Abstract
Context:
Anaplastic thyroid cancer (ATC) is the most lethal of all thyroid cancers and one of the most aggressive human carcinomas. In the search for effective treatment options, research toward targeted, personalized therapies is proving to be a path with great potential. As we gain a deeper understanding of the genetic (eg, BRAFV600E, PIK3CA, TP53, hTERT mutations, etc) and epigenetic (eg, histone methylation, histone de-acetylation, microRNA regulatory circuits, etc) alterations driving ATC, we are able to find targets when developing novel therapies to improve the lives of patients. Beyond development, we can look into the effectiveness of already approved targeted therapies (eg, anti-BRAFV600E selective inhibitors, tyrosine kinase inhibitors, histone deacetylase inhibitors, inhibitors of DNA methylation, etc) to potentially test in ATC after learning the molecular mechanisms that aid in tumor progression.
Design:
We performed a literature analysis in Medline through the PubMed web site for studies published between 2003 and 2014 using the following main keywords: anaplastic thyroid cancer, genetic and epigenetic alterations.
Objective:
Here, we outlined the common pathways that are altered in ATC, including the BRAFV600E/ERK1/2-MEK1/2 and PI3K-AKT pathways. We then examined the current research looking into personalized, potential targeted therapies in ATC, mentioning those that have been tentatively advanced into clinical trials and those with the potential to reach that stage. We also reviewed side effects of the current and potential targeted therapies used in patients with advanced thyroid cancer.
Conclusions:
DNA and RNA next-generation sequencing analysis will be fundamental to unraveling a precise medicine and therapy in patients with ATC. Indeed, given the deep biological heterogeneity/complexity and high histological grade of this malignancy and its tumor microenvironment, personalized therapeutic approaches possibly based on the use of combinatorial targeted therapy will provide a rational approach when finding the optimal way to improve treatments for patients with ATC.
Anaplastic thyroid cancer (ATC) is the least common but the most aggressive of all thyroid cancers, with a median survival rate of 3–5 months (1, 2). It is thought to develop from existing papillary thyroid cancer (PTC) or follicular thyroid cancer (FTC); once ATC is established, it has an extremely high proliferative rate, it can quickly invade the neck structures and metastasize to other organs, and, more importantly, it shows resistance to radioiodine treatment (1, 3–6). ATC displays a multitude of morphological patterns, commonly presenting itself with bizarre spindle, giant, and squamoid tumor cells (7). When observed either through a biopsy or tracheostomy, it is found to be a “rock hard” mass, testing positive for keratin (1). Given the severity of the disorder, understanding genetic alterations that drive tumor progression is important when determining targets for treatment.
Genetics of ATC
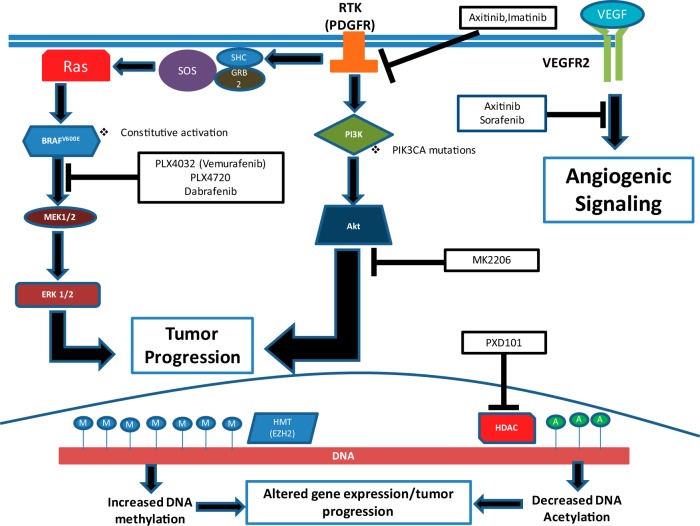
There have been a multitude of genetic alterations associated with ATC, most often causing dysfunction in the ERK1/2-MEK1/2 and PI3K-AKT signaling pathways (Figure 1) (8). A mutation that has been the focus of a lot of research is BRAFV600E. This point mutation leaves BRAF, a serine/threonine kinase involved in ERK1/2-MEK1/2 signaling, constitutively active, and it is thought to be involved with the progression of PTC to ATC and angioinvasion (6, 9–16). To cause this progression, BRAFV600E induces changes in the tumor microenvironment, promoting tumor invasion and metastasis (17, 18). The BRAFV600E mutation occurs in about 38% of ATC (13), and recent reports indicate that BRAFV600E alters ATC tumor microenvironment through extracellular matrix (ECM) protein such as thrombospondin-1 (TSP-1) and ECM receptors (ie, integrins). Thrombospondin-1 is fundamental in ECM remodeling, and it has prometastatic properties in ATC, making research focusing on its mechanisms in thyroid cancers an important endeavor (11, 19).
Figure 1.
Genetic and epigenetic alterations in human ATC are potential targets for treatment options. The BRAFV600E/ERK1/2-MEK1/2 and PI3K-AKT pathways are commonly mutated in ATC, along with epigenetic alterations on the histone proteins associated with DNA. PLX4720, PLX4032 (vemurafenib), and dabrafenib can inhibit the BRAFV600E oncoprotein. MK2206 is an AKT inhibitor, halting downstream proliferative signaling. Imatinib works on the level of receptor tyrosine kinases (RTK) such as PDGFR. Axitinib and sorafenib inhibit angiogenic signaling through VEGFR2. PXD101 inhibits HDACs, which alter gene expression to promote tumor progression.
There are a multitude of other mutations that lead to increased PI3K-AKT signaling pathway activity. PIK3CA, which encodes the p110α catalytic subunit of PI3K, has been found to be mutated in 12–23% of ATC and in 6–13% of FTC (20), whereas this mutation is rarely seen in PTC (1–3%) (10, 20, 21). Importantly, the overactivation of the PI3K-AKT pathway can be induced by PTEN inactivation, including PTEN promoter methylation, deletion, or point mutations that occur in 10–20% of ATC (10). Furthermore, RAS mutations seem to preferentially activate the PI3K-AKT pathway and were found to be mutated in ATC 17% (NRAS mutations) and 6% (HRAS) of the time (13).
In 11% of ATC patients, a mutation in anaplastic lymphoma kinase (ALK), a protein involved with the activation of both the ERK1/2-MEK1/2 and PI3K-AKT pathways, is observed. Specifically, two point mutations have been identified (ALKL1198F and ALKG1201E) that result in increased tyrosine kinase activity and subsequent overactivation of the aforementioned pathways (22). On the opposite end of the spectrum, a negative modulator of these pathways, RASAL1, has been found to be mutated in about 16% of ATC, and the mutations lead to its subsequent inactivation (8). This gene is also found to be inactivated by epigenetic processes. When used in vitro and in vivo, RASAL1 inhibited thyroid cancer growth. Importantly, this was observed in human ATC cells (8). Specifically, the levels of phosphorylated AKT and ERK were decreased, indicating that RASAL1 has a direct effect in the pathways mentioned above (8). The report of this inactivation is significant because it helps bring validity to the hypothesis that RASAL1 is a true tumor suppressor gene in thyroid cancers (8).
The TP53 gene is another gene commonly mutated/inactivated in a variety of advanced human cancers and strongly involved in the ATC pathogenesis. It is mutated (12–83%) in ATC and rarely in well-differentiated thyroid carcinomas (eg, PTC), and the protein is also aberrantly overexpressed in ATC (20), causing inactivation of apoptosis and cell cycle progression. Importantly, a recent development in our understanding of ATC genetics is the mutations of the human telomerase reverse transcriptase (hTERT) promoter. This gene encodes for the catalytic domain of telomerase (23), and there are two different mutations that have been reported: C228T and C250T. About 33–50% of ATC harbor the C228T mutation in their hTERT promoter, and this is believed to result in an increase in telomerase activity (24), suggesting that it can play a role in the aggressiveness of thyroid carcinomas (24–28). The C228T mutation has been found to be associated with BRAFV600E (29), and coexistence of these two genetic markers is a way to define the most aggressive subgroup of PTC (27–29). In PTC, the rate of recurrence is eight times greater when harboring both of these mutations, as compared to patients who lack both mutations (28). Also, the co-occurrence of hTERT C228T mutation and BRAFV600E mutation confers a significant PTC aggressiveness and worst clinicopathological outcome (28). The synergy between the hTERT C228T and BRAFV600E mutations may cause overexpression of an overactive hTERT, which by lengthening telomeres helps thyroid cancer cells evade apoptosis and promote cell proliferation through dysfunctional ERK1/2-MEK1/2 signaling. Given the correlation with tumor aggressiveness observed in PTC, the findings with this mutation coexistence of hTERT and BRAF bring about the possibility that the BRAFV600E mutation with hTERT promoter mutations could be a genetic mechanism that contributes in the tumor progression from PTC to ATC.
Finally, it has also been recently described that thioredoxin-interacting protein (TXNIP) can play a role as a tumor suppressor in thyroid cancer cells. The low expression level of TXNIP in ATC is distinct when compared to differentiated thyroid cancers because they express high endogenous levels of the protein (30). Its down-regulation could, therefore, be important in the transition from differentiated thyroid cancer to ATC (30). The difference in TXNIP expression is because of the tumor suppressor's negative regulator peroxisome proliferator-activated receptor-γ (PPAR-γ). High PPAR-γ levels are observed in ATC, whereas low expression is seen in differentiated thyroid cancer (30). By contrast, the forkhead box protein M1, a member of the forkhead box family of transcription factors, has been recently identified as a novel oncogene involved in the control of ATC cell proliferation, invasion, and metastasis (31). These discoveries in ATC represent an important achievement that will improve and personalize therapy in patients with this type of aggressive malignancy.
Overall, understanding the genetic alterations of ATC is important when looking for targeted therapies that will be effective in patients who harbor this aggressive malignancy. Insights to this subject will enable us to accurately target dysfunctional signaling pathways, which could lead to improved clinical prognoses for patients.
Epigenetics and MicroRNAs (miRNAs) in ATC
Examination beyond genomic DNA alterations shows us that there are epigenetic factors that contribute to tumor progression in ATC. Enhancer of Zeste homolog 2 (EZH2) is a histone lysine-methyltransferase and is overexpressed in ATC (32). EZH2 is a member of the polycomb family of proteins, which in general are essential for the regulation of cell proliferation and differentiation. When EZH2 is overexpressed, histone methylation is altered, silencing the PAX8 gene and leading to an aggressive phenotype and poor clinical outcome (32, 33). Increased histone methylation (Figure 1) has been found to alter the expression of other genes in ATC cell lines, including p16INK4A, DAPK, UCHL1, MGMT, TSHR, PTEN, and MAGE-A4 (20, 32, 34, 35). Many of the genes mentioned above play roles in tumor suppression, apoptosis, cell cycle regulation, and DNA repair, so with aberrant methylation at these sites, cells lose proper regulation in a multitude of areas (20, 32). Overmethylation of the TSHR gene leaves the cell incapable of concentrating iodine, rendering it resistant to radioiodide therapy and thus making the malignancy more difficult to treat (32). Additionally, histone deacetylation plays a role in ATC pathogenesis. Most ATC tumors show overexpression of histone deacetylases (HDACs), and less acetylation of histones leads to an altered expression of proteins controlling the cell cycle and proliferation (Figure 1) (36). Specifically, an overexpression of HDACs contributes to the dysregulation of both the ERK1/2-MEK1/2 and PI3K-AKT pathways (36), suggesting how these important pathways are not only altered by the aforementioned genetic mutations.
Similar to histone modifications, miRNA molecules also have the capability to alter the gene expression of ATC cells, thus making them worthy of discussion when looking into targeted therapies. miRNAs are a group of small (19–25 nucleotides) RNA molecules that can alter gene expression in a post-transcriptional manner by eliciting regulatory circuits. They function as negative regulators of the expression of protein-encoding genes involved in major processes such as development, apoptosis, cell proliferation, immune response, and hematopoiesis (32). With such a biological role, improper regulation of miRNAs can contribute to tumor progression, and this has been observed in ATC. Specifically, there are two families of miRNAs that contribute to a tumor's invasiveness via down-regulation (miR-200 and miR-30) (32, 37, 38) or up-regulation (miR-20a) (39). For example, miR-30d, a negative regulator of beclin-1, shows reduced expression in some cases of ATC, contributing to the tumor cell's inability to undergo autophagy. A down-regulation of miR-30d can also indicate insensitivity to cisplatin, a platinum-based chemotherapeutic agent that induces apoptosis in tumor cells (40).
Collectively, studies of miRNAs in thyroid tumorigenesis hold promise to improve our understanding of the biology of ATC as well as making progress in clinical prognosis.
Targeted Therapy Against Human ATC
There have been many different targeted therapies used in ATC, some not showing any success but others demonstrating a significant amount of potential. Sorafenib, a broad spectrum kinase inhibitor (multi-tyrosine kinase inhibitor [TKI]), including vascular endothelial growth factor receptor 2 (VEGFR2) inhibition (Figure 1), was recently approved by the US Food and Drug Administration (FDA) for radioiodine-resistant metastatic differentiated thyroid cancer (41). However, it did not show a significant therapeutic effect in patients with ATC, leading to additional side effects that include cardiovascular toxicity and dyspnea (Table 1) (42, 43). In particular, patients with PTC or FTC treated with sorafenib in phase II clinical trials showed either stable disease or partial responses (44, 45), whereas patients with ATC showed rapid clinical progressive disease after drug administration, making it less useful for this malignancy (44, 45). Axitinib (Figure 1), a VEGFR2 inhibitor (46), showed therapeutic efficacy only in one of two patients with ATC (42). Pazopanib, another TKI, similarly to sorafenib, also failed to significantly improve the clinical outcome of patients with ATC (42, 47). It should be noted that some of these TKIs have shown efficacy in other thyroid malignancies (eg, PTC and FTC), suggesting that their effectiveness could be limited to some histological subtypes of thyroid cancer and, more importantly, could be related to their molecular profile (ie, genetic and epigenetic alterations) (42, 48), likely because in differentiated thyroid cancers we do not observe the vast complexity of genetic and epigenetic alterations witnessed in ATC. Also, one factor that might be a reason for these TKIs to show little clinical significance in ATC is the inability to administer doses at high enough concentrations (20). Dosing of these drugs, in general, is something that needs to be worked out because different plasma levels of administered TKIs are seen among patients who received the same doses (42). With such a complex pathogenesis, many signaling pathways lose proper regulation, giving the tumor the ability to gain drug resistance. To help mitigate the lack of TKI responses in ATC, future preclinical work can test combinational therapies as a way to target many of the deregulated pathways at once, giving greater odds of responsiveness. That being said, detailed descriptions of how such drug resistance comes about are yet to be revealed. Research exploring this could give insight to disease progression that leads to an ATC phenotype.
Table 1.
Common Adverse Events of Prospective Targeted Therapies in Patients With Advanced Thyroid Cancers
| Targeted Therapy (Agent) | Common Side Effects | First Author, Year (Ref) |
|---|---|---|
| Genetic | ||
| Sorafenib | Dyspnea, cardiovascular toxicity, diarrhea, weight loss, squamous cell carcinoma | Harris, 2011 (42); Cabanillas, 2010 (62) |
| Axitinib | Fatigue, weight loss, diarrhea, nausea, hypertension, mucositis, stomatitis, proteinuria | Harris, 2011 (42); Cohen, 2008 (46) |
| Vemurafenib | Fatigue, nausea, diarrhea, arthralgia, dermatitis, squamous cell carcinoma, alopecia | Swaika, 2014 (63) |
| Imatinib | Edema, fatigue, nausea, myalgia, hyperglycemia, lymphopenia, anemia, hyponatremia, abnormal liver function | Ha, 2010 (58) |
| Selumetinib | Fatigue, maculopapular rash, acneiform rash, edema, elevated liver enzymes | Ho, 2013 (59); Hayes, 2012 (64) |
| Dabrafenib | Fatigue, squamous cell carcinoma, pyrexia | Azijli, 2014 (60) |
| MK-2206 | Rash, nausea, vomiting, fatigue, hyperglycemia | Yap, 2011 (65) |
| Epigenetic | ||
| PXD101 (belinostat) | Nausea, vomiting, dyspnea, edema, hematological toxicities, flushing | McDermott, 2014 (66) |
Current research into potential targeted therapies has begun using specific mouse models that can more closely mimic the disease than previous in vivo systems. First, there are genetically engineered mouse (GEM) models that have been used in an effort to define the specific role a mutation plays in tumorigenesis (49). Specifically, GEM models have been used to help understand the role of BRAFV600E. The BRAFV600E GEM model developed tumors that appeared similar to poorly differentiated PTC (50). When this transgenic mouse was treated with BRAFV600E inhibitors (ie, PLX4720), iodine uptake was detected (50). GEM models are useful when trying to understand the biology of specific mutations, even if they have high costs for their development (49). Another preclinical strategy to validate targeted therapy can be to develop orthotopic/xenograft mouse models that allow the study of human thyroid tumors in vivo (49). Orthotopic models of ATC that have been devised also have the capability to develop metastases, both local and distant, to more closely mimic clinical presentation (49, 51). This model has been used in successful preclinical trials aiming to inhibit BRAFV600E with very specific inhibitors (12, 18, 51). PLX4720 is one of these very specific inhibitors, and in 2010 it was reported to inhibit tumor growth and metastasis in vivo using a human ATC cell line harboring the BRAFV600E and TP53 mutations (12). PLX4720 has also been shown to significantly reduce tumor size in both early and late stages (tumor regression model) of human ATC in vivo (52, 53) (Figure 1). Another treatment option that has a very similar inhibitory effect is PLX4032, also named vemurafenib. When discovered in 2010, it was shown to be effective in most metastatic melanoma patients with the BRAFV600E mutation (54). A recent study used vemurafenib in an in vivo system similar to an orthotopic model that used human ATC cells transfected with a luciferase reporter gene to make metastases more easily detectable (51). The researchers demonstrated that vemurafenib was able to slow tumor progression in their model, indicating that it could be an effective agent against ATC (51). There is yet to be any preclinical work using an orthotopic mouse model with this treatment, but such a study could help validate this as a functional therapeutic in ATC. The most promising results in humans with the inhibitor to date were in a report that indicated the treatment significantly improved (eg, by reducing lung metastasis) the outcome of an ATC patient, demonstrating potential clinical significance (55). Beyond the BRAFV600E mutation, researchers have found success with this model looking into inhibiting the Src family kinases with dasatinib, a potential targeted therapy for ATC that is already FDA approved for patients with imatinib-resistant chronic myelogenous leukemia (56). Treating both ATC and PTC cell lines with dasatinib caused a significant induction of caspase-3/-7, indicating the agent's ability to induce apoptosis (56). Mechanistically, it also inhibited ERK1/2 activation by decreasing phosphorylation (56), considered as a potential first readout of many therapies used to treat thyroid malignancies, and partially blocked ATC proliferation and growth. The results from this study provided the first evidence that Src is a central mediator of PTC growth and metastasis, indicating that Src inhibitors may have a higher therapeutic efficacy in PTC as both antitumor and antimetastatic agents (56).
Future Directions
Currently, there are some targeted therapies being tested for their effectiveness in ATC, but they have not yet reached a clinical setting. Specifically, there has been an attempt to target the epigenetic alterations that come about with ATC. PXD101 (also known as belinostat) is an HDAC inhibitor that antagonized tumor progression of ATC in vivo, giving another prospective route for improving the lives of patients (36). Clinical validity of this drug is yet to be determined, and given the primary results, clinical trials with PXD101 could be a viable option. Another possible route would be to inhibit histone-lysine methyl-transferases, and this option should be explored when trying to alter chromatin structure in ATC. Some targeted therapies have begun to be tested in ATC, but their value is yet to be accurately assessed because of a lack of data. Imatinib, a TKI that acts on c-ABL and platelet-derived growth factor receptor (PDGFR) tyrosine kinases, was reported to have antitumor activity in ATC cell lines, making it a prospective treatment option for patients with this type of cancer (57). When used in a phase 2 clinical trial, this treatment showed some antitumor activity, with two of eight patients showing partial responsiveness; however, further trials with a larger group of patients will help determine its validity as a targeted therapy in ATC (58). There are other targeted therapies that have been tested in other types of human thyroid cancers, but these are yet to be assessed in ATC; eg, the MEK1/2 inhibitor selumetinib was used in patients with differentiated thyroid cancers that were refractory to radioiodine treatment and the effectiveness of radioiodine uptake was found to increase specifically in patients who harbored RAS mutations (59). There is also a new BRAFV600E inhibitor that has been approved by the FDA, ie, dabrafenib, which has shown success in melanoma patients, inhibiting tumor progression and even acting on metastases (60). There is yet to be any research with this treatment in any type of thyroid cancer, but given its success in melanoma, work needs to be done to determine whether it is a viable option for ATC patients. Another target that should be considered in ATC is the PI3K-AKT pathway. MK2206, an AKT inhibitor, was shown to inhibit thyroid cancer cells in vitro (61). Future work could use this inhibitor in an in vivo ATC model to determine whether it has clinical potential. The search for new therapies could also look toward inhibiting overactive hTERT, another genetic alteration recently observed in ATC. Overactive hTERT correlates with tumor aggressiveness, and thus targeting it is a reasonable option for future research. For the current and potential targeted therapies mentioned above, side effects must be considered to determine the true potential in patients, and these are summarized in Table 1.
In summary, using specific targeted therapies in preclinical models against this lethal human carcinoma (ie, ATC) has shown to be a promising path for treatment in patients with ATC. Continuing translational research that will be crucial to make deep genomic analyses and optimize combinatorial targeted therapy approaches in patients with ATC will help develop a precise medicine and decrease the currently dismal mortality rates.
Acknowledgments
C.N. (Human Thyroid Cancers Preclinical and Translational Research at the Beth Israel Deaconess Medical Center/Harvard Medical School) was supported by the National Cancer Institute/National Institutes of Health (Grants NIH R21CA165039-01A1 and 1R01CA181183-01A1). C.N. was also supported by the American Thyroid Association and ThyCa:Thyroid Cancer Survivors Association Inc (USA) for Thyroid Cancer Research. C.N. was also recipient of the 2013 Guido Berlucchi “Young Investigator” research award (Italy) and Beth Israel Deaconess Medical Center (BIDMC) and Chief Academic Officer (CAO) grants.
We thank those authors whom we were not able to cite because of limited space.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ALK
- anaplastic lymphoma kinase
- ATC
- anaplastic thyroid cancer
- ECM
- extracellular matrix
- EZH2
- enhancer of Zeste homolog 2
- GEM
- genetically engineered mouse
- HDAC
- histone deacetylase
- hTERT
- human telomerase reverse transcriptase
- miRNA
- microRNA
- PDGFR
- platelet-derived growth factor receptor
- TKI
- tyrosine kinase inhibitor
- TXNIP
- thioredoxin-interacting protein
- VEGFR2
- vascular endothelial growth factor receptor 2.
References
- 1. Smallridge RC. Approach to the patient with anaplastic thyroid carcinoma. J Clin Endocrinol Metab. 2012;97:2566–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nikiforov YE. Editorial: anaplastic carcinoma of the thyroid–will aurora B light a path for treatment? J Clin Endocrinol Metab. 2005;90:1243–1245. [DOI] [PubMed] [Google Scholar]
- 3. Nucera C, Eeckhoute J, Finn S, et al. FOXA1 is a potential oncogene in anaplastic thyroid carcinoma. Clin Cancer Res. 2009;15:3680–3689. [DOI] [PubMed] [Google Scholar]
- 4. Smallridge RC, Ain KB, Asa SL, et al. American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid. 2012;22:1104–1139. [DOI] [PubMed] [Google Scholar]
- 5. Deshpande HA, Roman S, Sosa JA. New targeted therapies and other advances in the management of anaplastic thyroid cancer. Curr Opin Oncol. 2013;25:44–49. [DOI] [PubMed] [Google Scholar]
- 6. Phay JE, Ringel MD. Metastatic mechanisms in follicular cell-derived thyroid cancer. Endocr Relat Cancer. 2013;20:R307–R319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bible KC, Foote RL, Smallridge RC. Toward improved outcomes in patients with anaplastic thyroid cancer. Oncology (Williston Park). 2012;26:398, 401,, 406. [PubMed] [Google Scholar]
- 8. Liu D, Yang C, Bojdani E, Murugan AK, Xing M. Identification of RASAL1 as a major tumor suppressor gene in thyroid cancer. J Natl Cancer Inst. 2013;105:1617–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. [DOI] [PubMed] [Google Scholar]
- 10. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013;13:184–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duquette M, Sadow PM, Lawler J, Nucera C. Thrombospondin-1 silencing down-regulates integrin expression levels in human anaplastic thyroid cancer cells with BRAF(V600E): new insights in the host tissue adaptation and homeostasis of tumor microenvironment. Front Endocrinol (Lausanne). 2013;4:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nucera C, Porrello A, Antonello ZA, et al. B-Raf(V600E) and thrombospondin-1 promote thyroid cancer progression. Proc Natl Acad Sci USA. 2010;107:10649–10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ricarte-Filho JC, Ryder M, Chitale DA, et al. Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res. 2009;69:4885–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Franco AT, Malaguarnera R, Refetoff S, et al. Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA. 2011;108:1615–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shaik S, Nucera C, Inuzuka H, et al. SCF(β-TRCP) suppresses angiogenesis and thyroid cancer cell migration by promoting ubiquitination and destruction of VEGF receptor 2. J Exp Med. 2012;209:1289–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Montero-Conde C, Ruiz-Llorente S, Dominguez JM, et al. Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discov. 2013;3:520–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nucera C. Targeting thyroid cancer microenvironment: basic research and clinical applications. Front Endocrinol (Lausanne). 2013;4:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nehs MA, Nagarkatti S, Nucera C, Hodin RA, Parangi S. Thyroidectomy with neoadjuvant PLX4720 extends survival and decreases tumor burden in an orthotopic mouse model of anaplastic thyroid cancer. Surgery. 2010;148:1154–1162; discussion 1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nucera C, Lawler J, Parangi S. BRAF(V600E) and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res. 2011;71:2417–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Smallridge RC, Marlow LA, Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Smallridge RC, Copland JA. Anaplastic thyroid carcinoma: pathogenesis and emerging therapies. Clin Oncol (R Coll Radiol). 2010;22:486–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res. 2011;71:4403–4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vinagre J, Almeida A, Pópulo H, et al. Frequency of TERT promoter mutations in human cancers. Nat Commun. 2013;4:2185. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013;20:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–E1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99:E754–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu R, Xing M. Diagnostic and prognostic TERT promoter mutations in thyroid fine-needle aspiration biopsy. Endocr Relat Cancer. 2014;21:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J Clin Oncol. 2014;32:2718–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu X, Qu S, Liu R, et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J Clin Endocrinol Metab. 2014;99:E1130–E1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morrison JA, Pike LA, Sams SB, et al. Thioredoxin interacting protein (TXNIP) is a novel tumor suppressor in thyroid cancer. Mol Cancer. 2014;13:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bellelli R, Castellone MD, Garcia-Rostan G, et al. FOXM1 is a molecular determinant of the mitogenic and invasive phenotype of anaplastic thyroid carcinoma. Endocr Relat Cancer. 2012;19:695–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Catalano MG, Fortunati N, Boccuzzi G. Epigenetics modifications and therapeutic prospects in human thyroid cancer. Front Endocrinol (Lausanne). 2012;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Borbone E, Troncone G, Ferraro A, et al. Enhancer of zeste homolog 2 overexpression has a role in the development of anaplastic thyroid carcinomas. J Clin Endocrinol Metab. 2011;96:1029–1038. [DOI] [PubMed] [Google Scholar]
- 34. Hou P, Ji M, Xing M. Association of PTEN gene methylation with genetic alterations in the phosphatidylinositol 3-kinase/AKT signaling pathway in thyroid tumors. Cancer. 2008;113:2440–2447. [DOI] [PubMed] [Google Scholar]
- 35. Gunda V, Cogdill AP, Bernasconi MJ, Wargo JA, Parangi S. Potential role of 5-aza-2′-deoxycytidine induced MAGE-A4 expression in immunotherapy for anaplastic thyroid cancer. Surgery. 2013;154:1456–1462; discussion 1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin SF, Lin JD, Chou TC, Huang YY, Wong RJ. Utility of a histone deacetylase inhibitor (PXD101) for thyroid cancer treatment. PLoS One. 2013;8:e77684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Braun J, Hoang-Vu C, Dralle H, Hüttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. [DOI] [PubMed] [Google Scholar]
- 38. Zhang Z, Liu ZB, Ren WM, Ye XG, Zhang YY. The miR-200 family regulates the epithelial-mesenchymal transition induced by EGF/EGFR in anaplastic thyroid cancer cells. Int J Mol Med. 2012;30:856–862. [DOI] [PubMed] [Google Scholar]
- 39. Xiong Y, Zhang L, Kebebew E. MiR-20a is upregulated in anaplastic thyroid cancer and targets LIMK1. PLoS One. 2014;9:e96103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Yang WQ, Zhu H, et al. Regulation of autophagy by miR-30d impacts sensitivity of anaplastic thyroid carcinoma to cisplatin. Biochem Pharmacol. 2014;87:562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thomas L, Lai SY, Dong W, et al. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist. 2014;19:251–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Harris PJ, Bible KC. Emerging therapeutics for advanced thyroid malignancies: rationale and targeted approaches. Expert Opin Investig Drugs. 2011;20:1357–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cohen EE, Rosen LS, Vokes EE, et al. Axitinib is an active treatment for all histologic subtypes of advanced thyroid cancer: results from a phase II study. J Clin Oncol. 2008;26:4708–4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bible KC, Suman VJ, Menefee ME, et al. A multiinstitutional phase 2 trial of pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin Endocrinol Metab. 2012;97:3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Guerra A, Di Crescenzo V, Garzi A, et al. Genetic mutations in the treatment of anaplastic thyroid cancer: a systematic review. BMC Surg. 2013;13(suppl 2):S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Antonello ZA, Nucera C. Orthotopic mouse models for the preclinical and translational study of targeted therapies against metastatic human thyroid carcinoma with BRAFV600E or wild-type BRAF [published online December 23, 2013]. Oncogene. doi: 10.1038/onc.2013.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chakravarty D, Santos E, Ryder M, et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J Clin Invest. 2011;121:4700–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang L, Gaskins K, Yu Z, Xiong Y, Merino MJ, Kebebew E. An in vivo mouse model of metastatic human thyroid cancer. Thyroid. 2014;24:695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nucera C, Nehs MA, Nagarkatti SS, et al. Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist. 2011;16:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nehs MA, Nucera C, Nagarkatti SS, et al. Late intervention with anti-BRAF(V600E) therapy induces tumor regression in an orthotopic mouse model of human anaplastic thyroid cancer. Endocrinology. 2012;153:985–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosove MH, Peddi PF, Glaspy JA. BRAF V600E inhibition in anaplastic thyroid cancer. N Engl J Med. 2013;368:684–685. [DOI] [PubMed] [Google Scholar]
- 56. Chan CM, Jing X, Pike LA, et al. Targeted inhibition of Src kinase with dasatinib blocks thyroid cancer growth and metastasis. Clin Cancer Res. 2012;18:3580–3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kurebayashi J, Okubo S, Yamamoto Y, et al. Additive antitumor effects of gefitinib and imatinib on anaplastic thyroid cancer cells. Cancer Chemother Pharmacol. 2006;58:460–470. [DOI] [PubMed] [Google Scholar]
- 58. Ha HT, Lee JS, Urba S, et al. A phase II study of imatinib in patients with advanced anaplastic thyroid cancer. Thyroid. 2010;20:975–980. [DOI] [PubMed] [Google Scholar]
- 59. Ho AL, Grewal RK, Leboeuf R, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med. 2013;368:623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Azijli K, Stelloo E, Peters GJ, VAN DEN Eertwegh AJ. New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res. 2014;34:1493–1505. [PubMed] [Google Scholar]
- 61. Liu R, Liu D, Trink E, Bojdani E, Ning G, Xing M. The Akt-specific inhibitor MK2206 selectively inhibits thyroid cancer cells harboring mutations that can activate the PI3K/Akt pathway. J Clin Endocrinol Metab. 2011;96:E577–E585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cabanillas ME, Waguespack SG, Bronstein Y, et al. Treatment with tyrosine kinase inhibitors for patients with differentiated thyroid cancer: the M. D. Anderson experience. J Clin Endocrinol Metab. 2010;95:2588–2595. [DOI] [PubMed] [Google Scholar]
- 63. Swaika A, Crozier JA, Joseph RW. Vemurafenib: an evidence-based review of its clinical utility in the treatment of metastatic melanoma. Drug Des Devel Ther. 2014;8:775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hayes DN, Lucas AS, Tanvetyanon T, et al. Phase II efficacy and pharmacogenomic study of selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res. 2012;18:2056–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yap TA, Yan L, Patnaik A, et al. First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors. J Clin Oncol. 2011;29:4688–4695. [DOI] [PubMed] [Google Scholar]
- 66. McDermott J, Jimeno A. Belinostat for the treatment of peripheral T-cell lymphomas. Drugs Today (Barc). 2014;50(5):337–345. [DOI] [PubMed] [Google Scholar]