Abstract
Context:
Vemurafenib, a selective BRAF inhibitor, appears to have promising clinical activity in patients with papillary thyroid cancer (PTC) harboring the BRAFV600E mutation.
Objective:
To determine the efficacy and safety of vemurafenib when used outside of a clinical trial.
Design:
A retrospective review at MD Anderson Cancer Center.
Methods:
The best responses were evaluated using RECIST v1.1. A single radiologist reviewed all images. Adverse events (AEs) were evaluated using CTCAE v.4.0.
Results:
We identified 17 patients with advanced PTC harboring the BRAFV600E mutation who were treated with vemurafenib outside of a clinical trial. Median age at diagnosis was 63 years, and 53% were male. At vemurafenib start, 3 (18%) patients had disease confined to the neck, and 14 (72%) had distant metastases. Tyrosine kinase inhibitors had been previously administered to 4 (24%) patients. Two (12%) patients discontinued vemurafenib because of AEs before restaging. Best response: partial response (PR) in 7/15 (47%) and stable disease (SD) in 8/15(53%) patients. The rate of durable response (PR plus SD ≥ 6 months) was 67%. Median time to treatment failure was 13 months. There was no association between change in thyroglobulin and tumor size. Drug discontinuation, drug interruptions, and dose reductions were needed in 5 (29%), 13 (76%), and 10 (59%) patients, respectively. Most common AEs were fatigue (71%), weight loss (71%), anorexia (65%), arthralgias (59%), hair loss (59%), rash (59%), hand-foot syndrome (53%), calluses (47%), diarrhea (47%), fever (41%), dry mouth (35%), nausea (35%), and verrucous keratosis (35%). Grade ≥ 3 AEs were present in 8 (47%) patients.
Conclusions:
Vemurafenib is a potentially effective and well-tolerated treatment strategy in patients with advanced PTC harboring the BRAFV600E mutation. Our results are similar to those reported in a phase II clinical trial and support the potential role of vemurafenib in this patient population.
Patients with differentiated thyroid cancer who develop metastatic, radioactive iodine (RAI)-refractory, progressive disease have a poor prognosis (1). Sorafenib is the only approved targeted agent for these patients, however, there are other emerging interventions.
The BRAFV600E mutation is the most common genetic alteration in papillary thyroid cancer (PTC) and is the most potent activator of the MAPK pathway, which plays a key role in thyroid carcinogenesis. Its presence correlates with aggressive tumor characteristics (2, 3). It is also associated with decreased ability of tumors to take up RAI (4), which is the only known cure for distant metastatic disease.
BRAF kinase inhibition has been of interest for advanced PTC treatment because of the BRAF mutation's oncogenic role in this disease. The response to sorafenib, a weak BRAF inhibitor, and VEGFR inhibitor, has been described previously. The phase 3 trial showed that sorafenib significantly improved progression free survival (PFS) over that of placebo (10.8 months with sorafenib vs 5.8 months with placebo) and patients benefited from sorafenib independent of BRAF mutation status (5). PR rates in the sorafenib and placebo arms were 12.2% and 0.5% and rates of stable disease (SD) ≥ 6 months were 42% and 33%, respectively. The selective, potent BRAF inhibitor, dabrafenib, has also shown clinical activity. The phase 1 study included thyroid cancer patients, most of which had tumor shrinkage (6).
Vemurafenib, another selective, potent BRAF inhibitor, is approved for adult patients with BRAFV600E mutated, unresectable or metastatic melanoma. A phase 1 study of vemurafenib yielded encouraging results in 3 patients with metastatic PTC harboring the BRAFV600E mutation (7). On the basis of these results, a phase 2 trial of vemurafenib was performed in patients with progressive metastatic, RAI-refractory BRAFV600E-positive PTC (8).
Before sorafenib's approval, for those patients who could not participate in a clinical trial, a common approach was to offer “off-label” treatment with commercially available tyrosine kinase inhibitors (TKIs) as per the American Thyroid Association and National Comprehensive Cancer Network (9, 10). This study reviews the use of vemurafenib in patients with metastatic, progressive, RAI-refractory, BRAFV600E mutation-positive PTC who were treated outside of a clinical trial.
Materials and Methods
Study population
Under an Institutional Review Board-approved protocol, we retrospectively collected data on adult patients with BRAFV600E mutated PTC who received vemurafenib outside of a clinical trial at The University of Texas MD Anderson Cancer Center (MDA) from August 2012 until November 2013. Assessment of the BRAFV600E mutation was determined by the Molecular Diagnostic Laboratory at MDA, a CLIA-compliant and accredited laboratory.
Evaluations and definitions
A single radiologist reviewed all cross-sectional images prior and during treatment with vemurafenib. The response was defined using Response Evaluation Criteria in Solid Tumors Version 1.1 [RECIST v1.1 (11, 12)]. PFS was defined as the time elapsed between treatment initiation and tumor progression, as determined by objective tumor measurements in evaluable patients. Time to failure (TTF) was defined as the time from the start of treatment until disease progression or unacceptable toxicity leading to drug discontinuation. Adverse events (AEs) were evaluated using Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v.4.0). Each visit included restaging images, laboratory, and assessment of AEs using specific guidelines designed by our institution (13).
Statistical analysis
Descriptive statistics were used to summarize patient characteristics and AEs. The best responses were plotted graphically using a waterfall plot. Linear mixed effects models were used to assess the effect of thyroglobulin (Tg) on tumor size. P values were compared with a significance level of 0.05. Statistical analyses were performed using SPSS version 17 (IBM).
Results
Study population
We identified 17 patients who met our inclusion criteria. Median age at diagnosis was 63 years, and 53% of patients were men. Most patients (65%) had conventional type PTC. Fifteen (88%) patients had undergone primary thyroid surgery and two (12%) patients started vemurafenib for locally advanced, unresectable disease. At the initiation of vemurafenib, 3 (18%) patients had localized disease confined to the neck and 14 (82%) patients had evidence of distant metastases. The most common sites of distant metastases included the lung in 11 (65%) and bone in 6 (35%) patients followed by mediastinal and hilar adenopathy (18%), pleura (12%), liver (6%), and subcutaneous tissue (6%). Four (24%) patients were previously treated with TKIs, which included sorafenib alone in 3 patients and multiple TKIs (sorafenib, sunitinib, and pazopanib) in 1 patient. The choice of vemurafenib was based on multiple factors, including ineligibility or unavailability of a clinical trial, concerns about use of antiangiogenic TKIs [eg, diverticulitis, bleeding, or high risk for fistula formation in patients with invasive tracheal or esophageal disease and a history of prior radiotherapy to the neck (14)]. Vemurafenib was administered orally at a starting dose of 960 mg twice daily (13 patients), 720 mg twice daily (1), 480 mg twice daily (2), and 240 mg twice daily (1). Patients were evaluated at frequent time intervals ranging from 1 to 5 months, mostly at around 3-month intervals.
Efficacy
Fifteen patients were evaluable for best response as two patients discontinued vemurafenib due to toxicity that occurred before restaging. Thirteen of the 15 patients had disease progression according to RECIST criteria within 12 months of starting vemurafenib (20–230% increase). Two patients (#9 and #11 on the waterfall plot) had advanced neck disease in a life threatening location or threatening the vocal cord and the disease was deemed inoperable without tracheostomy, which both patients declined.
Best response rates with vemurafenib were PR in 7/15 (47%) patients and SD in 8/15 (53%) patients (Figure 1A). Five of the 7 patients had a confirmed PR and 5/8 patients had SD ≥ 6 months. The durable response rate (PR plus SD ≥ 6 months) was 67%. Neither progressive disease nor complete responses were observed as a best response. All 4 patients whose disease progressed while on previous antiangiogenic TKIs responded to vemurafenib (2 confirmed PR, 2 SD ≥ 6 months). The median time from start of treatment to best response was 3 months (range: 0.25–10 months; Figure 1B).
Figure 1.
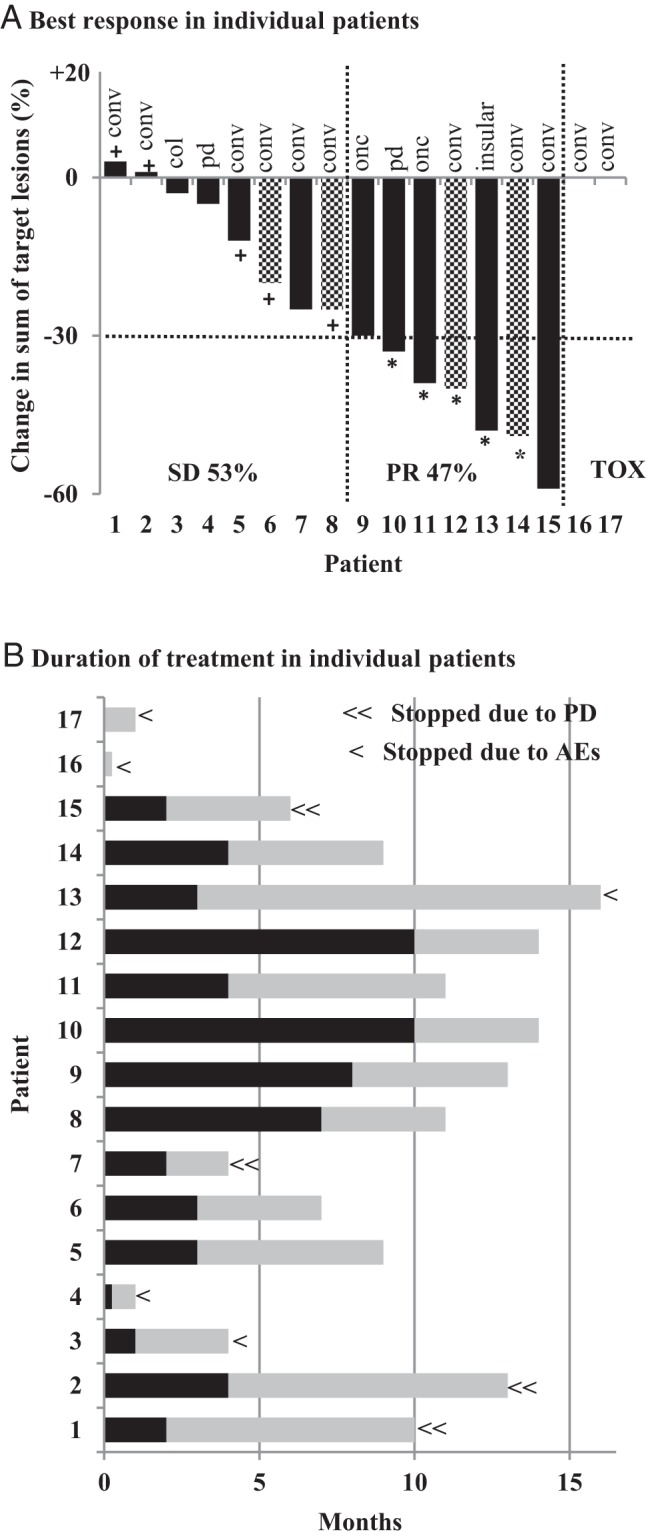
Patients (n = 17) are in the same order on the two graphs. (A) Best response in individual patients. The percent change from baseline in the sum of the diameters of the target lesions is shown on the y-axis. Black solid bars indicate no prior systemic therapy, and pattern bars indicate prior TKIs. Partial response (PR) in 7/15 (47%) evaluable patients, SD in 8/15 (53%) patients, and discontinuation due to toxicity prior to the first restaging in 2 patients. Five of 7 patients had confirmed PR (*) and 5/8 patients had SD ≥ 6 months (+). conv = papillary thyroid cancer - conventional type; pd = poorly differentiated; onc = oncocytic; col = columnar. (B) Duration of treatment in individual patients. Each bar represents the duration of treatment with vemurafenib for an individual patient. The time from vemurafenib initiation to the date of best response is represented in black and the time from best response to discontinuation or last follow-up is represented in gray. Four patients discontinued treatment due to progressive disease (PD) (≪) and 5 due to adverse events (AEs) (<). Treatment is ongoing in 8 patients.
At the time of this analysis, treatment is ongoing in 8 patients, who have been on treatment for a median of 11 months (range: 7–14). In addition to the 4 patients who discontinued vemurafenib because of disease progression after 4, 6, 10, and 13 months of treatment, 5 patients discontinued treatment due to toxicity. During the study period, the appearance of new lung metastases and bone metastases was noted in 1 patient, respectively. For the entire cohort, median, TTF was 13 months (95% CI = 10–15.9). Median PFS was not reached at the time of this analysis (Supplemental Figure 1).
Correlation of log Tg with tumor measurements
In 10 patients included in this analysis, there was no association between log (Tg) and change in tumor size (P = .22). Three patients had evidence of tumor size reduction despite continuous increase in Tg.
Safety and tolerability
AEs and their grades and frequencies are described in Table 1. Drug discontinuation owing to toxicity was needed in 5 (29%) patients. The reasons for discontinuation were skin lesions in 3 patients (generalized skin rash with blistering, generalized pruritic rash, and numerous squamous cell carcinomas which were intolerable to the patient), fatigue in 1 patient, and atrial fibrillation (AF) in 1 patient. Treatment interruptions and dose reductions were necessary in 13 (76%) and 10 (59%) patients, respectively.
Table 1.
Adverse Events by CTCAE Version 4 Classification in 17 Patients Treated with Vemurafenib
| Adverse event | All grades N (%) | Grade 1 N (%) | Grade 2 N (%) | Grade 3 N (%) | Grade 4 N (%) |
|---|---|---|---|---|---|
| Overall (at least one AE) | 17 (100) | 15 (88) | 16 (94) | 7 (41) | 1 (6) |
| Fatigue | 12 (71) | 9 (53) | 2 (12) | 1 (6) | - |
| Weight loss | 12 (71) | 8 (47) | 4 (24) | - | - |
| Anorexia | 11 (65) | 5 (29) | 6 (35) | - | - |
| Arthralgias | 10 (59) | 5 (29) | 5 (29) | - | - |
| Hair loss | 10 (59) | 9 (53) | 1 (6) | - | - |
| Rash | 10 (59) | 5 (29) | 5 (29) | - | - |
| Hand-foot syndrome | 9 (53) | 5 (29) | 3 (18) | 1 (6) | - |
| Calluses | 8 (47) | 8 (47) | - | - | - |
| Diarrhea | 8 (47) | 6 (35) | 2 (12) | - | - |
| Fever | 7 (41) | 7 (41) | - | - | - |
| Dry mouth | 6 (35) | 4 (24) | 2 (12) | - | - |
| Nausea | 6 (35) | 5 (29) | 1 (6) | - | - |
| Verrucous keratosis | 6 (35) | - | 6 (35) | - | - |
| Photosensitivity | 4 (24) | 1 (6) | 2 (12) | 1 (6) | - |
| Mucositis | 3 (18) | 2 (12) | 1 (6) | - | - |
| Weakness | 3 (18) | 2 (12) | 1 (6) | - | - |
| Squamous cell skin carcinoma | 3 (18) | - | - | 3 (18) | - |
| Blurry vision | 2 (12) | 2 (12) | - | - | - |
| Dysgeusia | 2 (12) | - | 2 (12) | - | - |
| Hoarseness | 2 (12) | 2 (12) | - | - | - |
| HTN | 2 (12) | 1 (6) | 1 (6) | - | - |
| Vomiting | 2 (12) | 2 (12) | - | - | - |
| Constipation | 1 (6) | 1 (6) | - | - | - |
| Headache | 1 (6) | 1 (6) | - | - | - |
| Nail changes | 1 (6) | 1 (6) | - | - | - |
| Palmar warts | 1 (6) | 1 (6) | - | - | - |
| QT prolongation | 1 (6) | 1 (6) | - | - | - |
| Melanocytic nevi | 1 (6) | - | 1 (6) | - | - |
| Atrial fibrillation | 1 (6) | - | 1 (6) | - | - |
| Gum lesion | 1 (6) | - | 1 (6) | - | - |
| Uveitis | 1 (6) | - | - | 1 (6) | - |
| Elevated serum lipase | 1 (6) | - | - | - | 1 (6) |
Abbreviations: HTN, hypertension; -, not observed.
Discussion
This is the first report to describe the efficacy and tolerability of vemurafenib used outside a clinical trial for BRAFV600E mutated PTC. The best response to vemurafenib was PR in 47% and SD in 53%, and the rate of durable response (PR plus SD ≥ 6 months) was 67%. Most patients had tumor shrinkage ranging from 3% to 58%. The median TTF was 13 months. Despite the fact that our patients were treated outside of a clinical trial, the results are consistent with the preliminary results of the phase 2 trial of vemurafenib in patients with PTC (8). In that trial, in TKI naive patients (n = 26), the PR rate was 35%, the rate of durable response was 58%, and median PFS was 15.6 months. The cohort previously treated with antiangiogenic TKIs (n = 25) had a PR rate of 29%, a durable response rate of 38%, and median PFS was 6.3 months. Our study also included 4 patients treated previously with antiangiogenic TKIs and we also found that those patients responded to vemurafenib even after prior therapies had failed. A prospective head-to-head comparison between sorafenib (the only approved targeted drug) and vemurafenib has not been performed to date. Preliminary results comparing sorafenib and vemurafenib in patients with BRAFV600E positive PTC showed a similar benefit in terms of PFS prolongation, but a larger tumor size reduction in vemurafenib treated patients (15). Patients who failed first line sorafenib could benefit from vemurafenib salvage therapy (8, 16).
In our study, 3 patients had evidence of advanced disease localized to the neck, invading into the trachea and/or esophagus, and 2 of those patients had not undergone primary surgery. All 3 patients had evidence of tumor shrinkage. This may represent a valuable approach for patients with aggressive locoregional disease. A clinical trial to assess the efficacy of vemurafenib as neoadjuvant therapy is ongoing (NCT 01709292).
In our study, there was no evidence of an association between change in Tg and tumor size. The number of patients in our study is small, but interpretation of an increase in the serum Tg level in patients treated with vemurafenib requires caution, as it may not indicate disease progression. This may represent tumor redifferentiation and increased RAI uptake. This is an important consideration that warrants further investigation, as RAI is the only known cure for metastatic disease. In human studies using the BRAF inhibitor, dabrafenib or MEK inhibitor, selumetinib, increased RAI uptake in a subset of thyroid cancers (17, 18).
Although vemurafenib has shown encouraging response rates, one must take into account the toxicity profile of the drug, understanding that thyroid cancer patients are also likely to require treatment for a prolonged period of time and that some toxicities may not become apparent over the short term. The results of the present study are similar to those reported in the vemurafenib phase 2 trial in PTC, in which the rates of AEs requiring drug discontinuation, drug interruption, and dose reductions were 23%, 73%, and 23%, respectively. Also, the most common AEs were very similar to those previously reported in studies of patients with advanced melanoma (19). As noted in our study and also in a previous reported experience in melanoma, vemurafenib is associated with numerous skin reactions (19). Cutaneous squamous cell carcinoma was present in 3 (18%) patients, and this rate was similar to those seen in melanoma patients (18%) (19). Patient education and implementation of preventive measures, thorough evaluations for toxicity and immediate measures should be performed to minimize treatment interruption or discontinuation (Supplemental Table).
This study has several limitations related to its retrospective design, small sample size, different intervals between visits and short follow-up time.
In summary, our results indicate that vemurafenib is a promising treatment that should be further evaluated in patients with PTC harboring the BRAFV600E mutation. Further studies are required to better understand long-term clinical outcomes, mechanisms of tumor resistance to selective BRAF kinase inhibition, and the safety of chronic medical therapy in this population of patients.
Acknowledgments
This work was supported by the NIH/NCI under Award No. P30CA016672.
Disclosure Summary: R.D., K.S., S.G.W., M.I.H., C.J., M.A.H., A.K.Y., R.B. have nothing to disclose. N.L.B. has received research funding from Bayer/Onyx. S.I.S. is a consultant to Bayer, Eisai, and Exelixis. M.E.C. has received research funding from Roche, Eisai, and Exelixis and has served on advisory boards for Eisai and Exelixis.
Footnotes
- AEs
- adverse events
- AF
- atrial fibrillation
- PFS
- progression free survival
- PTC
- papillary thyroid cancer
- RAI
- radioactive iodine
- SD
- stable disease
- TKIs
- tyrosine kinase inhibitors
- Tg
- thyroglobulin.
References
- 1. Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–2899. [DOI] [PubMed] [Google Scholar]
- 2. Elisei R, Ugolini C, Viola D, et al. Braf(v600e) mutation and outcome of patients with papillary thyroid carcinoma: A 15-year median follow-up study. J Clin Endocrinol Metab. 2008;93:3943–3949. [DOI] [PubMed] [Google Scholar]
- 3. Xing M. Braf mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–262. [DOI] [PubMed] [Google Scholar]
- 4. Durante C, Puxeddu E, Ferretti E, et al. Braf mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J Clin Endocrinol Metab. 2007;92:2840–2843. [DOI] [PubMed] [Google Scholar]
- 5. Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Falchook GS, Long GV, Kurzrock R, Kim KB, Arkenau TH, Brown MP, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet. 2012;379:1893–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim KB, Cabanillas ME, Lazar AJ, et al. Clinical responses to vemurafenib in patients with metastatic papillary thyroid cancer harboring BRAF(V600E) mutation. Thyroid. 2013;23:1277–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brose MS, Cabanillas ME, Cohen EE, et al. An open-label, multi-center phase 2 study of the BRAF inhibitor vemurafenib in patients with metastatic or unresectable papillary thyroid cancer positive for the BRAF v600 mutation and resistant to radioactive iodine. Proceedings of the European Cancer Congress, Amsterdam, 2013 2013;oral abstr 28. [Google Scholar]
- 9. Tuttle RM, Ball DW, Byrd D, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2010;8:1228–1274. [DOI] [PubMed] [Google Scholar]
- 10. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 11. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. [DOI] [PubMed] [Google Scholar]
- 12. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur J Cancer. 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 13. Carhill AA, Cabanillas ME, Jimenez C, et al. The noninvestigational use of tyrosine kinase inhibitors in thyroid cancer: Establishing a standard for patient safety and monitoring. J Clin Endocrinol Metab. 2013;98:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blevins DP, Dadu R, Hu M, et al. Aerodigestive fistula formation as a rare side effect of antiangiogenic tyrosine kinase inhibitor therapy for thyroid cancer. Thyroid. 2014;24:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dadu R, Shah K, Bassett R, et al. Is there an alternative treatment option for patients with advanced papillary thyroid cancer (PTC) harboring the brafv600e mutation? ICE/ENDO Meeting. 2014. [Google Scholar]
- 16. Dadu R, Devine C, Hernandez M, et al. Role of salvage targeted therapy in differentiated thyroid cancer patients who failed first-line sorafenib. J Clin Endocrinol Metab. 2014;99:2086–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rothenberg SM, McFadden DG, Palmer E, et al. Re-differentiation of radioiodine-refractory BRAFV600E-mutant thyroid carcinoma with dabrafenib: A pilot study. J Clin Oncol. 2013;31;(suppl; abstr 6025). [Google Scholar]
- 18. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with braf v600e mutation. N Engl J Med. 2011;364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]


