Abstract
Background
Parents often want to provide support to their children during medical procedures, but not all parents are effective in providing distraction after brief training.
Objective
The aim of this study was to investigate the effects of three doses of distraction intervention for children at high and medium risk for procedure-related distress.
Methods
Children undergoing scheduled intravenous insertions for diagnostic or treatment purposes and their parents participated. A computerized application, Children, Parents and Distraction, was used to predict distress risk. Doses of intervention were basic (parents trained on providing distraction), enhanced (basic training plus tailored instructions, environmental modifications, and support and guidance from the research assistant), and professional (a trained research assistant provided distraction). Outcome measures were Observational Scale of Behavioral Distress-Revised for behavioral distress, Oucher for self-reported pain, parent report of child distress, and salivary cortisol for physiological distress.
Results
A total of 574 children, ages 4–10, and their parents participated. The Children, Parents and Distraction predicted that the risk for distress was high for 156 children, medium for 372, and low for 46. Children predicted to have higher risk for distress displayed more behavioral distress (p < .01). Children in the medium-risk group who had the professional intervention displayed significantly less behavioral distress (p < .001). Children in the high-risk group tended to have less behavioral distress when receiving the professional intervention (p = .07). There were no significant group differences for self-report of pain, parent report of distress, or cortisol levels.
Discussion
Some parents may need additional training in providing distraction to their children during procedures, and some children at medium and high risk for distress may need professional support. Parents should be asked about their preferences in acting as the distraction coach and, if willing, be provided as much training and support as possible in the clinical situation.
Keywords: children, distraction, nursing, pain, pain management, parents, procedures
Children are among the most vulnerable to pain and the least likely to be adequately treated compared to other populations (Institute of Medicine, 2011). By the age of 6 years, most children have experienced over 20 painful procedures associated with routine immunizations (American Academy of Pediatrics, 2014). Children who are sick, in accidents, or hospitalized may experience significantly more painful procedures, including venipuncture and intravenous (IV) insertions. A recent Canadian study found in a 24-hour period 78.2% of hospitalized children required at least one painful procedure—a mean 6.3 painful procedures—and less than one third had documented interventions for pain (Stevens et al., 2011). Likewise, in a U.S. emergency department, investigators found 13% (859/6545) of children encountered had an IV insertion or venipuncture, but less than 1% (7/859) were pretreated with a topical anesthetic (MacLean, Obispo, & Young, 2007).
Children can vividly recall the pain and distress of medical procedures (Cohen et al., 2001; Salmon, Price, & Pereira, 2002). Inadequate management of procedure-related pain and distress could lead to anticipatory fear or anxiety in the child that may be manifested by difficulties in coping with future procedures (Kennedy, Luhmann, & Zempsky, 2008). Repeated medical procedures can lead to posttraumatic stress syndrome (Bronner, Knoester, Bos, Last, & Gootenhuis, 2008), maladaptive behaviors, such as noncompliance with medical care (Taddio et al., 2009), and fear of accessing the health system for care in later life (von Baeyer, Marche, Rocha, & Salmon, 2004).
Distraction is a relatively simple, yet effective, intervention for pain and distress associated with procedures that involves drawing attention away from the painful stimulus and onto a pleasurable diversion, such as interactive games or books. The efficacy of distraction for needle-related procedural pain has been supported by research for over a decade and is summarized in several recent critical reviews of the research (Buscemi, Vanermeer, & Curtis, 2008; Koller & Goldman, 2012; Stinson, Yamada, Dickson, Lamba, & Stevens, 2008; Uman et al., 2013). Although child life specialists and other healthcare providers are usually the distraction coaches for children, these resources are not always available in all settings. Teaching parents to be distraction coaches for their children is a logical solution to this problem.
Studies on the effectiveness of parents as distraction coaches for children’s procedural pain and distress began in the 1980s. Manne et al. (1990) reported that distraction provided by parents resulted in decreased behavioral distress, but not decreased pain. Blount and his colleagues (1992) compared parents and children who were trained in distraction and coping to parents and children who were untrained. The observed distress of experimental group children during a vaccination was lower on the Behavioral Approach-Avoidance Distress Scale, but not on the Observational Scale of Behavioral Distress (OSBD). Two groups compared the effects of parent distraction to parent reassurance during childhood immunizations (Gonzalez, Routh, & Armstrong, 1993; Manimala, Blount, & Cohen, 2000), and both studies reported significantly less behavioral distress in the children assigned to the distraction condition children. Kleiber, Craft-Rosenberg, and Harper (2001) studied children undergoing an IV catheter insertion. Parents of children in the experimental group received brief training in distraction techniques, and the children received no training. There were no significant differences in pain or OSBD scores between the two groups, but children in the experimental group displayed less behavioral distress over the course of the procedure. The results from these small studies provide some support for using parent distraction coaches to reduce child distress, but the mixed findings also indicate that some parents are effective, whereas others are not.
Concurrently, other researchers were investigating covariates that might influence the effectiveness of distraction. As summarized in the conceptual model by McCarthy and Kleiber (2006), potential covariates identified in the literature included children’s age, gender, diagnosis, ethnicity, experience, temperament, anxiety, coping style, ability to attend to the distracter, and genotype; parent characteristics were ethnicity, gender, experience, credibility of the distraction intervention, parenting style, and anxiety; and environmental factors include the difficulty of the medical procedure and use of topical anesthetics.
In order to tease out the most powerful predictors of successful parent distraction coaching, our research team developed a study of children undergoing an IV with a sufficiently large sample size to account for the covariates. A new model emerged from the data and included child age, typical coping style, parent expectation of child distress, and the quality and frequency of the parent distraction coaching (McCarthy et al., 2010a). The results of the experimental part of the study showed that not all parent coaches were successful in distracting their children after receiving brief distraction training (McCarthy et al., 2010b). This led us to hypothesize that some parents need more support and guidance to be successful distraction coaches, and some children would respond better to distraction provided by a professional. We also wanted to concentrate our research efforts on children most in need of distress-reducing intervention—those who showed the highest levels of distress despite the use of distraction during the procedure. Therefore, we used our study data to develop the Children, Parents and Distraction (CPaD), a computerized decision support application for categorizing children at risk for low, medium, and high procedural distress when their parents are the distraction coaches. One purpose of the study reported here was to provide additional information on the validity of the CPaD for determining distress risk. We also tested the effects of three doses of distraction on the distress responses (i.e., observed behavioral distress, physiological distress response, child self-report of pain, and parent report of child distress [PRCD]) of children at high and medium risk for procedure-related distress. The specific aims of this study were as follows:
To further validate the CPaD for predicting children’s risk for distress during a scheduled IV catheter insertion while parents provided distraction coaching.
To evaluate the effectiveness of professional and enhanced distraction interventions for children at high risk for distress. It was hypothesized that children predicted to be at high risk for distress would have less distress with professional intervention compared to the enhanced intervention.
To evaluate the effectiveness of professional, enhanced, and basic distraction interventions for children at medium risk for distress. It was hypothesized that children predicted to be at medium risk for distress would have less distress with professional intervention compared to the enhanced intervention and less distress with the professional or enhanced intervention compared to the basic intervention.
METHODS
Design
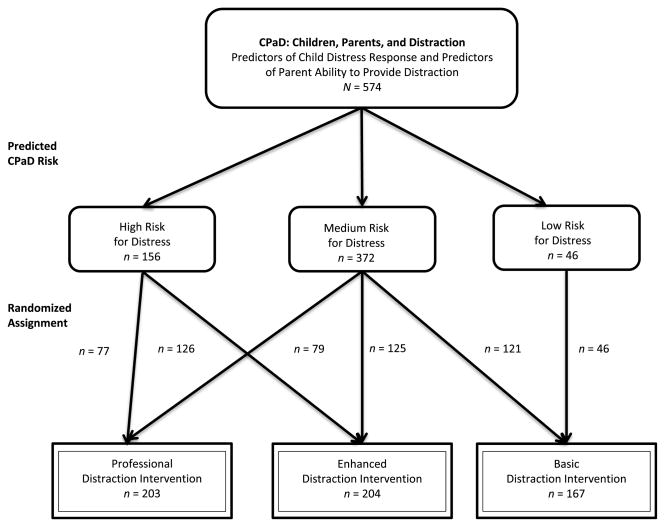
A computer-based decision support system, the CPaD, was used to predict a child’s level of distress when the parent received basic distraction training. Then a stratified randomization design was used to test the dose of distraction intervention (basic, enhanced, or professional) appropriate for each risk group (see Figure 1). Dyads in the high risk for distress group were assigned to either enhanced or professional intervention. Dyads in the high distress group were not randomized to basic because previous research indicated that children at high risk for distress do not respond well when their parents receive basic distraction training only (McCarthy et al., 2010b). Dyads in the medium risk for distress group were assigned to basic, enhanced, or professional intervention. All parent–child dyads predicted to be in the low distress group received the basic distraction intervention because previous research indicated that children at low risk for distress respond well when their parents receive basic distraction training (McCarthy et al., 2010b).
FIGURE 1.
Study protocol with subject assignments.
Setting and Sample
This study took place at three children’s hospitals in the Midwest. Children who were undergoing scheduled IV insertions for diagnostic or treatment purposes and their parents participated. Inclusion criteria for children were ages 4–10 years, English speaking, all medical diagnoses except cancer, and without major cognitive disabilities. Parents who were able to speak and read English and who planned to be present during the IV insertion were included.
On the basis of estimates from a previous study, it was expected that the distribution of children in the predicted high, medium, and low distress categories would be 22%, 65%, and 13%, respectively. With 582 total subjects, the expected number with high distress was 128 (64 per intervention group) and with medium distress 378 (126 per intervention group). Assuming a coefficient of variation of 115% for Observational Scale of Behavioral Distress-Revised (OSBD-R), the two-sample t test would be able to detect at the .05 significance level with .80 power at least a 44% smaller mean OSBD-R with professional intervention compared to enhanced in the predicted high distress group. If the mean OSBD-R for enhanced intervention was 4.0, then the detectable OSBD-R mean difference is at least 1.76. For the predicted medium distress group, the one-way analysis of variance (ANOVA) would be able to detect at least a 37% smaller mean OSBD-R with professional or enhanced, compared to basic intervention. If the mean OSBD-R for basic intervention was 2.4, the detectable OSBD-R mean difference would be at least 0.89.
Intervention Doses
Three doses of distraction intervention were tested: basic, enhanced, and professional. Research assistants (RAs) with education and experience in using distraction with children during medical procedures provided instruction to parents in the basic and enhanced intervention or were the distraction coaches for the professional intervention. The RAs at each site were evaluated with the Distraction Coaching Inventory (DCI) to ensure that they provided a professional level of distraction (Kleiber, McCarthy, Hanrahan, Myers, & Weathers, 2007). For all three interventions, healthcare providers were instructed to focus on the IV insertion and allow the parent or RA to take the role of distraction coach.
Basic
Parents of children assigned to the basic intervention learned to use distraction by first viewing a 7-minute video about distraction on a laptop computer. Parents then received general nstruction from a trained RA distraction coach on how to use distraction with children. During the IV insertion, the parent lead distraction and the RA observed without interfering.
Enhanced
This intervention included the basic intervention plus tailored directions provided to the parent. For example, if on the CPaD the child reported a preference to look away from the procedure, the parent directions might suggest using a large book to distract the child and, at the same time, to shield the child from viewing the procedure. The directions were on the computer screen to read—then printed so that the parent could refer to them later—and discussed with the parent by the trained RA distraction coach. During the procedure, as needed, parents in this group received direction and support from the RA, including (a) environmental modifications, such as repositioning of the child or parent to facilitate interaction; (b) timely prompts from the trained coach, such as providing developmentally appropriate toys, responding to the child’s physical cues, or staying focused on providing distraction; and (c) assistance with selecting distracters.
Professional
The only treatment condition in which the parent did not provide the child with distraction was professional intervention. For this group, the trained RA provided distraction to the child during the IV procedure. Parents could be present during the procedure and provide support (e.g., holding hands) to their child if they chose. Similar to the enhanced intervention, tailored directions (e.g., child’s preference for books, games, toys) were provided to the trained coach. Environmental modifications to support distraction, such as repositioning the child, were directed by the coach.
Instruments
Instruments used in the study included the CPaD and the DCI. In addition, four measures of the child distress outcome were used to capture the full range of the child’s response: observed behavioral distress, physiological distress response, child self-report of pain, and PRCD.
Children, Parents and Distraction
The CPaD was used to predict which parents could successfully provide high-quality distraction to their children after receiving brief standard distraction training (basic distraction) and which children would respond well to their parents’ distraction efforts. Questions for predicting parent’s ability to effectively provide distraction include items about parenting style, anxiety, and previous experiences. Questions for predicting child distress include items about coping, anxiety, and previous experience.
Development and validation of the CPaD was based on data from 542 parent–child dyads who participated in a previous study (McCarthy et al., 2010a, 2010b). Using automatic feature selection and support vector machine regression models, three predictive models were identified: (a) parent ability to use distraction effectively, (b) child risk for behavioral distress, and (c) child risk for physiologic distress during a medical procedure (e.g., a scheduled IV insertion). Taken together, these three models predict the child’s risk for distress, identified as high, medium, or low risk for distress, when a parent provides distraction after completing basic distraction training. Validation of the models involved multiple cross-validation tests and comparisons between explanatory variables identified with traditional regression and those identified with support vector regression (Hanrahan et al., 2012).
The CPaD includes 44 predictive items and 40 additional items that support tailoring of distraction for the child or that answer other research questions. The parent completes most items, although the child completes a few items, such as self-report of anxiety and coping.
Distraction Coaching Index
Performance of distraction coaching by parents and trained RA distraction coaches was evaluated using the DCI in order to document treatment fidelity. The DCI is a behavioral observation scale that measures the frequency and quality of distraction coaching. Distraction is defined as any verbalization or action by the coach directed toward the child that is meant to focus the child’s attention away from the medical procedure.
The behaviors of the distraction coach and child were videotaped from the time the child was positioned on the exam table until 2 minutes after the IV was secured. Videotapes were divided into 10-second intervals and were coded at a later time using Adobe Premier software. Time samplings of two 2-minute procedural phases were coded for both frequency and quality of distraction. Phase 1, which represents the nonpainful but anxiety-provoking part of the procedure, included the 2 minutes prior to needle insertion; Phase 2, the potentially painful part of the procedure, lasted from the time of the IV needle stick until 2 minutes following the insertion.
While viewing the videotapes, both frequency of distraction and the quality of distraction are scored. Frequency of distraction coaching is a percentage determined by the number of intervals in which distraction coaching is evident, divided by the total number of intervals. Quality of distraction coaching is assessed using five quality indicators: (a) sensitive to the child’s developmental level, (b) sensitive to the child’s cues, (c) demonstrates focus on using distraction, (d) makes an effort to engage child in distraction, and (e) encourages child’s use of distraction.
Total DCI scores range from 0 to 40. Previous validation work found that DCI scores of professional child life specialists tend to be close to 40—indicating excellent distraction coaching. Parents who received basic training as distraction coaches scored around 20, and parents who received no training scored less than 10 (Kleiber et al., 2007).
Three members of the research team were trained to code the videotapes. After training on how to score the DCI, inter-rater reliability was obtained by having 20% of the videotapes coded by two coders. Interrater agreement, measured by intraclass correlation, was 0.88 (95% CI [0.83, 0.92]).
Observation Scale of Behavioral Distress-Revised
Assessment of the child’s behavioral distress during IV insertion was evaluated with the OSBD-R (Elliott, Jay, & Woody, 1987; Jay & Elliott, 1986), an objective observation scale that consists of operationally defined behaviors indicative of distress in children during medical procedures. It consists of eight behavioral categories that demonstrate anxiety and/or pain in children (e.g., cry, scream, flail, restraint). Time samplings of the child’s behavior are recorded, and each behavioral category is weighted according to set intensity scores (e.g., scream is weighted more than cry). A total distress score is calculated by adding together the weighted values of each behavior at each interval, with higher scores reflecting more distress. Scores range from 0 to 21.5. The developers of the OSBD-R reported a reliability of .72 using Cronbach’s alpha and interrater reliability of .89 (Jay & Elliot, 1986). We obtained a reliability estimate of .76 using Cronbach’s alpha for a previous study (McCarthy et al., 2010a) and .78 for this study.
In this study, observations were divided into the two phases described above for the DCI. Prior to coding, RAs were trained in the OSBD-R scoring system using tapes obtained during previous studies until interrater reliabilities greater than .90 were reached. Interrater reliability was obtained by having 20% of the videotapes coded by two coders with an intraclass correlation of .95 (95% CI [0.93, 0.96]).
Salivary Cortisol
The physiological indicator of child distress was salivary cortisol. Four salivary samples were collected from each child: two on the day of the procedure (one on arrival to the clinic [Time 1-Clinic] and one 20 minutes after the IV insertion [Time 2-Clinic]) and two on a baseline day (baseline samples collected at the same times of day as the clinic samples [Time 1-Home and Time 2-Home]). For example, on the clinic day, if a child’s first sample on arriving at the clinic was at 10 A.M., and the second was at 11:45 A.M., parents were asked to collect two samples at home—on a typical Saturday—at 10 A.M. and 11:45 A.M. The baseline samples collected at home provided a comparison for the child’s response in the clinic. Standardized procedures were used for sample collection and storage. Sample collection and storage is described elsewhere (Hanrahan, McCarthy, Kleiber, Lutgendorf, & Tsalikian, 2006). A chemiluminescence immunoassay was used to perform assays that had a high analytic sensitivity (0.16 ng/ml) and good specificity with low-cross reactivity to related metabolites, good precision at low concentrations (intra-assay coefficient of variance = 2.9%–7.7% at 0.1–0.9 mcg/dl, interassay = 5.7%–11.7% at 0.1–0.7 mcg/dl), and small sample size required for testing (20–50 μl).
Oucher
Child perception of procedural pain was measured using the Oucher Scale. The Oucher is a self-report of pain intensity for children ages 3–12 years and includes two separate scales (Aradine, Beyer, & Tompkins, 1988). One scale is a series of six photographs showing a child in varying degrees of discomfort and should be used by children who are unable to count by number. In this study, seriation screening items were used to determine the appropriate scale for each subject. Children who are able to identify the larger of two numbers use the vertical numeric scale (0–10) that is printed next to the faces. The range of scores is 0–10 for both scales. Discriminate validity has been shown by investigating relationships between the Oucher and two fear scales for children (Beyer & Aradine, 1986).
Parent Report of Child Distress
One question from the Perception of Procedures Questionnaire (Kazak, Penati, Waibel, & Blackall, 1996) was used to measure parent perception of child distress. The question “How distressed was your child today during the IV procedure?” was anchored from “not at all” (1) to “extremely distressed” (7). Parents answered this question immediately after the IV procedure was completed. The other questions from the questionnaire were not used as part of the outcome measure of distress for this study because they focus on child behavior prior to this medical procedure—not in response to this medical procedure.
Procedures
Family recruitment began after institutional review board approval was obtained at each of the three sites. Standardized research protocols for enrollment and topical anesthetic use were used at all three sites. Nurses or physicians from each specialty clinic at the three sites identified potential participants 2–3 weeks prior to a scheduled clinic visit. A letter was sent to the families explaining the study and inviting them to participate. Families were routinely scheduled to come to the clinic 1 hour before the IV was to be placed so that a topical anesthetic could be applied. The RA met the family in the waiting room, accompanied them to an exam room, and obtained informed consent from the parents and assent from the children. Families were asked to specify which parent would participate in the study. A salivary sample from the child for Time 1-Clinic cortisol level was obtained at this time.
Next, parents and children were asked to complete the CPaD questions on a laptop computer. The CPaD identified the appropriate risk group for each parent–child dyad and then randomly assigned the dyad to an intervention arm of the study. For parent–child dyads randomized to either basic or enhanced intervention, parents completed the appropriate training. For parent–child dyads randomized to the professional intervention, parents viewed the basic distraction teaching video and were offered the opportunity to review the tailored directions and be with their child while the professional provided distraction. Training for all groups was completed in approximately 15 minutes.
The IV was inserted according to the clinic’s usual standard of care. The procedure was videotaped from the time the child was placed on the exam table to the time the IV catheter was secured in place. The videotapes were coded for the OSBD-R and DCI scores at a later time.
Following IV insertion, the child and parent were asked to complete the remaining aspects of data collection. Children completed the Oucher as soon as possible—following the IV insertion—and the parents answered the PRCD question. The child’s Time 2-Clinic salivary cortisol sample was obtained 20–30 minutes after the IV insertion. Instructions and tubes for collecting the two salivary cortisol samples from the child on a baseline day were given to the family with instructions on how to obtain the samples and mail them back to the researchers. Each family received $30 compensation for their participation.
Data Management and Analyses
Responses to the questions on the CPaD were automatically entered directly into a Microsoft Access database, and demographic data were maintained in a separate database. Both databases were housed on a secured university server. Descriptive statistics (median, mean, percentage, interquartile range, and standard deviation) were computed for the treatment groups in each risk group. Participant characteristics were compared using t-test or one-way ANOVA for age, Wilcoxon rank-sum test or Kruskal–Wallis test for ordinal scale variables, and Pearson’s chi-square test for the categorical variables. Wilcoxon rank-sum test was used to compare OSBD-R scores for validation of the CPaD and the DCI index for assessment of treatment fidelity.
Scores on Oucher (child self-report of pain), PRCD, and OSBD-R were compared between enhanced and professional distraction in the high-risk group using Wilcoxon rank-sum test and among basic, enhanced, and professional interventions in the medium-risk group using Kruskal–Wallis test. When there were differences in treatment group characteristics, a nonparametric method was used for comparing Oucher and PRCD between treatment groups adjusted for covariates. This analysis was done by fitting an ANOVA model with covariates on the rank transform of the data.
For OSBD-R, natural log transformation was first applied to the data to normalize the data distribution, and analysis of covariance was then performed to adjust for covariates.
For cortisol levels—which measured physiological distress—natural log transformation was applied to normalize the distribution, and linear mixed-model analysis was then used to test for treatment group differences. The fixed effects in the mixed model included treatment group—which is a between-subject effect—location (home or clinic), and time (prior to IV or during IV)—which are within-subject effects. The model also included all two-factor and three-factor interactions. To account for covariates, the linear mixed model was expanded to include the covariates. Estimates of treatment group mean cortisol levels in the original scale were computed by back-transformation of the natural log means. Treatment effects—which were based on differences of natural log means—were expressed after back transformation as mean percent difference.
RESULTS
Participants
A total of 582 children and their parents from three Midwestern children’s hospitals were enrolled. From those enrolled, eight withdrew and 574 participated, including 273 (48%) boys and 301 (52%) girls—primarily White (84%) with a mean age of 7.3 years (SD = 1.9). Parent coaches were primarily mothers (83%) and most had some college education (79%). Children were seen primarily in the GI clinics (45%), followed by other (21%), endocrine (17%), urology (8%), pulmonary (5%), and renal (4%).
Child assignment to risk groups included 156 (27%) in the high risk for distress group (enhanced, n = 79; professional, n = 77) and 372 (65%) in the medium risk for distress group (basic = 121; enhanced, n = 125; professional, n = 126; see Table 1 and Figure 1). Children in the low risk for distress group (n = 46, 8%) were assigned only to the basic intervention. As noted previously, children at low risk for distress do well with the basic level of intervention (McCarthy et al., 2010a, 2010b), so testing the effectiveness of higher doses of distraction is not indicated. Data from the low-risk group were used to further examine the validity of the CPaD.
TABLE 1.
Participant Characteristics by Risk for Distress and Intervention Group
| Characteristic | Low distress risk
|
Medium distress risk
|
High distress risk
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basic
|
Basic
|
Enhanced
|
Professional
|
Enhanced
|
Professional
|
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Gender (male) | 31 | 67 | 48 | 40 | 62 | 50 | 65 | 52 | 29 | 37 | 38 | 49 |
| Race (White) | 43 | 93 | 97 | 80 | 103 | 82 | 103 | 82 | 70 | 89 | 64 | 83 |
| ADHD (yes) | 6 | 13 | 8 | 7 | 19 | 15 | 15 | 12 | 15 | 19 | 13 | 17 |
| Anxiety disorder (yes) | 0 | 0 | 4 | 3 | 2 | 2 | 9 | 7 | 5 | 6 | 5 | 6 |
| CT experience (yes) | 14 | 30 | 44 | 36 | 35 | 28 | 31 | 25 | 16 | 20 | 18 | 23 |
| Previous difficult IV insertion (yes) | 17 | 37 | 46 | 38 | 48 | 38 | 52 | 42 | 24 | 30 | 27 | 35 |
|
| ||||||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
|
| ||||||||||||
| Age (years) | 8.4 | 1.3 | 7.3 | 2.0 | 7.3 | 1.8 | 7.6 | 1.8 | 7.1 | 2.2 | 6.4 | 2.0 |
|
| ||||||||||||
| Mdn | [LQ, UQ] | Mdn | [LQ, UQ] | Mdn | [LQ, UQ] | Mdn | [LQ, UQ] | Mdn | [LQ, UQ] | Mdn | [LQ, UQ] | |
|
| ||||||||||||
| Distress during previous procedures | 3.5 | [2, 5] | 5 | [4, 6] | 5 | [3, 6] | 5 | [3, 6] | 6 | [5, 7] | 6 | [5, 7] |
Note. CT = child therapist; IV = intravenous; LQ = lower quartile; Mdn = median; SD = standard deviation; UQ = upper quartile.
Sample sizes for analyses varied by the number available for each outcome measure. For example, not all videos could be scored for the OSBD-R and not all children provided four cortisol samples.
Aim 1: CPaD Validation
Two preliminary analyses were carried out for further validation of the CPaD and assurance of treatment fidelity (see Table 2). OSBD-R scores—indicative of behavioral distress—were significantly higher in the medium compared to the low distress risk group (p = .006) for those who received basic intervention—a consistent intervention for both groups. Children in the high distress risk group had significantly higher OSBD-R scores compared to the medium distress risk group for those in the enhanced intervention arm (p = .004) and in the professional intervention arm (p = .002). As noted earlier, treatment fidelity was monitored with the DCI by scoring the distraction provided by parents and the trained distraction coaches. As expected, the trained distraction coaches had significantly higher DCI scores (p < .0001) compared to parents within each distress risk group, indicating high-quality distraction (see Table 3).
TABLE 2.
Validation of Children, Parents and Distraction: Observational Scale of Behavioral Distress-Revised Scores by Distress Category Within Intervention Group
| Intervention | Low distress risk
|
Medium distress risk
|
High distress risk
|
p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | Mdn | [LQ, UQ] | n | % | Mdn | [LQ, UQ] | n | % | Mdn | [LQ, UQ] | ||
| Basic | 45 | 100.0 | 0.67 | [0.0, 1.63] | 114 | 33.0 | 1.32 | [0.50, 4.67] | .006 | ||||
| Enhanced | 113 | 33.0 | 1.04 | [0.21, 2.52] | 72 | 50.0 | 2.94 | [0.63, 5.29] | .004 | ||||
| Professional | 117 | 34.0 | 0.50 | [0.0, 2.08] | 73 | 50.0 | 1.33 | [0.46, 5.00] | .002 | ||||
Note. LQ = lower quartile; Mdn = median; UQ = upper quartile.
TABLE 3.
Treatment Fidelity Assessment Using Distraction Coaching Inventory Scores
| Intervention | Low distress risk
|
Medium distress riska
|
High distress riskb
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mdn | [LQ, UQ] | n | Mdn | [LQ, UQ] | n | Mdn | [LQ, UQ] | |
| Basic | 45 | 26.0 | [17.3, 31.2] | 114 | 24.5 | [15.0, 31.6] | |||
| Enhanced | 118 | 26.5 | [20.0, 32.4] | 73 | 28.0 | [20.1, 33.0] | |||
| Professional | 119 | 38.0 | [34.5, 40.0] | 72 | 38.3 | [32.5, 40.0] | |||
Note. LQ = lower quartile; Mdn = median; UQ = upper quartile.
In the medium distress risk group, professional is significantly greater than both basic and enhanced (p < .0001).
In the high distress risk group, professional is significantly greater than enhanced (p < .0001).
Aim 2: High Distress Risk Group Outcomes
The second aim for this study was to compare the effectiveness of the professional and enhanced interventions for children in the high distress risk group. Comparison of the outcome measures for those at risk for high distress between professional and enhanced interventions showed no significant difference in Oucher (p = .48), PRCD (p = .11), and total OSBD-R (p = .24) scores (see Table 4). However, it was noted that these two intervention groups differed in gender and age, with more boys and younger children in the professional intervention group. After adjusting for these covariates, there were no statistically significant differences. Cortisol levels at clinic compared to home (baseline) increased by 85% in the enhanced group (p = .002) and by 37% in the professional group (p = .16; see Table 5). However, the cortisol change was not significantly different between the two groups (p = .23).
TABLE 4.
Child Report (Oucher), Parent Report, and Behavioral Distress by Intervention Group for Medium and High Distress Risk
| Distress risk | Measure | Intervention | n | Mdn | [LQ, UQ] | M | SD | p | Adjusted pa |
|---|---|---|---|---|---|---|---|---|---|
| High | Oucher | Enhanced | 76 | 4.00 | [0.50, 6.00] | 3.79 | 3.31 | .48 | .48 |
| Professional | 70 | 2.00 | [0.00, 6.00] | 3.51 | 3.60 | ||||
| PRCD | Enhanced | 79 | 4.00 | [2.00, 5.00] | 3.82 | 1.91 | .11 | .11 | |
| Professional | 75 | 3.00 | [2.00, 5.00] | 3.33 | 1.89 | ||||
| OSBD-R | Enhanced | 72 | 2.94 | [0.63, 5.29] | 3.90 | 4.99 | .24 | .07 | |
| Professional | 73 | 1.33 | [0.46, 5.00] | 3.08 | 3.83 | ||||
| Medium | Oucher | Basic | 116 | 2.00 | [0.00, 6.00] | 3.55 | 3.61 | .86 | .76 |
| Enhanced | 116 | 2.00 | [0.00, 5.00] | 3.22 | 3.32 | ||||
| Professional | 124 | 2.00 | [1.00, 4.00] | 3.09 | 3.11 | ||||
| PRCD | Basic | 116 | 3.50 | [2.00, 5.00] | 3.48 | 1.98 | .18 | .11 | |
| Enhanced | 117 | 3.00 | [2.00, 5.00] | 3.26 | 1.86 | ||||
| Professional | 121 | 3.00 | [2.00, 4.00] | 2.97 | 1.7 | ||||
| OSBD-R | Basic | 112 | 1.38 | [0.50, 4.81] | 3.16 | 4.06 | .0005 | .0006 | |
| Enhanced | 112 | 1.05 | [0.23, 2.55] | 2.57 | 4.35 | ||||
| Professional | 117 | 0.50 | [0.00, 2.08] | 1.65 | 2.65 |
Note. LQ = lower quartile; Mdn = median; PRCD = Parent Report of Child Distress; OSBD-R = Observational Scale of Behavioral Distress-Revised; UQ = upper quartile.
Adjusting for gender and age.
TABLE 5.
Average Cortisol Changes by Intervention Group for High and Medium Distress Risk
| Distress risk | Intervention | na | Clinic
|
Home
|
Clinic vs. Home
|
pb | |||
|---|---|---|---|---|---|---|---|---|---|
| Percent change (%) | SE | Percent change (%) | SE | Percent change (%) | SE | ||||
| High | Enhancedc | 28 | 9.68 | 1.10 | 5.22 | 0.74 | 85.4 | 33.0 | .002 |
| Professionalc | 27 | 7.98 | 0.94 | 5.84 | 0.80 | 36.7 | 24.3 | .16 | |
| Medium | Basicd | 46 | 8.32 | 1.43 | 7.11 | 1.29 | 17.0 | 17.0 | .84 |
| Enhancedd | 53 | 7.27 | 1.22 | 7.23 | 1.21 | 0.5 | 13.6 | .99 | |
| Professionald | 55 | 8.10 | 1.28 | 6.84 | 1.03 | 18.3 | 15.7 | .62 | |
Note. Changes for the clinic involved the difference between the cortisol collected when the child arrived at the clinic (Time 1-Clinic) and the sample collected 20 minutes after the IV insertion (Time 2-Clinic). Changes for home involved the difference between the cortisol collected at home on a typical Saturday, the first (Time 1-Home) obtained at the same time of day that the first sample was obtained in clinic and the second (Time 2-Home) at the same time of day as the second one in clinic.
Data presented only for subjects with a complete set of four samples.
Adjusted for age, gender, ADHD, and anxiety disorder.
No significant difference in mean percent change from home to clinic among the groups, p = .64.
No significant differences in mean percent change from home to clinic, enhanced vs. professional, p = .23.
Aim 3: Medium Distress Risk Group Outcomes
The third aim was to evaluate the effectiveness of the professional, enhanced, and basic interventions for children in the medium distress risk group. The Oucher and PRCD were not statistically different for the three interventions. Total OSBD-R scores were significantly lower (p = .0005) for the professional compared to the enhanced or basic interventions (see Table 4). Similar conclusions were found after adjusting for covariates (gender, ADHD, anxiety disorder). There were no significant differences in cortisol measures between groups (see Table 5).
DISCUSSION
An important outcome of this study was validation of the CPaD for identifying parent–child dyads at low, medium, and high risk for distress during a medical procedure. This study demonstrates that—as predicted by the CPaD—27% of the children were at risk for high distress, the majority (65%) of children fell in the medium risk for distress group, and few were predicted to have low distress (8%). Also, OSBD-R scores, which represent behavioral distress, decreased within a risk group as the dose of distraction increased, and within each distraction dose increased as the risk for distress increased. By using the CPaD to predict distress, clinicians may be able to identify child–parent dyad’s need for additional support in a clinical setting.
Research with the early childhood and early school age children report positive effects of parent distraction coaching on children’s distress behavior (Blount et al., 1992; Gonzalez et al., 1993; Manne et al., 1990; Manimala et al., 2000). The study adds to the literature by attempting to match doses of distraction (parent coach, parent coach with support, or professional coach) to children’s predicted risk for distress during a medical procedure. Our goal is to provide appropriate interventions for children predicted to be at high or medium risk for distress. These are the children who need to be identified prior to the procedure so that additional support services can be scheduled.
Treatment effects were analyzed separately for the high-and medium-risk groups. Children at high risk for distress were randomized to the enhanced or professional interventions. There were no significant differences for self-report of pain, PRCD, or cortisol change (clinic vs. home). The mean OSBD-R score for the professional intervention was 3.08 versus 3.90 for the enhanced group. It is apparent that some high risk children responded very well to these interventions, but others did not. This is an important area for study because more than one quarter of the children in this study were predicted to be at high risk for distress. We know from previous research that children who have poor experiences with healthcare procedures are at risk for long-term negative sequelae (Bronner et al., 2008; Kennedy et al., 2008; Taddio et al., 2009). In addition, high behavioral distress is disruptive to procedures and may be costly in terms of repeated procedures.
About two thirds of the children were predicted to be in the medium-risk group. Children in this category were randomized to basic, enhanced, or professional interventions. There was a significant group difference for OSBD-R, with lower behavioral distress in the group receiving the professional intervention. The enhanced intervention does not appear to be superior to the basic intervention for children in the medium-risk group. We had hypothesized that having a “helper” to prompt parents in the use of distraction, as provided in the enhanced intervention, would result in higher quality of distraction and improved outcomes for the children. However, the DCI scores of parents in the enhanced group were not significantly better than those in the basic group.
Other research has questioned the effectiveness of parent-provided distraction compared to professional intervention. A systematic review of psychological interventions for reducing immunization pain and distress concluded that there is insufficient evidence to support the use of parent-led distraction (Chambers, Taddio, Uman, & McMurtry, 2009). However, half of the studies in that review investigated the effect of parent distraction on infant distress during immunization. We propose that the effectiveness of distraction is very different for the age group of 4–10 years presented in the current study versus the infant population and that combining evidence for the two age groups may be misleading.
A few comments about the other outcome measures are in order. The children assigned to the enhanced intervention in the high-risk group had a significantly higher clinic versus home cortisol level, but the difference for children in the professional intervention group was not significant. This discrepancy most likely reflects the wide variability in day-to-day cortisol fluctuations. The percent change from clinic to home was not significantly different between intervention groups. For the effect of interventions on pain, we were not surprised that there were no differences in Oucher scores between intervention groups. Application of a topical analgesic was part of the standard research protocol for all sites. A possible explanation for the lack of differences between groups is that topical analgesics work so well that the needle stick is not perceived as painful.
Limitations
Limitations of this study are acknowledged. The three participating hospitals were from the United States Midwest, limiting the ethnic diversity of the participants. The results cannot be generalized to more diverse ethnic groups. We strove to standardize the three sites as much as possible through rigorous training of the RAs, mandatory team meetings, and routine weekly phone contact between the primary site and the secondary sites. As with all clinical research, we were unable to control for the behaviors of nurses and physicians who were not part of our research team. In retrospect, the enhanced intervention was difficult to deliver in a consistent and timely manner and the differences between the basic and enhanced treatment arms may not have been sufficient to find treatment effects.
Implications
There are no easy “checklists” that we know of to accurately predict children’s risk for distress. Computerized tools such as the CPaD and predictive programs that take in and analyze data from prospective parent distraction coaches and children are the best hope for identifying children at risk for high distress during procedures. If such applications were available, nurses could prescreen children and then plan for additional staff to be available or plan for time to teach parents to act as distraction coaches.
High-quality distraction provided by either a parent or a professional can decrease a child’s distress response during a stressful medical procedure. The availability of a professional to provide distraction for all children undergoing a procedure is costly, whereas involvement of a parent is more cost effective and preferred by some families. This study adds to the body of knowledge on the use of distraction by examining how children in different distress risk groups responded to coaching from parents with basic training, coaching from parents with basic training and additional support, and coaching from a trained professional. The findings of this study indicate that children who are predicted to have low distress with procedures will most likely do well with distraction coaching provided by a parent after basic training. Children who are predicted to have high distress may display less behavioral distress when paired with a professional distraction coach. Most children will fall into the medium risk for distress category. If professional distraction coaching is not available, we recommend that parents be asked about their preferences in acting as the distraction coach and, if willing, be provided as much training and support as possible in the clinical situation.
Conclusions
The CPaD proved useful for identifying risk of distress and matching dose of distraction intervention to risk for distress. Some parents may need additional training in providing distraction to their children during procedures, and some children at medium and high risk for distress may need professional support. Parents should be asked about their preferences in acting as the distraction coach and, if willing, be provided as much training and support as possible in the clinical situation. Additional studies are needed to identify those children who do not respond well to professional distraction coaching as they may benefit from an alternative intervention such as preprocedural desensitization.
Acknowledgments
The authors would like to thank the children, families, healthcare providers, and nursing students that participated in this study.
The study was funded by R01 Grant NR05269-01A2 from the National Institute for Nursing Research to the first author.
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Ann Marie McCarthy, College of Nursing, University of Iowa.
Charmaine Kleiber, College of Nursing, University of Iowa.
Kirsten Hanrahan, University of Iowa Health Care, University of Iowa.
M. Bridget Zimmerman, Department of Biostatistics, University of Iowa.
Anne Ersig, College of Nursing, University of Iowa.
Nina Westhus, School of Nursing, Saint Louis University, Missouri.
Susan Allen, Nursing Manager of Emergency Department, PICU, Internal Resource Team, Blank Children’s Hospital, Des Moines, Iowa.
References
- American Academy of Pediatrics. 2014 immunization schedules. 2014 Retrieved from http://www2.aap.org/immunization/izschedule.html.
- Aradine CR, Beyer JE, Tompkins JM. Children’s pain perception before and after analgesia: A study of instrument construct validity and related issues. Journal of Pediatric Nursing. 1988;3:11–23. [PubMed] [Google Scholar]
- Beyer JE, Aradine CR. Content validity of an instrument to measure young children’s perceptions of the intensity of their pain. Journal of Pediatric Nursing. 1986;1:386–395. [PubMed] [Google Scholar]
- Blount RL, Bachanas PJ, Powers SW, Cotter MC, Franklin A, Chaplin W, Blount SD. Training children to cope and parents to coach them during routine immunizations: Effects on child, parent and staff behaviors. Behavior Therapy. 1992;23:689–705. [Google Scholar]
- Bronner MB, Knoester H, Bos AP, Last BF, Gootenhuis MA. Posttraumatic stress disorder (PTSD) in children after paedi-atric intensive care treatment compared to children who survived a major fire disaster. Child and Adolescent Psychiatry and Mental Health. 2008;2:9. doi: 10.1186/1753-2000-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi N, Vandermeer B, Curtis S. The Cochrane library and procedural pain in children: An overview of reviews. Evidence-Based Child Health. 2008;3:260–279. doi: 10.1002/ebch.225. [DOI] [Google Scholar]
- Chambers CT, Taddio A, Uman LS, McMurtry CM. Psychological interventions for reducing pain and distress during routine childhood immunizations: A systematic review. Clinical Therapeutics. 2009;31:S77–S103. doi: 10.1016/j.clinthera.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Cohen LL, Blount RL, Cohen RJ, Ball CM, McClellan CB, Bernard RS. Children’s expectations and memories of acute distress: Short- and long-term efficacy of pain management interventions. Journal of Pediatric Psychology. 2001;26:367–374. doi: 10.1093/jpepsy/26.6.367. [DOI] [PubMed] [Google Scholar]
- Elliott CH, Jay SM, Woody P. An observation scale for measuring children’s distress during medical procedures. Journal of Pediatric Psychology. 1987;12:543–551. doi: 10.1093/jpepsy/12.4.543. [DOI] [PubMed] [Google Scholar]
- Gonzalez JC, Routh DK, Armstrong FD. Effects of maternal distraction versus reassurance on children’s reactions to injections. Journal of Pediatric Psychology. 1993;18:593–604. doi: 10.1093/jpepsy/18.5.593. [DOI] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Ataman K, Street WN, Zimmerman MB, Ersig AL. Building a computer program to support children, parents, and distraction during healthcare procedures. Computers, Informatics, Nursing. 2012;30:554–561. doi: 10.1097/NXN.0b013e31825e211a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Applied Nursing Research. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, care, education and research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- Jay SM, Elliott C. Unpublished scoring manual. Los Angeles, CA: Psychosocial Program, Children’s Hospital of Los Angeles; 1986. Observation scale of behavioral distress—Revised. [Google Scholar]
- Kazak AE, Penati B, Waibel MK, Blackall GF. The Perception of Procedures Questionnaire: Psychometric properties of a brief parent report measure of procedural distress. Journal of Pediatric Psychology. 1996;21:195–207. doi: 10.1093/jpepsy/21.2.195. [DOI] [PubMed] [Google Scholar]
- Kennedy RM, Luhmann J, Zempsky WT. Clinical implications of unmanaged needle-insertion pain and distress in children. Pediatrics. 2008;122:S130–S133. doi: 10.1542/peds.2008-1055e. [DOI] [PubMed] [Google Scholar]
- Kleiber C, Craft-Rosenberg M, Harper DC. Parents as distraction coaches during IV insertion: A randomized study. Journal of Pain and Symptom Management. 2001;22:851–861. doi: 10.1016/S0885-3924(01)00316-5. [DOI] [PubMed] [Google Scholar]
- Kleiber C, McCarthy AM, Hanrahan K, Myers L, Weathers N. Development of the distraction coaching index. Children’s Health Care. 2007;36:219–235. doi: 10.1080/02739610701377897. [DOI] [Google Scholar]
- Koller D, Goldman RD. Distraction techniques for children undergoing procedures: A critical review of pediatric research. Journal of Pediatric Nursing. 2012;27:652–681. doi: 10.1016/j.pedn.2011.08.001. [DOI] [PubMed] [Google Scholar]
- MacLean S, Obispo J, Young KD. The gap between pediatric emergency department procedural pain management treatments available and actual practice. Pediatric Emergency Care. 2007;23:87–93. doi: 10.1097/PEC.0b013e31803. [DOI] [PubMed] [Google Scholar]
- Manimala MR, Blount RL, Cohen LL. The effects of parental reassurance versus distraction on child distress and coping during immunizations. Children’s Health Care. 2000;29:161–177. doi: 10.1207/S15326888CHC2903_2. [DOI] [Google Scholar]
- Manne SL, Redd WH, Jacobsen PB, Gorfinkle K, Schorr O, Rapkin B. Behavioral intervention to reduce child and parent distress during venipuncture. Journal of Consulting and Clinical Psychology. 1990;58:565–572. doi: 10.1037/0022-006X.58.5.565. [DOI] [PubMed] [Google Scholar]
- McCarthy AM, Hanrahan K, Kleiber C, Zimmerman MB, Lutgendorf S, Tsalikian E. Normative salivary cortisol values and responsivity in children. Applied Nursing Research. 2009;22:54–62. doi: 10.1016/j.apnr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, Kleiber C. A conceptual model of factors influencing children’s responses to a painful procedure when parents are distraction coaches. Journal of Pediatric Nursing. 2006;21:88–98. doi: 10.1016/j.pedn.2005.06.007. [DOI] [PubMed] [Google Scholar]
- McCarthy AM, Kleiber C, Hanrahan K, Zimmerman MB, Westhus N, Allen S. Factors explaining children’s responses to intravenous needle insertions. Nursing Research. 2010a;59:407–416. doi: 10.1097/NNR.0b013e3181f80ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy AM, Kleiber C, Hanrahan K, Zimmerman MB, Westhus N, Allen S. Impact of parent-provided distraction on child responses to an IV insertion. Children’s Health Care. 2010b;39:125–141. doi: 10.1080/02739611003679915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon K, Price M, Pereira JK. Factors associated with young children’s long-term recall of an invasive medical procedure: A preliminary investigation. Journal of Developmental & Behavioral Pediatrics. 2002;23:347–352. doi: 10.1097/00004703-200210000-00008. [DOI] [PubMed] [Google Scholar]
- Stevens BJ, Abbott LK, Yamada J, Harrison D, Stinson J, Taddio A, Finley GA. Epidemiology and management of painful procedures in children in Canadian hospitals. Canadian Medical Association Journal. 2011;183:E403–E410. doi: 10.1503/cmaj.101341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson J, Yamada J, Dickson A, Lamba J, Stevens B. Review of systematic reviews on acute procedural pain in children in the hospital setting. Pain Research & Management. 2008;13:51–57. doi: 10.1155/2008/465891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddio A, Chambers CT, Halperin SA, Ipp M, Lockett D, Rieder MJ, Shah V. Inadequate pain management during routine childhood immunizations: The nerve of it. Clinical Therapeutics. 2009;31:S152–S167. doi: 10.1016/j.clinthera.2009.07.022. [DOI] [PubMed] [Google Scholar]
- Uman LS, Birnie KA, Noel M, Parker JA, Chambers CT, McGrath PJ, Kisely SR. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database of Systematic Reviews. 2013;10 doi: 10.1002/14651858.CD005179.pub3. [DOI] [PubMed] [Google Scholar]
- von Baeyer CL, Marche TA, Rocha EM, Salmon K. Children’s memory for pain: Overview and implications for practice. Journal of Pain. 2004;5:241–249. doi: 10.1016/j.jpain.2004.05.001. [DOI] [PubMed] [Google Scholar]