Abstract
Context:
Telomere biology plays a fundamental role in genomic integrity, cellular regeneration, physiology, aging, disease risk, and mortality. The initial setting of telomere length (TL) in early life has important implications for telomere maintenance and related disorders throughout the life span. However, little is known about the predictors of this initial setting.
Objective:
Given the established role of estrogen on adult TL and the role of estriol (E3) in the context of fetal development, the goal of this study was to test the hypothesis that higher maternal E3 concentration during early pregnancy is associated with longer infant telomere length.
Design, Participants, and Setting:
Study participants comprised a cohort of N = 100 infants followed prospectively from intrauterine life and birth through early childhood from a population-based, representative sample of pregnant mothers recruited in early pregnancy at university-based obstetric clinics in Southern California. Maternal unconjugated E3 concentrations were assessed in plasma in early gestation (around wk 15). Infant TL was assessed in buccal cells at approximately 15 months of age.
Results:
After accounting for the effects of potential confounding maternal and infant variables, there was a significant, independent effect of maternal E3 concentration on infant TL (unstandardized β = 0.297; P = .001; 95% Cl, 0.121–0.473). Specifically, a one-multiple-of-the-median (MoM) increase in maternal E3 concentration during early pregnancy was associated with a 14.42% increase in infant TL.
Conclusions:
This study supports the concept of developmental plasticity of the telomere biology system and highlights specifically the role of a potentially modifiable intrauterine factor for additional mechanistic and clinical investigation.
Telomere biology has emerged in recent years as playing a pervasive and potentially causal role in key aspects of genomic integrity, cell physiology, regeneration, aging, and disease risk (1). Telomeres are tracts of noncoding tandem repeats of DNA sequences and their bound proteins that cap the ends of linear chromosomes, thereby protecting from degradation and deleterious recombination. Human cell telomeres shorten with each cell division, and their decline to critical length leads to loss of telomere function and consequent cellular malfunctions. Telomere shortness is associated with the occurrence and progression of common chronic disorders such as cardiovascular disease, hypertension, atherosclerosis, heart failure, type 2 diabetes (2), and with earlier mortality (3).
The initial setting of telomere length (TL) represents a critically important characteristic of an individual's telomere biology system (4). It constitutes one of two major determinants of TL at any subsequent age [the other determinant is TL attrition over time (1, 5)]. A recent prospective study of the natural life span in zebra finches established that TL in early life was a very strong predictor of realized life span (6). Moreover, a recent study in human adults reported that although TL varies within individuals across different tissues (blood, muscle, skin, and fat) the rate of age-dependent TL shortening is similar across different somatic tissues, thereby supporting the notion that the observed TL differences across tissues are established in early life (7). Despite the presumed importance of the initial or early life setting of TL for human health, little is known about the determinants of the initial setup, as recently reviewed (8).
Despite relatively high heritability estimates, known genetic variants account for only a small proportion of the variance in TL [eg, (4, 9)]. Because heritability estimates represent a combined effect of genetic makeup and environmental conditions in utero, this observation highlights the importance of a better understanding of the intrauterine milieu that may contribute to early life TL (8). Our own work and that of other groups suggests that adverse or suboptimal conditions in intrauterine life such as intrauterine growth restriction, preeclampsia, and maternal stress are associated with shorter offspring TL [summarized in (8)], thereby supporting the concept that TL may, in part, be programmed in utero.
Telomeres are longer in adult women than in men (10). It is believed that estrogens account, in part, for this consistently observed sex difference. In vitro, estrogens stimulate the transcription of the gene encoding the telomerase reverse transcriptase enzyme that adds telomere repeats to chromosome ends, thereby slowing the rate of telomere attrition (11–14). Furthermore, estrogens reduce oxidative stress, which is know to shorten telomeres via several pathways [summarized in (15)] and to indirectly influence TL. In postmenopausal women, duration of endogenous estrogen exposure (menses to menopause) is associated with longer TL (16).
The estrogen estriol (E3) plays a particularly prominent role in primates in the context of pregnancy and fetal development. Dehydroepiandosterone produced by the fetal adrenals and modified in the fetal liver by 16-hydroxylation is converted into E3 in the placenta. The maternal liver lacks the capacity to express 16-hydroxylase, and dehydroepiandrosterone (DHEA) produced by the maternal adrenal is converted by the placenta into estradiol but not E3. Thus, E3 arises solely from fetal precursors (17). Once produced by the placenta, E3 is released into the maternal and fetal compartments, and there is a significant correlation between maternal and fetal E3 (18). Because the production of E3 depends on an intact maternal-placental-fetal unit, E3 levels in maternal blood are used in research and clinical practice to monitor fetal status during intrauterine life. In clinical practice maternal blood unconjugated estiol (uE3) is one of the components of the quad marker screen, and a low uE3 level in women suggests the possibility of fetal loss, fetal chromosomal abnormalities or fetal adrenal insufficiency (19, 20). In contrast, an elevated maternal blood uE3 can be an indication of pending labor or adverse pregnancy outcomes (21–23).
Given 1) the importance of achieving a better understanding of the initial setting of TL, 2) the possibility that the intrauterine milieu influences the initial setting of TL, 3) the established role of estrogen on adult TL via its effects on telomerase expression and oxidative stress, and 4) the clinically established role of E3 in the context of fetal development, the goal of this study was to test the hypothesis that higher maternal E3 concentrations during early pregnancy are associated with longer telomeres in infants.
Materials and Methods
Participants
Study participants comprised a cohort of children followed prospectively from intrauterine life and birth through infancy and into early childhood. Pregnant mothers were recruited in early gestation from obstetric care provider clinics at the University of California, Irvine, School of Medicine in Orange, California, and the Cedars Sinai Medical Center in Los Angeles, California. Exclusionary criteria at the time of recruitment of pregnant mothers included tobacco, alcohol, or other drug use in pregnancy, uterine or cervical abnormalities, or presence of conditions associated with dysregulated neuroendocrine and immune function such as endocrine, hepatic, or renal disorders or corticosteroid medication use. Exclusion criteria for children were preterm delivery less than 34 weeks gestation, perinatal complications associated with neurological consequences (eg, hypoxia), and congenital, genetic, or neurologic disorders (eg, fetal alcohol syndrome, Down syndrome, or other aneuploidy, fragile X syndrome). All pregnant mothers that met the inclusion/exclusion criteria at the study clinics were approached consecutively. The final sample consisted of 100 mother-child pairs. Forty-three percent of the children were girls.
At the time of TL assessment, children were, on average, 14.6 months old (mean ± SEM, 14.6 ± 1.08 mo; range, 0–46 mo). There were no differences in age at TL assessment based on sex (F [1,99] = 2.21, P = .35). The sociodemographic, pregnancy, and concurrent maternal and child characteristics of the study population are presented in Table 1. All the study procedures were approved by the institutional review boards of both institutions, and all mothers provided written informed consent.
Table 1.
Maternal and Infant Characteristics
| Maternal Characteristics | n = 100 Mother-Infant Dyads |
|---|---|
| Sociodemographic | |
| Age, y, mean ± sd | 29.1 ± 5.8 |
| Race/Ethnicity | |
| Hispanic White, % | 34 |
| Non-Hispanic White, % | 41 |
| Non-Hispanic African American, % | 13 |
| Non-Hispanic other race, % | 12 |
| Years of school completed, mean ± sd | 14.2 ± 2.4 |
| Presence of obstetric risk conditions during index pregnancy, % | 21 |
| Child characteristics | |
| Sex (female) | 43 |
| Gestational age at birth wk, mean ± sd | 38.8 ± 1.9 |
| Birth weight, g, mean ± sd | 3378 ± 549 |
| Age at time of TL assessment mo, mean ± sd | 14.6 ± 11.5 |
Maternal E3 concentrations
A maternal blood sample was collected by venipuncture at the first pregnancy visit to our research clinic (mean ± SD gestational age at visit, 15 ± 0.7 wk). This coincides with the time at which standard clinical screening tests are performed to assess maternal E3 and other markers of fetal development. The maternal blood sample was transferred to heparin tubes and centrifuged at 2000× g at 4 C° for 15 minutes. Plasma was separated and kept at −20°C until assayed. uE3 concentrations were determined by ELISA (IBL-America).
Obstetric risk, length of gestation, and birth weight
Obstetric risk conditions were ascertained by medical chart review, and obstetric risk was defined as the presence of any major medical complications in the index pregnancy (gestational diabetes, vaginal bleeding, placenta abruptio, pregnancy-induced hypertension, preeclampsia, or infection) and coded as a binary variable, as previously described (24).
Gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry before 20 weeks using standard clinical criteria (25). Birth outcomes were abstracted from the medical record, and birth weight percentiles were computed using national norms (26).
Maternal sociodemographic characteristics
At the first pregnancy visit standardized, structured interviews were administered for ascertainment of maternal sociodemographic characteristics (maternal age, education, race/ethnicity).
Infant TL
TL was assessed in buccal cells collected at the time of infant follow-up by brushing the oral mucosa with a sterile nylon bristle cytology brush using a separate brush and 10 strokes for each cheek. Samples were processed and DNA was purified using the Puregene DNA isolation kid (Gentra Systems, now QIAGEN) for analyzing DNA with buccal cells (27). Measurement of relative TLs (telomere repeat copy number to single gene copy number [T/S ratio]) by qPCR were adapted from a validated, published method (28) and performed as previously described (29).
Statistical analyses
The distributions of continuous variables included in the analyses were tested for normality (Kolmogorov-Smirnov test). All continuous variables were normally distributed (P > .200 for null-hypothesis). Pearson product moment correlations were used to test bivariate associations between E3 concentrations and other variables (maternal prepregnancy body mass index [BMI], maternal BMI, weight gain during pregnancy, obstetric risk, maternal educational level, gestational age at birth, birth weight, infant sex). Differences in E3 concentrations between different racial/ethnic groups was tested using an univaraite ANOVA. TL between boys and girls was compared using univariate ANOVA, adjusting for the effect of age. Multiple linear regression was used to quantify the association between maternal E3 concentrations (entered as a continuous variable) and infant TL with adjustment for the effects of other potential determinants. Because of assay method variation from laboratory to laboratory, E3 concentrations are typically expressed as multiples of the median (MoM) for that gestational age. Thus, for comparability and to determine the clinical significance of findings, we transformed maternal E3 concentrations into MoM before entering into the regression model.
Approach to covariate adjustment
We sought to ensure parsimonious models and to avoid inappropriate adjustment. All models were adjusted for gestational age at blood draw for E3 assessment, infant age, and sex. Additional adjustment for covariates that have been previously associated with either maternal E3 concentrations during pregnancy or child or adult TL (ie, maternal educational level, race/ethnicity, maternal prepregnancy BMI, presence of obstetric risk factors, gestational age at birth, birth weight, and infant weight-for-length ratio) had no influence on associations, so the findings of the final model reported here omit these variables.
Regression diagnostics were performed to assess the validity of standard linear regression assumptions and diagnose potential influential points. No extreme departures from general assumptions were observed and no observations were removed from the analysis. All statistical analyses were performed using SPSS v21 (IBM), and the statistical significance level was set at α = 0.05.
Results
The median maternal E3 concentration in early gestation was 1.57 ng/ml and ranged from 0.40–5.26 ng/ml (median and range for women carrying male vs female fetuses, 1.58 [0.40–5.26] vs 1.54 [0.44–3.92] ng/ml). Expressed as MoM, the E3 values ranged between 0.25 and 3.35 MoM. These concentrations (and MoM) are consistent with those reported in previous research and clinical studies of human pregnancy (30). In bivariate analyses, maternal E3 concentration was not associated with maternal prepregnancy BMI (r = −0.01, P = .93), maternal BMI during pregnancy (r = 0.03, P = .84) or weight gain during pregnancy (r = 0.05, P = .87), obstetric risk (r = −0.05, P = .65), maternal educational level (r = −0.08, P = .47), maternal race/ethnicity (F(3,99) = 1.26, P = .29), gestational age at birth (r = 0.05, P = .65), birth weight (r = −0.001, P = .99), or infant sex (r = −0.05, P = .59).
The mean ± SEM child buccal cell TL as measured by T/S ratio was 2.05 ± 0.05 and ranged from 1.03–3.69. Race/ethnicity was not associated with child TL (P = .11). Girls had significantly longer telomeres than boys (2.18 vs 1.95 T/S ratio, P = .03), and there was a trend for TL to decrease with infant age (T/S ratio decreased by 0.096 per year, P = .07).
After accounting for the effects of gestational age at maternal blood draw during pregnancy, infant sex, and infant age, there was a significant, independent effect of maternal E3 concentrations (expressed as MoM) on infant TL (unstandardized β = 0.297, P = .001; 95% Cl, 0.121–0.473, see Figure 1 and Table 2). Specifically, a 1-MoM increase in maternal E3 concentrations during early pregnancy was associated with a 14.42% increase in infant TL.
Figure 1.
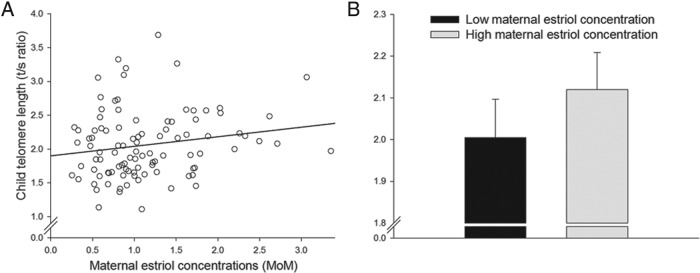
A, Scatterplot depicting the association between maternal estriol concentrations expressed as MoM and child TL (T/S ratio). B, Mean adjusted TL (T/S ratio ± SEM) for infants of mothers who fall in the lowest quartile (“low maternal estriol concentration”) vs infants of mothers in the highest estriol quartile (“high maternal estriol concentration”).
Table 2.
Fully Adjusted Regression Model Predicting Infant TL (T/S Ratio)
| Unstandardized β [95% CI] | Standardized β | Variance Inflection Factor | |
|---|---|---|---|
| Maternal E3 concentrations, MoM | 0.297a [0.121–0.473] | 0.375 | 1.328 |
| Infant sex (female .vs male) | 0.248b [0.121–0.473] | 0.244 | 1.048 |
| Infant age, mo | −0.014a [−0.023–−0.005] | −0.322 | 1.187 |
| Gestational age at E3 assessment, wk | −0.126 [−0.267–0.014] | −0.195 | 1.265 |
P ≤ 0.01.
P ≤ 0.05.
Discussion
To the best of our knowledge this finding represents the first report linking E3 concentration in early pregnancy with offspring TL. The study thus supports the concept of developmental plasticity of the telomere biology system in early life. This protective effect of E3 on infant TL in this report is consistent with the literature in adults. Because maternal E3 is an indicator of fetal E3 exposure in utero, our findings suggests the early life setting of TL may be influenced by variation in E3 exposure, possibly acting via the mechanisms previously discussed [ie, unbound (free) E3 stimulates the transcription of the gene encoding the telomerase reverse transcriptase enzyme that adds telomere repeats to chromosome ends, thereby slowing the rate of telomere attrition and reduces oxidative stress (11–14)]. Epigenetic modifications have been discussed in the context of fetal programming as a process of how developmental exposures can affect the fetal gene expression and subsequent disease risk (eg, Ref. 31). The telomere system seems to be under tight epigenetic control, and in particular methylation changes in subtelomeric loci (32) and epigenetic modulation of the core promoter region of the telomerase reverse transciptase gene that regulates telomerase activity (33) seem to be involved in regulation of telomere maintenance and TL. Thus, it is possible that intrauterine factors program the developing telomere system by producing such epigenetic alterations in subtelomeric and other regions.
Our study finding highlights two issues regarding the role of E3 in the context of human fetal development. First, it supports a new (previously unrecognized) role for E3 (ie, in the specific context of the development of the fetal telomere biology system). Moreover, this effect is apparent for E3 variation within the normal, physiological range (as opposed to at only the very low or very high E3 levels established for other outcomes). Thus, if replicated and validated in future studies, this would suggest that the threshold for the effect of E3 on infant TL is substantially lower than that for other currently established effects of E3 on pregnancy and fetal complications, further emphasizing the importance of E3 in the development of this critical biological system regulating telomere maintenance and function. Second, the magnitude of the effect of E3 on infant TL is quite considerable. From a study population range of 3.1 uE3 MoM units (0.25–3.35), each 1 unit MoM increase of maternal E3 was associated with an approximately 15% increase in infant TL.
Although TL was longer in female vs male infants and there was an effect of maternal E3 concentrations on infant TL, maternal E3 concentrations in early pregnancy were not different in women carrying a female vs male fetus. The strength of the association between maternal E3 and infant TL also was not different in girls compared with boys. We note that several large studies of maternal E3 concentrations assessed as a part of routine pregnancy screening tests (these assessments are performed at approximately the same gestational age as when we obtained our maternal samples) also have found no differences in E3 between women carrying a male vs female fetus (34, 35), whereas maternal blood and cord blood levels of E3 at birth are reported to be higher in girls compared with boys (36). This suggests that sex differences in E3 seem to emerge only later in gestation. The establishment of TL occurs in early gestation, and it is possible that the initial protective effect of E3 on telomere maintenance is similar in female and male fetuses. The higher E3 levels observed in girls in later gestation may have an additional protective effect on subsequent TL attrition, perhaps by sensitizing the system to the effects of estrogens. Interestingly, and in line with our study findings, a recent study of leukocyte TL in opposite-sex and same-sex cotwins (37) found that within same-sex twins leukocyte TL was longer in females than in males, and that the sex difference in TL was ablated in opposite-sex cotwins (the female twin's leukocyte TL was indistinguishable from that of the male twin). This finding implies that among opposite-sex twins the masculinization of the female fetus that is known to occur during intrauterine life alters female TL, thereby more generally suggesting that the observed sex difference in leukocyte TL in the population may be largely determined in utero by the intrauterine hormonal environment (37).
Some of our study design and methodological considerations warrant discussion. Infant TL was assessed in our study in buccal cell DNA at one time point. Despite some controversy in the literature regarding the correlation between TL assessed in leukocytes and buccal cells (38, 39), both buccal cell and blood/leukocyte TL have been shown to predict age-related disease outcomes in adults (38, 39). Moreover, several studies have reported effects of conditions in early life (eg, exposure to early life stress) on child buccal cell TL (40, 41), and one study (42) has found that a more stress-reactive temperament is associated with shorter buccal cell TL. Yet another recent study has reported that although adult TL varies within individuals across different tissues, TL within individuals is strongly correlated across tissues and the rates of telomere shortening within individuals are very similar across different somatic tissues (7). TL attrition occurs in somatic tissues over the life span and is particularly high in the first few years of life (43, 44). Because of variation in the age range of the infants in our study and the observed statistical trend that older infants had shorter TL all analyses were adjusted for the effects of infant age. Finally, because E3 levels are known to change over the course of gestation, we adjusted for the potential effect of gestational age at sample collection for E3 measurement.
As discussed earlier, the effect of estrogen on telomere maintenance is believed to be mediated via effects on telomerase expression and/or oxidative stress. Because measures of telomerase expression or oxidative stress were not available in the current study we were unable to perform mediational analyses to test these putative pathways. Clearly, future studies are warranted that incorporate these features and also conduct longitudinal follow up of telomere dynamics from birth into infancy, childhood and beyond.
To conclude, maternal E3 concentrations in early pregnancy (within the normal physiological range) were significantly and positively associated with infant TL. Questions remain regarding the molecular mechanism(s) underlying this effect and potential interactions with other biological and environmental processes. However, in so far as the initial setting of TL is an important determinant of subsequent telomere biology-related processes and health outcomes, the current finding represents an important step because it adds evidence to support the concept of developmental plasticity of the telomere biology system.
Acknowledgments
This work was supported in part by US PHS (National Institutes of Health) Grants RO1 HD-060628 and PO1 HD-047609 to P.D.W., and R01 HD-065825 to S.E.
Disclosure Summary: S.E., C.B., H.N.S., and P.D.W. have nothing to declare. E.S.E., J.L., and E.H.B. are cofounders of Telomere Health, Inc, a company focused on telomere measurement. Assays and all other activity for the current report are, however, unrelated to this company.
Footnotes
- BMI
- body mass index
- E3
- estriol
- MoM
- multiple of the median
- TL
- telomere length
- uE3
- unconjugated estriol.
References
- 1. Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu H, Belcher M, van der Harst P. Healthy aging and disease: Role for telomere biology? Clin Sci (Lond). 2011;120(10):427–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–5. [DOI] [PubMed] [Google Scholar]
- 4. Aviv A. Genetics of leukocyte telomere length and its role in atherosclerosis. Mutat Res. 2012;730(1–2):68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hewitt G, Jurk D, Marques FD, et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun. 2012;3:708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109(5):1743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniali L, Benetos A, Susser E, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Entringer S, Buss C, Wadhwa PD. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci Signal. 2012;5:pt12. [DOI] [PubMed] [Google Scholar]
- 9. Prescott J, Kraft P, Chasman DI, et al. Genome-wide association study of relative telomere length. PLoS One. 2011;6(5):e19635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aviv A, Shay J, Christensen K, Wright W. The longevity gender gap: Are telomeres the explanation? Sci Aging Knowledge Environ. 2005;2005(23):pe16. [DOI] [PubMed] [Google Scholar]
- 11. Calado RT. Telomeres and marrow failure. Hematology Am Soc Hematol Educ Program. 2009:338–343. [DOI] [PubMed] [Google Scholar]
- 12. Cha Y, Kwon SJ, Seol W, Park KS. Estrogen receptor-alpha mediates the effects of estradiol on telomerase activity in human mesenchymal stem cells. Mol Cells. 2008;26(5):454–8. [PubMed] [Google Scholar]
- 13. Grasselli A, Nanni S, Colussi C, et al. Estrogen receptor-alpha and endothelial nitric oxide synthase nuclear complex regulates transcription of human telomerase. Circ Res. 2008;103(1):34–42. [DOI] [PubMed] [Google Scholar]
- 14. Misiti S, Nanni S, Fontemaggi G, et al. Induction of hTERT expression and telomerase activity by estrogens in human ovary epithelium cells. Mol Cell Biol. 2000;20(11):3764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin J, Epel E, Blackburn E. Telomeres and lifestyle factors: Roles in cellular aging. Mutat Res. 2012;730(1–2):85–9. [DOI] [PubMed] [Google Scholar]
- 16. Lin J, Kroenke CH, Epel E, et al. Greater endogenous estrogen exposure is associated with longer telomeres in postmenopausal women at risk for cognitive decline. Brain Res. 2011;1379:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith R, Paul J, Maiti K, Tolosa J, Madsen G. Recent advances in understanding the endocrinology of human birth. Trends Endocrinol Metab. 2012;23(10):516–23. [DOI] [PubMed] [Google Scholar]
- 18. Troisi R, Potischman N, Roberts JM, et al. Correlation of serum hormone concentrations in maternal and umbilical cord samples. Cancer Epidemiol Biomarkers Prev. 2003;12(5):452–6. [PubMed] [Google Scholar]
- 19. Dugoff L, Society for Maternal-Fetal M. First- and second-trimester maternal serum markers for aneuploidy and adverse obstetric outcomes. Obstet Gynecol. 2010;115(5):1052–61. [DOI] [PubMed] [Google Scholar]
- 20. Huang T, Hoffman B, Meschino W, Kingdom J, Okun N. Prediction of adverse pregnancy outcomes by combinations of first and second trimester biochemistry markers used in the routine prenatal screening of Down syndrome. Prenat Diagn. 2010;30(5):471–7. [DOI] [PubMed] [Google Scholar]
- 21. Smith R, Smith JI, Shen X, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocrinol Metab. 2009;94(6):2066–74. [DOI] [PubMed] [Google Scholar]
- 22. Talge NM, Holzman C, Senagore PK, Klebanoff M, Fisher R. Biological indicators of the in-utero environment and their association with birth weight for gestational age. J Dev Orig Health Dis. 2011;2(5):280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Metcalfe A, Langlois S, Macfarlane J, Vallance H, Joseph KS. Prediction of obstetrical risk using maternal serum markers and clinical risk factors. Prenat Diagn. 2014;34(2):172–9. [DOI] [PubMed] [Google Scholar]
- 24. Wadhwa PD, Garite TJ, Porto M, et al. Placental corticotropin-releasing hormone (CRH), spontaneous preterm birth, and fetal growth restriction: A prospective investigation. Am J Obstet Gynecol. 2004;191(4):1063–9. [DOI] [PubMed] [Google Scholar]
- 25. O'Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139(5):540–5. [DOI] [PubMed] [Google Scholar]
- 26. Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Puregene DNA Purification Kit: DNA purification from 1 buccal brush. Gentra Systems; 2004. [Google Scholar]
- 28. Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin J, Epel E, Cheon J, et al. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. J Immunol Methods. 2010;352(1–2):71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Benn PA, Clive JM, Collins R. Medians for second-trimester maternal serum alpha-fetoprotein, human chorionic gonadotropin, and unconjugated estriol; differences between races or ethnic groups. Clin Chem. 1997;43(2):333–7. [PubMed] [Google Scholar]
- 31. Hanson M, Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD. Developmental plasticity and developmental origins of non-communicable disease: Theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol. 2011;106(1):272–80. [DOI] [PubMed] [Google Scholar]
- 32. Buxton JL, Suderman M, Pappas JJ, et al. Human leukocyte telomere length is associated with DNA methylation levels in multiple subtelomeric and imprinted loci. Sci Rep. 2014;4:4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Daniel M, Peek GW, Tollefsbol TO. Regulation of the human catalytic subunit of telomerase (hTERT). Gene. 2012;498(2):135–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mueller VM, Huang T, Summers AM, Winsor SH. The effect of fetal gender on the false-positive rate of Down syndrome by maternal serum screening. Prenat Diagn. 2005;25(13):1258–61. [DOI] [PubMed] [Google Scholar]
- 35. Bazzett LB, Yaron Y, O'Brien JE, et al. Fetal gender impact on multiple-marker screening results. Am J Med Genet. 1998;76(5):369–71. [PubMed] [Google Scholar]
- 36. Troisi R, Potischman N, Roberts J, et al. Associations of maternal and umbilical cord hormone concentrations with maternal, gestational and neonatal factors (United States). Cancer Causes Control. 2003;14(4):347–55. [DOI] [PubMed] [Google Scholar]
- 37. Benetos A, Dalgard C, Labat C, et al. Sex difference in leukocyte telomere length is ablated in opposite-sex co-twins. Int J Epidemiol. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Thomas P, O'Callaghan NJ, Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mech Ageing Dev. 2008;129(4):183–90. [DOI] [PubMed] [Google Scholar]
- 39. Gadalla SM, Cawthon R, Giri N, Alter BP, Savage SA. Telomere length in blood, buccal cells, and fibroblasts from patients with inherited bone marrow failure syndromes. Aging. 2010;2(11):867–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shalev I, Moffitt TE, Sugden K, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: A longitudinal study. Mol Psychiatry. 2013(5):576–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Drury SS, Theall K, Gleason MM, et al. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Mol Psychiatry. 2012;17(7):719–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kroenke CH, Epel E, Adler N, et al. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom Med. 2011;73(7):533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zeichner SL, Palumbo P, Feng Y, et al. Rapid telomere shortening in children. Blood. 1999;93(9):2824–30. [PubMed] [Google Scholar]
- 44. Frenck RW, Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95(10):5607–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


