Abstract
It has been determined that there is extensive communication between the immune system and the central nervous system (CNS). Proinflammatory cytokines play a key role in this communication. There is an emerging realization that glia and microglia, in particular, (which are the brain’s resident macrophages), are an important source of inflammatory mediators and may have fundamental roles in CNS disorders. Microglia respond also to proinflammatory signals released from other non-neuronal cells, principally those of immune origin, such as mast cells. Mast cells reside in the CNS and are capable of migrating across the blood-brain barrier (BBB) in situations where the barrier is compromised as a result of CNS pathology. Mast cells are both sensors and effectors in communication among nervous, vascular, and immune systems. In the brain, they reside on the brain side of the BBB, and interact with astrocytes, microglia, and blood vessels via their neuroactive stored and newly synthesized chemicals. They are first responders, acting as catalysts and recruiters to initiate, amplify, and prolong other immune and nervous responses upon activation. Mast cells both promote deleterious outcomes in brain function and contribute to normative behavioral functioning, particularly cognition and emotion. Mast cells may play a key role in treating systemic inflammation or blockade of signaling pathways from the periphery to the brain.
MeSH Keywords: Blood-Brain Barrier, Inflammation, Mast Cells, Microglia, Neurodegenerative Diseases
Background
Fundamentally, inflammation is a protective physiologic response to injury and infection and is necessary for removing detrimental stimuli and initiating tissue healing. A similar process that occurs in the CNS in response to injury or disease is termed neuroinflammation. In acute neuroinflammation, microglia become reactive and limit the area of injury through phagocytosing dying cells and releasing pro-inflammatory cytokines [1]. However, when neuroinflammation is prolonged, it overwhelms the bounds of physiological control and produce deleterious effects involving pro-inflammatory signaling pathways, increased oxidative stress, and death of nearby neurons. Neuroinflammation is a common mechanism influencing the severity and progression of neurodegenerative disease and is, therefore, an important target for neuroprotective therapies [2–6].
Central Inflammation
Inflammation and degenerative diseases (AD, PD, HD)
Neurodegeneration, the progressive dysfunction and loss of neurons in the CNS, is the major cause of cognitive and motor dysfunction. Emerging evidence now points to a more active role of neuroinflammation in pathophysiology onset and progression, with glia having key roles in neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease, and Huntington’s disease, and may even contribute to multiple sclerosis, amyotrophic lateral sclerosis, stroke, and neuropsychiatric disorders. The initiation and propagation of neuroinflammation appear to rely very much on the interaction between glia, immune cells, and neurons, although the glia-immune cell connection remained to be fully explored. Microglia are the major resident immune cells in the brain and play a pivotal role in the immune surveillance of the central nervous system (CNS). Consequently, these cells are likely to have an important role in either the development of protective immune responses or the progression of damaging inflammation during CNS disease states. In the steady state, microglia protect the nervous system by acting as scavengers of debris and microbial pathogens and by regulating the innate and adaptive immune responses. Pathological states within the nervous system, including injury, ischemic stroke [7], and infection [8], can lead to activation of microglia. This in turn can cause release of inflammatory molecules that trigger astrocytes and cells of the immune system to respond to the injury [9]. In the disease state, activated microglia mediate neuronal injury and death through production of proinflammatory factors like cytokines and chemokines, glutamate, and reactive oxygen species and help mobilize the adaptive immune response and cell chemotaxis, leading to transendothelial migration of immune cells across the BBB and even perpetuation of neuronal damage [10].
Sustained inflammation resulting in tissue pathology implies persistence of an inflammatory stimulus or a failure in normal resolution mechanisms. A persistent stimulus may result from environmental factors or the formation of endogenous factors that are perceived by the immune system as “stranger” or “danger” signals, such as misfolded protein aggregates, which are present in neurodegenerative diseases (AD, PD, and HD). Inflammatory responses that establish feed-forward loops may overwhelm normal resolution mechanisms. Although some inflammatory stimuli induce beneficial effects (e.g., phagocytosis of debris and apoptotic cells), and inflammation is linked to tissue repair processes, uncontrolled inflammation may result in production of neurotoxic factors that amplify underlying disease states. The cytokines released by necrotic neurons induce further activation of microglia and astrocytes, resulting in positive feedback loops that can become independent of the original inducing molecules that are required to initiate inflammatory responses.
Inflammation and anesthesia
Some anesthetic agents have been shown to have anti-inflammation and immune-modulatory properties. Propofol, an intravenous anesthetic agent, was demonstrated that have neuroprotective effects. It is reported that propofol could inhibit LPS-induced microglial activation [11,12]. Propofol also exerted neuroprotection against ischemic brain damage through inhibited COX-2 and TNF-α expression and NF-κB activation in rat [13]. Volatile anesthetics have organ-protective effects against ischemia-reperfusion injury. Sevoflurane has protective action against cerebral ischemia reperfusion-induced brain injury by regulating cerebral antioxidant enzymes activities, Bcl-2, Bax, c-Fos, and Caspase-3 protein positive expression levels, and Bcl-2, Bax, TNF-α, and Caspase-3 mRNA expression [14].
Mast Cells
Mast cells were first characterized in 1878 by the Nobel-prize winning immunologist Paul Ehrlich, who described the histochemical properties and distinct morphological phenotypes of these small (6–12 mm) cells [15]. Mast cells originate in the bone marrow arising, in humans, from CD34+/CD117+ pluripotent progenitor cells [16]. Mast cells circulate in the blood as committed precursors and complete their differentiation in tissues where they reside. They are infamous and well-studied for their role in immunoglobulin type E (IgE)-associated allergic and inflammatory disorders. Efficient activation of MCs is via IgE binding to FceRI (high-affinity surface receptors for the Fc region of IgE), although MC activation can also occur through ligand binding to other receptors, including FcgRIIa, TrkA, complement component receptors, Toll-like receptors (TLRs), and interleukin (IL) receptors [17,18].
Mast cells and inflammation
General inflammation of mast cells activation
Mast cells are effector cells of the innate immune system. They are distributed in virtually all organs and vascularized tissues [19]. They are present in the skin, the gastrointestinal tract, and the airways, where they are in close contact with the outside environment. Mast cells are also normal residents of the peritoneum, synovium, hair follicles, and many other organs.
Mast cells produce a plethora of mediators, including biogenic amines (histamine and serotonin), cytokines [interleukin (IL)-1 to IL-6, leukemia inhibitory factor, tumor necrosis factor (TNF) α, interferon-γ, transforming growth factor-β, and granulocyte-microphage colony-stimulating factor], enzymes (acid hydrolyzes, chymase, phospholipases, rat mast-cell protease I and II, and tryptase), lipid metabolites (prostaglandins, leukotrienes, and platelet-activating factor), ATP, neuropeptides (vasoactive intestinal peptide), growth factors [nerve growth factor (NGF)], nitric oxide, and heparin [20]. It should be kept in mind that while mediator release via degranulation is a very rapid process, longer lasting activation results in the release of de novo formed mediators [21]. Mast cells as a class are heterogeneous, and no single mast cell makes all of these substances. Their immune regulatory role includes participation in switching of IgE by B cells [22] and the release of chemoattractants that recruit eosinophils [23] and monocytes [24].
Mast cells and central inflammation
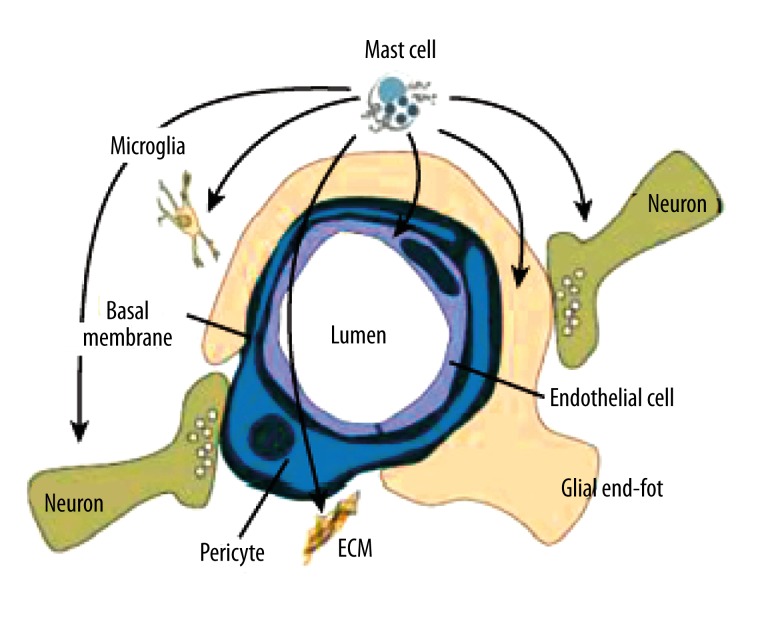
Mast cells are also found in the brain, on the brain side of the blood-brain barrier (BBB) and in the leptomeninges [25,26]. Almost 97% of mast cells reside on the abluminal (brain) side of the blood vessels, thus, they are able communicate with neurons, astrocytes, microglia, extra cellular matrix, and blood vessels. Under baseline conditions, in the absence of stress, disease, or trauma, the numbers of mast cells is considerably smaller than that of neurons, microglia, and other brain-resident cells. Despite their small numbers, activated mast cells can impact the BBB, neurons, microglia, and astrocytes (Figure 1). The multiphasic response pattern of mast cells, wherein they release preformed granular material within minutes and newly synthesized mediators for the next several hours, enables their actions as catalysts that amplify and prolong numerous vasoactive, neuroactive, and immunoactive cellular and molecular response.
Figure 1.
The picture shows the close proximity of the mast cells to other cellular elements in nerve tissue, and points to its ability to signal blood vessel components, neurons, glia, and microglia. Abbreviation: ECM, extracellular matrix. Reproduced from Silver and Curley [27].
Blood-brain barrier
There is substantial evidence that mast cells can penetrate the BBB and break its integrity. Mature mast cells themselves can migrate from blood to brain [28]. Intramuscular injection of the mast cell degranulator, compound 48/80, results in vastly increased Evans Blue tracer leakage in mast cell-rich, but not mast cell-lacking brain regions bearing fenestrated capillaries. These findings have been confirmed with various techniques in several species [29,30]. Mast cell effects on vascular permeability are blocked by mast cell stabilizers and are absent in mast cell-deficient W/Wv mice [31]. The mechanism of mast cell disruption of the BBB and basal lamina degradation may involve the vasoactive and matrix degrading components of mast cells, such as heparin, histamine, serotonin, nitric oxide, vasoactive intestinal peptide, calcitonin gene-related peptide (CGRP), vascular endothelial growth factor, and cytokines, including TNF-a, which in turn induces the expression of intercellular adhesion molecule-1 (ICAM-1) and permits leukocytes to enter the affected tissues [32–34]. Although other resident cells in the CNS produce TNF-a, most notably microglia/macrophages and endothelial cells, the presence and release of TNF-a from mast cells precedes its detection in other cells [35]. Proteolytic gelatinase enzymes, most importantly matrix metalloproteinases (MMP)-2 and MMP-9, are considered to be central mediators of ischemic BBB disruption; this is because of their ability to degrade components of microvascular basal lamina, especially collagen type IV [36,37], and to disrupt tight junction proteins [38]. A recent study demonstrated that cerebral mast cells participate in regulation of acute microvascular MMP-2 and -9 activation and consequent BBB disruption following transient cerebral ischemia [39]. Notably, mast cells can contribute approximately 90% of thalamic histamine and up to 50% of total brain histamine in rats [40]. Several reports indicate that BBB permeability is regulated by brain histamine. Infusion of histamine into the carotid artery enhances the penetration of albumin through the capillary and increases the cortical water content by activating H2 receptors [41,42]. Furthermore, serine proteases such as the mast cell-specific protease cause not only microvascular leakage, but also neuronal hyperexcitability and inflammation through protease-activated receptors [43–46].
Microglia and astrocytes
Besides releasing proinflammatory mediators, microglia also responds to proinflammatory signals released from other non-neuronal cells of immune origin. In this context, mast cells are of particular relevance. Several molecular mechanisms for potential interactions between mast cells and microglia have been determined in vitro [47]. For instance, activation of P2 receptors on microglia by ATP stimulates the release of IL-33, which binds to mast cell receptors, triggering the release of Il-6, IL-13, and monocyte chemo-attractant protein 1, which in turn may regulate microglia activity. Similarly, mast cell tryptase activates microglial-activated receptor 2 (PAR2) receptors, facilitating the release of pro-inflammatory mediators such as TNF-α, IL-6, and reactive oxygen species (ROS), which consequently upregulate the expression of PAR2 receptors on mast cells [48,49]. Mast cell activation leads to the upregulation of purinergic receptor P2X, ligand-gated ion, 4 (PR2X4) receptors on microglia, resulting in the release of brain-derived neurotrophic factor (BDNF) [50]. Furthermore, a new study now demonstrates that microglia can constitutively express all 4 histamine receptors (H1R, H2R, H3R, and H4R) and the expression of H1R and H4R can be selectively upregulated in primary cultured microglia in a dose-dependent manner by histamine. Histamine can also dose-dependently stimulate microglia activation and, subsequently, production of pro-inflammatory factors TNF-alpha and IL-6. The antagonists of H1R and H4R, but not H2R and H3R, reduced histamine-induced TNF-alpha and IL-6 production and MAPK and PI3K/AKT pathway activation, and mitochondrial membrane potential loss in microglia, suggesting that the actions of histamine are via H1R and H4R [51]. Numerous other molecules and receptors such as complement component 5a receptor (C5aR), chemokine (C-X-C motif) receptor 4/12 (CXCr4 and CXCL12) and TLRs provide even more opportunities for microglia-MC interaction [47], suggesting that MCs have a large role in the normative functioning of microglia, as well as modulating host responses to infection, inflammation, trauma, and stress.
Besides microglia, mast cells also have a dynamic relationship with astrocytes. Mast cells and astrocytes are co-localized in the perivasculature and thalamus [52]. In vitro, astrocytes can be activated by mast cells via activation of CD40-CD40 ligand interactions, and mast cells also are stimulated to release histamine, leukotrienes, and cytokines [53]. Interestingly, astrocytes also have histamine receptors (H1R and H2R) [54], and cytokines released from astrocytes can induce mast cell degranulation [55].
Mast cells and degenerative diseases
For their heterogeneity, first-responder capability, and positioning to impact on each of the elements of brain, it is perhaps not surprising that mast cells have been considered as a participant in almost all major CNS disease states, including MS, cerebral ischemia, AD, and PD (Table 1) [56,57]. In this section we summarize findings in 3 diseases from among the many that have been implicated in mast cell function: multiple sclerosis (MS), cerebral ischemia, and AD.
Table 1.
Mast cells and degenerative diseases.
| Disease species | Manipulation used | Findings |
|---|---|---|
| MS | EAE | Mast cell stabilizer reduced the severity of EAE |
| Mast cell-deficient mice develop a less severe EAE | ||
| Stroke | Hypoxic-ischemic brain damage | Rapid increase in cerebral mast cell number and activation |
| Significant neuroprotection by inhibition of the early mast cell response | ||
| Mast cell-degranulating increased the ischemic brain edema | ||
| AD | Fibrillar amyloid beta peptides trigger concentration-dependent exocytosis and histamine secretion from mast cells | |
| Tryptase-containing mast cells have been infiltrated in the brains of AD pateints | ||
| Masitinib slowed the cognitive decline in AD patients in phase II clinical trial |
MS
Large numbers of mast cells are found at sites of inflammatory demyelination in the brain and spinal cord of MS patients [58,59], and mast cell tryptase is elevated in their cerebrospinal fluid [60]. Experimental autoimmune encephalomyelitis (EAE) is a widely studied rodent model of MS. As in MS, significant of destruction of myelin and oligodendrocytes, the myelin-producing cells, occurs due to T cell-mediated autoimmune response against proteins in the myelin sheath following an influx of immune cells into the CNS through the BBB [61]. Rodents with EAE exhibit increased degranulation of their brain mast cells [62] and elevated levels of the demyelinating mast cell protease [63]. Perivascular mast cells are among the earliest participants in MS, which produce TNF and other cytokines, break down the BBB, and elicit an early influx of neutrophils into myelin [64]. The proinflammatory mediators released from mast cells also aggravate the development of MS. Treatment is with the mast cell stabilizer picroximil, the H1 receptor antagonist hydroxyzine, or intracisternal administration of c48/80 [32]. The most direct evidence for mast cell action in EAE comes from studies utilizing W/Wv mice in a model of primary progressive (PP) MS [65]. This model disease is induced by immunization with MOG35–55 in CFA along with pertussis toxin. Mast cell deficiency leads to significantly less clinical disease that is associated with loss of BBB permeability and inflammatory cell infiltration into the parenchyma of the CNS [65,66].
Cerebral ischemia
The function of mast cells in ischemic brain injury is well documented and amply reviewed in both the adult and the immature brain [57]. In brief, the inflammatory response triggered by stroke is associated with the influx of many inflammatory cells, including leukocytes, neutrophils, and macrophages [67]. Mast cells differ from other hematopoietic cells is that they are resident in the brain and meninges and are first responders, even before microglia [68]. They can act rapidly on the cerebral vessels and other CNS compartments during the very earliest phase of acute cerebral ischemia and hemorrhage by releasing their preformed cytoplasmic granules containing a host of readily available vasoactive and neuroactive mediators. Mast cells act on the basal membrane, promoting BBB damage, brain edema, prolonged extravasation, and hemorrhage. Indeed, mast cell stabilization inhibits hypoxic ischemic-induced brain damage [69]. Furthermore, following even acute ischemic brain injury, cerebral mast cells amplify and prolong the endothelial expression of adhesion molecules and the continued breakdown of the BBB, thereby enabling the infiltration of other blood-borne cells and signals [57,70].
AD
The role of mast cells in AD remains elusive, but there have been studies suggesting the linkage between mast cells and AD. In this respect, fibrillar amyloid beta peptides trigger concentration-dependent exocytosis and histamine secretion from mast cells [71]. It was demonstrated that tryptase-containing mast cells have been infiltrated in the brains of AD patients, and these mast cells are colocalized with amyloid plaques [72]. Recently, a study showed that administration of masitinib mesylate, a selective tyrosine kinase inhibitor efficient in controlling the survival, differentiation, and degranulation of mast cells, slowed the cognitive decline in AD patients in a phase II clinical trial [73].
Conclusions
Neuroinflammatory disorders are conditions in which components of the nervous system are damaged by inflammatory effectors derived from the innate and adaptive immune systems, as well as glia within the CNS. Microglia, in particular, act as sensor cells for disturbed brain tissue homeostasis and accumulate locally in response to neuron injury [74]. Few studies until now have focused on resident cell types capable of mounting immediate host responses in the brain. Mast cells are effector cells of the innate immune system, and represent the ‘first responders’ to injury rather than microglia. Potent preformed mediators of mast cell release are very rapid, while de novo-formed mediators are slower in activating [75].
The puzzling differences in the findings between distinct mast cell-deficient mouse models, as well as those between different laboratories, indicate that the role of mast cells in inflammatory CNS diseases is far from clear. Systematic analyses should include strictly controlled parameters such as sex and age of the animals, different immunization protocols, and EAE type (ie, onset and disease course). The development of new mast cell-deficient mouse models [76,77] and comparison of those with existing models such as KitW-sh/W-sh mice and KitW/W-v mice will help to unravel the true mast cell functions and distinguish them from other mechanisms resulting from mast cell-independent c-kit effects or CRE toxicity. A key role of mast cells in neuroinflammation can be expected from the known pro- and anti-inflammatory activities in the periphery. However, because of the contradictory results from experiments in rodents, very little is actually known about the exact role of mast cells in neuroinflammation.
Footnotes
Conflict of interests
The authors declare that they have no competing interests.
Source of support: This project was sponsored by the National Natural Science Foundation of China (No. 81270429) and a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD)
References
- 1.Graeber MB, Li W, Rodriguez ML. Role of microglia in CNS inflammation. Febs Lett. 2011;585:3798–805. doi: 10.1016/j.febslet.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 2.Ownby RL. Neuroinflammation and cognitive aging. Curr Psychiatry Rep. 2010;12:39–45. doi: 10.1007/s11920-009-0082-1. [DOI] [PubMed] [Google Scholar]
- 3.Pimplikar SW. Neuroinflammation in Alzheimer’s disease: from pathogenesis to a therapeutic target. J Clin Immunol. 2014;34(Suppl 1):S64–69. doi: 10.1007/s10875-014-0032-5. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S210–12. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- 5.Moller T. Neuroinflammation in Huntington’s disease. J Neural Transm. 2010;117:1001–8. doi: 10.1007/s00702-010-0430-7. [DOI] [PubMed] [Google Scholar]
- 6.Frohman EM, Racke MK, Raine CS. Multiple sclerosis – the plaque and its pathogenesis. N Engl J Med. 2006;354:942–55. doi: 10.1056/NEJMra052130. [DOI] [PubMed] [Google Scholar]
- 7.Nilupul PM, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi: 10.1016/j.jocn.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 8.De Chiara G, Marcocci ME, Sgarbanti R, et al. Infectious agents and neurodegeneration. Mol Neurobiol. 2012;46:614–38. doi: 10.1007/s12035-012-8320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilhardt F. Microglia: phagocyte and glia cell. Int J Biochem Cell Biol. 2005;37:17–21. doi: 10.1016/j.biocel.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Gendelman HE. Neural immunity: Friend or foe? J Neurovirol. 2002;8:474–79. doi: 10.1080/13550280290168631. [DOI] [PubMed] [Google Scholar]
- 11.Gui B, Su M, Chen J, et al. Neuroprotective effects of pretreatment with propofol in LPS-induced BV-2 microglia cells: role of TLR4 and GSK-3beta. Inflammation. 2012;35:1632–40. doi: 10.1007/s10753-012-9478-x. [DOI] [PubMed] [Google Scholar]
- 12.Peng M, Ye JS, Wang YL, et al. Posttreatment with propofol attenuates lipopolysaccharide-induced up-regulation of inflammatory molecules in primary microglia. Inflamm Res. 2014;63:411–18. doi: 10.1007/s00011-014-0713-9. [DOI] [PubMed] [Google Scholar]
- 13.Shi SS, Yang WZ, Chen Y, et al. Propofol reduces inflammatory reaction and ischemic brain damage in cerebral ischemia in rats. Neurochem Res. 2014;39:793–99. doi: 10.1007/s11064-014-1272-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu HG, Hua Z, Zhang Y, et al. Effect of Sevoflurane postconditioning on gene expression in brain tissue of the middle cerebral artery occlusion rat model. Mol Biol Rep. 2012;39:10505–13. doi: 10.1007/s11033-012-1935-y. [DOI] [PubMed] [Google Scholar]
- 15.Beaven MA. Our perception of the mast cell from Paul Ehrlich to now. Eur J Immunol. 2009;39:11–25. doi: 10.1002/eji.200838899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2–12. doi: 10.1007/978-1-4419-9533-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. Faseb J. 2012;26:3103–17. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- 18.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125:S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilfillan AM, Austin SJ, Metcalfe DD. Mast cell biology: introduction and overview. Adv Exp Med Biol. 2011;716:2–12. doi: 10.1007/978-1-4419-9533-9_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson D, Krenger W. Interactions of mast cells with the nervous system – recent advances. Neurochem Res. 1992;17:939–51. doi: 10.1007/BF00993271. [DOI] [PubMed] [Google Scholar]
- 21.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–42. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 22.Gauchat JF, Henchoz S, Mazzei G, et al. Induction of human IgE synthesis in B cells by mast cells and basophils. Nature. 1993;365:340–43. doi: 10.1038/365340a0. [DOI] [PubMed] [Google Scholar]
- 23.Wardlaw AJ, Moqbel R, Cromwell O, Kay AB. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986;78:1701–06. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry VH, Andersson PB, Gordon S. Macrophages and inflammation in the central nervous system. Trends Neurosci. 1993;16:268–73. doi: 10.1016/0166-2236(93)90180-t. [DOI] [PubMed] [Google Scholar]
- 25.Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J Comp Neurol. 2000;424:651–69. [PubMed] [Google Scholar]
- 26.Khalil M, Ronda J, Weintraub M, et al. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007;1171:18–29. doi: 10.1016/j.brainres.2007.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver R, Curley JP. Mast cells on the mind: new insights and opportunities. Trends Neurosci. 2013;36:513–21. doi: 10.1016/j.tins.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J Neurosci. 2000;20:401–8. doi: 10.1523/JNEUROSCI.20-01-00401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silver R, Silverman AJ, Vitkovic L, Lederhendler II. Mast cells in the brain: evidence and functional significance. Trends Neurosci. 1996;19:25–31. doi: 10.1016/0166-2236(96)81863-7. [DOI] [PubMed] [Google Scholar]
- 30.Silverman AJ, Asarian L, Khalil M, Silver R. GnRH, brain mast cells and behavior. Prog Brain Res. 2002;141:315–25. doi: 10.1016/S0079-6123(02)41102-8. [DOI] [PubMed] [Google Scholar]
- 31.Theoharides TC, Donelan J, Kandere-Grzybowska K, Konstantinidou A. The role of mast cells in migraine pathophysiology. Brain Res Brain Res Rev. 2005;49:65–76. doi: 10.1016/j.brainresrev.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Brown MA, Hatfield JK. Mast Cells are Important Modifiers of Autoimmune Disease: With so Much Evidence, Why is There Still Controversy? Front Immunol. 2012;3:147. doi: 10.3389/fimmu.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelissen S, Lemmens E, Geurts N, et al. The role of mast cells in neuroinflammation. Acta Neuropathol. 2013;125:637–50. doi: 10.1007/s00401-013-1092-y. [DOI] [PubMed] [Google Scholar]
- 35.Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40:3107–12. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- 36.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–52. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 37.Del ZG. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–82. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, Estrada EY, Thompson JF, et al. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab. 2007;27:697–709. doi: 10.1038/sj.jcbfm.9600375. [DOI] [PubMed] [Google Scholar]
- 39.Mattila OS, Strbian D, Saksi J, et al. Cerebral mast cells mediate blood-brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke. 2011;42:3600–5. doi: 10.1161/STROKEAHA.111.632224. [DOI] [PubMed] [Google Scholar]
- 40.Goldschmidt RC, Hough LB, Glick SD. Rat brain mast cells: contribution to brain histamine levels. J Neurochem. 1985;44:1943–47. doi: 10.1111/j.1471-4159.1985.tb07191.x. [DOI] [PubMed] [Google Scholar]
- 41.Dux E, Joo F. Effects of histamine on brain capillaries. Fine structural and immunohistochemical studies after intracarotid infusion. Exp Brain Res. 1982;47:252–58. doi: 10.1007/BF00239384. [DOI] [PubMed] [Google Scholar]
- 42.Gross PM, Teasdale GM, Graham DI, et al. Intra-arterial histamine increases blood-brain transport in rats. Am J Physiol. 1982;243:H307–17. doi: 10.1152/ajpheart.1982.243.2.H307. [DOI] [PubMed] [Google Scholar]
- 43.Corvera CU, Dery O, McConalogue K, et al. Thrombin and mast cell tryptase regulate guinea-pig myenteric neurons through proteinase-activated receptors-1 and -2. J Physiol. 1999;517:741–56. doi: 10.1111/j.1469-7793.1999.0741s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forsythe P, Bienenstock J. The mast cell-nerve functional unit: a key component of physiologic and pathophysiologic responses. Chem Immunol Allergy. 2012;98:196–221. doi: 10.1159/000336523. [DOI] [PubMed] [Google Scholar]
- 45.Reed DE, Barajas-Lopez C, Cottrell G, et al. Mast cell tryptase and proteinase-activated receptor 2 induce hyperexcitability of guinea-pig submucosal neurons. J Physiol. 2003;547:531–42. doi: 10.1113/jphysiol.2002.032011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Steinhoff M, Vergnolle N, Young SH, et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat Med. 2000;6:151–58. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- 47.Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. Faseb J. 2012;26:3103–17. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- 48.Zhang S, Zeng X, Yang H, et al. Mast cell tryptase induces microglia activation via protease-activated receptor 2 signaling. Cell Physiol Biochem. 2012;29:931–40. doi: 10.1159/000171029. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Yang H, He S. TNF increases expression of IL-4 and PARs in mast cells. Cell Physiol Biochem. 2010;26:327–36. doi: 10.1159/000320556. [DOI] [PubMed] [Google Scholar]
- 50.Yuan H, Zhu X, Zhou S, et al. Role of mast cell activation in inducing microglial cells to release neurotrophin. J Neurosci Res. 2010;88:1348–54. doi: 10.1002/jnr.22304. [DOI] [PubMed] [Google Scholar]
- 51.Dong H, Zhang W, Zeng X, et al. Histamine induces upregulated expression of histamine receptors and increases release of inflammatory mediators from microglia. Mol Neurobiol. 2014;49:1487–500. doi: 10.1007/s12035-014-8697-6. [DOI] [PubMed] [Google Scholar]
- 52.Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol. 2010;185:273–83. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- 53.Kim DY, Hong GU, Ro JY. Signal pathways in astrocytes activated by cross-talk between of astrocytes and mast cells through CD40-CD40L. J Neuroinflammation. 2011;8:25. doi: 10.1186/1742-2094-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosli L, Hosli E, Schneider U, Wiget W. Evidence for the existence of histamine H1- and H2-receptors on astrocytes of cultured rat central nervous system. Neurosci Lett. 1984;48:287–91. doi: 10.1016/0304-3940(84)90052-1. [DOI] [PubMed] [Google Scholar]
- 55.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–90. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 56.Walker ME, Hatfield JK, Brown MA. New insights into the role of mast cells in autoimmunity: evidence for a common mechanism of action? Biochim Biophys Acta. 2012;1822:57–65. doi: 10.1016/j.bbadis.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindsberg PJ, Strbian D, Karjalainen-Lindsberg ML. Mast cells as early responders in the regulation of acute blood-brain barrier changes after cerebral ischemia and hemorrhage. J Cereb Blood Flow Metab. 2010;30:689–702. doi: 10.1038/jcbfm.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bebo BJ, Yong T, Orr EL, Linthicum DS. Hypothesis: a possible role for mast cells and their inflammatory mediators in the pathogenesis of autoimmune encephalomyelitis. J Neurosci Res. 1996;45:340–48. doi: 10.1002/(SICI)1097-4547(19960815)45:4<340::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 59.Ibrahim MZ, Reder AT, Lawand R, et al. The mast cells of the multiple sclerosis brain. J Neuroimmunol. 1996;70:131–38. doi: 10.1016/s0165-5728(96)00102-6. [DOI] [PubMed] [Google Scholar]
- 60.Rozniecki JJ, Hauser SL, Stein M, et al. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann Neurol. 1995;37:63–66. doi: 10.1002/ana.410370112. [DOI] [PubMed] [Google Scholar]
- 61.Rao P, Segal BM. Experimental autoimmune encephalomyelitis. Methods Mol Biol. 2012;900:363–80. doi: 10.1007/978-1-60761-720-4_18. [DOI] [PubMed] [Google Scholar]
- 62.Kim DY, Jeoung D, Ro JY. Signaling pathways in the activation of mast cells cocultured with astrocytes and colocalization of both cells in experimental allergic encephalomyelitis. J Immunol. 2010;185:273–83. doi: 10.4049/jimmunol.1000991. [DOI] [PubMed] [Google Scholar]
- 63.Rouleau A, Dimitriadou V, Trung TM, et al. Mast cell specific proteases in rat brain: changes in rats with experimental allergic encephalomyelitis. J Neural Transm. 1997;104:399–417. doi: 10.1007/BF01277659. [DOI] [PubMed] [Google Scholar]
- 64.Christy AL, Walker ME, Hessner MJ, Brown MA. Mast cell activation and neutrophil recruitment promotes early and robust inflammation in the meninges in EAE. J Autoimmun. 2013;42:50–61. doi: 10.1016/j.jaut.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 65.Secor VH, Secor WE, Gutekunst CA, Brown MA. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J Exp Med. 2000;191:813–22. doi: 10.1084/jem.191.5.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sayed BA, Christy AL, Walker ME, Brown MA. Meningeal mast cells affect early T cell central nervous system infiltration and blood-brain barrier integrity through TNF: a role for neutrophil recruitment? J Immunol. 2010;184:6891–900. doi: 10.4049/jimmunol.1000126. [DOI] [PubMed] [Google Scholar]
- 67.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jin Y, Silverman AJ, Vannucci SJ. Mast cells are early responders after hypoxia-ischemia in immature rat brain. Stroke. 2009;40:3107–12. doi: 10.1161/STROKEAHA.109.549691. [DOI] [PubMed] [Google Scholar]
- 69.Jin Y, Silverman AJ, Vannucci SJ. Mast cell stabilization limits hypoxic-ischemic brain damage in the immature rat. Dev Neurosci. 2007;29:373–84. doi: 10.1159/000105478. [DOI] [PubMed] [Google Scholar]
- 70.Mattila OS, Strbian D, Saksi J, et al. Cerebral mast cells mediate blood-brain barrier disruption in acute experimental ischemic stroke through perivascular gelatinase activation. Stroke. 2011;42:3600–5. doi: 10.1161/STROKEAHA.111.632224. [DOI] [PubMed] [Google Scholar]
- 71.Niederhoffer N, Levy R, Sick E, et al. Amyloid beta peptides trigger CD47-dependent mast cell secretory and phagocytic responses. Int J Immunopathol Pharmacol. 2009;22:473–83. doi: 10.1177/039463200902200224. [DOI] [PubMed] [Google Scholar]
- 72.Maslinska D, Laure-Kamionowska M, Maslinski KT, et al. Distribution of tryptase-containing mast cells and metallothionein reactive astrocytes in human brains with amyloid deposits. Inflamm Res. 2007;56:S17–18. doi: 10.1007/s00011-006-0508-8. [DOI] [PubMed] [Google Scholar]
- 73.Piette F, Belmin J, Vincent H, et al. Masitinib as an adjunct therapy for mild-to-moderate Alzheimer’s disease: a randomised, placebo-controlled phase 2 trial. Alzheimers Res Ther. 2011;3:16. doi: 10.1186/alzrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 2011;12:388–99. doi: 10.1038/nrn3053. [DOI] [PubMed] [Google Scholar]
- 75.Metcalfe DD. Mast cells and mastocytosis. Blood. 2008;112:946–56. doi: 10.1182/blood-2007-11-078097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feyerabend TB, Weiser A, Tietz A, et al. Cre-mediated cell ablation contests mast cell contribution in models of antibody- and T cell-mediated autoimmunity. Immunity. 2011;35:832–44. doi: 10.1016/j.immuni.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 77.Sharma N, Kumar V, Everingham S, et al. SH2 domain-containing phosphatase 2 is a critical regulator of connective tissue mast cell survival and homeostasis in mice. Mol Cell Biol. 2012;32:2653–63. doi: 10.1128/MCB.00308-12. [DOI] [PMC free article] [PubMed] [Google Scholar]