Abstract
Context:
The sensitivity of the placenta to maternal insulin remains controversial. Early pregnancy may be a time of increased placental sensitivity to maternal insulin because insulin receptors are abundant on the syncytiotrophoblast in the first trimester but are far fewer at term.
Hypothesis:
Maternal insulin secretory response in early, but not late, pregnancy is positively associated with placental growth.
Design:
This is a secondary analysis of a cohort of women (n = 40) recruited before pregnancy.
Outcome Measures:
An iv glucose tolerance test was administered before pregnancy and in early (12–14 weeks) and late (34–36 weeks) pregnancy. Placental volume throughout gestation (in a subset of women via 3-dimensional ultrasound) and weight at birth were recorded.
Results:
Total insulin secretory response in early pregnancy was positively associated with placental volume in early pregnancy (R = 0.79, P = 0.04) and placental weight at term (R = 0.42, P = 0.007). Insulin secretory response before and in late pregnancy was not significantly associated with placental growth. Although neonatal fat mass was strongly correlated with placental weight at term (R = 0.449, P = 0.0003), maternal insulin secretory response was related to neonatal fat mass only at birth in male offspring (R = 0.59, P = 0.008).
Conclusions:
Maternal insulin secretory response in early pregnancy was strongly related to placental weight at birth. Thus, in early pregnancy, increased maternal insulin response as seen in obesity and gestational diabetes mellitus may be a key influence on placental growth, possibly due to the enhanced presence of placental insulin receptors on the maternal villous membrane early in gestation.
Babies born small or large for gestational age have been shown in numerous studies to be at higher risk for chronic disease and poor outcomes later in life (1–3). The weight of the placenta at birth is strongly associated with neonatal birth weight and explains the highest degree of variability in neonatal weight (4) and adiposity at term (5). In addition to placental size at birth, placental volume (measured by ultrasound) as early as the second trimester is also positively associated with birth weight in normal healthy pregnancies (6, 7). Thus, early influences on placental growth may profoundly affect fetal growth and adiposity.
Obese women who develop gestational diabetes have greater insulin responses during early pregnancy (8), larger placentas, and offspring with higher fat mass at birth (9, 10). Insulin is an important growth factor (11), and early pregnancy is a time of increased insulin sensitivity in most women (8, 12). Insulin has been shown to increase placental nutrient uptake in vitro (13, 14), but the sensitivity of the placenta to maternal insulin in vivo remains controversial (15–17). Early pregnancy may be a time of increased placental sensitivity to maternal insulin because insulin receptors are abundant on the syncytiotrophoblast, the cellular layer in contact with maternal blood and largely responsible for nutrient uptake and metabolism, in the first trimester (17–21). There are fewer insulin receptors on the maternal surface of the placenta later in pregnancy, whereas numbers are increased on the placental vascular endothelium (in contact with fetal blood), signaling a shift in responsiveness from maternal to fetal insulin levels (18). Thus, early pregnancy may be a time of heightened placental sensitivity to maternal insulin.
We hypothesized that maternal insulin secretory response in early, rather than late, pregnancy would be positively associated with placental weight at term due to effects on placental growth early in pregnancy.
Subjects and Methods
Experimental design
This is a secondary analysis of lean and obese women with and without gestational diabetes mellitus (GDM) (n = 40) recruited before pregnancy (8, 12). The original studies were conducted in the General Clinical Research Center at the University of Vermont (n = 19) and MetroHealth Medical Center, Case Western Reserve University (n = 21). The studies were approved by the hospitals' institutional review boards, and each subject provided informed consent before participating.
An iv glucose tolerance test was administered before pregnancy and in early (12–14 weeks) and late (34–36 weeks) pregnancy as previously described (8, 12). Briefly, for subjects <120% ideal body weight, we infused 0.5 g/kg glucose as a bolus over 3 minutes. Samples for glucose and insulin measurements were collected at baseline (0 minutes) and at 1, 3, 5, 10, 30, 45, and 60 minutes after completion of infusion. For subjects >120% ideal body weight, the glucose bolus was 19 g/m2 body surface area.
Upon delivery, placentas were trimmed of membranes and cord, drained of excess blood, and weighed. Neonatal fat mass was measured using skinfolds (n = 28) and total-body electrical conductivity (using a pediatric scanner: model HP-2; EM-SCAN, Inc) (n = 12) as previously described (22). Calculation of fat mass from skinfolds was accomplished using an equation validated against total body electrical conductivity measurements (22).
Serial ultrasound measures
In a subset of women (n = 7), placental volume was measured at 12–14, 20, 30, and 36 weeks of gestation. A 3-dimensional volume data set was acquired for each placenta using transabdominal sonography (RAB 4–8 probe, Voluson 730 Expert; GE Medical Systems). Sweep angle was adjusted to ensure the entire placenta was contained in each volume data set. Volumes were determined using the VOCAL volume technique (Virtual Organ Computer-aided AnaLysis; GE) employing a manual tracing of 6 consecutive outlines using a 30° rotation. The ultrasound machine then calculates the volume, in cubic centimeters, and displays a computed reconstruction on the screen (see Figure 1). Volumes were obtained in triplicate, and a mean of 3 values was taken when volumes were found to be within 10% of each other.
Figure 1.
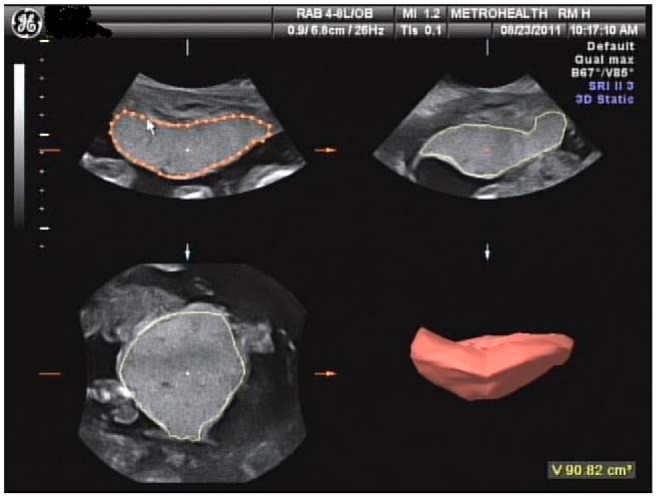
Representative placental sonogram presenting manual placental tracings along with the subsequent 3-dimensional reconstruction of placental volume. The ultrasound shown was completed at 20 weeks of gestation.
Metabolic measurements
Maternal fasting glucose was measured on the YSI Glucose Analyzer. Insulin (HI-14K RIA Kit; Millipore) was measured following manufacturer's instructions. Via the trapezoidal rule, the first-phase insulin response was calculated as the area under the insulin curve from 0 to 5 minutes and the second phase response was calculated as the insulin area under the curve (AUC) from 5 to 60 minutes. Total insulin response was the sum of the first- and second-phase AUC.
Statistical analysis
Spearman's rank correlation analysis was used to minimize the effect of extreme data points. Spearman correlation coefficients (R) between 1) placental weight at term and 2) neonatal fat mass and first-phase (0–5 minutes), second-phase (5–60 minutes), and total (0–60 minutes) insulin secretory capacity before and throughout pregnancy were calculated. Spearman correlations between ultrasound measures of placental volume and growth during gestation and early pregnancy insulin secretory capacity were calculated in a subset of women. The placental volume rate of growth was estimated by calculating 1) the slope of the line (via linear regression, assuring linearity via the runs test) describing the relationship between placental volume and gestational age for each individual, and 2) the AUC for each relationship using the trapezoid rule. A P value of <.05 was considered statistically significant. All statistical analysis was performed using STATA version 10.1.
Results
Table 1 shows maternal and neonatal characteristics of the cohort. Fourteen women developed GDM during the late second or early third trimester of pregnancy, as diagnosed by an abnormal oral glucose tolerance test (OGTT) using Carpenter-Coustan criteria (two or more values during a 3-hour 100-g OGTT exceeding the thresholds of 95 mg/dL for fasting glucose, 180 mg/dL at 1 hour, 155 mg/dL at 2 hours and 145 mg/dL at 3 hours) (23). These women had normal OGTT results in early pregnancy. Two women with GDM received insulin therapy, and GDM in the remainder was controlled by diet. We found no significant differences in the relationships presented below (ie, insulin secretory response with placental weight or volume or neonatal body fat) between GDM and normal glucose tolerant women or between lean and obese women (data not shown); thus, they were grouped together for subsequent analysis. One subject had notably high insulin responses (>8000 μU/mL), and all analyses were done with and without her. All statistically significant relationships presented below remained valid in her absence.
Table 1.
Maternal and Neonatal Characteristics
| n | Median ± IQRa | Range | |
|---|---|---|---|
| Prepregnancy | |||
| Maternal BMI, kg/m2 | 40 | 22.1 ± 6.0 | 17.7–41.0 |
| Fasting glucose, mg/dL | 40 | 90 ± 6 | 69–99 |
| Fasting insulin, μU/mL | 40 | 7.5 ± 5.4 | 1.9–50.5 |
| Insulin response, μU/mLb | 40 | 1645 ± 1024 | 347–8459 |
| Early pregnancy | |||
| Fasting glucose, mg/dL | 40 | 83 ± 10 | 70–97 |
| Fasting insulin, μU/mL | 40 | 6.3 ± 3.6 | 2.4–25.8 |
| Insulin response, μU/mLb | 40 | 2370 ± 1852 | 1142–9481 |
| Late pregnancy | |||
| Fasting glucose, mg/dL | 34 | 82 ± 10 | 69–102 |
| Fasting insulin, μU/mL | 34 | 8.9 ± 8.6 | 3.9–47.1 |
| Insulin response, μU/mLb | 34 | 4043 ± 2817 | 1785–10 299 |
| Gestational weight gain, kg | 40 | 14.3 ± 3.4 | 6.3–28.7 |
| Placental weight, g | 40 | 461.5 ± 168.0 | 300.0–805.0 |
| Gestational diabetes (yes/no) | 40 | 14/26 | |
| Gestational age, wk | 40 | 39.0 ± 1.0 | 37.0–41.0 |
| Birth weight, kg | 40 | 3.40 ± 0.60 | 2.49–4.93 |
| Body fat, % | 38 | 11.3 ± 5.6 | 3.5–21.3 |
| Fat-free mass, kg | 38 | 3.0 ± 0.4 | 2.4–3.9 |
| Fat mass, kg | 38 | 0.38 ± 0.25 | 0.11–1.05 |
| Sex (male/female) | 40 | 19/21 |
Abbreviation: IQR, interquartile range.
Unless indicated otherwise.
Represents insulin secretory response area under the curve from 0 to 60 minutes.
The total (Figure 2B) insulin secretory response in early pregnancy was positively associated with placental weight at term. Insulin secretory response before (Figure 2A) and in late pregnancy (Figure 2C) was not correlated with placental weight. Second-phase insulin response results were similar to total insulin response (data not shown). First-phase insulin response before or during pregnancy was not associated with placental weight (before: R = 0.10, P = .52; early: R = 0.16, P = .34; late: R = −0.14, P = .29). Adjusting for gestational age did not change the results (data not shown).
Figure 2.
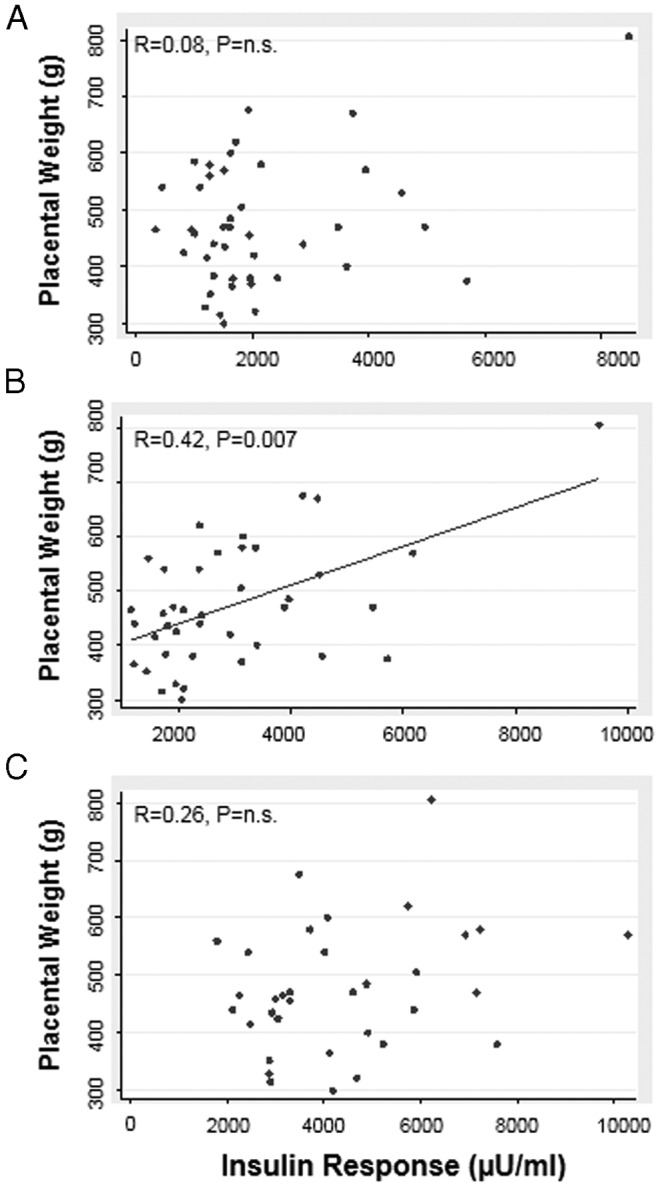
Association between placental weight at term and the insulin secretory response (AUC from 0–60 minutes) before pregnancy (A) and in early (B) and late (C) pregnancy. Results were not altered by 1) removal of subject with high insulin response (>8000 μU/mL) or 2) adjustment for gestational age. Spearman R values are shown. P < .05 was considered statistically significant.
Placental weight was not correlated with maternal fasting insulin or glucose before (insulin: R = 0.28, P = .07; glucose: R = 0.11, P = .52) or during early (insulin: R = 0.20, P = .14; glucose: R = −0.17, P = .20) or late (insulin: R = 0.24, P = .08; glucose: R = −0.07, P = .60) pregnancy.
Neonatal fat mass was moderately correlated with placental weight at term in all infants (R = 0.43, P = .007). Placental weight was also correlated with neonatal lean body mass (R = 0.54, P = .0004) and percent body fat (R = 0.37, P = .02) at birth. Maternal total insulin secretory response was not related to neonatal percent body fat at birth (prepregnancy AUC: R = −0.02, P = .91; early pregnancy AUC: R = 0.03, P = .86; late pregnancy AUC: R = 0.04, P = .81). When analyzed by fetal sex, however, maternal total insulin response in early pregnancy was positively associated with percent fat in male, but not female, offspring (Figure 3B). Maternal insulin secretory response before or in late pregnancy was not related to infant body composition at birth in males or females (Figure 3, A and C).
Figure 3.
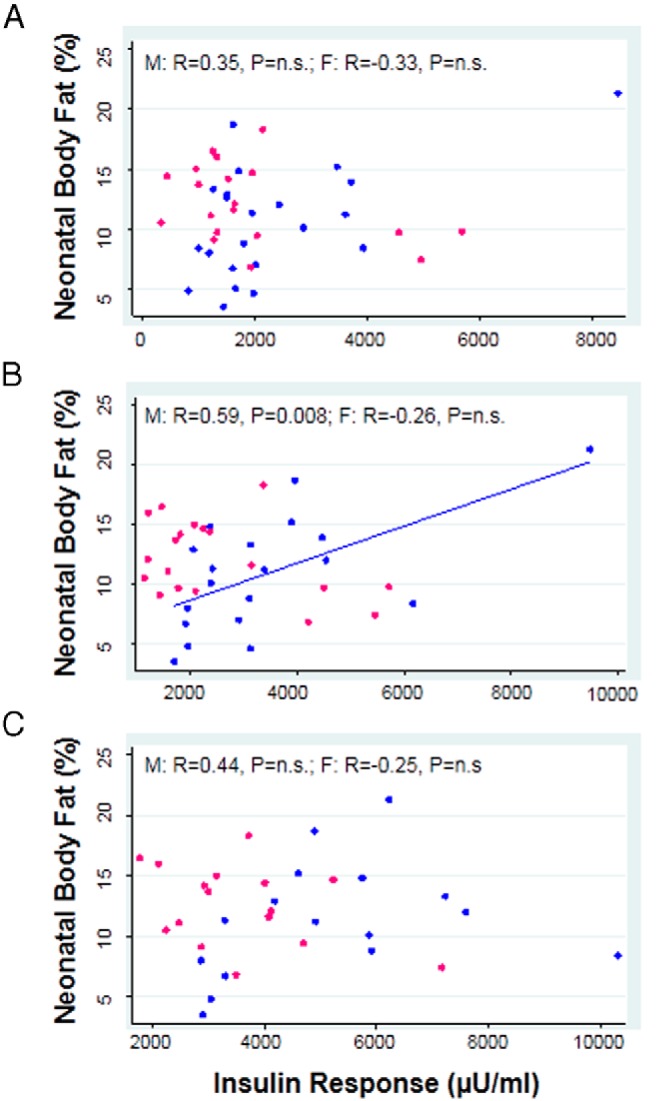
Association between neonatal percent body fat in males (blue markers) and females (pink markers), and the insulin secretory response (AUC from 0–60 minutes) before pregnancy (A) and in early (B) and late (C) pregnancy. Results were not altered by 1) removal of subject with high insulin response (>8000 μU/mL) or 2) adjustment for gestational age. M represents male offspring; F represents female offspring. Spearman R values are shown. P < .05 was considered statistically significant.
In a subset of nondiabetic women (n = 7), placental volume was measured at 12–14, 20, 30, and 36 weeks of gestation using 3-dimensional ultrasound. Figure 1 shows an example of the ultrasound tracings and 3-dimensional rendering for 1 subject at 20 weeks of gestation. Maternal insulin response in early pregnancy (12–14 weeks) was the only measure of insulin response that was significantly correlated with placental volume at 20 weeks of pregnancy (Figure 4). Although not significant, the trend for a positive correlation between early pregnancy insulin response and placental volume throughout pregnancy was strong (12–14 weeks: R = 0.75, P = .052; 30 weeks: R = 0.50, P = .25; 36 weeks: R = 0.71, P = .07). We were unable to detect a relationship between early maternal insulin response and the estimated placental volume rate of growth over gestation using the AUC (R = 0.64, P = .12) or the slope of the line representing cubic centimeters per week gestation (R = 0.14, P = 0. 76).
Figure 4.
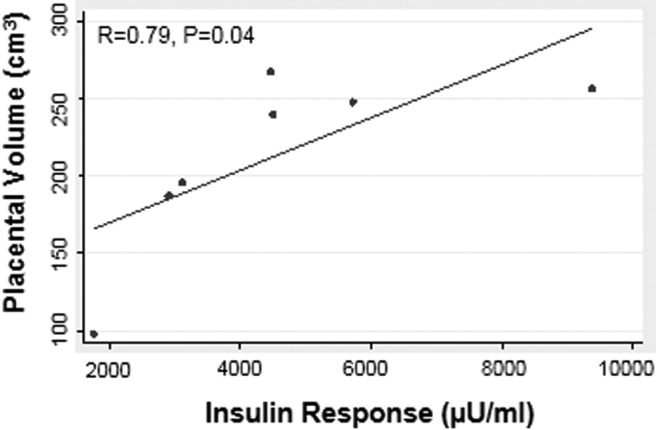
The insulin secretory response (AUC from 0–60 minutes) in early pregnancy was positively associated with placental volume as measured by ultrasound at 20 weeks of gestation. Spearman R values are shown. P < .05 was considered statistically significant.
Discussion
Our key finding was that maternal insulin response to an iv glucose load in early, but not late, pregnancy was positively correlated with placental volume in midpregnancy and placental weight at term. Additionally, neonatal adiposity in male, but not female, infants was related to this early pregnancy insulin response. Our findings suggest that maternal insulin is an important growth factor for the placenta in early, but not late, pregnancy. This may be due to the relative abundance of insulin receptors on the syncytiotrophoblast in early pregnancy, which shift to the endothelial lining of the placental vasculature in late pregnancy.
Catalano et al (8) previously found that maternal insulin response in early pregnancy was higher in obese women who developed GDM as compared with controls. Women with these conditions also have larger placentas and offspring with a higher fat mass at birth (9, 10), data consistent with our findings. The correlation between early pregnancy insulin response and placental weight did not differ between GDM and non-GDM, lean or obese women in our diverse cohort. This suggests that maternal insulin response in early pregnancy, regardless of maternal body mass index (BMI) or later glucose tolerance, is the primary driver for placental weight at term.
Furthermore, early pregnancy insulin response was strongly associated with placental volume at week 20 of pregnancy in a small number of patients. This finding supports the concept that maternal insulin stimulates placental growth during early pregnancy and underlies the relationship with placental weight, and possibly function, at term. Clapp et al (7) established the strong positive relationship between placental volumes in the second trimester and birth weight, which further emphasizes the significance of early pregnancy growth factors on fetal development. Thus, from a clinical perspective, interventions initiated in early pregnancy are likely to have a more profound effect on fetal outcome than those begun in late pregnancy, particularly when dealing with chronic conditions such as diabetes, obesity, or undernutrition.
The insulin secretory response represents the ability of maternal pancreatic β-cells to respond to a glucose load (or meal) (24). The second-phase, but not first-phase, insulin response was associated with placental weight at birth, indicating that the initial release of insulin (dependent on pancreatic insulin stores and basal insulin production) does not determine placental size, but rather the total response of the pancreas to a glucose challenge. This is consistent with our finding and others' (16) that fasting insulin levels do not correlate with placental weight.
Transient increases in maternal insulin in early pregnancy were also associated with higher placental weights and increased fetal growth in rats (17). Others have found that placental nutrient handling was sensitive to insulin in early pregnancy, a possible mechanism for effects on fetal growth. In first-trimester human placental explants, insulin treatment was found to increase glucose uptake by 180%, whereas it had no effect on term placental tissue (21). These findings were consistent with the absence of the insulin-sensitive glucose transporter, GLUT4, in term placental tissue but its presence in first-trimester placenta (21). Glucose uptake in isolated first-trimester trophoblast cells was also stimulated by insulin treatment in a dose-dependent fashion (25). Thus, our data are consistent with previous in vivo and in vitro studies showing that the placenta is sensitive to maternal insulin in early, but not late, pregnancy.
The increased sensitivity of the placenta to maternal insulin in early pregnancy is likely related to the location of the insulin receptors throughout pregnancy. During the first trimester, they are abundant on the syncytiotrophoblast, specifically syncytial sprouts and mesenchymal villi, both structures known to drive placental growth (19), whereas later in pregnancy, the insulin receptors are found mainly on the endothelial cells of the placental vasculature, exposed to fetal, rather than maternal, growth factors (18).
Although placental weight at term was strongly related to neonatal adiposity, we did not find a significant relationship between maternal insulin response and adiposity at birth in our cohort when male and female infants were analyzed together. We previously reported that placental size accounts for much of the variability in neonatal adiposity (5); however, maternal anthropometrics and insulin resistance also independently affect neonatal weight and body composition (26). The impact on fetal growth of maternal nutrient availability in late pregnancy, influenced by diet and maternal insulin resistance (27), is not accounted for by early placental growth factors, which may explain why we are unable to detect a strong relationship between maternal insulin response in early pregnancy and neonatal adiposity. Consistent with our previous findings, this relationship did not differ between GDM and non-GDM or lean and obese women.
Interestingly, when the sexes were analyzed separately, percent body fat of males, but not females, was associated with maternal insulin secretion in early pregnancy. This association is unlikely to depend on placental growth because we did not observe significant sex differences in the relationship between placental weight and early pregnancy insulin response. We have previously found that adiposity of male, but not female, neonates was sensitive to maternal BMI independent of placental weight (5), and we speculate that a higher insulin response as seen in obese women may mediate this relationship. These secondary analyses may also be affected by the small cohort size and should be interpreted with caution.
Our study was based upon a relatively small number of subjects (∼40). Our time-intensive protocol (ie, prepregnancy recruitment, multiple iv glucose tolerance tests, and serial ultrasounds) required highly committed individuals that may not be representative of the population as a whole. We were underpowered to thoroughly analyze the effect of maternal insulin response on placental volumes and growth throughout pregnancy, as observed with our strong, but nonsignificant trends, but despite this limitation, we were able to detect at 20 weeks gestation the strongest of the relationships with statistical significance. To the best of our knowledge, this is the first study to examine the effects of maternal insulin response before and throughout pregnancy on placental growth and neonatal adiposity at birth.
Conclusion
Maternal insulin secretory response in early pregnancy was strongly related to placental weight at birth and this may be initiated by growth effects in early pregnancy. Thus, in early pregnancy increased maternal insulin response as seen in obese women and those who go on to develop GDM, may be a key influence on placental growth, possibly due to the enhanced presence of placental insulin receptors on the maternal villous membrane early in gestation.
Acknowledgments
This work was supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants NICHD R00HD062841 (to P.O.-G.) and R01HD022965 (to P.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- GDM
- gestational diabetes mellitus
- OGTT
- oral glucose tolerance test
References
- 1. Frankel S, Elwood P, Sweetnam P, Yarnell J, Smith GD. Birthweight, body-mass index in middle age, and incident coronary heart disease. Lancet. 1996;348(9040):1478–1480. [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia. 1993;36(1):62–67. [DOI] [PubMed] [Google Scholar]
- 3. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–e296. [DOI] [PubMed] [Google Scholar]
- 4. Salafia CM, Zhang J, Charles AK, et al. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008;22(3):229–239. [DOI] [PubMed] [Google Scholar]
- 5. O'Tierney-Ginn P, Presley L, Minium J, Hauguel deMouzon S, Catalano PM. Sex-specific effects of maternal anthropometrics on body composition at birth. Am J Obstet Gynecol. 2014;211(3):292 e291–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Effendi M, Demers S, Giguère Y, et al. Association between first-trimester placental volume and birth weight. Placenta. 2014;35(2):99–102. [DOI] [PubMed] [Google Scholar]
- 7. Clapp JF, 3rd, Rizk KH, Appleby-Wineberg SK, Crass JR. Second-trimester placental volumes predict birth weight at term. J Soc Gynecol Investig. 1995;2(1):19–22. [PubMed] [Google Scholar]
- 8. Catalano PM, Huston L, Amini SB, Kalhan SC. Longitudinal changes in glucose metabolism during pregnancy in obese women with normal glucose tolerance and gestational diabetes mellitus. Am J Obstet Gynecol. 1999;180(4):903–916. [DOI] [PubMed] [Google Scholar]
- 9. Strøm-Roum EM, Haavaldsen C, Tanbo TG, Eskild A. Placental weight relative to birthweight in pregnancies with maternal diabetes mellitus. Acta Obstet Gynecol Scand. 2013;92(7):783–789. [DOI] [PubMed] [Google Scholar]
- 10. Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189(6):1698–1704. [DOI] [PubMed] [Google Scholar]
- 11. Straus DS. Growth-stimulatory actions of insulin in vitro and in vivo. Endocr Rev. 1984;5(2):356–369. [DOI] [PubMed] [Google Scholar]
- 12. Catalano PM, Tyzbir ED, Wolfe RR, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol. 1993;264(1 Pt 1):E60–E67. [DOI] [PubMed] [Google Scholar]
- 13. Jansson N, Greenwood SL, Johansson BR, Powell TL, Jansson T. Leptin stimulates the activity of the system A amino acid transporter in human placental villous fragments. J Clin Endocrinol Metab. 2003;88(3):1205–1211. [DOI] [PubMed] [Google Scholar]
- 14. Karl PI, Alpy KL, Fisher SE. Amino acid transport by the cultured human placental trophoblast: effect of insulin on AIB transport. Am J Physiol. 1992;262(4 Pt 1):C834–C839. [DOI] [PubMed] [Google Scholar]
- 15. Friis CM, Qvigstad E, Paasche Roland MC, et al. Newborn body fat: associations with maternal metabolic state and placental size. PLoS One. 2013;8(2):e57467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bomba-Opon DA, Wielgos M, Horosz E, Bartkowiak R, Kosinski P, Bochenska K. Placental first trimester's measurements in relation to maternal plasma adiponectin, leptin and insulin concentrations. Neuroendocrinol Lett. 2010;31(4):573–576. [PubMed] [Google Scholar]
- 17. Ericsson A, Saljo K, Sjostrand E, et al. Brief hyperglycaemia in the early pregnant rat increases fetal weight at term by stimulating placental growth and affecting placental nutrient transport. J Physiol. 2007;581(Pt 3):1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hiden U, Maier A, Bilban M, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia. 2006;49(1):123–131. [DOI] [PubMed] [Google Scholar]
- 19. Desoye G, Hartmann M, Jones CJ, et al. Location of insulin receptors in the placenta and its progenitor tissues. Microsc Res Tech. 1997;38(1–2):63–75. [DOI] [PubMed] [Google Scholar]
- 20. Desoye G, Hartmann M, Blaschitz A, et al. Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern. Histochemistry. 1994;101(4):277–285. [DOI] [PubMed] [Google Scholar]
- 21. Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod. 2005;20(2):521–530. [DOI] [PubMed] [Google Scholar]
- 22. Catalano PM, Thomas AJ, Avallone DA, Amini SB. Anthropometric estimation of neonatal body composition. Am J Obstet Gynecol. 1995;173(4):1176–1181. [DOI] [PubMed] [Google Scholar]
- 23. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982;144(7):768–773. [DOI] [PubMed] [Google Scholar]
- 24. Caumo A, Luzi L. First-phase insulin secretion: does it exist in real life? Considerations on shape and function. Am J Physiol Endocrinol Metab. 2004;287(3):E371–E385. [DOI] [PubMed] [Google Scholar]
- 25. Gordon MC, Zimmerman PD, Landon MB, Gabbe SG, Kniss DA. Insulin and glucose modulate glucose transporter messenger ribonucleic acid expression and glucose uptake in trophoblasts isolated from first-trimester chorionic villi. Am J Obstet Gynecol. 1995;173(4):1089–1097. [DOI] [PubMed] [Google Scholar]
- 26. Catalano PM, Drago NM, Amini SB. Maternal carbohydrate metabolism and its relationship to fetal growth and body composition. Am J Obstet Gynecol. 1995;172(5):1464–1470. [DOI] [PubMed] [Google Scholar]
- 27. Catalano PM, Kirwan JP, Haugel-de MS, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133(5 Suppl 2):1674S–1683S. [DOI] [PubMed] [Google Scholar]


