Abstract
Context:
Pituitary stalk interruption syndrome (PSIS) is a rare, congenital anomaly of the pituitary gland characterized by pituitary gland insufficiency, thin or discontinuous pituitary stalk, anterior pituitary hypoplasia, and ectopic positioning of the posterior pituitary gland (neurohypophysis). The clinical presentation of patients with PSIS varies from isolated growth hormone (GH) deficiency to combined pituitary insufficiency and accompanying extrapituitary findings. Mutations in HESX1, LHX4, OTX2, SOX3, and PROKR2 have been associated with PSIS in less than 5% of cases; thus, the underlying genetic etiology for the vast majority of cases remains to be determined.
Objective:
We applied whole-exome sequencing (WES) to a consanguineous family with two affected siblings who have pituitary gland insufficiency and radiographic findings of hypoplastic (thin) pituitary gland, empty sella, ectopic neurohypophysis, and interrupted pitiutary stalk—characteristic clinical diagnostic findings of PSIS.
Design and Participants:
WES was applied to two affected and one unaffected siblings.
Results:
WES of two affected and one unaffected sibling revealed a unique homozygous missense mutation in GPR161, which encodes the orphan G protein–coupled receptor 161, a protein responsible for transducing extracellular signals across the plasma membrane into the cell.
Conclusion:
Mutations of GPR161 may be implicated as a potential novel cause of PSIS.
Pituitary stalk interruption syndrome (PSIS, ORPHA95496) is a congenital defect of the pituitary gland mainly characterized by the triad of a very thin/interrupted pituitary stalk, an ectopic (or absent) posterior pituitary gland, and hypoplasia or aplasia of the anterior pituitary gland visible on magnetic resonance imaging (MRI) (1, 2). Patients with PSIS may present with a heterogeneous clinical picture resulting from either isolated or a combination of hypothalamic-pituitary hormone deficiencies. In severe cases, it may present during the neonatal period with hypoglycemia, congenital genitourinary malformations such as micropenis, and cryptorchidism, all of which are suggestive of hypothalamic-pituitary deficiency.
Due to the high frequency of associated perinatal events such as low Apgar scores, trauma at delivery has been proposed as a potential underlying etiologic event responsible for PSIS. However, the existence of familial cases, the presence of accompanying abnormalities—especially midline defects, and eye abnormalities—all suggest that a genetic disorder involving developmental processes rather than trauma underlies at least some portion of cases. Thus far, mutations and/or single nucleotide variants (SNVs) in HESX1, LHX4, OTX2, SOX3, and PROKR2 have been associated with PSIS (1, 3–6). Furthermore, GLI2, a mutation known to cause holoprosencephaly type 9, was also shown to be associated with ectopic neurohypophysis (7). However, most genetic causes (∼95%) remain unknown.
We applied whole-exome sequencing (WES) to a family with PSIS presenting with the classical triad of PSIS MRI findings as well as growth hormone (GH) deficiency. WES analysis revealed a homozygous mutation in the GPR161 gene. Further molecular and functional studies strongly suggest that GPR161 mutation is responsible for the observed clinical phenotype in this family with recessive PSIS.
Materials and Methods
Patients
Two female siblings with short stature and PSIS were referred to Duzce University Hospital, Turkey. Informed consent from all participants was obtained prior to their participation in this study.
WES and haplotype block analysis
We applied WES to both affected siblings and one healthy sister at Baylor College of Medicine Human Genome Sequencing Center through the Baylor-Hopkins Center for Mendelian Genomics research initiative. During the analyses of candidate variants/mutations, we used external publicly available databases such as the 1000 Genomes Project (http://www.1000genomes.org) and other large-scale exome-sequencing projects including the Exome variant server, NHLBI GO Exome Sequencing Project (ESP), Seattle, Washington (http://evs.gs.washington.edu/EVS/), our “in-house-generated” exomes (from ∼3000 individuals) at Baylor College of Medicine Human Genome Sequencing Center, and the Atherosclerosis Risk in Communities Study (ARIC) Database (http://drupal.cscc.unc.edu/aric/). All experiments and analyses were performed according to previously described methods (8).
Briefly, samples underwent whole-exome capture using Human Genome Sequencing Center core design (52Mb, Roche NimbleGen), followed by sequencing on the HiSeq platform (Illumina, Inc) with an ∼150× depth of coverage. Sequence data were aligned and mapped to the human genome reference sequence (hg19) using the Mercury in-house bioinformatics pipeline. Variants were called using the ATLAS (an integrative variant analysis pipeline optimized for variant discovery) and the Sequence Alignment/Map (SAMtools) suites and annotated with an in-house-developed annotation pipeline that uses Annotation of Genetic Variants (ANNOVAR) and additional tools and databases (9–11).
There are a total of eight transcripts of GPR161 in the University of California, Santa Cruz genome database. The reference transcript in the exome pipeline for our case is uc010pln.3 (RefSeq:NM_001267609.1), in which our variant is translated. To be able to examine for AOH regions surrounding GPR161, single nucleotide polymorphisms (SNPs) were analyzed using WES data to calculate a B-allele frequency revealing the absence of heterozygous loci.
PCR Confirmation
To confirm the mutation detected by exome sequencing and to perform segregation analysis, standard PCR was carried out as previously described (12), by using the GPR161F1: 5′-GAACTGGGTGATGATGACGC-3′ and GPR161R1: 5′-TCTTTTCCTGTCCCCTGGTC-3′ primer pair. Amplification products were electrophoresed on 0.7% agarose gels. PCR products were purified using ExoSAP-IT (Affymetrix) and analyzed by standard Sanger dideoxy nucleotide sequencing (DNA Sequencing Core Facility at Baylor College of Medicine, Houston, Texas).
Results
Clinical features
The proband (BAB4787), a 16-year-10-month-old Turkish female, was referred due to growth retardation and short stature. She was born at term at the end of an uneventful pregnancy with a body weight of 3300 g (50 p) (height and occipitofrontal circumference not available). By parental history, parents first admitted her to a different hospital in a different city because of short stature when she was 4 years 8 months old. Available records showed that the anthropometric measures at that age were 14 kg (5th percentile [p]) body weight and 94 cm (<3p) height (Supplemental Figure 1, A and B). She underwent GH stimulation tests because of her short stature and the maximum values detected at that time were 3.2 ng/ml (l-DOPA test) and 2 ng/ml (clonidine test). Pituitary MRI study revealed hypoplastic pituitary and an empty sella turcica. Therefore, she was diagnosed with GH deficiency and hormone replacement therapy was started (at age 5 years 3 months). Laboratory tests and clinical findings did not show any additional pituitary hormone deficiency. However, during a follow-up visit at age 7 years 3 months she was diagnosed with diabetes insipidus and desmopressin was added to the treatment. She was first admitted to Duzce University Hospital when she was 11 years 10 months of age. At admission, her height was 136.5 cm (<3 p; z-score, −2.87) and her body weight was 48.7 kg (75 p). In addition to GH deficiency and diabetes insipidus, she was diagnosed with central hypothyroidism (free T4, 0.638 ng/dl [reference interval, 0.8–1.9 ng/dl]; TSH, 3.69 ulU/ml [reference interval, 0.4–4 ulU/ml]) in November 2011. The most recent MRI study also confirmed the findings of a previous MRI and demonstrated a normal-sized sella turcica and an ectopically placed posterior pituitary lobe (neurohypophysis) consistent with PSIS (Figure 1, E and F). Currently, the patient is treated with GH, desmopressin, and levo T4. Additional clinical findings include ptosis of the left eye, congenital alopecia of the left frontal region, broad nasal root, thick ala nasi, short fifth finger, partial syndactyly of second and third toes with nail hypoplasia similar to her younger sibling (Figure 1, A, B, I–K).
Figure 1.
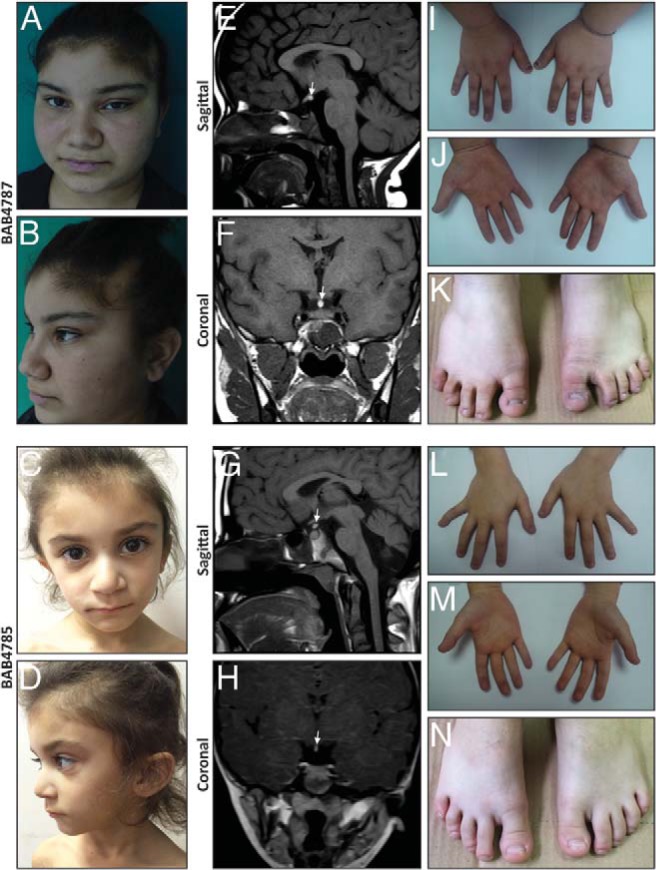
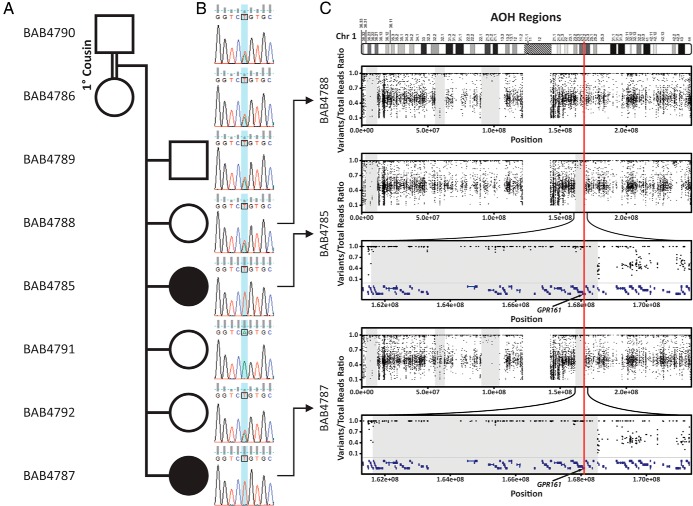
Facial images, extremity pictures, and hypophysis MRIs of the patients. A–D, Pictures of both affected siblings show hypotelorism, sparse hair on the frontal region, broad nasal root, and thick ala nasi. E–H, Hypophysis MRI reveals thin pituitary gland together with ectopic neurohypophysis and interrupted stalk. I–N, Pictures of hands and feet show partial syndactyly of second and third toes and hypoplastic nails.
The younger sibling (BAB4785), a 4-year-5-month-old female, was first referred from a local community hospital to Duzce University Hospital, Turkey, because of the history of several hypoglycemic episodes and short stature. She was born at term at the end of an uneventful pregnancy with a body weight of 2750 g (25–50 p) (length and occipitofrontal circumference measurements are not available). She first developed a hypoglycemic episode during the neonatal period and her last bout of significant hypoglycemia was 5 days before her first admission to university hospital. At the time of clinical hypoglycemia attack, blood glucose was measured as 17 mg/dl, cortisol as 42.57 ug/dl (reference interval, 6.2–19.4 mg/dl), and GH as 0.67 ng/ml. At age 4 years 5 months her height was 91.2 cm (<3 p, z-score, −3.4) (Supplemental Figure 1, A and B). She underwent further endocrinologic and radiologic investigations. Thyroid function test and pituitary hormone profile were normal except for GH. The maximum GH values after stimulation tests were 1.7 ng/ml (l-DOPA test) and 2.45 ng/ml (clonidine test). Pituitary MRI study revealed thin pituitary gland along with ectopic neurohypophysis and pituitary stalk interruption (Figure 1, G and H). Based on these results, the patient was diagnosed with GH deficiency and PSIS. She is currently receiving GH replacement therapy. Dysmorphologic evaluation revealed hypotelorism, sparse hair on the frontal region, broad nasal root, thick ala nasi, short fifth finger, partial syndactyly of second and third toes with nail hypoplasia (milder than the elder sister) (Figure 1, C, D, L–N).
Both siblings were further investigated by cerebral MRI, orbital and abdominal ultrasound studies, and chromosome analysis to detect additional visceral findings and cytogenetic aberrations; all were normal for both patients.
Molecular and in silico findings
Whole exome capture identified a homozygous c.56T>A; p.Leu19Gln; (RefSeq:NM_001267609; chr1:g.168 074 093A>T [hg19]) nonsynonymous substitution in the GPR161 gene located on 1q24.2 in both affected siblings. This variant has not been reported in the homozygous state in either our large-scale in-house-generated or public databases. Segregation studies revealed that both parents and two unaffected children had this variant in the heterozygous state and one healthy sister was wild-type; consistent with Mendelian expectations for recessive inheritance (Figure 2, A and B). Rare variants in PSIS, holoprosencephaly- and pituitary hormone deficiency–associated genes including HESX1, LHX4, OTX2, SOX3, and PROKR2, as well as GLI2, GLI3, and SHH were screened using the WES data and no deleterious variants were identified. Copy number variation call by using coding Single Nucleotide Polymorphism data did not detect any pathological copy number variation.
Figure 2.
Pedigree of the family, segregation study, and AOH regions. A, Pedigree of the family. Black filled boxes indicate affected individuals. Individual identification numbers are written in the left column starting with BAB. B, Sanger chromatographs of the entire family for segregation analyses. Affected individuals have homozygous mutation whereas unaffected individuals are heterozygous or wild type, which is consistent with Mendelian recessive expectations. C, AOH study based on data culled from WES. Gray shaded areas indicate AOH regions. Note that the GPR161 mutation is located in the ∼7.2 Mb block of AOH region in both affecteds, but not in the unaffected sibling.
The GPR161 homozygous mutation is predicted as “probably damaging” by Polyphen2. This residue, leucine, is conserved in human, mouse, rhesus, dog, and chicken. The residue change occurs in the extracellular region of the protein, which may play a role in assisting ligand binding by being a part of the receptor structure (Figure 3). According to PSIPRED (Protein Sequence Analysis Workbench, http://bioinf.cs.ucl.ac.uk/psipred/), secondary structure prediction server, the extracellular region, from the N-terminus to the mutated residue, has a coiled structure. Leucine is a hydrophobic residue with a long aliphatic side chain, whereas glutamine is a polar residue. Thus, losing the hydrophobic feature due to this mutation might disturb ligand recognition or can potentially change binding activity of the receptor.
Figure 3.
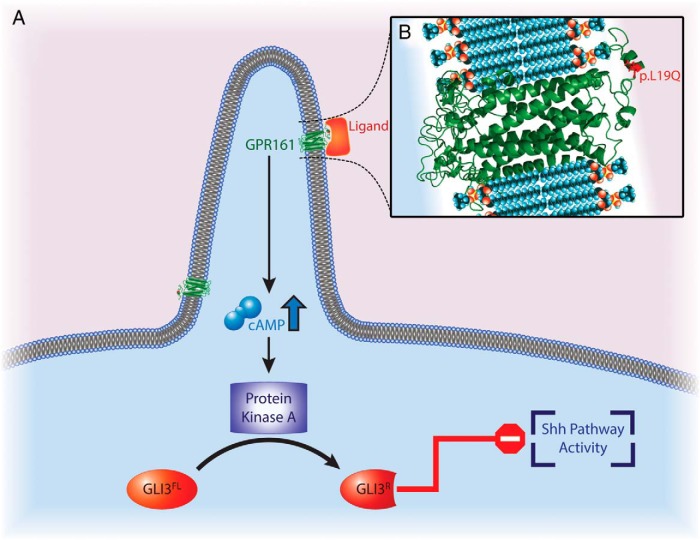
Role of GPR161 receptor in connection with GLI-Kruppel family member proteins and Shh signaling. A, GPR161 acts in the Shh signaling pathway; it is a regulator of the PKA-dependent basal repression machinery and functions by increasing cAMP levels. This eventually leads to proteolytic processing of Gli transcription factors (GLI2 and GLI3) into Gli repressor forms, which repress the Shh signaling pathway. B, p.Leu19Gln is predicted as “probably damaging” by Polyphen2; p.L19Q occurs at the extracellular region of the protein potentially assisting ligand binding. GPR161's protein structure was modeled by GPCR-I-TASSER server.
Gpr161 mRNA is expressed in pituitary gland and hypothalamus of both mouse and human (BioGPS, http://biogps.org/). These expression data are consistent with the role of Gpr161 function in the hypothalamo-pituitary region.
Given the consanguinity between the parents, we investigated the hypothesis that an absence of heterozygosity (AOH) region might encompass GPR161 and segregate as a haplotype block with the disease phenotype within the family. Haplotype block analysis based on SNP data culled from WES revealed that both affected individuals had an ∼7.2-Mb block of AOH, whereas healthy family members did not have the same AOH block, consistent with the segregation within the family (Figure 2C).
The GPR161 gene has not been associated with any disease phenotype. However, interaction of the protein encoded by this gene with the GLI2, GLI3 and Shh pathway, in which mutations can be associated with midline defects including pituitary gland abnormalities in humans, are well known.
Discussion
We identified a consanguineous family having two affected siblings with PSIS, in whom we found a homozygous missense mutation (c.56T>A; p.Leu19Gln, (RefSeq:NM_001267609.1; chr1:g.168 074 093A>T [hg19])) in GPR161. Endocrinologic and radiologic evaluations of both siblings exhibit GH insufficiency and structural abnormalities of the pituitary gland including hypoplastic (thin) pituitary gland, empty sella, ectopic neurohypophysis, and pituitary stalk interruption, which are characteristic findings of PSIS. Both siblings are receiving GH replacement therapy to compensate for GH deficiency.
Several etiological factors have been proposed for PSIS. Birth trauma and pathogenic alterations in the genes related to pituitary development and genes associated with midline defects have been suspected; however, the underlying mechanism remains elusive in most cases (3–5, 13, 14). HESX1, LHX4, OTX2, SOX3, and PROKR2 have been associated with PSIS in less than 5% of the cases (1, 3–6). Also, several GLI2- and GLI3-related disorders (Holoprosencephaly, type 9 [MIM#610829]; Pallister-Hall syndrome, type 1 [PHS1; MIM#146510], and Pallister-Hall syndrome, type 2 [PHS2, MIM# 615849]) were shown to be associated with thin pituitary gland and ectopic neurohypophysis pituitary aplasia and dysplasia (7, 15). In addition to mutations in single genes, pituitary stalk interruption was identified in patients with 2p25 duplication, 2q37 deletion, and 17q21.31 microdeletion (16, 17).
Alterations in genes encoding transcription factors that function during pituitary gland embryogenesis have been considered the most plausible explanation and, therefore, have been tested in many cohorts with congenital pituitary insufficiency. Among these, pathogenic alterations in HESX1, which is one of the early-expressed genes during pituitary development (7), were identified in patients with PSIS showing both recessive and dominant inheritance (5, 18). The only homozygous (nonsense) mutation was detected in a Turkish patient by Reynaud et al (5); this patient had PSIS on MRI together with recurrent hypoglycemia, growth retardation, micropenis, and cryptorchidism. In contrast, researchers also found heterozygous variants in this gene; however, their pathogenic role is not clear and it remains unproven whether they are disease-causing variants or not (5, 18). LHX4 plays a critical role in genesis and development of Rathke's pouch (3). LHX4 alterations can lead to a diversity of phenotypes varying even in the same family, as was observed in the familial case described by Reynaud et al (5) in 2011.
Recently, Tatsi et al (1) tested holoprosencephaly-related genes (SHH, TGIF, SIX3) in 30 patients with PSIS and isolated pituitary hypoplasia to explore potential causative variants based on their observation that a single incisor was found in three cases in the cohort. They found heterozygous nonsense mutation in the TGIF gene in one patient with PSIS and a single central incisor. They suggested that PSIS or isolated pituitary hypoplasia constitute mild forms of an expanded holoprosencephaly spectrum. This hypothesis correlates with the data of Davis et al 2010 (7), in which they showed mutations in GLI2, which is known to cause holoprosencephaly type 9 (MIM#610829), can be associated with morphological aberrations of the pituitary gland in addition to holoprosencephaly, cleft lip, central incisor, and polydactyly.
G protein–coupled receptors (GPCRs) play a crucial regulatory role in the developing embryo participating in nearly all essential processes beginning from the maturation of oocyte and continuing through gastrulation and organogenesis (19–27). GPR161 is an orphan member of this receptor family and was first described in a vacuolated lens mutant mouse presenting with congenital cataracts and neural tube defects. Expression studies in this mouse model suggested that the gpr161 signaling regulates the pathway crucial for neural fold apposition and fusion (25). Expression studies in zebrafish documented an important role for gpr161 throughout embryonic development beginning in the early stages (26). GPCRs are located in ciliary organelles, which are “sensory antenna” of many types of cells. Receptors located within the membrane of this organelle are organizing signaling receptors which are crucial for sensation, as seen in olfactory neuronal cilia. The primary cilia also play fundamental roles in the developing nervous system during normal embryogenesis, through orchestrated pattern of signaling processes.
G protein–coupled receptor activation has been implicated in pituitary hyperplasia and GH excess in two clinical conditions: McCune Albright Syndrome (MIM#174800) via GNAS1 activation and in Carney Complex (MIM#160980) via mutations in PRKAR1A. GNAS1 (guanine nucleotide-binding protein, α-stimulating activity polypeptide 1) couples with GPCRs and leads to increased cAMP levels, whereas PRKAR1A is a key component of type 1 protein kinase (PKA), which mediates cAMP in mammals (28, 29). Therefore, the activation of both are associated with a down-regulated Shh pathway. These two examples, at least, suggest the existence of a close relation between GPCRs' function and pituitary development.
GPR161 is a key negative regulator of Shh signaling (Figure 3) (27). Mukhopadhyay et al (27) recently demonstrated that GPR161 is gathered to primary cilia by the joint work of TULP3 (tubby family protein Tulp3) and the IFT-A complex (a member of intraflagellar transport involved in retrograde transport and protein trafficking to the cilia). The role of GPR161 in Shh signaling pathway seems to be as a regulator of the PKA-dependent basal repression machinery, by increasing cAMP levels, which eventually leads to proteolytic processing of Gli transcription factors (GLI2 and GLI3) into Gli repressor forms and represses Shh signaling (Figure 3) (30, 31). Moreover, Mukhopadhyay et al (27) demonstrated, in Gpr161 knockout mouse embryos, elevated levels of Shh pathway activity, which was previously shown to lead increased transcription of Gli1, Ptch1, and Hhip1, as well as to down-regulation of Gli3 RNA expression (27, 31, 32).
Interestingly, mutations in GLI2, GLI3, and SHH have been documented to be associated with pituitary aplasia/dysplasia and midline defect disorders or holoprosencephaly spectrum phenotypes (Table 1) (Holoprosencephaly, type 9 [MIM#610829]; PHS2 [MIM# 615849]; PHS1 [MIM#146510]; hypothalamic hamartomas [MIM#241800]; holoprosencephaly type 3 [MIM# 142945], and single-median maxillary central incisor [MIM#147250)]). In addition, PSIS is now considered in the spectrum of midline defects (1). Nonsynonymous mutation Leu19Gln, which is predicted as “probably damaging” by Polyphen2, occurs at the extracellular region of the protein potentially assisting ligand binding. In this sense, we speculate that this homozygous mutation harbored by both affected siblings disrupted the balance between Shh pathway activity and expression of Gli transcription factors, probably by affecting the ligand-receptor (GPR161) interaction (Figure 3).
Table 1.
Clinical Features of Our Patients and Comparison with a Group of GLI2-, GLI3-, and SHH-Related Disorders
| Gene |
GLI2 |
GLI3 |
SHH |
GPR161 | |||
|---|---|---|---|---|---|---|---|
| Disorder/Syndrome | HPE9 | PHS2 | PHS1 | HH | HPE3 | SMMCI | |
| Clinical feature | |||||||
| Broad nasal root | + | ||||||
| Hypopituitarism | + | + | + | + | − | + | + |
| Hypotelorism | + | + | − | − | + | + | + |
| Intellectual disability | + | + | − | − | − | − | + |
| Nail hypoplasia | − | − | + | − | − | − | + |
| Partial alopecia/sparse hair on the frontal region | − | − | − | − | − | − | + |
| Pituitary gland abnormality (hypoplasia, dysplasia, PSIS) | + | + | + | − | − | − | + |
| Short fifth finger | − | − | − | − | − | − | + |
| Syndactyly | − | − | + | − | − | − | + |
| Thick alae nasi | − | − | − | − | − | − | + |
Abbreviations: HPE9, holoprosencephaly 9; PHS1, Pollister-Hall syndrome, type 1; PHS2, Pollister-Hall syndrome, type 2; HH, Hypothalamic Hamartomas; HPE3, holoprosencephaly 3; SMMC1: Single median maxillary central incisor.
Several experiments were performed to assay the potential functional consequences of this GPR161 mutation. We tested its effect on the stability of GPR161 proteins as well as the amount and cleavage ratio of Gli proteins by Western blot using fibroblasts from this family (father, mother, two affected children, and an unaffected sister; data not shown). Unfortunately, the specific Gpr161 antibody generated by Mukhopadhyay et al (27) does not work well in Western blot for endogenous protein (personal communication with authors) and we are not able to detect Gpr161 in fibroblasts. In addition, we also tried to detect Gli1, 2, and 3 with antibodies used by Mukhopadhyay et al (27). However, the amounts of all three proteins are extremely low in fibroblast cells. Therefore, fibroblasts may not be the ideal cells to study the effect of this mutation on Hh signaling. We also measured cAMP levels in these fibroblasts as an indicator of activity of Gpr161 with cAMP Parameter Assay Kit (R&D; KGE002B; data not shown). The cAMP level was low in all fibroblasts tested and there was no significant difference between the affected siblings and parents or the unaffected sister (P = .1019). Given that Hh signaling is not a dominant pathway in the fibroblast cells, the cAMP level may not be a proper indicator for Gpr161 activity because cAMP is the secondary messenger in multiple signaling pathways.
Based on what we know for holoprosencephaly, that it results from loss of function of Hh signaling, and given that PSIS shares some features with this disorder, we suggest that the mutation we identified causes a loss of function of Hh signaling. Because Gpr161 is reported as a negative regulator in this pathway, it is reasonable to consider this identified mutation as generating a gain of function of Gpr161. The position of the mutation in the receptor suggests that the interaction with its ligand may be affected. However, the ligand that regulates Gpr161 in Hh signaling is currently unknown. In addition, whether Hh can directly bind to Gpr161 to regulate its function is also unknown. It is likely that this mutation increases the binding between ligand (agonist) and Gpr161, leads to its overactivation, and eventually causes a loss of function of Hh signaling.
In conclusion, our findings, together with the previously given animal model, suggest GPR161 as one of the potential causative genes for PSIS. As seen in this case, rare variants in families with rare Mendelian phenotypes may provide novel insights into human biology. Additional examples of GPR161 variants and clinical PSIS cases are needed to explore the function of GPR161 and its interactions during human embryogenesis and organogenesis.
Acknowledgments
We thank all the family members and collaborators that participated in this study. We also thank Saikat Mukhopadhyay, Jonathan Eggenschwiler, and Genentech, for their kind gift of reagents that were used during the revision process of the manuscript.
This work was supported by the US National Human Genome Research Institute (NHGRI)/National Heart Lung and Blood Institute (NHLBI) Grant No. U54HG006542 to the Baylor-Hopkins Center for Mendelian Genomics.
Disclosure Summary: J.R.L. has stock ownership in 23andMe and Ion Torrent Systems and is a coinventor on multiple United States and European patents related to molecular diagnostics for inherited neuropathies, eye diseases, and bacterial genomic fingerprinting. The Department of Molecular and Human Genetics at Baylor College of Medicine derives revenue from the chromosomal microarray analysis and clinical exome sequencing offered in the Medical Genetics Laboratory (http://www.bcm.edu/geneticlabs/). E.K., R.B., D.P., W.-L.C., K.O.Y., Y.B., T.G., M.W., M.M.A., I.A., S.B., S.E., A.B., E.Y., S.N.J., D.M.M., and R.A.G. have no disclosures relevant to the article.
Footnotes
- AOH
- absence of heterozygosity
- GPCR
- G protein–coupled receptor
- MRI
- magnetic resonance imaging
- PHS1
- Pallister-Hall syndrome type 1
- PHS2
- Pallister-Hall syndrome, type 2
- PSIS
- pituitary stalk interruption syndrome
- SNP
- single nucleotide polymorphism
- WES
- whole-exome sequencing.
References
- 1. Tatsi C, Sertedaki A, Voutetakis A, et al. Pituitary stalk interruption syndrome and isolated pituitary hypoplasia may be caused by mutations in holoprosencephaly-related genes. J Clin Endocrinol Metab. 2013;98:E779–784. [DOI] [PubMed] [Google Scholar]
- 2. Pinto G, Netchine I, Sobrier ML, Brunelle F, Souberbielle JC, Brauner R. Pituitary stalk interruption syndrome: A clinical-biological-genetic assessment of its pathogenesis. J Clin Endocrinol Metab. 1997;82:3450–3454. [DOI] [PubMed] [Google Scholar]
- 3. Davis SW, Potok MA, Brinkmeier ML, et al. Genetics, gene expression and bioinformatics of the pituitary gland. Hormone Res. 2009;71 Suppl 2:101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis SW, Ellsworth BS, Peréz Millan MI, et al. Pituitary gland development and disease: From stem cell to hormone production. Curr Top Dev Biol. 2013;106:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reynaud R, Albarel F, Saveanu A, et al. Pituitary stalk interruption syndrome in 83 patients: Novel HESX1 mutation and severe hormonal prognosis in malformative forms. Eur J Endocrinol. 2011;164:457–465. [DOI] [PubMed] [Google Scholar]
- 6. Reynaud R, Jayakody SA, Monnier C, Saveanu A, Bouligand J, Guedj AM, Simonin G, Lecomte P, Barlier A, Rondard P, Martinez-Barbera JP, Guiochon-Mantel A, Brue T. PROKR2 variants in multiple hypopituitarism with pituitary stalk interruption. J Clin Endocrinol Metab. 2012;97:E1068–1073. [DOI] [PubMed] [Google Scholar]
- 7. Davis SW, Castinetti F, Carvalho LR, et al. Molecular mechanisms of pituitary organogenesis: In search of novel regulatory genes. Mol Cell Endocrinol. 2010;323:4–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bainbridge MN, Hu H, Muzny DM, et al. De novo truncating mutations in ASXL3 are associated with a novel clinical phenotype with similarities to Bohring-Opitz syndrome. Genome Med. 2013;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Challis D, Yu J, Evani US, et al. An integrative variant analysis suite for whole exome next-generation sequencing data. BMC Bioinformatics. 2012;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li H, Handsaker B, Wysoker A, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang K, Li M, Hakonarson H. : Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pehlivan D, Hullings M, Carvalho CM, et al. NIPBL rearrangements in Cornelia de Lange syndrome: Evidence for replicative mechanism and genotype-phenotype correlation. Genet Med. 2012;14:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maghnie M, Larizza D, Triulzi F, Sampaolo P, Scotti G, Severi F. Hypopituitarism and stalk agenesis: A congenital syndrome worsened by breech delivery? Hormone Res. 1991;35:104–108. [DOI] [PubMed] [Google Scholar]
- 14. Kelberman D, Dattani MT. Role of transcription factors in midline central nervous system and pituitary defects. Endocr Dev. 2009;14:67–82. [DOI] [PubMed] [Google Scholar]
- 15. Johnston JJ, Olivos-Glander I, Killoran C, et al. Molecular and clinical analyses of Greig cephalopolysyndactyly and Pallister-Hall syndromes: Robust phenotype prediction from the type and position of GLI3 mutations. Am J Hum Genet. 2005;76:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vetro A, Pagani S, Silengo M, et al. Severe growth hormone deficiency and pituitary malformation in a patient with chromosome 2p25 duplication and 2q37 deletion. Molecular cytogenetics. 2014;7:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. El Chehadeh-Djebbar S, Callier P, Masurel-Paulet A, et al. 17q21.31 microdeletion in a patient with pituitary stalk interruption syndrome. Eur J Med Genet. 2011;54:369–373. [DOI] [PubMed] [Google Scholar]
- 18. Yang Y, Guo QH, Wang BA, et al. Pituitary stalk interruption syndrome in 58 Chinese patients: Clinical features and genetic analysis. Clin Endocrinol. 2013;79:86–92. [DOI] [PubMed] [Google Scholar]
- 19. Romo X, Pastén P, Martínez S, et al. xRic-8 is a GEF for Gsalpha and participates in maintaining meiotic arrest in Xenopus laevis oocytes. J Cell Physiol. 2008;214:673–680. [DOI] [PubMed] [Google Scholar]
- 20. Fraser LR, Adeoya-Osiguwa SA, Baxendale RW. First messenger regulation of capacitation via G protein-coupled mechanisms: A tale of serendipity and discovery. Mol Hum Reprod. 2003;9:739–748. [DOI] [PubMed] [Google Scholar]
- 21. Lin F, Sepich DS, Chen S, et al. Essential roles of G{alpha}12/13 signaling in distinct cell behaviors driving zebrafish convergence and extension gastrulation movements. J Cell Biol. 2005;169:777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Griffin CT, Srinivasan Y, Zheng YW, Huang W, Coughlin SR. A role for thrombin receptor signaling in endothelial cells during embryonic development. Science. 2001;293:1666–1670. [DOI] [PubMed] [Google Scholar]
- 23. Kupperman E, An S, Osborne N, Waldron S, Stainier DY. A sphingosine-1-phosphate receptor regulates cell migration during vertebrate heart development. Nature. 2000;406:192–195. [DOI] [PubMed] [Google Scholar]
- 24. Zeng XX, Wilm TP, Sepich DS, Solnica-Krezel L. Apelin and its receptor control heart field formation during zebrafish gastrulation. Dev Cell. 2007;12:391–402. [DOI] [PubMed] [Google Scholar]
- 25. Matteson PG, Desai J, Korstanje R, et al. The orphan G protein–coupled receptor, Gpr161, encodes the vacuolated lens locus and controls neurulation and lens development. Proc Natl Acad Sci U S A. 2008;105:2088–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung T, Humbert JE, Stauffer AM, et al. The orphan G protein-coupled receptor 161 is required for left-right patterning. Dev Biol. 2008;323:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mukhopadhyay S, Wen X, Ratti N, et al. The ciliary G-protein–coupled receptor Gpr161 negatively regulates the Sonic hedgehog pathway via cAMP signaling. Cell. 2013;152:210–223. [DOI] [PubMed] [Google Scholar]
- 28. Schwindinger WF, Francomano CA, Levine MA. Identification of a mutation in the gene encoding the alpha subunit of the stimulatory G protein of adenylyl cyclase in McCune-Albright syndrome. Proc Natl Acad Sci U S A. 1992;89:5152–5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bossis I, Stratakis CA. Minireview: PRKAR1A: Normal and abnormal functions. Endocrinology. 2004;145:5452–5458. [DOI] [PubMed] [Google Scholar]
- 30. Jiang J, Struhl G. Regulation of the hedgehog and wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature. 1998;391:493–496. [DOI] [PubMed] [Google Scholar]
- 31. Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. [DOI] [PubMed] [Google Scholar]
- 32. Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103–115. [DOI] [PubMed] [Google Scholar]