2006;88(7):928–932. doi: 10.1302/0301-620X.88B7.17639. [DOI] [PubMed] [Google Scholar]
- 3.Zalavras CG, et al. Management of open fractures. Infect Dis Clin North Am. 2005;19(4):915–929. doi: 10.1016/j.idc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Webb JC, Spencer RF. The role of polymethylmethacrylate bone cement in modern orthopaedic surgery. J Bone Joint Surg Br. 2007;89(7):851–857. doi: 10.1302/0301-620X.89B7.19148. [DOI] [PubMed] [Google Scholar]
- 5.Jaeblon T. Polymethylmethacrylate: properties and contemporary uses in orthopaedics. J Am Acad Orthop Surg. 2010;18(5):297–305. doi: 10.5435/00124635-201005000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Beeching NJ, et al. Comparative in-vitro activity of antibiotics incorporated in acrylic bone cement. J Antimicrob Chemother. 1986;17(2):173–184. doi: 10.1093/jac/17.2.173. [DOI] [PubMed] [Google Scholar]
- 7.Li B, et al. Sustained release of vancomycin from polyurethane scaffolds inhibits infection of bone wounds in a rat femoral segmental defect model. Journal of controlled release : official journal of the Controlled Release Society. 2010;145(3):221–230. doi: 10.1016/j.jconrel.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 8.Brown KV, et al. Earlier debridement and antibiotic administration decrease infection. Journal of surgical orthopaedic advances. 2010;19(1):18–22. [PubMed] [Google Scholar]
- 9.Matsuno H, et al. A new antibacterial carrier of hyaluronic acid gel. J Orthop Sci. 2006;11(5):497–504. doi: 10.1007/s00776-006-1030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clasper JC, et al. Spread of infection, in an animal model, after intramedullary nailing of an infected external fixator pin track. J Orthop Res. 2001;19(1):155–159. doi: 10.1016/S0736-0266(00)00023-1. [DOI] [PubMed] [Google Scholar]
- 11.Nair MB, et al. Infection and tissue engineering in segmental bone defects--a mini review. Current opinion in biotechnology. 2011;22(5):721–725. doi: 10.1016/j.copbio.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reizner W, et al. A systematic review of animal models for Staphylococcus aureus osteomyelitis. Eur Cell Mater. 2014;27:196–212. doi: 10.22203/ecm.v027a15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, et al. Characterization of a chronic infection in an internally-stabilized segmental defect in the rat femur. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2005;23(4):816–823. doi: 10.1016/j.orthres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. 2005;19(4):765–786. doi: 10.1016/j.idc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Matthys R, Perren SM. Internal fixator for use in the mouse. Injury. 2009;40(Suppl 4):S103–S109. doi: 10.1016/j.injury.2009.10.044. [DOI] [PubMed] [Google Scholar]
- 16.Arciola CR, et al. Prevalence of cna, fnbA and fnbB adhesin genes among Staphylococcus aureus isolates from orthopedic infections associated to different types of implant. FEMS Microbiol Lett. 2005;246(1):81–86. doi: 10.1016/j.femsle.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 17.Li D, et al. Quantitative mouse model of implant-associated osteomyelitis and the kinetics of microbial growth, osteolysis, and humoral immunity. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(1):96–105. doi: 10.1002/jor.20452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green JM, et al. Anti-oxidation treatment of ultra high molecular weight polyethylene components to decrease periprosthetic osteolysis: evaluation of osteolytic and osteogenic properties of wear debris particles in a murine calvaria model. Curr Rheumatol Rep. 2013;15(5):325. doi: 10.1007/s11926-013-0325-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li D, et al. Effects of antiresorptive agents on osteomyelitis: novel insights into the pathogenesis of osteonecrosis of the jaw. Ann N Y Acad Sci. 2010;1192:84–94. doi: 10.1111/j.1749-6632.2009.05210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niska JA, et al. Vancomycin-rifampin combination therapy has enhanced efficacy against an experimental Staphylococcus aureus prosthetic joint infection. Antimicrob Agents Chemother. 2013;57(10):5080–5086. doi: 10.1128/AAC.00702-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Healy DP, et al. Comparison of steady-state pharmacokinetics of two dosage regimens of vancomycin in normal volunteers. Antimicrob Agents Chemother. 1987;31(3):393–397. doi: 10.1128/aac.31.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albrecht LM, et al. Vancomycin protein binding in patients with infections caused by Staphylococcus aureus. DICP. 1991;25(7–8):713–715. doi: 10.1177/106002809102500701. [DOI] [PubMed] [Google Scholar]
- 23.Crandon JL, Kuti JL, Nicolau DP. Comparative efficacies of human simulated exposures of telavancin and vancomycin against methicillin-resistant Staphylococcus aureus with a range of vancomycin MICs in a murine pneumonia model. Antimicrob Agents Chemother. 2010;54(12):5115–5119. doi: 10.1128/AAC.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pribaz JR, et al. Mouse model of chronic post-arthroplasty infection: noninvasive in vivo bioluminescence imaging to monitor bacterial burden for long-term study. J Orthop Res. 2012;30(3):335–340. doi: 10.1002/jor.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shandley S, et al. Hyperbaric oxygen therapy in a mouse model of implant-associated osteomyelitis. J Orthop Res. 2012;30(2):203–208. doi: 10.1002/jor.21522. [DOI] [PubMed] [Google Scholar]
- 26.Varrone JJ, et al. Passive immunization with anti-glucosaminidase monoclonal antibodies protects mice from implant-associated osteomyelitis by mediating opsonophagocytosis of Staphylococcus aureus megaclusters. J Orthop Res. 2014;32(10):1389–1396. doi: 10.1002/jor.22672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Current opinion in infectious diseases. 2006;19(4):349–356. doi: 10.1097/01.qco.0000235161.85925.e8. [DOI] [PubMed] [Google Scholar]
- 28.Bosse MJ, Gruber HE, Ramp WK. Internalization of bacteria by osteoblasts in a patient with recurrent, long-term osteomyelitis. A case report. J Bone Joint Surg Am. 2005;87(6):1343–1347. doi: 10.2106/JBJS.D.02649. [DOI] [PubMed] [Google Scholar]
- 29.Rani SA, et al. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J Bacteriol. 2007;189(11):4223–4233. doi: 10.1128/JB.00107-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gogia JS, et al. Local antibiotic therapy in osteomyelitis. Seminars in Plastic Surgery. 2009;23(2):100–107. doi: 10.1055/s-0029-1214162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferraris S, et al. In vitro comparison between commercially and manually mixed antibiotic-loaded bone cements. Journal of applied biomaterials & biomechanics : JABB. 2010;8(3):166–174. [PubMed] [Google Scholar]
- 32.Mariconda M, et al. Sonication of antibiotic-loaded cement spacers in a two-stage revision protocol for infected joint arthroplasty. BMC Musculoskelet Disord. 2013;14:193. doi: 10.1186/1471-2474-14-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmolders J, et al. Evidence of MRSE on a gentamicin and vancomycin impregnated polymethyl-methacrylate (PMMA) bone cement spacer after two-stage exchange arthroplasty due to periprosthetic joint infection of the knee. BMC Infect Dis. 2014;14:144. doi: 10.1186/1471-2334-14-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang G, et al. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44(11):3883–3886. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Darouiche RO, et al. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. J Infect Dis. 1994;170(3):720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2005;49(6):2467–2473. doi: 10.1128/AAC.49.6.2467-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerli W, Trampuz A, Ochsner PE. Prosthetic-joint infections. N Engl J Med. 2004;351(16):1645–1654. doi: 10.1056/NEJMra040181. [DOI] [PubMed] [Google Scholar]
- 38.Tang HJ, et al. In vitro efficacies and resistance profiles of rifampin-based combination regimens for biofilm-embedded methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2013;57(11):5717–5720. doi: 10.1128/AAC.01236-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mihailescu R, et al. High activity of Fosfomycin and Rifampin against methicillin-resistant staphylococcus aureus biofilm in vitro and in an experimental foreign-body infection model. Antimicrob Agents Chemother. 2014;58(5):2547–2553. doi: 10.1128/AAC.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Schematic of the infection-revision model and the evaluation time course. Images from each surgery and post-op X-rays demonstrate the procedural techniques. Note the use of sutures tied around the bone and plate in between each set of screws to improve mechanical stability of the fixation plate in cases of insufficient antibiotic treatment, which can result in dramatic osteolysis and implant loosening.
Figure S2. Less than 15% of the vancomycin loaded into the PMMA spacers is released during the first 6 days in vitro. The mouse-sized (1.8 mm diameter × 3 mm) PMMA spacers, loaded with 5 wt% vancomycin, were placed into 1 mL PBS. At each time point, the concentration of vancomycin in the PBS was measured by the optical density at 280 nm using a spectrophotometer.
Figure S3. Positive gram staining and empty osteocyte lacunae were not present in uninfected mice. Dark staining in Gram-stained sections from uninfected mice was artifact only (A-B). Bone necrosis, as indicated by empty osteocyte lacunae, in the infected mice is not attributable to the osteotomy considering that necrosis was not observed in the uninfected mice (C-D).