Abstract
Context:
Late-term pregnancy may lead to maternal and neonatal morbidity and mortality. Mice null for the progesterone receptor co-regulator Krüppel-like Factor 9 (KLF9) exhibit delayed parturition and increased incidence of neonatal deaths.
Objective:
Our aim is to evaluate the contribution of myometrial KLF9 to human parturition.
Design:
Myometrial biopsies were obtained from women with term (>37 to ≤41 wk) and late-term (>41 wk) pregnancies during cesarean delivery and assessed for gene and protein expression. Human myometrial cells transfected with nontargeting or KLF9 small interfering RNAs (siRNA) were treated with the progesterone antagonist RU486 and analyzed for pro-inflammatory chemokine/cytokine gene expression.
Setting:
The study took place in a University-affiliated tertiary care hospital and University research laboratory.
Patients:
Term patients (n = 8) were in spontaneous active labor whereas late-term patients (n = 5) were either in or were induced to active labor, prior to elective cesarean delivery.
Outcome Measures:
Steroid hormone receptor, contractility, and inflammation-associated gene expression in myometrial biopsies and in siKLF9-transfected, RU486-treated human myometrial cells was associated with KLF9 expression levels.
Results:
Myometrium from women with late-term pregnancy showed lower KLF9, total PGR, and PGR-A/PGR-B isoform expression. Transcript levels of select chemokines/cytokines were up- (CSF3, IL1, IL12A, TGFB2) and down- (CCL3, CCL5, CXCL1, CXCL5, IL15) regulated in late-term relative to term myometrium. Knock-down of KLF9 expression in RU486-treated human myometrial cells modified the expression of PGR and labor-associated cytokines, relative to control siRNA-treated cells.
Conclusions:
Myometrial KLF9 may contribute to the onset of human parturition through its regulation of PGR expression and inflammatory signaling networks.
Parturition is a complex physiologic process regulated by numerous signals originating from both the maternal and fetal units (1). These signals are responsible for the transformation of the progesterone (P4)-controlled quiescent myometrium to an active contractile state to initiate labor for successful birth (1, 2). Premature or delayed functional withdrawal of P4 signaling, respectively, leads to preterm or late/post-term pregnancies (3, 4) that have adverse effects on the wellbeing of the mother and her child (5, 6). Preterm birth (prior to 37 wk of pregnancy) affects nearly 1 of 8 infants born in the United States (www.cdc.gov). Further, preterm-related deaths account for approximately 35% of all infant deaths, and preterm infants who survived through childhood are often plagued with long-term physical and neurological disabilities including increased incidence of autism (7). Post-term births (>41 wk), although less prevalent than preterm births, have similar significant economic, emotional, and physical costs. Antepartum stillbirths account for more peri-natal deaths than either complications of prematurity or sudden infant syndrome (3, 5, 6). Neurologic complications are also highly associated with post-term pregnancy (8). Thus, understanding the complex mechanisms responsible for on-time labor has major consequences to population health in the United States and globally.
Progesterone responsiveness in target tissues is mediated by two major progesterone receptor (PGR) isoforms, PGR-A and PGR-B and various nuclear coregulators (9, 10). Ligand-bound PGR-B is the principal mediator of uterine myometrial quiescence. In humans, an increase in myometrial PGR-A to PGR-B ratio at term coincident with maintenance of high P4 levels initiates functional withdrawal of PGR-B action, resulting in the activation of estrogen receptor signaling and leading to induction of contraction-associated (oxytocin receptor [OXTR], gap junction alpha 1 [GJAI]) and nuclear factor κB (NF-κB) –mediated inflammatory cytokine/chemokine expression (4, 11, 12). The mechanism(s) responsible for the increase in PGR-A relative to PGR-B near term is not well-understood; nevertheless, in vitro evidence supporting PGR-A inhibition of PGR-B action (13) as well as the presence of distinct PGR-A vs PGR-B coregulators (9, 10) may contribute to predominance of PGR-A sensitivity and hence, action near term. In this regard, several corepressors of PGR-B signaling that target P4-sensitive contraction-associated genes have been described (14, 15).
Our group has previously identified the transcription factor Krüppel-like factor 9 (KLF9) as a PGR coregulator relevant to uterine biology and pathology (16–21). In vitro evidence suggested that in uterine endometrial cells, KLF9 selectively enhanced PGR-B-mediated transactivation (22, 23). Moreover, Klf9 null mutant mice exhibit delayed parturition, due in part to lower myometrial PGR-A levels, when compared with Klf9 wild type mice near term (24). Although KLF9 expression in the human endometrium has been previously demonstrated (19, 21), a systematic investigation of KLF9 expression in human myometrium under normal or pathological contexts is lacking. In the present study, we determined KLF9 expression in myometrium of women with term (>37 to ≤41 wk) and late-term (>41 wk) pregnancies and examined potential association between its expression and those of parturition-associated genes.
Materials and Methods
Study population and tissue collection
The case-study research design to explore the association between myometrial KLF9 expression and term pregnancy was approved by the Institutional Review Board of the Crozer-Chester Medical Center (Upland, Pennsylvania) and women signed informed consent to participate. Subject demographic data for women with term (>37 to ≤41 wk; n = 8) and late-term (>41 wk; n = 5) pregnancies are presented in Supplemental Table 1; there were no exclusion criteria except for age less than 17 years old. Term pregnancy patients presented to the Labor and Delivery Unit with spontaneous active labor, whereas late-term pregnancy patients were either in or were induced to active labor. All underwent elective cesarean surgery with obstetric indications (eg, arrest of labor progress, nonreassuring fetal heart rate, breech in active labor) and/or declined the option of vaginal birth after prior history of cesarean delivery. Biopsies (1 cm3) were obtained from the upper edge of uterine incisions (at the lower uterine segment) after delivery. Samples were snap-frozen in liquid nitrogen for subsequent analyses (below).
Western blot analyses
Nuclear and cytoplasmic proteins from isolated myometrial biopsies were prepared using NE-PER Nuclear and Cytoplasmic Extraction Kit (Pierce Biotechnology) and resolved by SDS-PAGE. Proteins were incubated with rabbit polyclonal antirat KLF9 (16), mouse monoclonal antihuman PGR (PGR-1294; Dako), and rabbit polyclonal antimouse estrogen receptor α (ERα) (MC-20; Santa Cruz Biotechnology) antibodies. The anti-KLF9 and anti-ERα antibodies were previously shown to recognize corresponding human and mouse proteins (19, 20, 25). Protein-antibody complexes were detected as described previously (18). Membranes were reprobed with rabbit antihuman Lamin A antibody (Sigma-Aldrich) as normalizing control.
Immunohistochemistry
Paraffin-embedded human myometrial samples were serially sectioned, dewaxed with xylene, and rehydrated through a graded alcohol series as previously described (18). Antigen unmasking was performed by boiling the sections in Citra Plus (Biogenex) for 30 minutes. After cooling to room temperature, sections were treated with 3% hydrogen peroxide to quench endogenous peroxidase activity and incubated in blocking solution with IgG (Vectastain ABC Kit, Vector Laboratories) for 1 hour. Sections were then incubated overnight at 4°C with rabbit polyclonal antimouse ERα antibody (MC-20; Santa Cruz Biotechnology) at 1:100 dilution or mouse monoclonal antihuman PGR antibody (PGR-1294; Dako) at 1:50 dilution. Following incubation with antirabbit or antimouse secondary antibodies (Vectastain ABC Kit) for 30 minutes, sections were stained with 3,3′-diaminobenzidine tetra-hydrochloride (Dako) and counterstained with hematoxylin. Control sections were processed similarly with omission of primary antibody. Results are expressed as % nuclear-immunopositive cells [(number of nuclei-staining cells/number of total cells counted) × 100].
RNA isolation and analyses
Total RNA was isolated from tissues or cells using TRIzol (Invitrogen) following the manufacturer's instructions. RNA (1 μg) was reverse-transcribed to cDNA using iScript cDNA synthesis kit (Bio-Rad Laboratories) and used for SYBR green-based real-time PCR (18). Primer sequences and amplicon sizes are provided in Supplemental Table 2. Transcript levels were normalized to corresponding levels of 18S and were calibrated to a standard curve generated using pooled cDNA stocks.
Focused gene array analyses
Focused qPCR array (Human Cytokines and Chemokines PCR Array; QIAGEN) analyses followed protocols described by the manufacturer, using cDNAs prepared from total RNAs isolated from myometrial tissues of women with term (>37 to ≤41 wk) and late-term (>41 wk) pregnancies. The array profiles the expression of 84 known secreted proteins related to immune and inflammatory signaling (QIAGEN). RNAs pooled from samples of three (term) and two (late-term) women, respectively constituted one biological replicate and were hybridized to one array; two independent replicates (arrays) were evaluated per patient group. qPCR of cDNAs prepared from individual RNAs (n = 5), each representing an individual subject, was used to confirm differential expression.
Cell culture treatments and RNA interference
The human uterine smooth muscle cell line was obtained from Lifeline Cell Technology. Cells were maintained and propagated following the company's protocol. Due to their limited life span (up to 15 passages), cells were used between passages 3–5 after receipt from the manufacturer and initial propagation. For the siRNA targeting experiments, cells were grown up to 70% confluence and treated with 17β-estradiol (E2, 10nM [Sigma]) and progestin (medroxyprogesterone acetate, P4; 1μM [Sigma]) in regular growth medium. After 48 hours, cells were transfected with siRNAs targeting human KLF9 (siGeNOME SMART pool) or nontargeting (siCONTROL) siRNAs (Dharmacon) using Dharmafect reagent 4 (ThermoFisher Scientific) in regular growth medium. Twenty-four hours later, cells were treated with the progestin antagonist RU486 (mifepristone, 100nM [Sigma]) and incubated for an additional 24 hours. Collected cells were processed for RNA analyses.
Statistical analysis
Data (mean ± SEM) were analyzed by two-tailed Student t test using SigmaStat 3.5 software (SPSS). P ≤ .05 was considered significant.
Results
Altered PGR and KLF9 expression in myometrium of women with late-term pregnancy
To examine a potential contribution of KLF9 to human parturition, we evaluated myometrial biopsies obtained from women with gestational age of >37 to ≤41 weeks (n = 8) and >41 weeks (n = 5), respectively during elective cesarean delivery (Supplemental Table 1). Patients had comparable body mass index (BMI) (Table 1), did not statistically differ in mean age, were ethnically diverse (Supplemental Table 1), and presented with obstetric indications. The infant birth weights for women with late-term pregnancies were higher (approaching significance at P = .07) than those of women with term pregnancies (Table 1).
Table 1.
Maternal and Fetal Birth Weights
| Parameter | ≤41 Weeks (n = 8) | >41 Weeks (n = 5) | P Value |
|---|---|---|---|
| Maternal age, y | 29.12 ± 2.92 | 22.60 ± 1.36 | .12 |
| BMI, kg/m2 | 32.01 ± 2.05 | 34.36 ± 2.05 | .51 |
| Birth weight, lb | 7.50 ± 0.34 | 8.56 ± 0.42 | .07 |
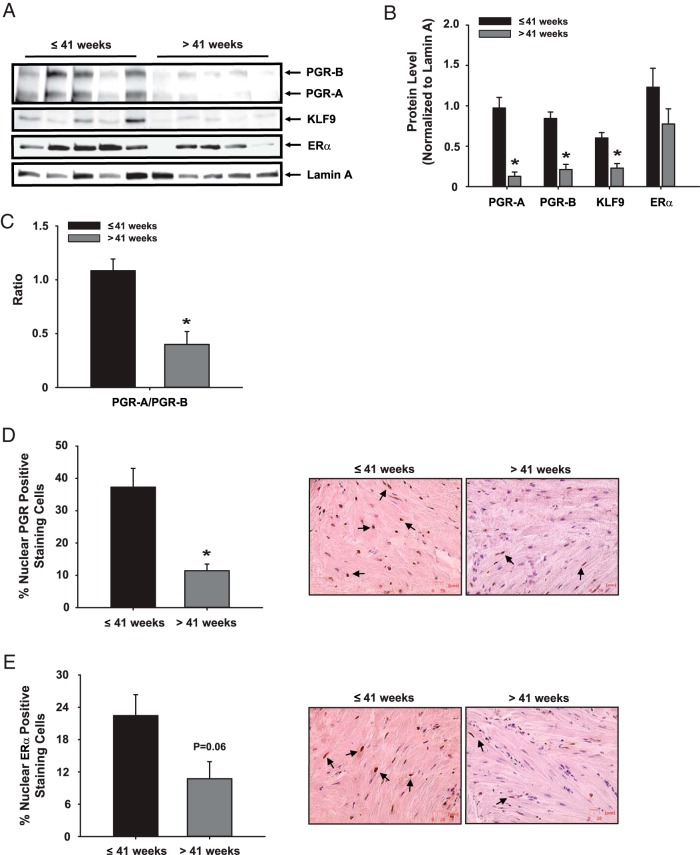
Myometrium of women with term pregnancies had lower protein levels of PGR-A, PGR–B and KLF9 but comparable ERα protein levels relative to those of women with late-term pregnancies (Figure 1, A and B). Notably, the expression ratio of PGR-A to PGR-B was lower in those with prolonged pregnancy (Figure 1C). The significant reduction in total PGR expression, which was not observed for ERα (P = .06) in late-term pregnancy myometrium was confirmed by immunohistochemistry (Figure 1, D and E).
Figure 1.
A–E, Expression of PGR isoforms, ERα, and KLF9 in term vs late-term pregnancy myometrium. A, Nuclear extracts prepared from myometrial biopsies of women with term and late-term pregnancies were evaluated for expression of PGR-A, PGR-B, KLF9, and ERα by Western blot analyses. Lamin A protein served as normalizing control. B, Immunoreactive bands were quantified by densitometry (each lane represents an individual patient) and normalized to those for Lamin A. C, PGR-A to PGR-B isoform expression ratio from data presented in panels A and B. Tissue sections of myometrial samples were stained with D, anti-PGR; or E, anti-ERα antibodies, and the percentage of stained cells was determined by counting the number of immunopositive nuclei over the total number of cells per field. The graphical data (mean ± SEM) represent analyses of tissue sections from n = 4 individual patients per group. Representative pictures of immunostained cells are shown. *, P < .05 by the Student t test.
Human myometrium at late-term pregnancy displayed normal expression levels of contractility-associated and clock-related genes
To expand the repertoire of labor-related genes that may be linked with reduced myometrial KLF9 expression and delayed parturition, the expression of select contractility-associated genes (OXTR, GJA1, RXFP1, NR3C1), KLF9 family members (KLF4, KLF11, KLF13), and clock genes related to timing of labor (BMAL1, CRY1, CRY2, PER1, PER2) were evaluated by qPCR in myometrium of women with term vs late-term pregnancy. Only relaxin receptor protein 1 (RXFP1) and KLF11 transcript levels differed (P < .05) between the two groups (Figure 2, A and B).
Figure 2.
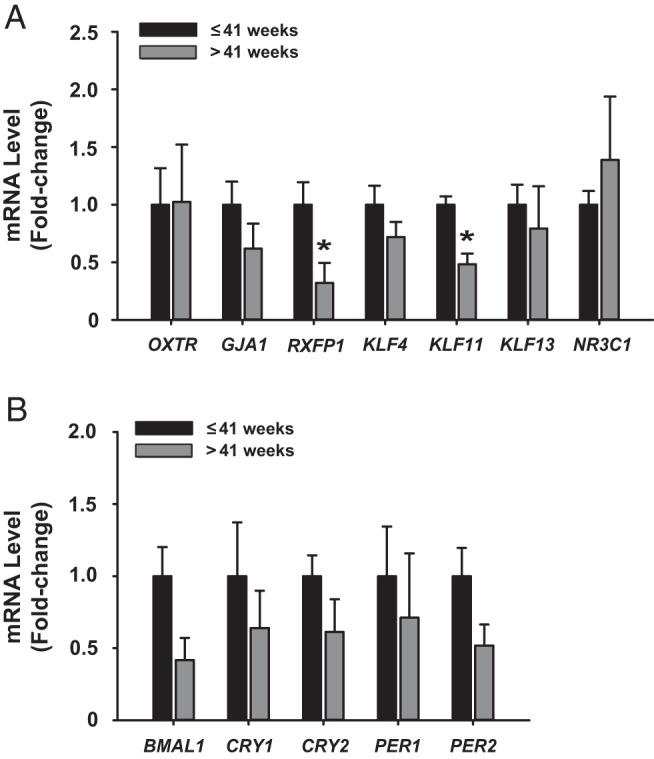
Expression of select genes in myometrium of women with term vs late-term pregnancy. Transcript levels of contractility-associated (OXTR, GJA1, RXFP1, NR3C1), KLF9 family members (KLF4, KLF11, KLF13), A; and clock-related (BMAL1, CRY1, CRY2, PER1, PER2), B genes were quantified by qPCR. Data (mean ± SEM) are expressed as fold-change and were obtained from n = 4–5 individual myometrial biopsy RNAs, each representing an individual patient. *, P < .05 by the Student t test.
Human myometrial cytokine/chemokine expression differed between term and late-term pregnancy
We used a Human Cytokine/Chemokine PCR Array to identify inflammatory mediators whose aberrant expression may be related to prolonged pregnancy. From a total of 84 known immune/inflammatory-related genes present in the Focused Array, six were up-regulated and 11 were down-regulated by at least 1.4-fold in late-term relative to term pregnancy myometrium (Table 2). qPCR using individual samples of the two patient groups demonstrated that transcript levels of CSF3, IL11, IL12A, and TGFB2 were up-regulated whereas those for CCL3, CCL5, CXCL1, CXCL5, and IL15 were down-regulated in late-term vs term pregnancy myometrium (Figure 3, A and B).
Table 2.
Differentially Expressed Immune Signaling Genes
| Gene | Fold Changea |
|---|---|
| Anti-inflammation | |
| CSF3 | 13.99 |
| IL1RN | 2.28 |
| IL6 | 4.49 |
| IL11 | 10.60 |
| IL12A | 3.57 |
| TGFB2 | 1.36 |
| Pro-inflammation | |
| CCL3 | −1.58 |
| CCL5 | −1.54 |
| CSF1 | −1.35 |
| CXCL1 | −2.03 |
| CXCL5 | −1.62 |
| CXCL9 | −1.83 |
| CXCL11 | −1.88 |
| IL1A | −3.71 |
| IL15 | −2.00 |
| IL5 | −1.88 |
| LTB | −1.34 |
Genes were identified using cytokines and chemokines qPCR array.
Late-term relative to term myometrium; negative fold-chain values denote down-regulation.
Figure 3.
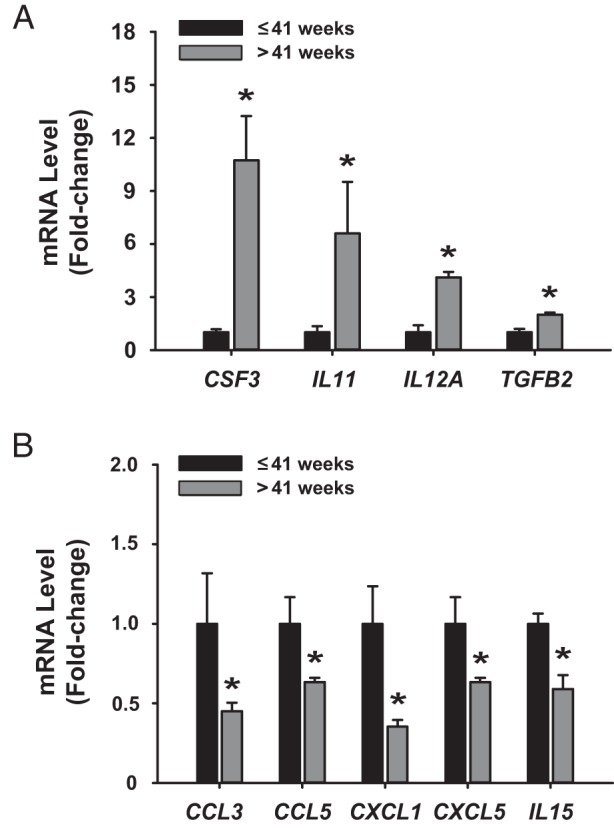
Term and late-term pregnancy myometria show distinct cytokine/chemokine expression profiles. Validation by qPCR of expression of A, up-regulated; and B, down-regulated cytokines/chemokines identified from Focused PCR array analyses of myometrium from women with term vs late-term pregnancies. Data (mean ± SEM) are expressed as fold-change and were obtained from n = 4–5 individual myometrial biopsy RNAs, each representing an individual patient. *, P < .05 by the Student t test.
KLF9 influenced antagonism between progesterone and proinflammatory signals in human myometrial cells in vitro
To determine whether KLF9 directly regulates myometrial progesterone sensitivity and induction of inflammatory signals in near-term myometria leading to normal labor onset, we used a human uterine smooth muscle cell line to evaluate response of myometrial cells to progesterone withdrawal under conditions of reduced KLF9 expression, the latter achieved by KLF9 siRNA targeting. Subconfluent cells were treated with E2 + P4 for 2 days to mimic pregnancy conditions and then transfected with nontargeting (control) or KLF9 siRNAs prior to treatment with RU486 (Figure 4A). By binding to PGR, RU486 initiates progesterone withdrawal and promotes inflammation, leading to parturition within 24 hours in vivo (26). RU486-treated cells with a significant reduction in KLF9 transcript levels (by 80%) had elevated total PGR, PGR-B, and ERα transcript levels; no significant changes were noted for contractility-associated (OXTR, GJA1) and Glucocorticoid receptor (NR3C1) transcript levels (Figure 4B). RU486-treated cells with KLF9 loss-of-expression also manifested decreased transcript levels for the proinflammatory cytokines/chemokines CCL3, CXCL1, CXCL5 and IL6 (Figure 4C). By contrast, Prostaglandin F receptor (PTGFR) gene expression was increased, consistent with the decline in its myometrial expression with labor onset (27).
Figure 4.
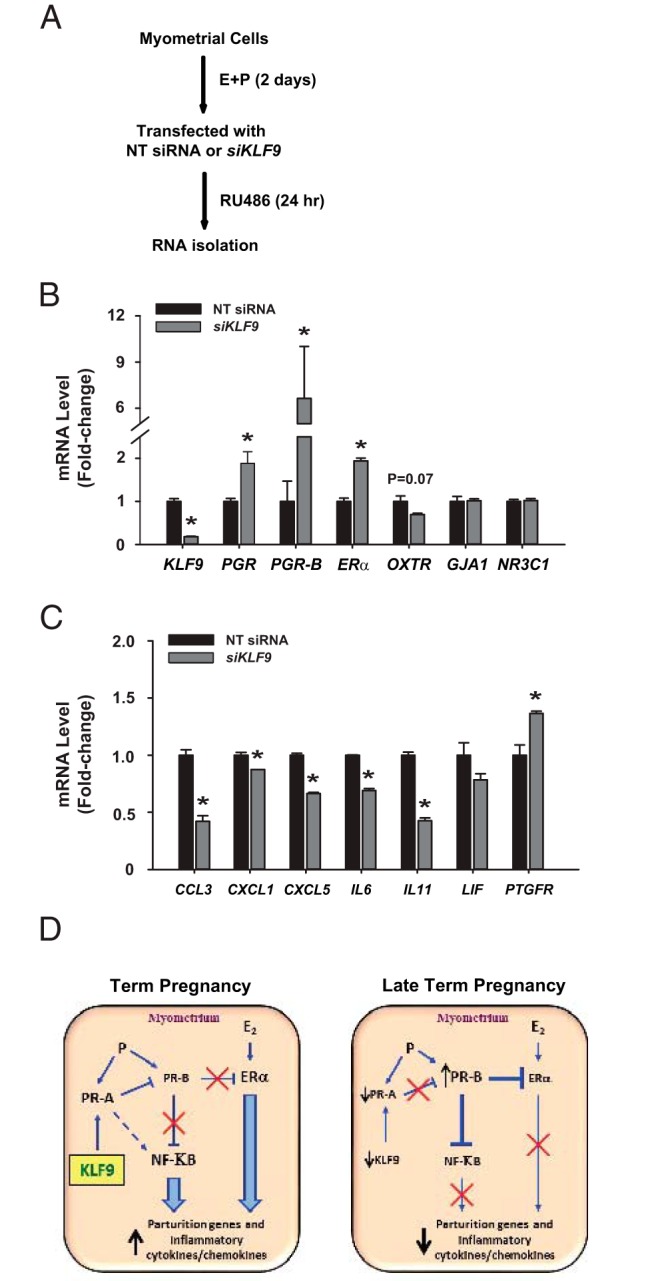
Potential function of KLF9 in human myometrial cells. A, Schematic of treatment protocols for human myometrial cells. Cells were pretreated with estradiol (E) and medroxyprogesterone acetate (P) for 2 days prior to transfection with control (nontargeting, NT) or KLF9 small interfering RNAs (siKLF9) and treatment with RU486. Cells were processed for RNA analyses. B and C, Transcript levels of select genes associated with labor onset were evaluated from RNAs of two independent experiments, by qPCR. Data (mean ± SEM) are expressed as fold-change relative to NT siRNA-treated cells. *, P < .05 by the Student t test. D, Model illustrating potential regulation by KLF9 of PGR isoforms to influence inflammatory cytokines/chemokines and parturition. This model incorporates results from previously published studies by other investigators (1, 2, 11, 12) and our studies with the Klf9 null mice (24).
Discussion
This study constitutes to our knowledge, the first report on transcription factor KLF9 expression in human myometrium of pregnancy and provides support for its potential role in regulating the timely onset of parturition in humans, as was previously demonstrated in Klf9 null mutant mice with delayed parturition (24). Using myometrial biopsies isolated during cesarean delivery from women in active labor with normal (term) and delayed (late-term) pregnancy, we found that prolonged pregnancy is characterized by reduced myometrial KLF9 and total PGR protein levels and lower PGR-A/PGR-B isoform expression ratio. We showed that myometria of women with prolonged pregnancy, relative to those with term pregnancy, had decreased expression of the chemokines/cytokines CCL5, CCL3, CXCL1, and CXCL5, all of which were reported previously to be elevated in preterm relative to term-labor myometrium (28) and of IL15, an interferon γ-induced protein produced by monocytes, which are implicated in labor onset (29). Further, using RU486-treated human myometrial cells with siRNA-mediated KLF9 knock-down, we demonstrated that KLF9 can influence two requisite events for initiation of parturition, namely functional progesterone withdrawal and promotion of inflammatory status. Specifically, loss of KLF9 expression in myometrial cells in vitro led to an increase in total PGR, specifically in PGR-B transcript levels, and attenuated the expression of a subset of proinflammatory cytokines, including IL6, an essential determinant of on-time parturition in mice (30). Our previous demonstration of reduced sensitivity of Klf9 null mice to RU486-induced parturition near term (24) is consistent with these results. These collective findings suggest that KLF9 is expressed in near-term human myometrium; that a deficiency in KLF9 expression is associated with the pathology of delayed labor onset in women; and that the loss of induction of proinflammatory cytokine expression that drives inflammation for labor onset may be due in part to KLF9 loss.
The present study raised the possibility that KLF9 may contribute to the timing of human parturition by maintaining higher PGR-A relative to PGR-B expression near term. Indeed, we found that whereas PGR-A and PGR-B protein levels were comparable in term myometrium, late-term myometrium showed reductions in both isoforms, with the decrease greater for PGR-A than for PGR-B as reflected in the lower PGR-A-to-PGR-B protein ratio. The latter is consistent with reduced PGR-A protein levels in myometrium of Klf9 null relative to wild-type mice (24) and with the higher PGR-B transcript levels in RU486-treated human myometrial cells with KLF9 knock-down. Another potential causative mechanism of KLF9 in the timing of parturition may occur through KLF9 regulation of the proinflammatory cytokine/chemokine CCL3, CXCL1, and CXCL5 gene expression, which we suggest are direct KLF9 targets based on the combined in vivo (using myometrial biopsies) and in vitro (siKLF9-targeted myometrial cells) experiments. In previous studies (18, 19), we reported on a number of KLF9-regulated genes in human endometrial stromal cells. Interestingly, we found no overlap in stromal KLF9-regulated genes with those identified here for myometrial cells. Although the mechanistic underpinnings of KLF9 regulation of PGR isoform and of specific cytokine/chemokine expression were not addressed in the present study, the suggested relevance of KLF9 in the proposed regulatory network (Figure 4C) may provide new insights into the mechanisms of dysfunctional labor onset.
Using a combination of targeted (qPCR) and discovery (Focused Array) approaches, we show here that myometria isolated during cesarean delivery from women with term and late-term pregnancies are characterized by distinct gene expression patterns. Although the expression levels of contractility and clock genes related to labor initiation did not differ between the two groups, those for a subset of inflammatory mediators were significantly altered. The increase in IL11, IL12A, and TGFB2 expression in late-term pregnancy myometria is consistent with reports that IL11 is a progesterone down-regulated cytokine (31); IL12A is associated with reduced risk of prematurity (32) and reductions in TGFB2 expression facilitate inflammation (33). Similarly, the lower transcript levels for the chemokines CXCL1, CCL5, and CXCL5 in late-term pregnancy myometrium are supported by findings that CXCL1 is a direct target of progesterone (34) and that preterm labor is associated with elevated CCL5 and CXCL5 expression (28). The lack of direct correlations among expression patterns of contractility, clock-related, and inflammatory genes suggests that the kinetics of parturition initiation in response to functional progesterone withdrawal may differ for labor-associated contractility genes and for proinflammatory genes. In this regard, myometrium-specific knockout of the clock gene Bmal1, which disrupted the timing of parturition in mutant mice, also showed no effect on uterine Oxtr and Gja1 expression (35). The reduced expression of the myometrial relaxin receptor RXFP1 and conversely, the increased expression of colony stimulating factor 3 (CSF3, also designated as granulocyte-colony stimulating factor) in women with late-term vs term pregnancy are unexpected, given previous demonstration that progesterone withdrawal mediates the decrease of this receptor (36) and the suggested critical role of CSF3 in driving inflammation (37). Thus, the numerous pathways orchestrating the timing of labor may not be interdependent.
A significant finding related to KLF9's function in the myometrium is the absence of parallel deregulated expression of KLF4 and KLF13 with delayed parturition. In a number of uterine pathologies such as endometriosis (19) and endometrial cancer (20), the expression levels of these highly-related members are coincidentally reduced. We previously reported that KLF9 and KLF13 act as PGR coregulators (22, 23) and KLF4 has been shown to mediate PGR action in uterine endometrial cells (38). Moreover, Klf13 null mice do not display the delayed parturition characteristic of Klf9 null mice (39). These findings, coupled with the lack of shared KLF9-regulated genes in endometrial and myometrial compartments (above), suggest that distinct regulatory networks are used by KLF9 in these uterine cell types. Interestingly, family member KLF11 showed lower myometrial expression in late-term relative to term pregnancy; the latter is in agreement with the reported anti-inflammatory action of KLF11 in endothelial cells (40). A linkage between KLF9 and KLF11 in the myometrium to regulate parturition is an intriguing possibility that we plan to address in future studies.
A recognized limitation of the present study is the relatively small patient numbers used in the analyses. Moreover, whereas the patient population showed no significant differences in BMI and age, five of eight women with term pregnancies are multiparous compared with none in women with late-term pregnancies. Nevertheless, the observed significant difference in KLF9 expression between the two groups, which was associated with well-acknowledged markers of timing of labor onset suggests the relevance of KLF9 to parturition biology and provides a rationale for its further evaluation involving a larger population.
In conclusion, our studies constitute the first report indicating the potential contribution of myometrial KLF9 to the timing of parturition. Aberrant myometrial expression of PGR isoforms and of proinflammatory mediators in women with late-term pregnancy may be linked to attenuated myometrial KLF9. Our studies raise the interesting possibility of enhancing myometrial KLF9 expression as a candidate strategy for parturition onset to reduce maternal and infant morbidity and mortality.
Acknowledgments
We thank members of our laboratories for helpful discussions during the course of this work and the surgical team at Crozer-Chester Medical Center for assistance with human myometrial sample collection.
This work was supported by the National Institutes of Health/Eunice Kennedy Shriver Institute of Child Health and Human Development (HD21961).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CCL
- chemokine/cytokine ligand
- CXCL
- C-X-C motif ligand
- CSF3
- colony stimulating factor 3
- ERα
- estrogen receptor α
- Gja1
- gap junction alpha 1
- KLF9
- Krüppel-like factor 9
- NF-κB
- nuclear factor κB
- OXTR
- oxytocin receptor
- P4
- progesterone
- PGR
- progesterone receptor
- siRNA
- small interfering RNA
- TGFB2
- transforming growth factor B2.
References
- 1. Mendelson CR. Minireview: Fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol. 2009;23:947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shynlova O, Tsui P, Jaffer S, Lye SJ. Integration of endocrine and mechanical signals in the regulation of myometrial functions during pregnancy and labour. Eur J Obstet Gynecol Reprod Biol. 2009;144 Suppl 1:S2–10. [DOI] [PubMed] [Google Scholar]
- 3. Norwitz ER, Snegovskikh VV, Caughey AB. Prolonged pregnancy: When should we intervene? Clin Obstet Gynecol. 2007;50:547–557. [DOI] [PubMed] [Google Scholar]
- 4. Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab. 2002;87:2924–2930. [DOI] [PubMed] [Google Scholar]
- 5. Hilder L, Costeloe K, Thilaganathan B. Prolonged pregnancy: Evaluating gestation-specific risks of fetal and infant mortality. Br J Obstet Gynaecol. 1998;105:169–173. [DOI] [PubMed] [Google Scholar]
- 6. Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal and obstetric complications of pregnancy are associated with increasing gestational age at term. Am J Obstet Gynecol 2007;196:155.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Healy E, Reichenberg A, Nam KW, et al. Preterm birth and adolescent social functioning-alterations in emotion-processing brain areas. J Pediatr. 2013;163:1596–1604. [DOI] [PubMed] [Google Scholar]
- 8. Chantry AA, Lopez E. Fetal and neonatal complications related to prolonged pregnancy. J Gynecol Obstet Biol Reprod. 2011;40:717–725. [DOI] [PubMed] [Google Scholar]
- 9. McEwan IJ. Nuclear receptors: One big family. Methods Mol Biol. 2009;505:3–18. [DOI] [PubMed] [Google Scholar]
- 10. Lonard DM, O'Malley BW. Nuclear receptor coregulators: Modulators of pathology and therapeutic targets. Nat Rev Endocrinol. 2012;8:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: Evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. [DOI] [PubMed] [Google Scholar]
- 12. Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and –B have opposite effects on pro-inflammatory gene expression in human myometrial cells: Implications for progesterone actions in human pregnancy and parturition. J Clin Endocrinol Metab. 2012;97:E719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O'Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–1255. [DOI] [PubMed] [Google Scholar]
- 14. Dong X, Yu C, Shynlova O, Challis JR, Rennie PS, Lye SJ. p54nrb is a transcriptional corepressor of the progesterone receptor that modulates transcription of the labor-associated gene, connexin 43 (Gja1). Mol Endocrinol. 2009;23:1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bodoor K, Lontay B, Safi R, et al. Smoothelin-like 1 protein is a bifunctional regulator of the progesterone receptor during pregnancy. J Biol Chem. 2011;286:31839–31851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simmen RC, Eason RR, McQuown JR, et al. Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Krüppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem. 2004;9:279, 29286–29294. [DOI] [PubMed] [Google Scholar]
- 17. Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC. Null mutation of Kruppel-like factor9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod. 2005;73:472–481. [DOI] [PubMed] [Google Scholar]
- 18. Pabona JM, Zeng Z, Simmen FA, Simmen RC. Functional differentiation of uterine stromal cells involves cross-regulation between bone morphogenetic protein 2 and Kruppel-like factor (KLF) family members KLF9 and KLF13. Endocrinology. 2010;151:3396–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pabona JMP, Simmen FA, Nikiforov MA, et al. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: Implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2012;97:E376–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Simmons CD, Pabona JM, Heard ME, et al. Krüppel-like factor 9 loss-of-expression in human endometrial carcinoma links altered expression of growth-regulatory genes with aberrant proliferative response to estrogen. Biol Reprod. 2011;85:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Heard ME, Simmons CD, Simmen FA, Simmen RC. Krüppel-like factor 9 deficiency in uterine endometrial cells promotes ectopic lesion establishment associated with activated notch and hedgehog signaling in a mouse model of endometriosis. Endocrinology. 2014;155:1532–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang D, Zhang XL, Michel FJ, Blum JL, Simmen FA, Simmen RC. Direct interaction of the Krüppel-like family (KLF) member, BTEB1, and PR mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology. 2002;143:62–73. [DOI] [PubMed] [Google Scholar]
- 23. Zhang XL, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC. Selective interactions of Kruppel-like factor 9/basic transcription element-binding protein with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem. 2003;278:21474–21482. [DOI] [PubMed] [Google Scholar]
- 24. Zeng Z, Velarde MC, Simmen FA, Simmen RC. Delayed parturition and altered myometrial progesterone receptor isoform A expression in mice null for Krüppel-like factor 9. Biol Reprod. 2008;78:1029–1037. [DOI] [PubMed] [Google Scholar]
- 25. Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RC. Kruppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor alpha signaling in Ishikawa endometrial adenocarcinoma cells. Mol Endocrinol. 2007;21:2988–3001. [DOI] [PubMed] [Google Scholar]
- 26. Baulieu EE. The steroid hormone antagonist RU486. Mechanism at the cellular level and clinical applications. Endocrinol Metab Clin North Am. 1991;20:873–891. [PubMed] [Google Scholar]
- 27. Hay A, Wood S, Olson D, Slater DM. Labour is associated with decreased expression of the PGF2alpha receptor (PTGFR) and a novel PTGFR splice variant in human myometrium but not decidua. Mol Hum Reprod. 2010;16:752–760. [DOI] [PubMed] [Google Scholar]
- 28. Hua R, Pease JE, Sooranna SR, Viney JM, Nelson SM, Myatt L, Bennett PR, Johnson MR. Stretch and inflammatory cytokines drive myometrial chemokine expression via NF-κB activation. Endocrinology. 2012;153:481–491. [DOI] [PubMed] [Google Scholar]
- 29. Lee N, Shin MS, Kang KS, et al. Human monocytes have increased IFN-γ-mediated IL-15 production with age alongside altered IFN-γ receptor signaling. Clin Immunol. 2014;152:101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson SA, Christiaens I, Dorian CL, et al. Interleukin-6 is an essential determinant of on-time parturition in the mouse. Endocrinology. 2010;151:3996–4006. [DOI] [PubMed] [Google Scholar]
- 31. Cordeaux Y, Tattersall M, Charnock-Jones DS, Smith GC. Effects of medroxyprogesterone acetate on gene expression in myometrial explants from pregnant women. J Clin Endocrinol Metab. 2010;95:437–447. [DOI] [PubMed] [Google Scholar]
- 32. Harmon QE, Engel SM, Olshan AF, et al. Association of polymorphisms in natural killer cell-related genes with preterm birth. Am J Epidemiol. 2013;178:1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siglienti I, Chan A, Kleinschnitz C, et al. Downregulation of transforming growth factor-beta2 facilitates inflammation in the central nervous system by reciprocal astrocyte/microglia interactions. J Neuropathol Exp Neurol. 2007;66:47–56. [DOI] [PubMed] [Google Scholar]
- 34. Kavandi L, Collier MA, Nguyen H, Syed V. Progesterone and calcitriol attenuate inflammatory cytokines CXCL1 and CXCL2 in ovarian and endometrial cancer cells. J Cell Biochem. 2012;113:3143–3152. [DOI] [PubMed] [Google Scholar]
- 35. Ratajczak CK, Asada M, Allen GC, et al. Generation of myometrium-specific Bmal1 knockout mice for parturition analysis. Reprod Fertil Dev. 2012;24:759–767. [DOI] [PubMed] [Google Scholar]
- 36. Vodstrcil LA, Shynlova O, Westcott K, et al. Progesterone withdrawal, and not increased circulating relaxin, mediates the decrease in myometrial relaxin receptor (RXFP1) expression in late gestation in rats. Biol Reprod. 2010;83:825–832. [DOI] [PubMed] [Google Scholar]
- 37. Lawlor KE, Campbell IK, Metcalf D, et al. Criticial role for granulocyte colony-stimulating factor in inflammatory arthritis. Proc Natl Acad Sci U.S.A. 2004;101:11398–11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shimizu Y, Takeuchi T, Mita S, Notsu T, Mizuguchi K, Kyo S. Krüppel-like factor 4 mediates anti-proliferative effects of progesterone with G0/G1 arrest in human endometrial epithelial cells. J Endocrinol Invest. 2010;33:745–750. [DOI] [PubMed] [Google Scholar]
- 39. Heard ME, Pabona JM, Clayberger C, Krensky AM, Simmen FA, Simmen RC. The reproductive phenotype of mice null for transcription factor Krüppel-Like Factor 13 suggests compensatory function of family member Krüppel-Like Factor 9 in the peri-implantation uterus. Biol Reprod. 2012;87:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fan Y, Guo Y, Zhang J, et al. Krüppel-like factor-11, a transcription factor involved in diabetes mellitus, suppresses endothelial cell activation via the nuclear factor-κB signaling pathway. Arterioscler Thromb Vasc Biol. 2012;32:2981–2988. [DOI] [PMC free article] [PubMed] [Google Scholar]