Abstract
Context:
The role of 11β-hydroxysteroid dehydrogenase types 1 (11β-HSD-1) and 2 (11β-HSD-2) enzymes in sc adipose tissue is controversial.
Objective:
The objective of the study was to determine the activity of 11β-HSD-1 and -2 enzymes in the abdominal and leg sc adipose tissue in obesity and diabetes.
Design:
11β-HSD-1 and -2 enzyme activities in abdominal and leg sc adipose tissue were measured by infusing [2,2,4,6,6,12,12-2H7] cortisone (D7 cortisone) and [9,12,12-2H3] cortisol (D3 cortisol) via microdialysis catheters placed in sc fat depots.
Setting:
The study was conducted at the Mayo Clinic Clinical Research Unit.
Participants:
Lean nondiabetic (n = 13), overweight/obese nondiabetic (n = 15), and overweight/obese participants with type 2 diabetes mellitus (n = 15) participated in the study.
Main Outcome Measures:
The conversion of infused D7 cortisone to D7 cortisol (via 11β-HSD reductase activity) and D3 cortisol to D3 cortisone (via 11β-HSD dehydrogenase activity) in sc adipose tissue.
Results:
Enrichment of D7 cortisone and D3 cortisol were similar in the effluents from both sites in all groups. D3 cortisone enrichment did not differ in the three cohorts, indicating that 11β-HSD-2 enzyme activity (conversion of cortisol to cortisone) occurs equally in all groups. However, D7 cortisol enrichment was detectable in abdominal sc fat of overweight/obese participants with type 2 diabetes mellitus only, implying 11β-HSD-1 reductase activity (conversion of cortisone to cortisol) occurs in obese subjects with type 2 diabetes.
Conclusions:
There is conversion of cortisone to cortisol via the 11β-HSD-1 enzyme pathway in abdominal sc fat depots in overweight/obese participants with type 2 diabetes mellitus. This observation has significant implications for developing tissue-specific 11β-HSD-1 inhibitors in type 2 diabetes mellitus.
Tissue specific conversion of cortisone to cortisol via the 11β-hydroxysteroid dehydrogenase type 1 enzyme pathway (11β-HSD-1) results in high local cortisol concentrations (1). 11β-HSD-1 is present in multiple tissues including the liver and adipose tissue (2–6). The 11β-HSD-1 pathway has attracted considerable attention both as a therapeutic target and a potential contributor to the pathogenesis of diabetes, obesity, and the so-called metabolic syndrome (1, 7, 8). 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD-2) enzyme is mostly present in the kidneys, adipose tissue and intestine and primarily converts cortisol to metabolically inactive cortisone (6). Evidence from animal studies shows that the knockout of 11β-HSD-1 enzyme activity prevents, whereas overexpression induces obesity and diabetes mellitus in mice (9). Transgenic mice selectively overexpressing 11β-HSD-1 in adipose tissue develop hyperglycemia and visceral obesity. In vitro studies measuring 11β-HSD-1 enzyme activity in the dehydrogenase direction (ie, cortisol to cortisone) in human abdominal sc fat have provided indirect evidence that increased reactivation of cortisone to cortisol could contribute to obesity in individuals with metabolic syndrome (10, 11). Previous studies have shown that expression of the mRNA encoding 11β-HSD-1 is increased in the adipose tissue of obese individuals (4, 12–16). Valsamakis et al (17) have shown that whole-body 11β-HSD-1 activity measured by urinary metabolites of cortisol and cortisone are unchanged in obese men with type 2 diabetes in comparison with obese men without diabetes.
The present study was undertaken to examine the tissue-specific interconversion of cortisone to cortisol via 11β-reductase and 11β-dehydrogenase activity of HSD-1 enzyme and cortisol to cortisone via 11β-HSD-2 enzyme pathways in the sc adipose tissue of the abdomen and leg in lean nondiabetic, obese nondiabetic, and obese subjects with type 2 diabetes. We did so by using sophisticated tracer methods coupled with the microdialysis technique.
Our previous studies have shown that the liver is the major source of extraadrenal cortisol production, with minimal contribution from visceral fat (6). However, the contribution from sc adipose tissue to extraadrenal cortisol production remains uncertain. Cortisol-cortisone interconversion in sc adipose tissue has not been measured using independent isotopes to concurrently estimate 11β-HSD-1 and 11β-HSD-2 enzyme activity.
Previous studies have focused only on abdominal sc fat and concluded perhaps incorrectly that sc fat depots at other sites (eg, leg) may have similar activity as in the abdominal sc fat. To our knowledge, this study is the first to provide novel insight with respect to the depot-specific (abdominal sc fat vs sc leg fat) activity of 11β-HSD-1 (both dehydrogenase and reductase) and 11β-HSD-2 in healthy and type 2 diabetic humans, which has previously not been studied.
Development of drugs for treatment of metabolic syndrome is an active area of investigation by several pharmaceutical companies. A comprehensive review by Anagnostis et al (8) and Stomby et al (18) have provided evidence of a need to develop tissue specific inhibitors of 11β-HSD-1 for treatment of metabolic syndrome. Our study further strengthens the requirement for development of site-specific inhibitors of 11β-HSD-1 as promising targets for treatment of type 2 diabetes.
Research Design and Methods
Participants
After receiving approval from the Mayo Clinic Institutional Review Board, we recruited 13 lean nondiabetic (LND) [body mass index (BMI) 19–24 kg/m2], 15 overweight/obese nondiabetic (OND) (BMI 27–40 kg/m2), and 15 overweight/obese individuals (ODM) with type 2 diabetes mellitus (BMI 27–40 kg/m2) and obtained informed written consent to participate in the study. All participants were in good health and at a stable weight. None regularly engaged in vigorous physical exercise. None of the first-degree relatives of the nondiabetic participants had a history of diabetes mellitus. Individuals with history of smoking or alcohol intake over and above American Diabetes Association guidelines, ie, two drinks per day for men and one drink per day for females, were excluded from participation in the study. At the time of the screening, two participants with type 2 diabetes were being treated with lifestyle modifications alone, seven with metformin alone, and the remaining six with a combination of a sulfonylurea and metformin. Individuals with diabetes who were taking thiazolidinediones were excluded because these agents have been reported to decrease 11β-HSD-1 activity in vitro (19).
All participants were instructed to maintain a constant body weight and follow a weight-maintenance diet (55% carbohydrate, 30% fat, and 15% protein) for at least 2 weeks before the study. Oral antihyperglycemic medications were discontinued 10 days before the study visit to prevent confounding effects of medications (metformin and sulfonylurea) on study outcomes. Body composition (total body fat and lean body mass) was measured before the study visit in the Center for Clinical and Translational Science body composition core using Lunar iDXA, software version 6.10 (GE Healthcare Technologies). Detailed baseline characteristics of participants are provided in Table 1. We did not exclude women of child-bearing potential; however, a negative pregnancy test was confirmed prior to study. Four of nine women in the LND, one of eight in OND, and none of four in ODM were younger than 50 years. This is a substudy of a larger previously published study (20). Because this was an optional substudy, subjects decided whether they wished to allow the insertion of microdialysis catheters and infusions at the time of the informed consent process, thereby preventing any selection bias by the study team.
Table 1.
Participant Characteristics
| Participant Characteristics | LND (n = 13) | OND (n = 15) | ODM (n = 15) |
|---|---|---|---|
| Age, y | 56 ± 14 | 56 ± 11 | 60 ± 10 |
| Sex, male/female | 4/9 | 7/8 | 11/4 |
| BMI, kg/m2 | 23 ± 1a | 32 ± 3 | 32 ± 4 |
| Waist circumference | 82 ± 7a | 97 ± 12 | 106 ± 9 |
| Lean body mass, kg | 45 ± 8a | 51 ± 12 | 55 ± 10 |
| Total body fat, % | 30 ± 5a | 39 ± 10 | 38 ± 6 |
| Fasting plasma glucose, mmol/L | 4.9 ± 0.3 | 4.9 ± 0.3 | 7.8 ± 2.3b |
| HbA1c, %, mmol/mol | 5.4 ± .4 (36 ± 4) | 5.5 ± .3 (37 ± 3) | 7.1 ± .8b (54 ± 8) |
Abbreviation: HbA1c, glycated hemoglobin. Data are mean ± SD.
P < .05 vs OND and ODM.
P < .001 vs LND and OND.
Experimental design
Participants were admitted to the Mayo Clinic Clinical Research Unit at 5:00 pm on the evening before the study. A standard 42-kJ/kg (10 kcal/kg) meal (55% carbohydrate, 30% fat, 15% protein) was eaten between 5:30 and 6:00 pm. Thereafter participants remained fasting until the end of the study. Sips of water were permitted ad libitum.
The following morning (∼6:00 am), 2 microdialysis catheters were placed, one in the abdominal sc adipose tissue and the other in the leg sc adipose tissue, for tracer infusion. After a 60-minute stabilization period, infusions of [2,2,4,6,6,12,12-2H7]cortisone (D7 cortisone) and [9,12,12-2H3]cortisol (D3 cortisol) were started via the microdialysis catheter at 2759 nmol/L (1 μg/mL; 1 μL/min) and continued for 4 hours. In preliminary in vitro studies at different microdialysis pump infusion rates, we had observed that the recovery of the cortisol tracer/s were optimal at a pump infusion rate of 1 μL/min. Microdialysate samples were collected to measure isotopic enrichment before and after 4 hours of infusion as well as recovery of isotopes in the effluent.
Analytical method
Enrichments of D3 cortisol, D3 cortisone, D7 cortisone, and D7 cortisol in the microdialysate effluent samples were analyzed using liquid chromatography-tandem mass spectrometry as previously described (14, 20–22).
Statistical analyses
Data in the text and figures are expressed as mean ± SD. One-way ANOVA models and Kruskal-Wallis tests were used to test the hypothesis that the conversion of cortisone to cortisol in sc adipose tissue differed among the LND, OND, and ODM participants. Values of P < .05 were considered statistically significant. A statistical analysis was conducted using the SAS system (version 9.3; SAS Institute Inc). Post hoc comparisons were done to test the effect of obesity (LND vs OND) and diabetes (OND vs ODM) using Wilcoxon rank sum testing.
Results
Enrichment of D7 cortisone and D7 cortisol in abdominal and leg sc adipose tissue
D7 cortisone recovered in effluent (Figure 1, A and B) from abdominal and leg sc adipose tissues were not different from infused D7 cortisone (Table 2) in LND, OND, and ODM participants. Concordance between infused and recovered isotope suggests that equilibration time provided was appropriate and adequate for the measurement of isotopic enrichment. The D7 cortisol enrichment in abdominal sc adipose tissue was significantly higher (P < .001) in ODM as compared with LND and OND participants. D7 cortisol enrichment did not differ from zero in LND and OND participants but was significantly higher (P = .001 vs zero) in ODM participants, suggesting that substantial 11β-HSD-1 enzyme activity occurs in abdominal sc adipose tissue of people with type 2 diabetes (Table 2 and Figure 1C). The D7 cortisol enrichment in effluent from leg sc adipose tissue was not different from zero (P = .23) in all 3 study groups, implying a lack of detectable 11β-HSD-1 enzyme activity in leg adipose tissue (Table 2 and Figure 1D).
Figure 1.
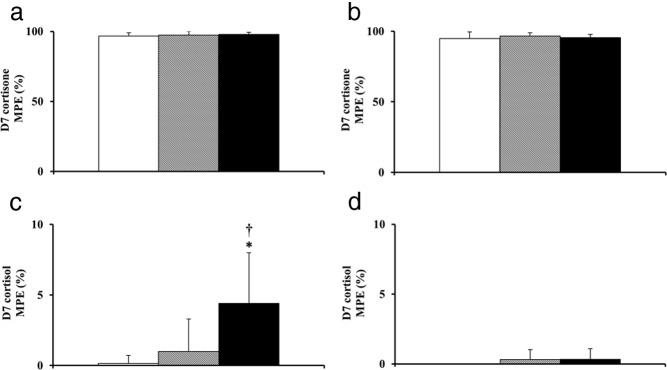
Enrichments of D7 cortisone (A and B) and D7 cortisol (C and D) observed in microdialysis effluent of abdominal (left panel) and leg (right panel) sc adipose tissue. Microdialysate samples were collected over 4 hours of D7 cortisone infusion in LND (white bar), OND (shaded bar), and ODM (black bar). *, P = .001 vs 0; †, P < .001 vs LND and OND. MPE, mole percent excess.
Table 2.
MPE of D7 cortisone, D7 cortisol, D3 cortisol, and D3 cortisone observed during the study
| Tracer Hormone | Mole Percent Excess |
Kruskal-Wallis P Value (Post Hoc Comparison With P < .05) | ||
|---|---|---|---|---|
| LND (n = 13) (P Valuea) | OND (n = 15) (P Valuea) | ODM (n = 15) (P Valuea) | ||
| Abdomen | ||||
| D7 cortisone (infused) | 98 ± 3 | 99 ± 3 | 100 ± 0 | .10 |
| D7 cortisone (recovered) | 97 ± 2 | 97 ± 4 | 98 ± 1 | .31 |
| D7 cortisol | 0.15 ± 0.56 (P = 1.0) | 0.98 ± 2.32 (P = .13) | 4.4 ± 5.58 (P = .001) | <.001 (LND, OND < ODM) |
| D3 cortisol (infused) | 95 ± 3 | 95 ± 3 | 96 ± 2 | .83 |
| D3 cortisol (recovered) | 86 ± 23 | 89 ± 13 | 95 ± 2 | .32 |
| D3 cortisone | 20 ± 16 (P = .002) | 20 ± 19 (P = .002) | 33 ± 21 (P < .001) | .14 |
| Leg | ||||
| D7 cortisone (infused) | 100 ± 0 | 100 ± 1 | 100 ± 0 | .45 |
| D7 cortisone (recovered) | 95 ± 5 | 97 ± 2 | 95 ± 2 | .67 |
| D7 cortisol | 0 ± 0 (P = NAb) | 0.32 ± 0.70 (P = .25) | 0.34 ± 0.75 (P = .25) | .23 |
| D3 cortisol (infused) | 94 ± 3 | 96 ± 2 | 95 ± 2 | .13 |
| D3 cortisol (recovered) | 93 ± 4 | 94 ± 3 | 93 ± 5 | .96 |
| D3 cortisone | 7 ± 6 (P = .008) | 6 ± 5 (P = .002) | 8 ± 7 (P = .001) | .62 |
Abbreviation: NA, not available.
P values are for a signed rank test for the hypothesis that the concentrations are equal to zero.
P value for D7 cortisol was not computed for LND due to lack of variability (all measurements were zero).
Enrichment of D3 cortisol and D3 cortisone in abdominal and leg sc adipose tissue
The D3 cortisol recovered in effluent (Figure 2, A and B) from abdominal and leg sc adipose tissues was not different from infused D3 cortisol (Table 2) in LND, OND, and ODM participants. The concordance between infused and recovered isotope suggests that the equilibration time provided was appropriate and adequate for the measurement of isotopic enrichment. D3 cortisol enrichment recovered in the microdialysis effluent was not different in LND, OND, and ODM participants in both abdominal (P = .32) and leg sc adipose tissue (P = .96) (Table 2 and Figure 2, A and B). The D3 cortisone enrichments obtained in microdialysis effluent from the abdominal and leg sc adipose tissue was significantly greater than zero (P < .01), suggesting the presence of detectable dehydrogenase activity of 11β-HSD enzyme in the sc adipose tissues of abdomen as well as the leg in LND, OND, and ODM participants (Table 2 and Figure 2, C and D). Additionally, the absolute mole percentage enrichment of D3 cortisone in the abdominal sc adipose tissue was high in all 3 groups, indicating substantial dehydrogenase activity compared with the reductase activity of 11β-HSD enzyme in the sc adipose tissues of humans. The D3 cortisone enrichments obtained in the abdominal and leg sc adipose tissue did not differ (P = .14 and P = .62) among study groups, respectively (Table 2).
Figure 2.
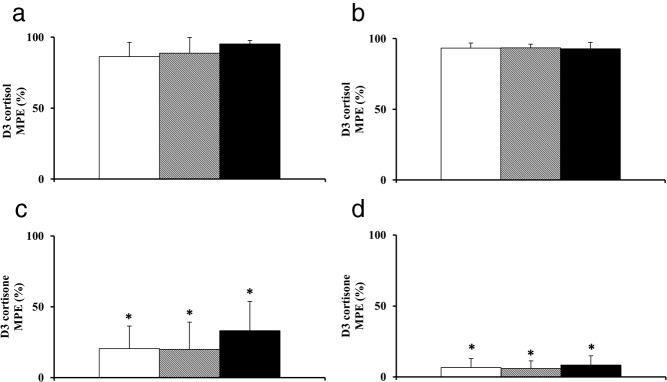
Enrichments of D3 cortisol (A and B) and D3 cortisone (C and D) observed in microdialysis effluent of abdominal (left panel) and leg (right panel) sc adipose tissue. Microdialysate samples were collected over 4 hours of D3 cortisol infusion in LND (white bar), OND (shaded bar), and ODM (black bar). *, P < .01 vs 0. MPE, mole percent excess.
Discussion
The present study used a combination of cortisol and cortisone tracers infused via microdialysis catheters to examine the interconversion of cortisone and cortisol by 11β-HSD isoenzymes in the sc adipose tissues of the abdomen and leg. Our results indicate that conversion of D7 cortisone to D7 cortisol (ie, 11β-reductase activity) is higher in overweight/obese participants with type 2 diabetes mellitus as compared with lean and overweight/obese nondiabetic participants and is limited to sc abdominal fat alone, with no contribution from leg adipose tissue. In contrast, conversion of D3 cortisol to D3 cortisone (ie, 11β-dehydrogenase activity) occurred equally in abdominal sc fat in all 3 groups studied, with minimal contribution from leg sc adipose tissue.
We had more female subjects in the LND cohort (9 of 13) and more male subjects in the ODM cohort (11 of 15), whereas there was sex balance in the OND cohort (seven males and eight females). However, we have found no statistical differences in cortisone to cortisol conversion or cortisol to cortisone conversion after accounting for sex in the cohorts studied. Taken together, these results are intriguing because they imply differential activity (ie, reductase vs dehydrogenase) of the 11β-HSD-1 enzyme in the abdominal and leg sc depots in overweight/obese participants with type 2 diabetes compared with lean and overweight/obese nondiabetic participants. 11β-HSD-1 is an intracellular enzyme. Infusion of D7 cortisone into the interstitial space resulted in conversion to D7 cortisol, indicating that D7 cortisone diffused into the cell and was converted by 11β-HSD-1 to D7 cortisol, which diffused back into the interstitial space. 11β-HSD-1 activity has been previously measured in vitro using sc fat biopsy. However, these studies have been limited to the measurement of the dehydrogenase activity (ie, cortisol to cortisone) since after the release of the enzyme from the intracellular compartment that is the preferred direction of reaction (11).
Our results showing comparable D3 cortisone enrichments in sc fat in all three cohorts are somewhat consistent with previous in vitro and in vivo studies (10, 11, 23–25) that reported enhanced 11β-HSD-2 enzyme activity in sc adipose tissue of lean individuals with or without type 2 diabetes and obese nondiabetic participants. However, these studies did not include obese diabetic individuals. Previous studies have shown increased expression of 11β-HSD-1 mRNA in sc abdominal adipose tissue of obese nondiabetic men and women (4, 12, 16, 26). Studies have also shown increased activity of 11β-HSD-2 (conversion of cortisol to cortisone) in the sc fat of obese nondiabetic men and indirectly suggested that increased reactivation of cortisone to cortisol in adipose tissues by virtue of increased substrate availability perhaps contributes to obesity and metabolic syndrome (10, 11, 24, 26). Walker and colleagues (14) infused 3H-cortisone alone via microdialysis catheters in only abdominal sc fat depots and concluded that 11β-HSD-1 activity was perhaps higher in all sc fat depots in obese individuals. Again it is important to emphasize that all of the previous studies did not include obese individuals with type 2 diabetes that are commonly encountered in clinical practice.
To our knowledge, our study is the first to systematically evaluate the role of both 11β-reductase and dehydrogenase enzyme activities in abdominal and leg sc fat using a combination of stable isotopes of cortisone and cortisol tracers that has not been directly estimated in obese individuals with or without type 2 diabetes. The relative contribution of 11β-HSD-2 (ie, dehydrogenase activity) appears to occur equally in all 3 cohorts, with reductase activity occurring predominantly in ODM via 11β-HSD-1.
Type 2 diabetic participants were taken off all their antihyperglycemic medications 10 days prior to the study visit, thereby mitigating the confounding effects of drugs such as metformin on study results. Livingstone et al (27) have shown that in an animal model, metformin had no effect on 11β-HSD-1 enzyme activity either in the fat or liver. Additionally, Valsamakis et al (17) have elegantly demonstrated that 11β-HSD-1 enzyme activity does not differ in people with diabetes under conditions of hyperglycemia when compared with BMI-matched controls. Hence, we do not anticipate that hyperglycemia as a result of stopping oral hypoglycemic agents would have negatively impacted our study because we and others have used this approach in numerous studies conducted over the course of many years to avoid confounding effects of medications on study results (17, 24, 28).
Our study suffers from certain limitations. We opted not to measure blood flow by 133Xe washout as previously done by others (29–32). Although there have been conflicting views about the xenon adipose-blood partition coefficient, most authors agree that the partition coefficient is the same for lean and obese individuals. Additionally, based on prior studies, we do not anticipate that blood flow changes would occur during our experimental conditions (33, 34). Because we were unable to measure blood flow in this study, we were not able to calculate cortisol production in the sc fat depots. However, we do not anticipate that substantial cortisol production would occur in the fasted state, and had we measured blood flow and calculated cortisol production, our conclusions would have likely remained unchanged. Additionally, because we are comparing data for each subject with their baseline in the fasted state, each subject serves as his or her own control. Further studies are required to tease out differences, if any, in cortisol production in the fasted vs fed state under conditions of variable hormonal milieu.
In our hands, the insertion of microdialysis catheters results in very minimal trauma. Additionally, we provide a 60-minute stabilization period before starting infusions via the catheter. Measurements were taken after 4 hours of infusion, and the concordant enrichments obtained in the recovery of infused isotopes suggest that the study results were unlikely to be affected by the minimal trauma induced during insertion.
Furthermore, the molar enrichment of isotopes presented provides a reliable index of 11β-HSD-1 (reductase) and 11β-HSD-2 (dehydrogenase) activities in vivo. We conducted the experiment over 4 hours only. It is plausible that the conversion of D7 cortisone to D7 cortisol and D3 cortisol to D3 cortisone might have been higher had we extended the study period for a longer duration. It is noteworthy that in a small subset of individuals (n = 12), ie, four from each cohort, we continued the experiment for another 6 hours and did not observe any differences in molar enrichment of the isotopes at 4 vs 10 hours (data not shown), thereby excluding the possibility of time-dependent change in 11β-reductase and -dehydrogenase activities under these experimental conditions.
In conclusion, these data, collected by using tracer techniques in combination with microdialysis, provide new insight into 11β-HSD reductase activity in regional adipose tissue depots in lean and overweight/obese nondiabetic individuals and overweight/obese individuals with type 2 diabetes mellitus. These results suggest that reductase activity of 11β-HSD-1 enzyme in abdominal adipose tissue contributes to extraadrenal cortisol production in obese people with diabetes and that selective inhibitors of 11β-HSD-1 enzyme may be beneficial as a novel targeted therapy for type 2 diabetes mellitus. Further studies are required to investigate the role of blocking this enzyme pathway in adipose tissue.
Acknowledgments
Our deepest appreciation and thanks go to Robert A. Rizza for his valuable and constructive suggestions with the study. We are deeply indebted to the research participants. Our sincere thanks go to the staff of the Mayo Clinic Center for Clinical and Translational Science (CCaTS), Clinical Research Unit, the CCaTS Immunochemical Core Laboratory, and the CCaTS Metabolomics Core facility (Mai T. Persson). We thank Mayo Clinic employees, Pamela A. Reich (research assistant), Betty Dicke (laboratory technician), and Michael Q. Slama (laboratory technician) for technical assistance; Brent W. McConahey (laboratory technician) for technical assistance and graphic design; and Linda Kvall Boynton (medical secretary) for assistance with the preparation of the manuscript.
This work was supported by National Institutes of Health Grant R01 DK29953 and Grant UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- D7 cortisone
- [D3 cortisol, 9,12,12-2H3]cortisol; [2,2,4,6,6,12,12-2H7]cortisone
- 11β-HSD-1
- 11β-hydroxysteroid dehydrogenase type 1
- 11β-HSD-2
- 11β-hydroxysteroid dehydrogenase type 2
- LND
- lean nondiabetic
- ODM
- overweight/obese
- OND
- overweight/obese nondiabetic.
References
- 1. Walker BR. Is “Cushing's disease of the omentum” an affliction of mouse and men? Diabetologia. 2004;47:767–769. [DOI] [PubMed] [Google Scholar]
- 2. Moore JS, Monson JP, Kaltsas G, et al. Modulation of 11β-hydroxysteroid dehydrogenase isozymes by growth hormone and insulin-like growth factor: in vivo and in vitro studies. J Clin Endocrinol Metab. 1999;84:4172–4177. [DOI] [PubMed] [Google Scholar]
- 3. Jamieson PM, Chapman KE, Edwards CRW, Seckl JR. 11β-hydroxysteroid dehydrogenase is an exclusive 11β-reductase in primary cultures of rat hepatocytes: effect of physicochemical and hormonal manipulations. Endocrinology. 1995;136:4754–4761. [DOI] [PubMed] [Google Scholar]
- 4. Paulmyer-Lacroix O, Boullu S, Oliver C, Alessi M-C, Grino M. Expression of the mRNA coding for 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue from obese patients: an in situ hybridization study. J Clin Endocrinol Metab. 2002;87:2701–2705. [DOI] [PubMed] [Google Scholar]
- 5. Bujalska IJ, Kumar S, Stewart PM. Does central obesity reflect “Cushing's disease of the omentum”? Lancet. 1997;349:1210–1213. [DOI] [PubMed] [Google Scholar]
- 6. Basu R, Basu A, Grudzien M, et al. Liver is the site of splanchnic cortisol production in obese non-diabetic humans. Diabetes. 2009;58:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Whorwood CB, Donovan SJ, Flanagan D, Phillips DIW, Byrne CD. Increased glucocorticoid receptor expression in human skeletal muscle cells may contribute to the pathogenesis of the metabolic syndrome. Diabetes. 2002;51:1066–1075. [DOI] [PubMed] [Google Scholar]
- 8. Anagnostis P, Katsiki N, Adamidou F, et al. 11β-Hydroxysteroid dehydrogenase type 1 inhibitors: novel agents for the treatment of metabolic syndrome and obesity-related disorders? Metabolism. 2013;62:21–33. [DOI] [PubMed] [Google Scholar]
- 9. Masuzaki H, Paterson J, Shinyama H, et al. A transgenic model of visceral obesity and the metabolic syndrome. Science. 2001;294:2166–2170. [DOI] [PubMed] [Google Scholar]
- 10. Rask E, Walker BR, Söderberg S, et al. Tissue-specific changes in peripheral cortisol metabolism in obese women: increased adipose 11β-hydroxysteroid dehydrogenase type 1 activity. J Clin Endocrinol Metab. 2002;87:3330–3336. [DOI] [PubMed] [Google Scholar]
- 11. Rask E, Olsson T, Söderberg S, et al. Tissue-specific dysregulation of cortisol metabolism in human obesity. J Clin Endocrinol Metab. 2001;86:1418–1421. [DOI] [PubMed] [Google Scholar]
- 12. Lindsay RS, Wake DJ, Nair S, et al. Subcutaneous adipose 11β-hydroxysteroid dehydrogenase type 1 activity and messenger ribonucleic acid levels are associated with adiposity and insulinemia in Pima Indians and Caucasians. J Clin Endocrinol Metab. 2003;88:2738–2744. [DOI] [PubMed] [Google Scholar]
- 13. Katz JR, Mohamed-Ali V, Wood PJ, Yudkin JS, Coppack SW. An in vivo study of the cortisol-cortisone shuttle in subcutaneous abdominal adipose tissue. Clin Endocrinol (Oxf). 1999;50:63–68. [DOI] [PubMed] [Google Scholar]
- 14. Sandeep TC, Andrew R, Homer NZ, Andrews RC, Smith K, Walker BR. Increased in vivo regeneration of cortisol in adipose tissue in human obesity and effects of the 11β-hydroxysteroid dehydrogenase type 1 inhibitor carbenoxolone. Diabetes. 2005;54:872–879. [DOI] [PubMed] [Google Scholar]
- 15. Sutinen J, Kannisto K, Korsheninnikova E, et al. In the lipodystrophy associated with highly active antiretroviral therapy, pseudo-Cushing's syndrome is associated with increased regeneration of cortisol by 11β-hydroxysteroid dehydrogenase type 1 in adipose tissue. Diabetologia. 2004;47:1668–1671. [DOI] [PubMed] [Google Scholar]
- 16. Kannisto K, Pietiläinen KH, Ehrenborg E, et al. Overexpression of 11β-hydroxysteroid dehydrogenase-1 in adipose tissue is associated with acquired obesity and features of insulin resistance: studies in young adult monozygotic twins. J Clin Endocrinol Metab. 2004;89:4414–4421. [DOI] [PubMed] [Google Scholar]
- 17. Valsamakis G, Anwar A, Tomlinson JW, et al. 11 β-Hydroxysteroid dehydrogenase type 1 activity in lean and obese males with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2004;89:4755–4761. [DOI] [PubMed] [Google Scholar]
- 18. Stomby A, Andrew R, Walker BR, Olsson T. Tissue-specific dysregulation of cortisol regeneration by 11βHSD1 in obesity: has it promised too much? Diabetologia. 2014;57:1100–1110. [DOI] [PubMed] [Google Scholar]
- 19. Draper N, Stewart PM. 11 β-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J Endocrinol. 2005;186:251–271. [DOI] [PubMed] [Google Scholar]
- 20. Dube S, Norby B, Pattan V, et al. Hepatic 11β-hydroxysteroid dehydrogenase type 1 activity in obesity and type 2 diabetes using a novel triple tracer cortisol technique. Diabetologia. 2014;57(7):1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor RL, Machacek D, Singh RJ. Validation of a high-throughput liquid chromatography-tandem mass spectrometry method for urinary cortisol and cortisone. Clin Chem. 2003;48:1511. [PubMed] [Google Scholar]
- 22. Basu R, Singh RJ, Basu A, et al. Splanchnic cortisol production occurs in humans: evidence for conversion of cortisone to cortisol via the 11-β hydroxysteroid dehydrogenase (11-β HSD) type 1 pathway. Diabetes. 2004;53:2051–2059. [DOI] [PubMed] [Google Scholar]
- 23. Hughes KA, Manolopoulos KN, Iqbal J, et al. Recycling between cortisol and cortisone in human splanchnic, subcutaneous adipose, and skeletal muscle tissues in vivo. Diabetes. 2012;61:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Andrews RC, Herlihy O, Livingstone DEW, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87:5593. [DOI] [PubMed] [Google Scholar]
- 25. Wake DJ, Homer NZ, Andrew R, Walker BR. Acute in vivo regulation of 11β-hydroxysteroid dehydrogenase type 1 activity by insulin and intralipid infusions in humans. J Clin Endocrinol Metab. 2006;91:4682–4688. [DOI] [PubMed] [Google Scholar]
- 26. Goedecke JH, Wake DJ, Levitt NS, et al. Glucocorticoid metabolism within superficial subcutaneous rather than visceral adipose tissue is associated with features of the metabolic syndrome in South African women. Clin Endocrinol (Oxf). 2006;65:81–87. [DOI] [PubMed] [Google Scholar]
- 27. Livingstone DE, Kenyon CJ, Walker BR. Mechanisms of dysregulation of 11β-hydroxysteroid dehydrogenase type 1 in obese Zucker rats. J Endocrinol. 2000;167:533–539. [DOI] [PubMed] [Google Scholar]
- 28. Basu R, Singh RJ, Chittilapilly EG, et al. Obesity and type 2 diabetes do not alter splanchnic cortisol production in humans. J Clin Endocrinol Metab. 2005;90:3919–3926. [DOI] [PubMed] [Google Scholar]
- 29. Nielsen SL. Adipose tissue blood flow determined by the washout of locally injected 133 Xenon. Scand J Clin Lab Invest. 1972;29:31–36. [DOI] [PubMed] [Google Scholar]
- 30. Bulow J, Jelnes R, Astrup A, Madsen J, Vilmann P. Tissue/blood partition coefficients for xenon in various adipose tissue depots in man. Scand J Clin Lab Invest. 1987;47:1–3. [DOI] [PubMed] [Google Scholar]
- 31. Coppack SW, Evans RD, Fisher RM, et al. Adipose tissue metabolism in obesity: lipase action in vivo before and after a mixed meal. Metabolism. 1992;41:264–272. [DOI] [PubMed] [Google Scholar]
- 32. Jansson PA, Lonnroth P. Comparison of two methods to assess the tissue/blood partition coefficient for xenon in subcutaneous adipose tissue in man. Clin Physiol. 1995;15:47–55. [DOI] [PubMed] [Google Scholar]
- 33. Samra JS, Clark ML, Humphreys SM, MacDonald IA, Bannister PA, Frayn KN. Effects of physiological hypercortisolemia on the regulation of lipolysis in subcutaneous adipose tissue. J Clin Endocrinol Metab. 1998;83:626–631. [DOI] [PubMed] [Google Scholar]
- 34. Hazlehurst JM, Gathercole LL, Nasiri M, et al. Glucocorticoids fail to cause insulin resistance in human subcutaneous adipose tissue in vivo. J Clin Endocrinol Metab. 2013;98:1631–1640. [DOI] [PubMed] [Google Scholar]


